Heme Oxygenase-1 as Therapeutic Target for Diabetic Foot Ulcers
Abstract
1. Introduction
2. Normal Wound Healing vs. Chronic Non-Healing Diabetic Wounds
3. Heme Oxygenase-1
| HO-1 Modulator | Model | Refs. | |
|---|---|---|---|
| HO-1 inducers | |||
| Oxidants | UV irradiation | Mouse | [67] |
| H2O2 | HaCat cells, Human primary melanocytes | [68,69] | |
| Menadione | Rat primary hepatocytes | [70] | |
| (superoxide donor) | |||
| Inflammatory stimuli | |||
| LPS | Mouse LPS-induced septic shock | [71] | |
| RAW264.7 cells | [72,73] | ||
| IL-6 | HEPG2 cell | [74] | |
| Metalloporphyrins | |||
| Heme | K562 cells | [75] | |
| Hemin | Diabetic rat wound healing | [26,76] | |
| CoPP | Aorta diabetic rats | [18] | |
| Pharmacological agent | |||
| Statins | HT-29 cells | [20] | |
| Aspirin | Human primary melanocytes | [69] | |
| Natural Phytochemical | |||
| Resveratrol | HaCat cells | [77] | |
| Curcumin | Human primary skin fibroblasts | [78] | |
| Quercetin | HDF cells, HEKC cells | [79] | |
| EGCG | BV2 microglia cells | [80] | |
| Chemicals | |||
| CoCl2 | HK-2 cells | [80] | |
| CdCl2 | HK-2 cells | [81] | |
| Physical stress | |||
| Heat stress | Heat stress-stimulated rat liver | [82] | |
| Oxygen levels | |||
| Hypoxia | Mice overexpressing HO-1 in lungs | [66] | |
| Hyperoxia | Rats | [83] | |
| Transcription factors | |||
| Nrf2 | Diabetic rat wound model | [84] | |
| AP-1 | Mouse model of sepsis | [73] | |
| STAT-3 | HEPG2 cell | [74] | |
| YY1 | Rat aortic smooth muscle cells | [85] | |
| HIF-1 alpha | UV irradiation in mice | [67] | |
| Gene therapy | |||
| Adenovirus mediated HO-1 transduction | Mouse, systemic administration | [86] | |
| Rat primary cardiomyocytes | [87] | ||
| HO-1 gene transfection | HEPG2 cell | [74] | |
| RBL2H3 cells | [88] | ||
| Mouse primary keratinocytes | [34] | ||
| HO-1 gene | Transgenic mice overexpressing HO-1 in keratinocytes | [34] | |
| HO-1 enzymatic inhibitors | |||
| Metalloporphyrins | SnPPIX | Diabetic rat wound healing | [26,33,76] |
| Rat VSMC, RAW264.7 cells | |||
| ZnPPIX | Rat VSMC, RAW264.7 cells | [33] | |
| HO-1 repressor | |||
| Transcription factor | Bach-1 | NIH/3T3 cells, murine embryonic fibroblasts and murine erythroleukemia cells | [89] |
4. HO-1 in Wound Healing
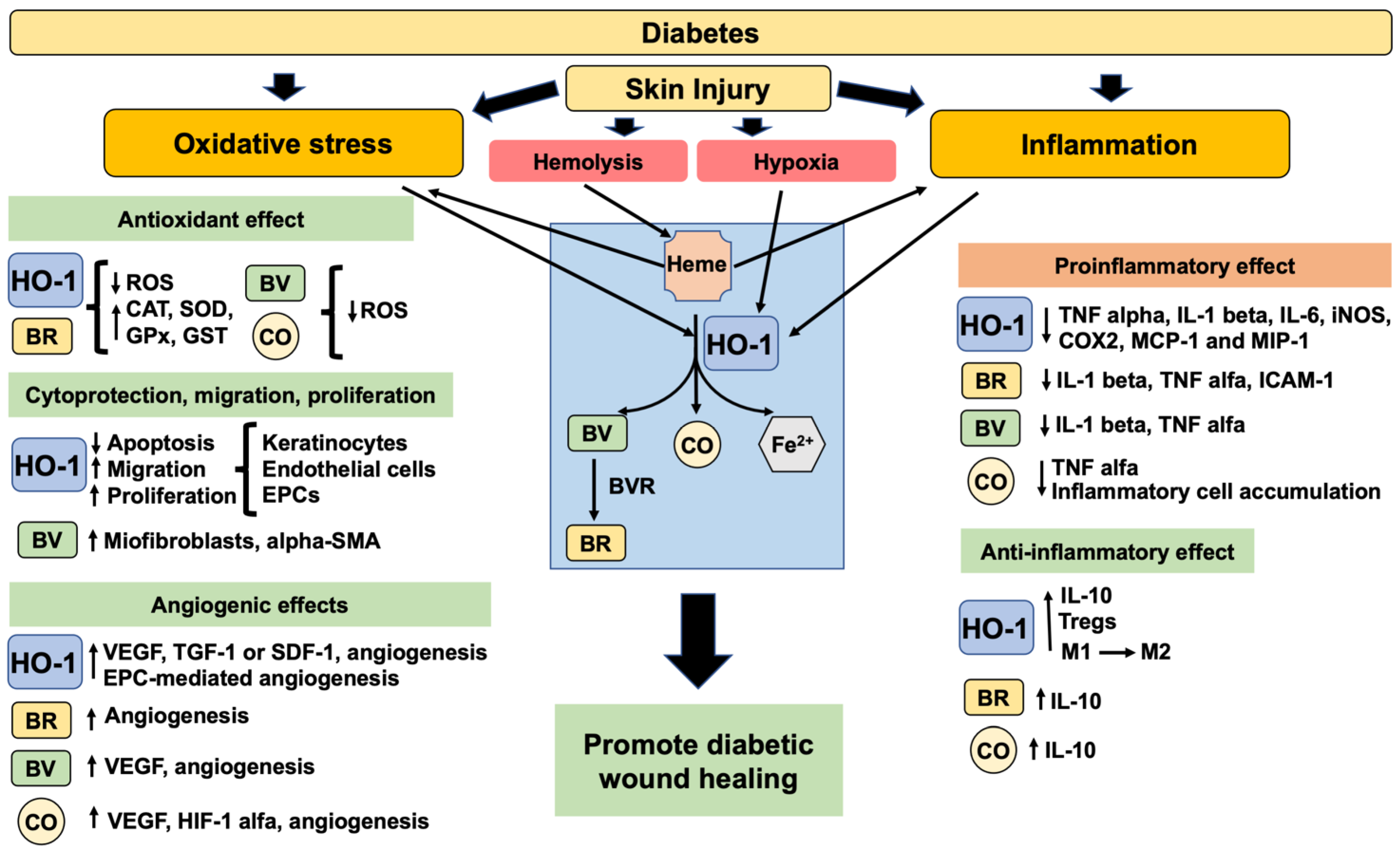
5. The Biological Properties of HO-1 Promoting Wound Healing
5.1. Antioxidant Properties
5.2. Anti-Inflammatory Properties
5.3. Cytoprotective, Migration, Proliferative and Angiogenic Properties
6. Therapeutic Potential of HO-1 Induction for DFU Treatment
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bakker, K.; Apelqvist, J.; Schaper, N.C.; International Working Group on the Diabetic Foot Editorial Board. Practical guidelines on the management and prevention of the diabetic foot 2011. Diabetes Metab. Res. Rev. 2012, 28 (Suppl. S1), 225–231. [Google Scholar]
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers and Their Recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar]
- Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Apelqvist, J. Diagnostics and treatment of the diabetic foot. Endocrine 2012, 41, 384–397. [Google Scholar] [CrossRef]
- Singh, N.; Armstrong, D.G.; Lipsky, B.A. Preventing Foot Ulcers in Patients with Diabetes. JAMA 2005, 293, 217–228. [Google Scholar] [CrossRef]
- Avishai, E.; Yeghiazaryan, K.; Golubnitschaja, O. Impaired wound healing: Facts and hypotheses for multi-professional considerations in predictive, preventive and personalised medicine. EPMA J. 2017, 8, 23–33. [Google Scholar] [CrossRef]
- Figueiredo, A.; Leal, E.C.; de Carvalho, E.M.L. Protein tyrosine phosphatase 1B inhibition as a potential therapeutic target for chronic wounds in diabetes. Pharmacol. Res. 2020, 159, 104977. [Google Scholar] [CrossRef]
- Leal, E.C.; Carvalho, E.; Tellechea, A.; Kafanas, A.; Tecilazich, F.; Kearney, C.; Kuchibhotla, S.; Auster, M.E.; Kokkotou, E.; Mooney, D.J.; et al. Substance P Promotes Wound Healing in Diabetes by Modulating Inflammation and Macrophage Phenotype. Am. J. Pathol. 2015, 185, 1638–1648. [Google Scholar] [CrossRef]
- Wetzler, C.; Kämpfer, H.; Stallmeyer, B.; Pfeilschifter, J.; Frank, S. Large and Sustained Induction of Chemokines during Impaired Wound Healing in the Genetically Diabetic Mouse: Prolonged Persistence of Neutrophils and Macrophages during the Late Phase of Repair. J. Investig. Dermatol. 2000, 115, 245–253. [Google Scholar] [CrossRef]
- Schönfelder, U.; Abel, M.; Wiegand, C.; Klemm, D.; Elsner, P.; Hipler, U.-C. Influence of selected wound dressings on PMN elastase in chronic wound fluid and their antioxidative potential in vitro. Biomaterials 2005, 26, 6664–6673. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Moura, L.I.; Dias, A.M.; Suesca, E.; Casadiegos, S.; Leal, E.C.; Fontanilla, M.R.; Carvalho, L.; de Sousa, H.C.; Carvalho, E. Neurotensin-loaded collagen dressings reduce inflammation and improve wound healing in diabetic mice. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2014, 1842, 32–43. [Google Scholar] [CrossRef]
- Salerno, L.; Pittalà, V.; Romeo, G.; Modica, M.N.; Siracusa, M.A.; Di Giacomo, C.; Acquaviva, R.; Barbagallo, I.; Tibullo, D.; Sorrenti, V. Evaluation of novel aryloxyalkyl derivatives of imidazole and 1,2,4-triazole as heme oxygenase-1 (HO-1) inhibitors and their antitumor properties. Bioorganic Med. Chem. 2013, 21, 5145–5153. [Google Scholar] [CrossRef]
- Abraham, N.G.; Kappas, A. Pharmacological and Clinical Aspects of Heme Oxygenase. Pharmacol. Rev. 2008, 60, 79–127. [Google Scholar] [CrossRef]
- Turkseven, S.; Kruger, A.; Mingone, C.J.; Kaminski, P.; Inaba, M.; Rodella, L.F.; Ikehara, S.; Wolin, M.S.; Abraham, N.G. Antioxidant mechanism of heme oxygenase-1 involves an increase in superoxide dismutase and catalase in experimental diabetes. Am. J. Physiol. Circ. Physiol. 2005, 289, H701–H707. [Google Scholar] [CrossRef]
- Barbagallo, I.; Galvano, F.; Frigiola, A.; Cappello, F.; Riccioni, G.; Murabito, P.; D’Orazio, N.; Torella, M.; Gazzolo, D.; Volti, G.L. Potential Therapeutic Effects of Natural Heme Oxygenase-1 Inducers in Cardiovascular Diseases. Antioxidants Redox Signal. 2013, 18, 507–521. [Google Scholar] [CrossRef]
- Jang, H.J.; Hong, E.M.; Kim, M.; Kim, J.H.; Jang, J.; Park, S.W.; Byun, H.W.; Koh, D.H.; Choi, M.H.; Kae, S.H.; et al. Simvastatin induces heme oxygenase-1 via NF-E2-related factor 2 (Nrf2) activation through ERK and PI3K/Akt pathway in colon cancer. Oncotarget 2016, 7, 46219–46229. [Google Scholar] [CrossRef]
- Maines, M.D.; Kappas, A. Regulation of heme pathway enzymes and cellular glutathione content by metals that do not chelate with tetrapyrroles: Blockade of metal effects by thiols. Proc. Natl. Acad. Sci. USA 1977, 74, 1875–1878. [Google Scholar] [CrossRef]
- Hanselmann, C.; Mauch, C.; Werner, S. Haem oxygenase-1: A novel player in cutaneous wound repair and psoriasis? Biochem. J. 2001, 353 Pt 3, 459–466. [Google Scholar]
- Kämpfer, H.; Kolb, N.; Manderscheid, M.; Wetzler, C.; Pfeilschifter, J.; Frank, S. Macrophage-derived heme-oxygenase-1: Expression, regulation, and possible functions in skin repair. Mol. Med. 2001, 7, 488–498. [Google Scholar] [CrossRef]
- Motterlini, R.; Otterbein, L.E. The therapeutic potential of carbon monoxide. Nat. Rev. Drug Discov. 2010, 9, 728–743. [Google Scholar] [CrossRef]
- Ahanger, A.A.; Leo, M.D.; Gopal, A.; Kant, V.; Tandan, S.K.; Kumar, D. Pro-healing effects of bilirubin in open excision wound model in rats. Int. Wound J. 2014, 13, 398–402. [Google Scholar] [CrossRef]
- Chen, Q.-Y.; Wang, G.-G.; Li, W.; Jiang, Y.-X.; Lu, X.-H.; Zhou, P.-P. Heme Oxygenase-1 Promotes Delayed Wound Healing in Diabetic Rats. J. Diabetes Res. 2015, 2016, 1–10. [Google Scholar] [CrossRef]
- Brandi, C.; Grimaldi, L.; Nisi, G.; Brafa, A.; Campa, A.; Calabrò, M.; Campana, M.; D’Aniello, C. The role of carbon dioxide therapy in the treatment of chronic wounds. In Vivo 2010, 24, 223–226. [Google Scholar]
- Jansen, T.; Hortmann, M.; Oelze, M.; Opitz, B.; Steven, S.; Schell, R.; Knorr, M.; Karbach, S.; Schuhmacher, S.; Wenzel, P.; et al. Conversion of biliverdin to bilirubin by biliverdin reductase contributes to endothelial cell protection by heme oxygenase-1—Evidence for direct and indirect antioxidant actions of bilirubin. J. Mol. Cell. Cardiol. 2010, 49, 186–195. [Google Scholar] [CrossRef]
- Ryter, S.W.; Choi, A.M. Heme oxygenase-1/carbon monoxide: From metabolism to molecular therapy. Am. J. Respir. Cell Mol. Biol. 2009, 41, 251–260. [Google Scholar]
- Gozzelino, R.; Jeney, V.; Soares, M.P. Mechanisms of Cell Protection by Heme Oxygenase-1. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 323–354. [Google Scholar] [CrossRef]
- Loboda, A.; Jazwa, A.; Grochot-Przeczek, A.; Rutkowski, A.J.; Cisowski, J.; Agarwal, A.; Jozkowicz, A.; Dulak, J. Heme Oxygenase-1 and the Vascular Bed: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid. Redox Signal. 2008, 10, 1767–1812. [Google Scholar] [CrossRef]
- Dulak, J.; Deshane, J.; Jozkowicz, A.; Agarwal, A. Heme oxygenase-1 and carbon monoxide in vascular pathobiology: Focus on angiogenesis. Circulation 2008, 117, 231–241. [Google Scholar]
- Jozkowicz, A.; Dulak, J. Effects of protoporphyrins on production of nitric oxide and expression of vascular endothelial growth factor in vascular smooth muscle cells and macrophages. Acta Biochim. Pol. 2003, 50, 69–79. [Google Scholar]
- Grochot-Przeczek, A.; Lach, R.; Mis, J.; Skrzypek, K.; Gozdecka, M.; Sroczynska, P.; Dubiel, M.; Rutkowski, A.; Kozakowska, M.; Zagorska, A.; et al. Heme Oxygenase-1 Accelerates Cutaneous Wound Healing in Mice. PLoS ONE 2009, 4, e5803. [Google Scholar] [CrossRef]
- Sindrilaru, A.; Scharffetter-Kochanek, K. Disclosure of the Culprits: Macrophages—Versatile Regulators of Wound Healing. Adv. Wound Care 2013, 2, 357–368. [Google Scholar] [CrossRef]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar]
- Ferrante, C.J.; Leibovich, S.J. Regulation of Macrophage Polarization and Wound Healing. Adv. Wound Care (New Rochelle) 2012, 1, 10–16. [Google Scholar]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in Wound Repair: Molecular and Cellular Mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef]
- Vannella, K.M.; Wynn, T.A. Mechanisms of Organ Injury and Repair by Macrophages. Annu. Rev. Physiol. 2017, 79, 593–617. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Chung, S.S.; Ho, E.C.; Lam, K.S.; Chung, S.K. Contribution of polyol pathway to diabetes-induced oxidative stress. J. Am. Soc. Nephrol. 2003, 14 (Suppl. S3), S233–S236. [Google Scholar]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Mechanistic Insight into Oxidative Stress-Triggered Signaling Pathways and Type 2 Diabetes. Molecules 2022, 27, 950. [Google Scholar] [CrossRef]
- Schleicher, E.D.; Weigert, C. Role of the hexosamine biosynthetic pathway in diabetic nephropathy. Kidney Int. 2000, 58, S13–S18. [Google Scholar] [CrossRef]
- Prattichizzo, F.; De Nigris, V.; Mancuso, E.; Spiga, R.; Giuliani, A.; Matacchione, G.; Lazzarini, R.; Marcheselli, F.; Recchioni, R.; Testa, R.; et al. Short-term sustained hyperglycaemia fosters an archetypal senescence-associated secretory phenotype in endothelial cells and macrophages. Redox Biol. 2017, 15, 170–181. [Google Scholar] [CrossRef]
- Rochette, L.; Zeller, M.; Cottin, Y.; Vergely, C. Diabetes, oxidative stress and therapeutic strategies. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 2709–2729. [Google Scholar] [CrossRef]
- Galkowska, H.; Wojewodzka, U.; Olszewski, W.L. Chemokines, cytokines, and growth factors in keratinocytes and dermal endothelial cells in the margin of chronic diabetic foot ulcers. Wound Repair Regen. 2006, 14, 558–565. [Google Scholar] [CrossRef]
- Jude, E.B.; Blakytny, R.; Bulmer, J.; Boulton, A.J.M.; Ferguson, M.W.J. Transforming growth factor-beta 1, 2, 3 and receptor type I and II in diabetic foot ulcers. Diabet. Med. 2002, 19, 440–447. [Google Scholar] [CrossRef]
- Blakytny, R.; Jude, E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet. Med. 2006, 23, 594–608. [Google Scholar] [CrossRef]
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef]
- Wunder, C.; Potter, R.F. The heme oxygenase system: Its role in liver inflammation. Curr. Drug Targets-Cardiovasc. Hematol. Disord. 2003, 3, 199–208. [Google Scholar]
- Ryter, S.W.; Alam, J.; Choi, A.M. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol. Rev. 2006, 86, 583–650. [Google Scholar]
- Han, Z.; Varadharaj, S.; Giedt, R.J.; Zweier, J.L.; Szeto, H.H.; Alevriadou, B.R. Mitochondria-Derived Reactive Oxygen Species Mediate Heme Oxygenase-1 Expression in Sheared Endothelial Cells. J. Pharmacol. Exp. Ther. 2009, 329, 94–101. [Google Scholar] [CrossRef]
- Motterlini, R.; Green, C.J.; Foresti, R. Regulation of Heme Oxygenase-1 by Redox Signals Involving Nitric Oxide. Antioxidants Redox Signal. 2002, 4, 615–624. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, L.; Xiong, Y.; Panayi, A.C.; Abududilibaier, A.; Hu, Y.; Yu, C.; Zhou, W.; Sun, Y.; Liu, M.; et al. Antioxidant Therapy and Antioxidant-Related Bionanomaterials in Diabetic Wound Healing. Front. Bioeng. Biotechnol. 2021, 9, 554. [Google Scholar] [CrossRef]
- Whitington, P.F.; Moscioni, A.D.; Gartner, L.M. The Effect of Tin (IV)-Protoporphyrin-IX on Bilirubin Production and Excretion in the Rat. Pediatr. Res. 1987, 21, 487–491. [Google Scholar] [CrossRef]
- Verman, H.J.; Ekstrand, B.C.; Stevenson, D.K. Selection of Metalloporphyrin Heme Oxygenase Inhibitors Based on Potency and Photoreactivity. Pediatr. Res. 1993, 33, 195–200. [Google Scholar] [CrossRef]
- Fernández-Fierro, A.; Funes, S.C.; Rios, M.; Covián, C.; González, J.; Kalergis, A.M. Immune Modulation by Inhibitors of the HO System. Int. J. Mol. Sci. 2020, 22, 294. [Google Scholar] [CrossRef]
- Huber, W.J., 3rd; Backes, W.L. Expression and characterization of full-length human heme oxygenase-1: The presence of intact membrane-binding region leads to increased binding affinity for NADPH cytochrome P450 reductase. Biochemistry 2007, 46, 12212–12219. [Google Scholar]
- Linnenbaum, M.; Busker, M.; Kraehling, J.R.; Behrends, S. Heme Oxygenase Isoforms Differ in Their Subcellular Trafficking during Hypoxia and Are Differentially Modulated by Cytochrome P450 Reductase. PLoS ONE 2012, 7, e35483. [Google Scholar] [CrossRef]
- Lin, Q.; Weis, S.; Yang, G.; Weng, Y.-H.; Helston, R.; Rish, K.; Smith, A.; Bordner, J.; Polte, T.; Gaunitz, F.; et al. Heme Oxygenase-1 Protein Localizes to the Nucleus and Activates Transcription Factors Important in Oxidative Stress. J. Biol. Chem. 2007, 282, 20621–20633. [Google Scholar] [CrossRef]
- Biswas, C.; Shah, N.; Muthu, M.; La, P.; Fernando, A.P.; Sengupta, S.; Yang, G.; Dennery, P.A. Nuclear Heme Oxygenase-1 (HO-1) Modulates Subcellular Distribution and Activation of Nrf2, Impacting Metabolic and Anti-oxidant Defenses. J. Biol. Chem. 2014, 289, 26882–26894. [Google Scholar] [CrossRef]
- Yachie, A.; Niida, Y.; Wada, T.; Igarashi, N.; Kaneda, H.; Toma, T.; Ohta, K.; Kasahara, Y.; Koizumi, S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J. Clin. Investig. 1999, 103, 129–135. [Google Scholar] [CrossRef]
- Poss, K.D.; Tonegawa, S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc. Natl. Acad. Sci. USA 1997, 94, 10919–10924. [Google Scholar] [CrossRef]
- Vulapalli, S.R.; Chen, Z.; Chua, B.H.; Wang, T.; Liang, C.S. Cardioselective overexpression of HO-1 prevents I/R-induced cardiac dysfunction and apoptosis. Am. J. Physiol. Heart. Circ. Physiol. 2002, 283, H688–H694. [Google Scholar]
- Minamino, T.; Christou, H.; Hsieh, C.-M.; Liu, Y.; Dhawan, V.; Abraham, N.G.; Perrella, M.A.; Mitsialis, S.A.; Kourembanas, S. Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular responses to hypoxia. Proc. Natl. Acad. Sci. USA 2001, 98, 8798–8803. [Google Scholar] [CrossRef]
- Cho, J.L.; Allanson, M.; Reeve, V.E. Hypoxia inducible factor-1alpha contributes to UV radiation-induced inflammation, epidermal hyperplasia and immunosuppression in mice. Photochem. Photobiol. Sci. 2012, 11, 309–317. [Google Scholar]
- Kim, E.; Han, S.Y.; Hwang, K.; Kim, D.; Kim, E.-M.; Hossain, M.A.; Kim, J.-H.; Cho, J.Y. Antioxidant and Cytoprotective Effects of (−)-Epigallocatechin-3-(3″-O-methyl) Gallate. Int. J. Mol. Sci. 2019, 20, 3993. [Google Scholar] [CrossRef]
- Jian, Z.; Tang, L.; Yi, X.; Liu, B.; Zhang, Q.; Zhu, G.; Wang, G.; Gao, T.; Li, C. Aspirin induces Nrf2-mediated transcriptional activation of haem oxygenase-1 in protection of human melanocytes from H2 O2 -induced oxidative stress. J. Cell Mol. Med. 2016, 20, 1307–1318. [Google Scholar]
- De la Rosa, L.C.; Vrenken, T.E.; Hannivoort, R.A.; Buist-Homan, M.; Havinga, R.; Slebos, D.-J.; Kauffman, H.F.; Faber, K.N.; Jansen, P.L.; Moshage, H. Carbon monoxide blocks oxidative stress-induced hepatocyte apoptosis via inhibition of the p54 JNK isoform. Free Radic. Biol. Med. 2008, 44, 1323–1333. [Google Scholar] [CrossRef]
- Lee, T.-S.; Chau, L.-Y. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat. Med. 2002, 8, 240–246. [Google Scholar] [CrossRef]
- Luo, J.F.; Shen, X.Y.; Lio, C.K.; Dai, Y.; Cheng, C.S.; Liu, J.X.; Yao, Y.D.; Yu, Y.; Xie, Y.; Luo, P.; et al. Activation of Nrf2/HO-1 Pathway by Nardochinoid C Inhibits Inflammation and Oxidative Stress in Lipopolysaccharide-Stimulated Macrophages. Front. Pharmacol. 2018, 9, 911. [Google Scholar]
- Morse, D.; Pischke, S.E.; Zhou, Z.; Davis, R.J.; Flavell, R.A.; Loop, T.; Otterbein, S.L.; Otterbein, L.E.; Choi, A.M.K. Suppression of Inflammatory Cytokine Production by Carbon Monoxide Involves the JNK Pathway and AP-1. J. Biol. Chem. 2003, 278, 36993–36998. [Google Scholar] [CrossRef]
- Chiang, K.-C.; Chang, K.-S.; Hsu, S.-Y.; Sung, H.-C.; Feng, T.-H.; Chao, M.; Juang, H.-H. Human Heme Oxygenase-1 Induced by Interleukin-6 via JAK/STAT3 Pathways Is a Tumor Suppressor Gene in Hepatoma Cells. Antioxidants 2020, 9, 251. [Google Scholar] [CrossRef]
- Georgiou-Siafis, S.K.; Tsiftsoglou, A.S. Activation of KEAP1/NRF2 stress signaling involved in the molecular basis of hemin-induced cytotoxicity in human pro-erythroid K562 cells. Biochem. Pharmacol. 2020, 175, 113900. [Google Scholar]
- Kumar, D.; Jena, G.R.; Ram, M.; Lingaraju, M.C.; Singh, V.; Prasad, R.; Kumawat, S.; Kant, V.; Gupta, P.; Tandan, S.K.; et al. Hemin attenuated oxidative stress and inflammation to improve wound healing in diabetic rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 1435–1445. [Google Scholar] [CrossRef]
- Cui, B.; Wang, Y.; Jin, J.; Yang, Z.; Guo, R.; Li, X.; Yang, L.; Li, Z. Resveratrol Treats UVB-Induced Photoaging by Anti-MMP Expression, through Anti-Inflammatory, Antioxidant, and Antiapoptotic Properties, and Treats Photoaging by Upregulating VEGF-B Expression. Oxidative Med. Cell. Longev. 2022, 2022, 1–19. [Google Scholar] [CrossRef]
- Lima, C.F.; Pereira-Wilson, C.; Rattan, S.I.S. Curcumin induces heme oxygenase-1 in normal human skin fibroblasts through redox signaling: Relevance for anti-aging intervention. Mol. Nutr. Food Res. 2010, 55, 430–442. [Google Scholar] [CrossRef]
- Kim, K.M.; Im, A.-R.; Lee, S.; Chae, S. Dual Protective Effects of Flavonoids from Petasites japonicus against UVB-Induced Apoptosis Mediated via HSF-1 Activated Heat Shock Proteins and Nrf2-Activated Heme Oxygenase-1 Pathways. Biol. Pharm. Bull. 2017, 40, 765–773. [Google Scholar] [CrossRef]
- Oh, S.W.; Lee, Y.M.; Kim, S.; Chin, H.J.; Chae, D.W.; Na, K.Y. Cobalt chloride attenuates oxidative stress and inflammation through NF-kappaB inhibition in human renal proximal tubular epithelial cells. J. Korean Med. Sci. 2014, 29 (Suppl. S2), S139–S145. [Google Scholar]
- Ou, Y.-C.; Li, J.-R.; Wu, C.-C.; Yu, T.-M.; Chen, W.-Y.; Liao, S.-L.; Kuan, Y.-H.; Chen, Y.-F.; Chen, C.-J. Cadmium induces the expression of Interleukin-6 through Heme Oxygenase-1 in HK-2 cells and Sprague-Dawley rats. Food Chem. Toxicol. 2022, 161, 112846. [Google Scholar] [CrossRef]
- Du, D.; Lv, W.; Jing, X.; Yu, C.; Wuen, J.; Hasi, S. Camel whey protein alleviates heat stress-induced liver injury by activating the Nrf2/HO-1 signaling pathway and inhibiting HMGB1 release. Cell Stress Chaperones 2022, 27, 449–460. [Google Scholar]
- Chu, X.; Zhang, X.; Gong, X.; Zhou, H.; Cai, C. Effects of hyperoxia exposure on the expression of Nrf2 and heme oxygenase-1 in lung tissues of premature rats. Mol. Cell. Probes 2020, 51, 101529. [Google Scholar] [CrossRef]
- Li, M.; Yu, H.; Pan, H.; Zhou, X.; Ruan, Q.; Kong, D.; Chu, Z.; Li, H.; Huang, J.; Huang, X.; et al. Nrf2 Suppression Delays Diabetic Wound Healing Through Sustained Oxidative Stress and Inflammation. Front. Pharmacol. 2019, 10, 1099. [Google Scholar] [CrossRef]
- Beck, K.; Wu, B.J.; Ni, J.; Santiago, F.S.; Malabanan, K.P.; Li, C.; Wang, Y.; Khachigian, L.M.; Stocker, R. Interplay Between Heme Oxygenase-1 and the Multifunctional Transcription Factor Yin Yang 1 in the Inhibition of Intimal Hyperplasia. Circ. Res. 2010, 107, 1490–1497. [Google Scholar] [CrossRef]
- Lin, H.H.; Chen, Y.H.; Yet, S.F.; Chau, L.Y. After vascular injury, heme oxygenase-1/carbon monoxide enhances re-endothelialization via promoting mobilization of circulating endothelial progenitor cells. J. Thromb. Haemost 2009, 7, 1401–1408. [Google Scholar]
- Lin, H.H.; Lai, S.C.; Chau, L.Y. Heme oxygenase-1/carbon monoxide induces vascular endothelial growth factor expression via p38 kinase-dependent activation of Sp1. J. Biol. Chem. 2011, 286, 3829–3838. [Google Scholar]
- Takamiya, R.; Murakami, M.; Kajimura, M.; Goda, N.; Makino, N.; Takamiya, Y.; Yamaguchi, T.; Ishimura, Y.; Hozumi, N.; Suematsu, M. Stabilization of mast cells by heme oxygenase-1: An anti-inflammatory role. Am. J. Physiol. Circ. Physiol. 2002, 283, H861–H870. [Google Scholar] [CrossRef]
- Zenke-Kawasaki, Y.; Dohi, Y.; Katoh, Y.; Ikura, T.; Ikura, M.; Asahara, T.; Tokunaga, F.; Iwai, K.; Igarashi, K. Heme Induces Ubiquitination and Degradation of the Transcription Factor Bach1. Mol. Cell. Biol. 2007, 27, 6962–6971. [Google Scholar] [CrossRef]
- Wagener, F.A.D.T.G.; van Beurden, H.E.; Hoff, J.W.V.D.; Adema, G.J.; Figdor, C.G. The heme-heme oxygenase system: A molecular switch in wound healing. Blood 2003, 102, 521–528. [Google Scholar] [CrossRef]
- Brogliato, A.R.; Moor, A.N.; Kesl, S.L.; Guilherme, R.F.; Georgii, J.L.; Peters-Golden, M.; Canetti, C.; Gould, L.J.; Benjamim, C.F. Critical Role of 5-Lipoxygenase and Heme Oxygenase-1 in Wound Healing. J. Investig. Dermatol. 2014, 134, 1436–1445. [Google Scholar] [CrossRef]
- Ahanger, A.A.; Prawez, S.; Leo, M.D.M.; Kathirvel, K.; Kumar, D.; Tandan, S.K.; Malik, J.K. Pro-healing potential of hemin: An inducer of heme oxygenase-1. Eur. J. Pharmacol. 2010, 645, 165–170. [Google Scholar] [CrossRef]
- Long, M.; de la Vega, M.R.; Wen, Q.; Bharara, M.; Jiang, T.; Zhang, R.; Zhou, S.; Wong, P.K.; Wondrak, G.T.; Zheng, H.; et al. An Essential Role of NRF2 in Diabetic Wound Healing. Diabetes 2015, 65, 780–793. [Google Scholar] [CrossRef]
- An, L.; Liu, C.-T.; Qin, X.-B.; Liu, Q.-H.; Liu, Y.; Yu, S.-Y. Protective effects of hemin in an experimental model of ventilator-induced lung injury. Eur. J. Pharmacol. 2011, 661, 102–108. [Google Scholar] [CrossRef]
- Taye, A.; Ibrahim, B.M. Activation of renal haeme oxygenase-1 alleviates gentamicin-induced acute nephrotoxicity in rats. J. Pharm. Pharmacol. 2013, 65, 995–1004. [Google Scholar] [CrossRef]
- Collino, M.; Pini, A.; Mugelli, N.; Mastroianni, R.; Bani, D.; Fantozzi, R.; Papucci, L.; Fazi, M.; Masini, E. Beneficial effect of prolonged heme oxygenase 1 activation in a rat model of chronic heart failure. Dis. Model. Mech. 2013, 6, 1012–1020. [Google Scholar] [CrossRef]
- Deshane, J.; Chen, S.; Caballero, S.; Grochot-Przeczek, A.; Was, H.; Calzi, S.L.; Lach, R.; Hock, T.D.; Chen, B.; Hill-Kapturczak, N.; et al. Stromal cell–derived factor 1 promotes angiogenesis via a heme oxygenase 1–dependent mechanism. J. Exp. Med. 2007, 204, 605–618. [Google Scholar] [CrossRef]
- Dos Santos, J.M.; Tewari, S.; Mendes, R.H. The Role of Oxidative Stress in the Development of Diabetes Mellitus and Its Complications. J. Diabetes Res. 2019, 2019, 1–3. [Google Scholar] [CrossRef]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxidative Med. Cell. Longev. 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- Cano Sanchez, M.; Lancel, S.; Boulanger, E.; Neviere, R. Targeting Oxidative Stress and Mitochondrial Dysfunction in the Treatment of Impaired Wound Healing: A Systematic Review. Antioxidants 2018, 7, 98. [Google Scholar] [CrossRef]
- Campbell, N.K.; Fitzgerald, H.K.; Dunne, A. Regulation of inflammation by the antioxidant haem oxygenase 1. Nat. Rev. Immunol. 2021, 21, 411–425. [Google Scholar] [CrossRef]
- Shukla, A.; Rasik, A.M.; Patnaik, G.K. Depletion of Reduced Glutathione, Ascorbic Acid, Vitamin E and Antioxidant Defence Enzymes in a Healing Cutaneous Wound. Free Radic. Res. 1997, 26, 93–101. [Google Scholar] [CrossRef]
- Ram, M.; Singh, V.; Kumar, D.; Kumawat, S.; Gopalakrishnan, A.; Lingaraju, M.C.; Gupta, P.; Tandan, S.K.; Kumar, D. Antioxidant potential of bilirubin-accelerated wound healing in streptozotocin-induced diabetic rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 387, 955–961. [Google Scholar] [CrossRef]
- Tellechea, A.; Leal, E.C.; Kafanas, A.; Auster, M.E.; Kuchibhotla, S.; Ostrovsky, Y.; Tecilazich, F.; Baltzis, D.; Zheng, Y.; Carvalho, E.; et al. Mast Cells Regulate Wound Healing in Diabetes. Diabetes 2016, 65, 2006–2019. [Google Scholar] [CrossRef]
- Vijayan, V.; Wagener, F.A.; Immenschuh, S. The macrophage heme-heme oxygenase-1 system and its role in inflammation. Biochem. Pharmacol. 2018, 153, 159–167. [Google Scholar] [CrossRef]
- Naito, Y.; Takagi, T.; Higashimura, Y. Heme oxygenase-1 and anti-inflammatory M2 macrophages. Arch. Biochem. Biophys. 2014, 564, 83–88. [Google Scholar] [CrossRef]
- Brouard, S.; Otterbein, L.E.; Anrather, J.; Tobiasch, E.; Bach, F.H.; Choi, A.M.; Soares, M. Carbon Monoxide Generated by Heme Oxygenase 1 Suppresses Endothelial Cell Apoptosis. J. Exp. Med. 2000, 192, 1015–1026. [Google Scholar] [CrossRef]
- Otterbein, L.E.; Bach, F.H.; Alam, J.; Soares, M.; Lu, H.T.; Wysk, M.A.; Davis, R.J.; Flavell, R.A.; Choi, A.M.K. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2000, 6, 422–428. [Google Scholar] [CrossRef]
- Kozakowska, M.; Pietraszek-Gremplewicz, K.; Ciesla, M.; Seczynska, M.; Bronisz-Budzynska, I.; Podkalicka, P.; Bukowska-Strakova, K.; Loboda, A.; Jozkowicz, A.; Dulak, J. Lack of Heme Oxygenase-1 Induces Inflammatory Reaction and Proliferation of Muscle Satellite Cells after Cardiotoxin-Induced Skeletal Muscle Injury. Am. J. Pathol. 2018, 188, 491–506. [Google Scholar] [CrossRef]
- Yao, Q.; Lan, Q.-H.; Jiang, X.; Du, C.-C.; Zhai, Y.-Y.; Shen, X.; Xu, H.-L.; Xiao, J.; Kou, L.; Zhao, Y.-Z. Bioinspired biliverdin/silk fibroin hydrogel for antiglioma photothermal therapy and wound healing. Theranostics 2020, 10, 11719–11736. [Google Scholar] [CrossRef]
- Fan, J.; Liu, H.; Wang, J.; Zeng, J.; Tan, Y.; Wang, Y.; Yu, X.; Li, W.; Wang, P.; Yang, Z.; et al. Procyanidin B2 improves endothelial progenitor cell function and promotes wound healing in diabetic mice via activating Nrf2. J. Cell. Mol. Med. 2020, 25, 652–665. [Google Scholar] [CrossRef]
- Ahanger, A.A.; Prawez, S.; Kumar, D.; Prasad, R.; Amarpal; Tandan, S.K.; Kumar, D. Wound healing activity of carbon monoxide liberated from CO-releasing molecule (CO-RM). Naunyn Schmiedebergs Arch. Pharm. 2011, 384, 93–102. [Google Scholar]
- Cepinskas, G.; Katada, K.; Bihari, A.; Potter, R.F. Carbon monoxide liberated from carbon monoxide-releasing molecule CORM-2 attenuates inflammation in the liver of septic mice. Am. J. Physiol. Liver Physiol. 2008, 294, G184–G191. [Google Scholar] [CrossRef]
- Moura, J.; Rodrigues, J.; Alves, J.M.; Amaral, C.; Lima, M.; Carvalho, E. Impaired T-cell differentiation in diabetic foot ulceration. Cell. Mol. Immunol. 2016, 14, 758–769. [Google Scholar] [CrossRef]
- Seraphim, P.M.; Leal, E.C.; Moura, J.; Gonçalves, P.; Gonçalves, J.P.; Carvalho, E. Lack of lymphocytes impairs macrophage polarization and angiogenesis in diabetic wound healing. Life Sci. 2020, 254, 117813. [Google Scholar] [CrossRef]
- Brusko, T.M.; Wasserfall, C.H.; Agarwal, A.; Kapturczak, M.H.; Atkinson, M.A. An Integral Role for Heme Oxygenase-1 and Carbon Monoxide in Maintaining Peripheral Tolerance by CD4+CD25+ Regulatory T Cells. J. Immunol. 2005, 174, 5181–5186. [Google Scholar] [CrossRef]
- Xia, Z.-W.; Xu, L.-Q.; Zhong, W.-W.; Wei, J.-J.; Li, N.-L.; Shao, J.; Li, Y.-Z.; Yu, S.-C.; Zhang, Z.-L. Heme Oxygenase-1 Attenuates Ovalbumin-Induced Airway Inflammation by Up-Regulation of Foxp3 T-Regulatory Cells, Interleukin-10, and Membrane-Bound Transforming Growth Factor-β1. Am. J. Pathol. 2007, 171, 1904–1914. [Google Scholar] [CrossRef]
- Xia, Z.W.; Zhong, W.W.; Xu, L.Q.; Sun, J.L.; Shen, Q.X.; Wang, J.G.; Shao, J.; Li, Y.Z.; Yu, S.C. Heme oxygenase-1-mediated CD4+CD25high regulatory T cells suppress allergic airway inflammation. J. Immunol. 2006, 177, 5936–5945. [Google Scholar]
- George, J.F.; Braun, A.; Brusko, T.M.; Joseph, R.; Bolisetty, S.; Wasserfall, C.H.; Atkinson, M.A.; Agarwal, A.; Kapturczak, M.H. Suppression by CD4+CD25+ Regulatory T Cells Is Dependent on Expression of Heme Oxygenase-1 in Antigen-Presenting Cells. Am. J. Pathol. 2008, 173, 154–160. [Google Scholar] [CrossRef]
- Soares, M.P.; Usheva, A.; Brouard, S.; Berberat, P.O.; Gunther, L.; Tobiasch, E.; Bach, F.H. Modulation of Endothelial Cell Apoptosis by Heme Oxygenase-1-Derived Carbon Monoxide. Antioxidants Redox Signal. 2002, 4, 321–329. [Google Scholar] [CrossRef]
- Li Calzi, S.; Purich, D.L.; Chang, K.H.; Afzal, A.; Nakagawa, T.; Busik, J.V.; Agarwal, A.; Segal, M.S.; Grant, M.B. Carbon monoxide and nitric oxide mediate cytoskeletal reorganization in microvascular cells via vasodilator-stimulated phosphoprotein phosphorylation: Evidence for blunted responsiveness in diabetes. Diabetes 2008, 57, 2488–2494. [Google Scholar]
- Bloch, W.; Huggel, K.; Sasaki, T.; Grose, R.; Bugnon, P.; Addicks, K.; Timpl, R.; Werner, S. The angiogenesis inhibitor endostatin impairs blood vessel maturation during wound healing. FASEB J. 2000, 14, 2373–2376. [Google Scholar] [CrossRef]
- Rossiter, H.; Barresi, C.; Pammer, J.; Rendl, M.; Haigh, J.; Wagner, E.F.; Tschachler, E. Loss of Vascular Endothelial Growth Factor A Activity in Murine Epidermal Keratinocytes Delays Wound Healing and Inhibits Tumor Formation. Cancer Res. 2004, 64, 3508–3516. [Google Scholar] [CrossRef]
- Józkowicz, A.; Huk, I.; Nigisch, A.; Weigel, G.; Dietrich, W.; Motterlini, R.; Dulak, J. Heme Oxygenase and Angiogenic Activity of Endothelial Cells: Stimulation by Carbon Monoxide and Inhibition by Tin Protoporphyrin-IX. Antioxidants Redox Signal. 2003, 5, 155–162. [Google Scholar] [CrossRef]
- Drukala, J.; Bandura, L.; Cieślik, K.; Korohoda, W. Locomotion of Human Skin Keratinocytes on Polystyrene, Fibrin, and Collagen Substrata and its Modification by Cell-to-Cell Contacts. Cell Transplant. 2001, 10, 765–771. [Google Scholar] [CrossRef]
- Hou, C.; Shen, L.; Huang, Q.; Mi, J.; Wu, Y.; Yang, M.; Zeng, W.; Li, L.; Chen, W.; Zhu, C. The effect of heme oxygenase-1 complexed with collagen on MSC performance in the treatment of diabetic ischemic ulcer. Biomaterials 2012, 34, 112–120. [Google Scholar] [CrossRef]
- Takagi, T.; Okayama, T.; Asai, J.; Mizushima, K.; Hirai, Y.; Uchiyama, K.; Ishikawa, T.; Naito, Y.; Itoh, Y. Topical application of sustained released-carbon monoxide promotes cutaneous wound healing in diabetic mice. Biochem. Pharmacol. 2022, 199, 115016. [Google Scholar] [CrossRef]
- Wu, B.J.; Midwinter, R.G.; Cassano, C.; Beck, K.; Wang, Y.; Changsiri, D.; Gamble, J.R.; Stocker, R. Heme Oxygenase-1 Increases Endothelial Progenitor Cells. Arter. Thromb. Vasc. Biol. 2009, 29, 1537–1542. [Google Scholar] [CrossRef]
- Sidhu, G.S.; Mani, H.; Gaddipati, J.P.; Singh, A.K.; Seth, P.; Banaudha, K.K.; Patnaik, G.K.; Maheshwari, R.K. Curcumin enhances wound healing in streptozotocin induced diabetic rats and genetically diabetic mice. Wound Repair Regen. 1999, 7, 362–374. [Google Scholar] [CrossRef]
- Fu, J.; Huang, J.; Lin, M.; Xie, T.; You, T. Quercetin Promotes Diabetic Wound Healing via Switching Macrophages From M1 to M2 Polarization. J. Surg. Res. 2019, 246, 213–223. [Google Scholar] [CrossRef]
- Kim, H.; Kawazoe, T.; Han, D.-W.; Matsumara, K.; Suzuki, S.; Tsutsumi, S.; Hyon, S.-H. Enhanced wound healing by an epigallocatechin gallate-incorporated collagen sponge in diabetic mice. Wound Repair Regen. 2008, 16, 714–720. [Google Scholar] [CrossRef]
- Senger, D.R.; Cao, S. Diabetic Wound Healing and Activation of Nrf2 by Herbal Medicine. J. Nat. Sci. 2016, 2, e247. [Google Scholar]
- Foresti, R.; Bains, S.K.; Pitchumony, T.S.; De Castro Brás, L.E.; Drago, F.; Dubois-Randé, J.-L.; Bucolo, C.; Motterlini, R. Small molecule activators of the Nrf2-HO-1 antioxidant axis modulate heme metabolism and inflammation in BV2 microglia cells. Pharmacol. Res. 2013, 76, 132–148. [Google Scholar] [CrossRef]
- Bomprezzi, R. Dimethyl fumarate in the treatment of relapsing–remitting multiple sclerosis: An overview. Ther. Adv. Neurol. Disord. 2015, 8, 20–30. [Google Scholar] [CrossRef]
- Racke, M.; Nicholas, J.; Boster, A.; Imitola, J.; O’Connell, C. Design of oral agents for the management of multiple sclerosis: Benefit and risk assessment for dimethyl fumarate. Drug Des. Dev. Ther. 2014, 8, 897–908. [Google Scholar] [CrossRef]
- Li, Y.; Ma, F.; Li, H.; Song, Y.; Zhang, H.; Jiang, Z.; Wu, H. Dimethyl fumarate accelerates wound healing under diabetic conditions. J. Mol. Endocrinol. 2018, 61, 163–172. [Google Scholar] [CrossRef]
- Anderson, K.E.; Collins, S. Open-Label Study of Hemin for Acute Porphyria: Clinical Practice Implications. Am. J. Med. 2006, 119, 801.e1–801.e6. [Google Scholar] [CrossRef]
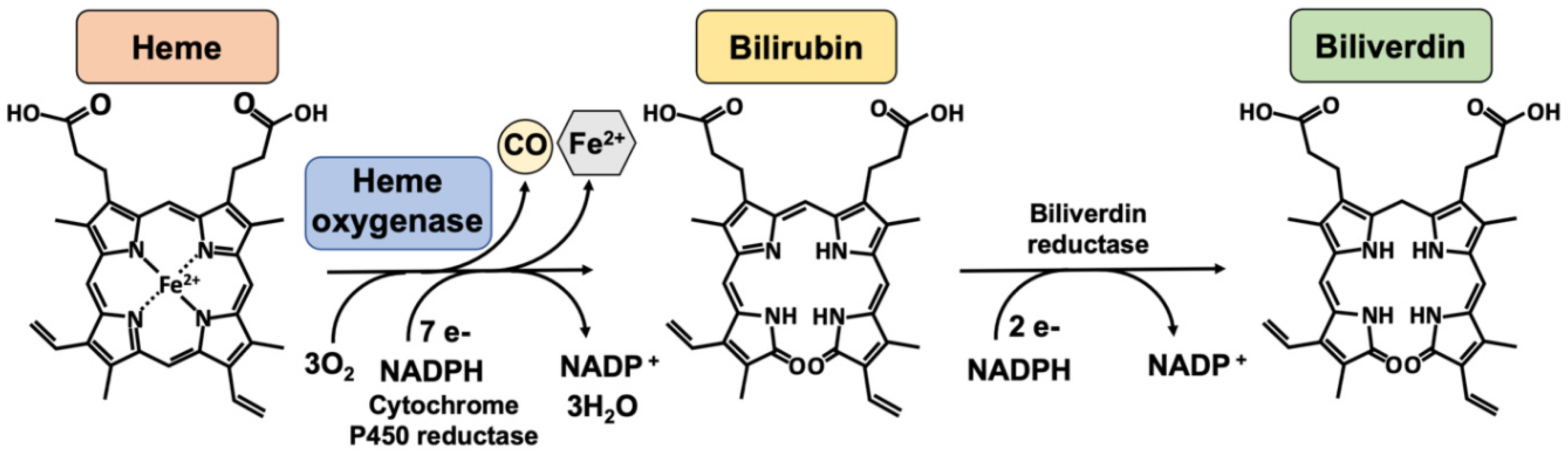

| Experimental Model | HO-1 Modulator/Agent | Findings | Refs. |
|---|---|---|---|
| HO-1 deficient mice | - | Delayed wound healing, impaired angiogenesis | [34] |
| Mice | SnPPIX (-) | Inhibition of HO-1 delay wound healing | |
| Transgenic mice | HO-1 overexpression in keratinocytes | Promote wound healing, increase angiogenesis | |
| Db/db mice | Adv HO-1 transduction | Promote wound healing, increase angiogenesis | |
| Mouse primary keratinocytes | Adv HO-1 transduction | Promote migration, increase VEGF expression | |
| - | |||
| Aortic rings from HO-1 deficient mice | CO | Impaired SDF1-mediated angiogenesis and migration | [97] |
| CO reversed impaired SDF1-mediated angiogenesis in aortic rings from HO-1 deficient mice | |||
| Diabetic rat | Hemin (+), SnPPIX (−) | HO-1 induction promotes wound healing, inhibit inflammatory cytokines, increase levels of antioxidant enzymes, and promote angiogenesis. | [26,76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leal, E.C.; Carvalho, E. Heme Oxygenase-1 as Therapeutic Target for Diabetic Foot Ulcers. Int. J. Mol. Sci. 2022, 23, 12043. https://doi.org/10.3390/ijms231912043
Leal EC, Carvalho E. Heme Oxygenase-1 as Therapeutic Target for Diabetic Foot Ulcers. International Journal of Molecular Sciences. 2022; 23(19):12043. https://doi.org/10.3390/ijms231912043
Chicago/Turabian StyleLeal, Ermelindo Carreira, and Eugenia Carvalho. 2022. "Heme Oxygenase-1 as Therapeutic Target for Diabetic Foot Ulcers" International Journal of Molecular Sciences 23, no. 19: 12043. https://doi.org/10.3390/ijms231912043
APA StyleLeal, E. C., & Carvalho, E. (2022). Heme Oxygenase-1 as Therapeutic Target for Diabetic Foot Ulcers. International Journal of Molecular Sciences, 23(19), 12043. https://doi.org/10.3390/ijms231912043






