Hydrogel-Assisted 3D Volumetric Hotspot for Sensitive Detection by Surface-Enhanced Raman Spectroscopy
Abstract
:1. Introduction
2. Results
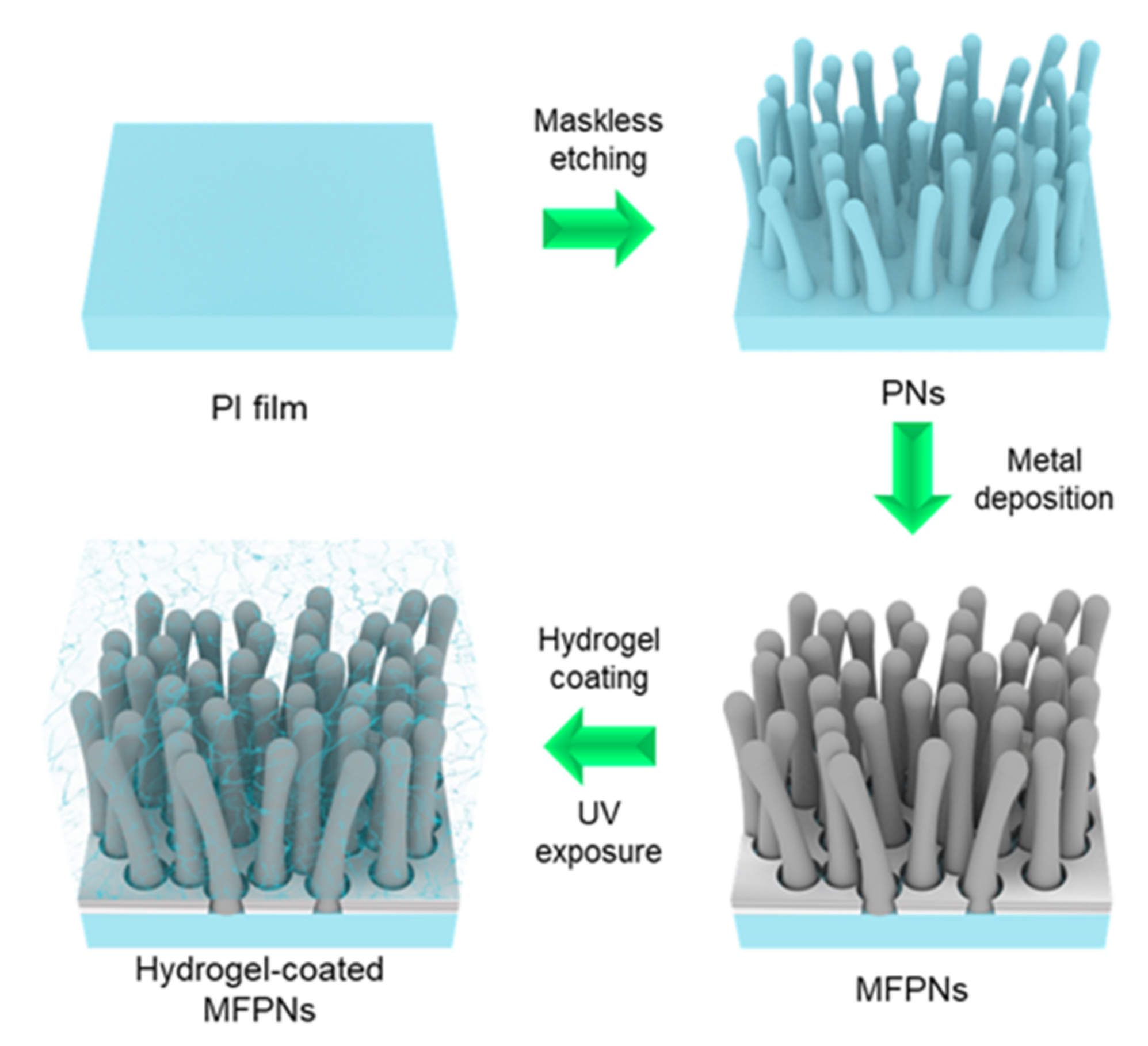
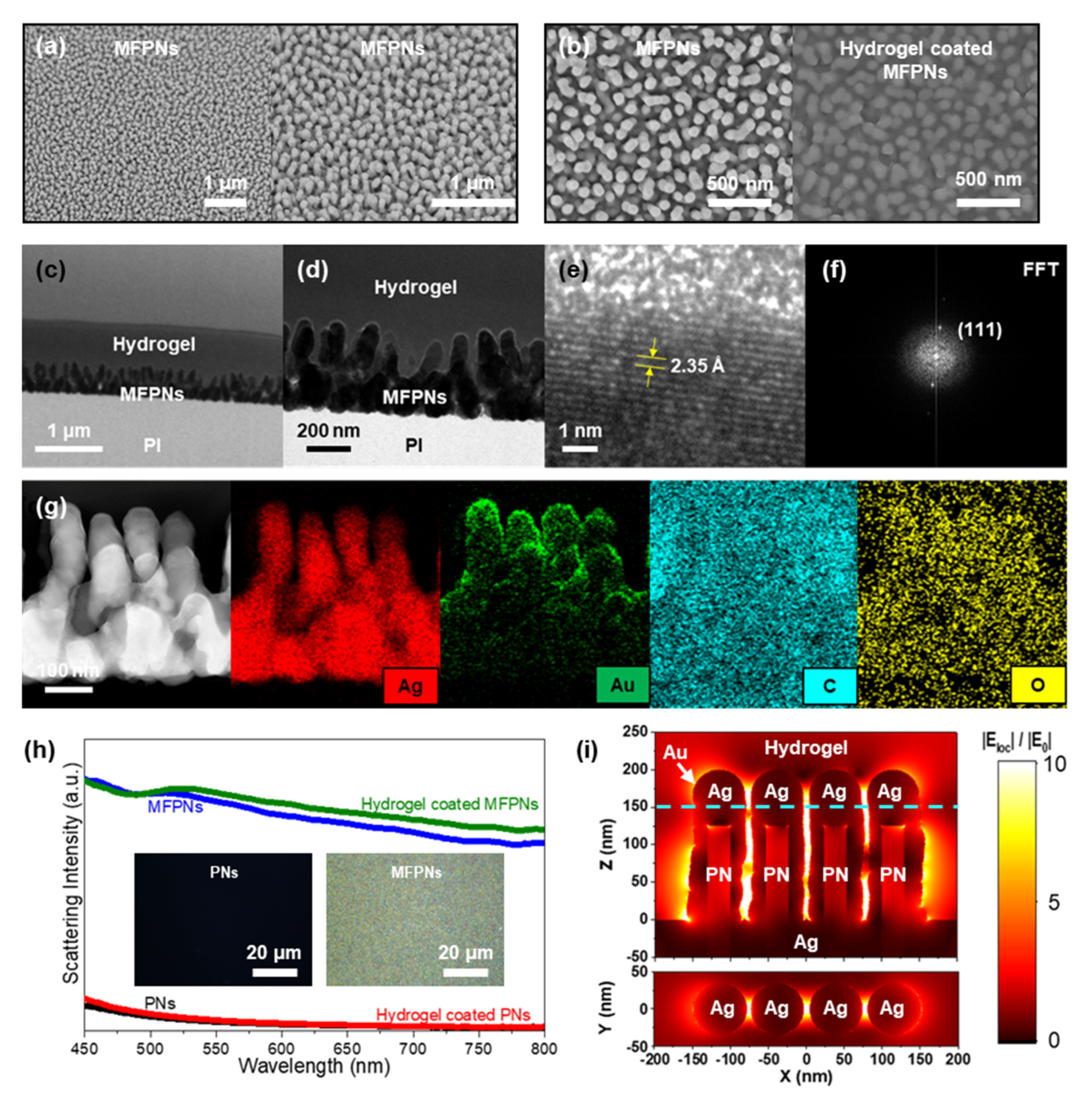
2.1. Structural and Morphological Properties of Hydrogel-Coated MFPNs
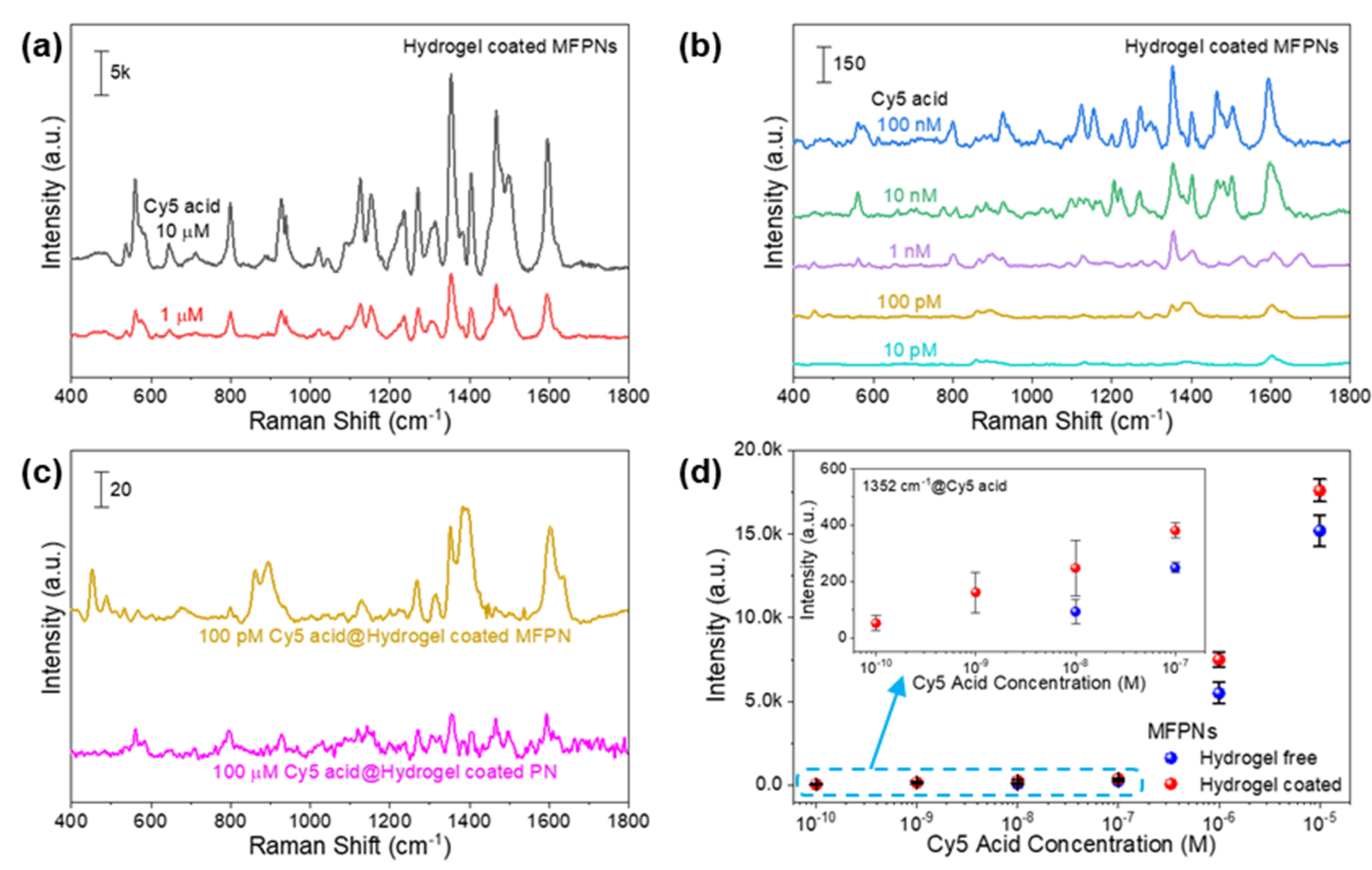
2.2. SERS Activities of Hydrogel-Coated MFPNs
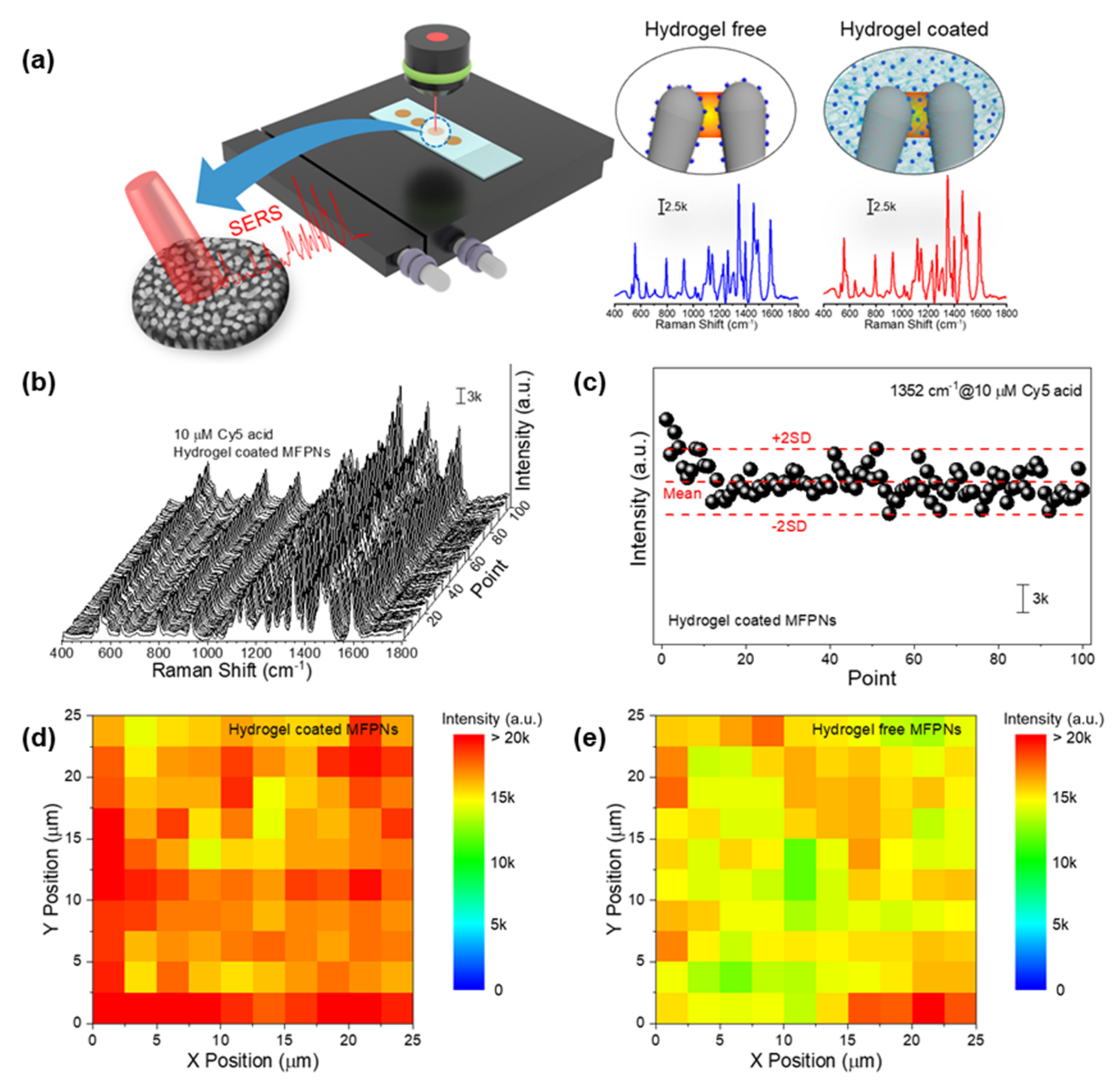
2.3. Reproducibility of Hydrogel-Coated MFPNs
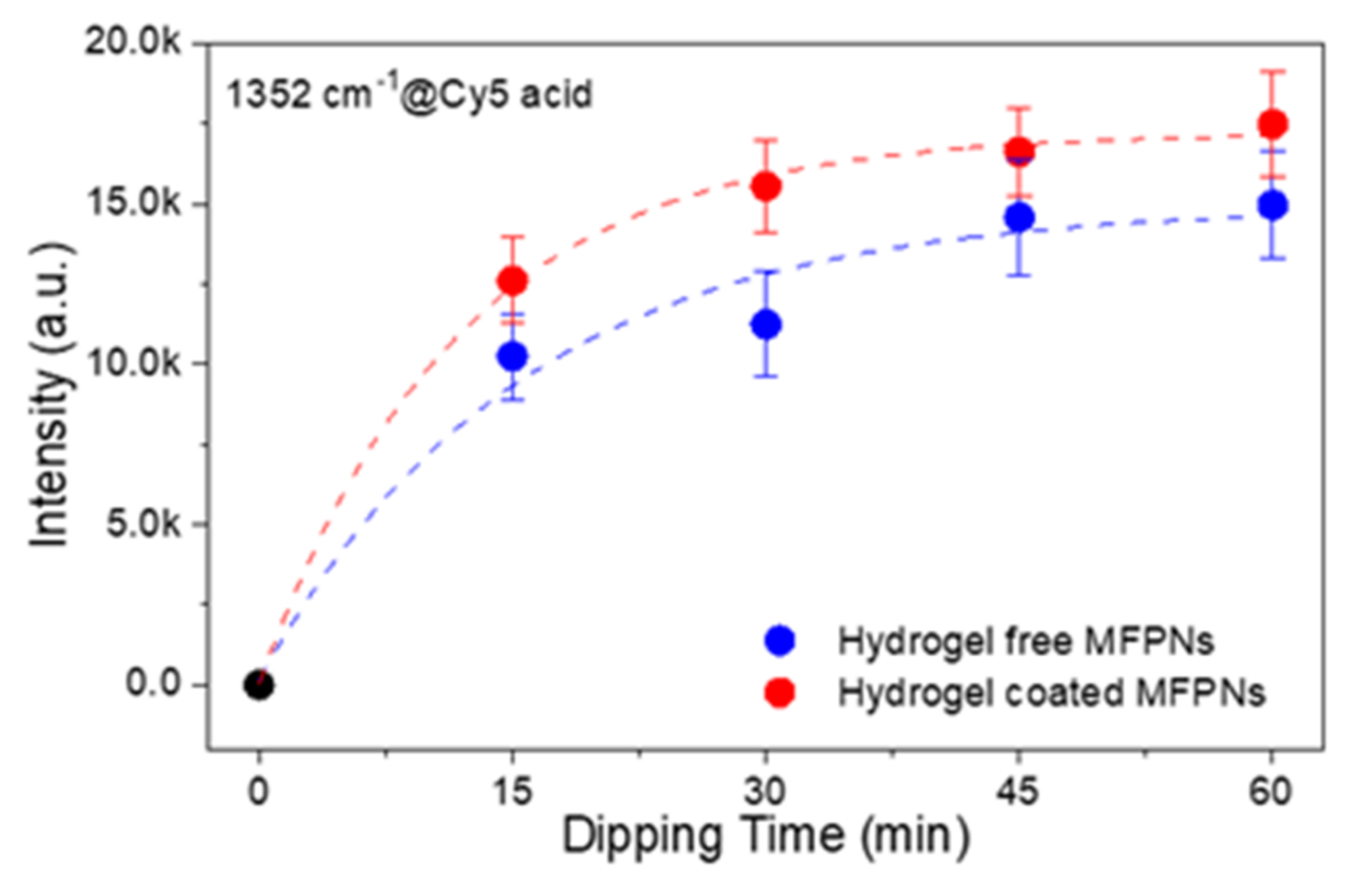
2.4. Optimization of Dipping Time for Efficient SERS Analysis
3. Discussion
4. Materials and Methods
4.1. Fabrication Procedures of Hydrogel-Coated MFPNs
4.2. Characterization
4.3. Theoretical Computation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laing, S.; Jamieson, L.E.; Faulds, K.; Graham, D. Surface-enhanced Raman spectroscopy for in vivo biosensing. Nat. Rev. Chem. 2017, 1, 0060. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, H.; Zhao, X.; Wu, J.; Muhammad, F.; Lin, S.; He, J.; Zhou, L.; Zhang, C.; Deng, Y.; et al. Surface-Enhanced Raman Scattering Active Gold Nanoparticles with Enzyme-Mimicking Activities for Measuring Glucose and Lactate in Living Tissues. ACS Nano 2017, 11, 5558–5566. [Google Scholar] [CrossRef]
- Hu, S.; Jiang, Y.; Wu, Y.; Guo, X.; Ying, Y.; Wen, Y.; Yang, H. Enzyme-Free Tandem Reaction Strategy for Surface-Enhanced Raman Scattering Detection of Glucose by Using the Composite of Au Nanoparticles and Porphyrin-Based Metal–Organic Framework. ACS Appl. Mater. Interfaces 2020, 12, 55324–55330. [Google Scholar] [CrossRef]
- Qin, X.; Si, Y.; Wu, Z.; Zhang, K.; Li, J.; Yin, Y. Alkyne/Ruthenium(II) Complex-Based Ratiometric Surface-Enhanced Raman Scattering Nanoprobe for In Vitro and Ex Vivo Tracking of Carbon Monoxide. Anal. Chem. 2020, 92, 924–931. [Google Scholar] [CrossRef]
- Rickard, J.J.S.; Di-Pietro, V.; Smith, D.J.; Davies, D.J.; Belli, A.; Oppenheimer, P.G. Rapid optofluidic detection of biomarkers for traumatic brain injury via surface-enhanced Raman spectroscopy. Nat. Biomed. Eng. 2020, 4, 610–623. [Google Scholar] [CrossRef]
- Neng, J.; Zhang, Q.; Sun, P. Application of surface-enhanced Raman spectroscopy in fast detection of toxic and harmful substances in food. Biosens. Bioelectron. 2020, 167, 112480. [Google Scholar] [CrossRef]
- Kim, W.; Lee, S.H.; Kim, J.H.; Ahn, Y.J.; Kim, Y.-H.; Yu, J.S.; Choi, S. Paper-Based Surface-Enhanced Raman Spectroscopy for Diagnosing Prenatal Diseases in Women. ACS Nano 2018, 12, 7100–7108. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.K.; Jeon, K.S.; Hwang, J.H.; Kim, H.; Kwon, S.; Suh, Y.D.; Nam, J.-M. Highly uniform and reproducible surface-enhanced Raman scattering from DNA-tailorable nanoparticles with 1-nm interior gap. Nature Nanotech. 2011, 6, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Thacker, V.V.; Herrmann, L.O.; Sigle, D.O.; Zhang, T.; Liedl, T.; Baumberg, J.J.; Keyser, U.F. DNA origami based assembly of gold nanoparticle dimers for surface-enhanced Raman scattering. Nat. Commun. 2014, 5, 3448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montjoy, D.G.; Bahng, J.H.; Eskafi, A.; Hou, H.; Kotov, N.A. Omnidispersible Hedgehog Particles with Multilayer Coatings for Multiplexed Biosensing. J. Am. Chem. Soc. 2018, 140, 7835–7845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Large, N.; Wang, H. Gold Nanoparticles with Tipped Surface Structures as Substrates for Single-Particle Surface-Enhanced Raman Spectroscopy: Concave Nanocubes, Nanotrisoctahedra, and Nanostars. ACS Appl. Mater. Interfaces 2014, 6, 17255–17267. [Google Scholar] [CrossRef]
- Tang, H.; Meng, G.; Huang, Q.; Zhang, Z.; Huang, Z.; Zhu, C. Arrays of Cone-Shaped ZnO Nanorods Decorated with Ag Nanoparticles as 3D Surface-Enhanced Raman Scattering Substrates for Rapid Detection of Trace Polychlorinated Biphenyls. Adv. Funct. Mater. 2012, 22, 218–224. [Google Scholar] [CrossRef]
- Lin, D.; Wu, Z.; Li, S.; Zhao, W.; Ma, C.; Wang, J.; Jiang, Z.; Zhong, Z.; Zheng, Y.; Yang, X. Large-Area Au-Nanoparticle-Functionalized Si Nanorod Arrays for Spatially Uniform Surface-Enhanced Raman Spectroscopy. ACS Nano 2017, 11, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Ruan, Q.; Bao, D.-L.; Luo, Y.; Huang, C.; Tang, S.; Shen, J.; Cheng, C.; Chu, P.K. Effects of Ion Energy and Density on the Plasma Etching-Induced Surface Area, Edge Electrical Field, and Multivacancies in MoSe2 Nanosheets for Enhancement of the Hydrogen Evolution Reaction. Small 2020, 16, 2001470. [Google Scholar] [CrossRef] [PubMed]
- Akinoglu, E.M.; Morfa, A.J.; Giersig, M. Understanding Anisotropic Plasma Etching of Two-Dimensional Polystyrene Opals for Advanced Materials Fabrication. Langmuir 2014, 30, 12354–12361. [Google Scholar] [CrossRef] [PubMed]
- Leem, J.W.; Yu, J.S. Broadband and wide-angle antireflection subwavelength structures of Si by inductively coupled plasma etching using dewetted nanopatterns of Au thin films as masks. Thin Solid Films 2011, 519, 3792–3797. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, J.; Lee, J.; Du, K.; Yang, E.-H.; Moon, M.-W.; Choi, C.-H. Nanotexturing of Conjugated Polymers via One-Step Maskless Oxygen Plasma Etching for Enhanced Tunable Wettability. Langmuir 2017, 33, 6885–6894. [Google Scholar] [CrossRef] [PubMed]
- Her, E.K.; Chung, H.-S.; Moon, M.-W.; Oh, K.H. An angled nano-tunnel fabricated on poly(methyl methacrylate) by a focused ion beam. Nanotechnology 2009, 20, 285301. [Google Scholar] [CrossRef]
- Dang, H.; Park, S.-G.; Wu, Y.; Choi, N.; Yang, J.-Y.; Lee, S.; Joo, S.-W.; Chen, L.; Choo, J. Reproducible and Sensitive Plasmonic Sensing Platforms Based on Au-Nanoparticle-Internalized Nanodimpled Substrates. Adv. Funct. Mater. 2021, 31, 2105703. [Google Scholar] [CrossRef]
- Yang, J.-Y.; Park, S.-G.; Jung, S.; Byeon, E.-Y.; Kim, D.-g.; Jung, H.S.; Kim, H.J.; Lee, S. SERS substrates based on self-organized dimple nanostructures on polyethylene naphthalate films produced via oxygen ion beam sputtering. Appl. Surf. Sci. 2022, 572, 151452. [Google Scholar] [CrossRef]
- Du, K.; Jiang, Y.; Liu, Y.; Wathuthanthri, I.; Choi, C.-H. Manipulation of the Superhydrophobicity of Plasma-Etched Polymer Nanostructures. Micromachines 2018, 9, 304. [Google Scholar] [CrossRef] [Green Version]
- Phan, L.T.; Yoon, S.M.; Moon, M.-W. Plasma-Based Nanostructuring of Polymers: A Review. Polymers 2017, 9, 417. [Google Scholar] [CrossRef]
- Ko, T.-J.; Oh, K.H.; Moon, M.-W. Plasma-Induced Hetero-Nanostructures on a Polymer with Selective Metal Co-Deposition. Adv. Mater. Interfaces 2015, 2, 1400431. [Google Scholar] [CrossRef]
- Kho, K.W.; Shen, Z.X.; Lei, Z.; Watt, F.; Soo, K.C.; Olivo, M. Investigation into a Surface Plasmon Related Heating Effect in Surface Enhanced Raman Spectroscopy. Anal. Chem. 2007, 79, 8870–8882. [Google Scholar] [CrossRef]
- Kang, T.; Hong, S.; Choi, Y.; Lee, L.P. The Effect of Thermal Gradients in SERS Spectroscopy. Small 2010, 6, 2649–2652. [Google Scholar] [CrossRef]
- Gouzman, I.; Grossman, E.; Verker, R.; Atar, N.; Bolker, A.; Eliaz, N. Advances in polyimide-based materials for space applications. Adv. Mater. 2019, 31, 1807738. [Google Scholar] [CrossRef]
- Ren, C.; Gao, Y.; Guan, Y.; Wang, Z.; Yang, L.; Gao, J.; Fan, H.; Liu, J. Carrier-Free Supramolecular Hydrogel Composed of Dual Drugs for Conquering Drug Resistance. ACS Appl. Mater. Interfaces 2019, 11, 33706–33715. [Google Scholar] [CrossRef] [PubMed]
- Lyu, D.; Chen, S.; Guo, W. Liposome Crosslinked Polyacrylamide/DNA Hydrogel: A Smart Controlled-Release System for Small Molecular Payloads. Small 2018, 14, 1704039. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Yan, Z.; Bauer, R.J.; Peng, J.; Schieker, M.; Nerlich, M.; Docheva, D. Functionalized thermosensitive hydrogel combined with tendon stem/progenitor cells as injectable cell delivery carrier for tendon tissue engineering. Biomed. Mater. 2018, 13, 034107. [Google Scholar] [CrossRef] [PubMed]
- Nele, V.; Wojciechowski, J.P.; Armstrong, J.P.K.; Stevens, M.M. Tailoring Gelation Mechanisms for Advanced Hydrogel Applications. Adv. Funct. Mater. 2020, 30, 2002759. [Google Scholar] [CrossRef]
- Liu, J.; Ma, Y.; Shao, J.; Zhang, S.; Chen, Y. Ultra-tall sub-wavelength gold nano pillars for high sensitive LSPR sensors. Microelectron. Eng. 2018, 196, 7–12. [Google Scholar] [CrossRef]
- Kim, S.; Choi, W.; Kim, D.J.; Jung, H.S.; Kim, D.-H.; Kim, S.-H.; Park, S.-G. Encapsulation of 3D plasmonic nanostructures with ultrathin hydrogel skin for rapid and direct detection of toxic small molecules in complex fluids. Nanoscale 2020, 12, 12942. [Google Scholar] [CrossRef]
- Herrmann, A.; Rödiger, S.; Schmidt, C.; Schierack, P.; Schedler, U. Spatial Separation of Microbeads into Detection Levels by a Bioorthogonal Porous Hydrogel for Size-Selective Analysis and Increased Multiplexity. Anal. Chem. 2019, 91, 8484–8491. [Google Scholar] [CrossRef]
- Sun, M.; Bai, R.; Yang, X.; Song, J.; Qin, M.; Suo, Z.; He, X. Hydrogel Interferometry for Ultrasensitive and Highly Selective Chemical Detection. Adv. Mater. 2018, 30, 1804916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mundo, R.D.; Troia, M.; Palumbo, F.; Trotta, M.; d’Agostino, R. Nano-texturing of Transparent Polymers with Plasma Etching: Tailoring Topography for a Low Reflectivity. Plasma Process. Polym. 2012, 9, 947–954. [Google Scholar] [CrossRef]
- Resnik, M.; Zaplotnik, R.; Mozetic, M.; Vesel, A. Comparison of SF6 and CF4 Plasma Treatment for Surface Hydrophobization of PET Polymer. Materials 2018, 11, 311. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Liu, M.; Wang, Y.; Chen, X. Structures and Energetics of Silver and Gold Nanoparticles. J. Phys. Chem. C 2011, 115, 11374–11381. [Google Scholar] [CrossRef]
- Grouchko, M.; Roitman, P.; Zhu, X.; Popov, I.; Kamyshny, A.; Su, H.; Magdassi, S. Merging of metal nanoparticles driven by selective wettability of silver nanostructures. Nat. Commun. 2014, 5, 2994. [Google Scholar] [CrossRef] [Green Version]
- Zhan, P.; Wen, T.; Wang, Z.-g.; He, Y.; Shi, J.; Wang, T.; Liu, X.; Lu, G.; Ding, B. DNA Origami Directed Assembly of Gold Bowtie Nanoantennas for Single-Molecule Surface-Enhanced Raman Scattering. Angew. Chem. Int. Ed. 2018, 57, 2846–2850. [Google Scholar] [CrossRef]
- Li, T.; Wu, X.; Liu, F.; Li, N. Analytical methods based on the light-scattering of plasmonic nanoparticles at the single particle level with dark-field microscopy imaging. Analyst 2017, 142, 248–256. [Google Scholar] [CrossRef]
- Haes, A.J.; Duyne, R.P.V. Preliminary studies and potential applications of localized surface plasmon resonance spectroscopy in medical diagnostics. Expert Rev. Mol. Diagn. 2004, 4, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Kawasaki, M.; Kasatani, K.; Katsumata, M.-a. Raman Spectra of Some Indo-, Thia- and Selena-carbocyanine Dyes. J. Raman Spectrosc. 1988, 19, 129–132. [Google Scholar] [CrossRef]
- Novara, C.; Chiadò, A.; Paccotti, N.; Catuogno, S.; Esposito, C.L.; Condorelli, G.; De Franciscis, V.; Geobaldo, F.; Rivolo, P.; Giorgis, F. SERS-active metal-dielectric nanostructures integrated in microfluidic devices for label-free quantitative detection of miRNA. Faraday Discuss. 2017, 205, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, W.; Lee, S.H.; Kim, S.H.; Lee, J.-C.; Moon, S.W.; Yu, J.S.; Choi, S. Highly Reproducible Au-Decorated ZnO Nanorod Array on a Graphite Sensor for Classification of Human Aqueous Humors. ACS Appl. Mater. Interfaces 2017, 9, 5891–5899. [Google Scholar] [CrossRef]
- Gebhardt, C.; Lehmann, M.; Reif, M.M.; Zacharias, M.; Gemmecker, G.; Cordes, T. Molecular and Spectroscopic Characterization of Green and Red Cyanine Fluorophores from the Alexa Fluor and AF Series. ChemPhysChem 2021, 22, 1566–1583. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.H.; Kim, S.; Yang, J.-Y.; Mun, C.; Lee, S.; Kim, S.-H.; Park, S.-G. Hydrogel-Assisted 3D Volumetric Hotspot for Sensitive Detection by Surface-Enhanced Raman Spectroscopy. Int. J. Mol. Sci. 2022, 23, 1004. https://doi.org/10.3390/ijms23021004
Lee SH, Kim S, Yang J-Y, Mun C, Lee S, Kim S-H, Park S-G. Hydrogel-Assisted 3D Volumetric Hotspot for Sensitive Detection by Surface-Enhanced Raman Spectroscopy. International Journal of Molecular Sciences. 2022; 23(2):1004. https://doi.org/10.3390/ijms23021004
Chicago/Turabian StyleLee, Soo Hyun, Sunho Kim, Jun-Yeong Yang, ChaeWon Mun, Seunghun Lee, Shin-Hyun Kim, and Sung-Gyu Park. 2022. "Hydrogel-Assisted 3D Volumetric Hotspot for Sensitive Detection by Surface-Enhanced Raman Spectroscopy" International Journal of Molecular Sciences 23, no. 2: 1004. https://doi.org/10.3390/ijms23021004
APA StyleLee, S. H., Kim, S., Yang, J.-Y., Mun, C., Lee, S., Kim, S.-H., & Park, S.-G. (2022). Hydrogel-Assisted 3D Volumetric Hotspot for Sensitive Detection by Surface-Enhanced Raman Spectroscopy. International Journal of Molecular Sciences, 23(2), 1004. https://doi.org/10.3390/ijms23021004






