A Fine Regulation of the Hippocampal Thyroid Signalling Protects Hypothyroid Mice against Glial Cell Activation
Abstract
1. Introduction
2. Results
2.1. Hypothyroid Status
2.2. Consequences of Hypothyroidism on Hippocampal Inflammation
2.3. Hypothyroidism Effect on Behavior and Cognition
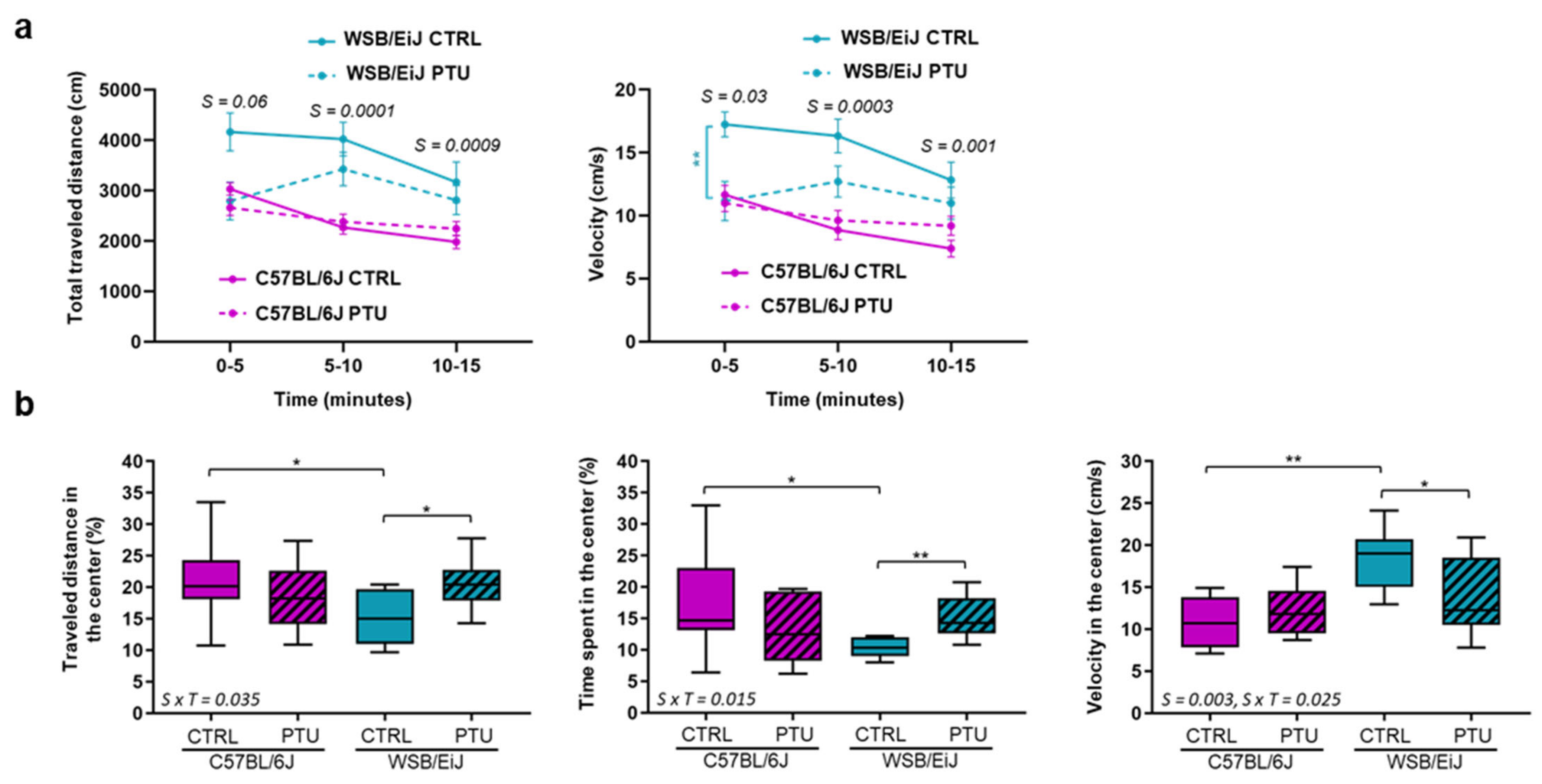
2.3.1. Open-Field Test
2.3.2. Barnes Maze Test
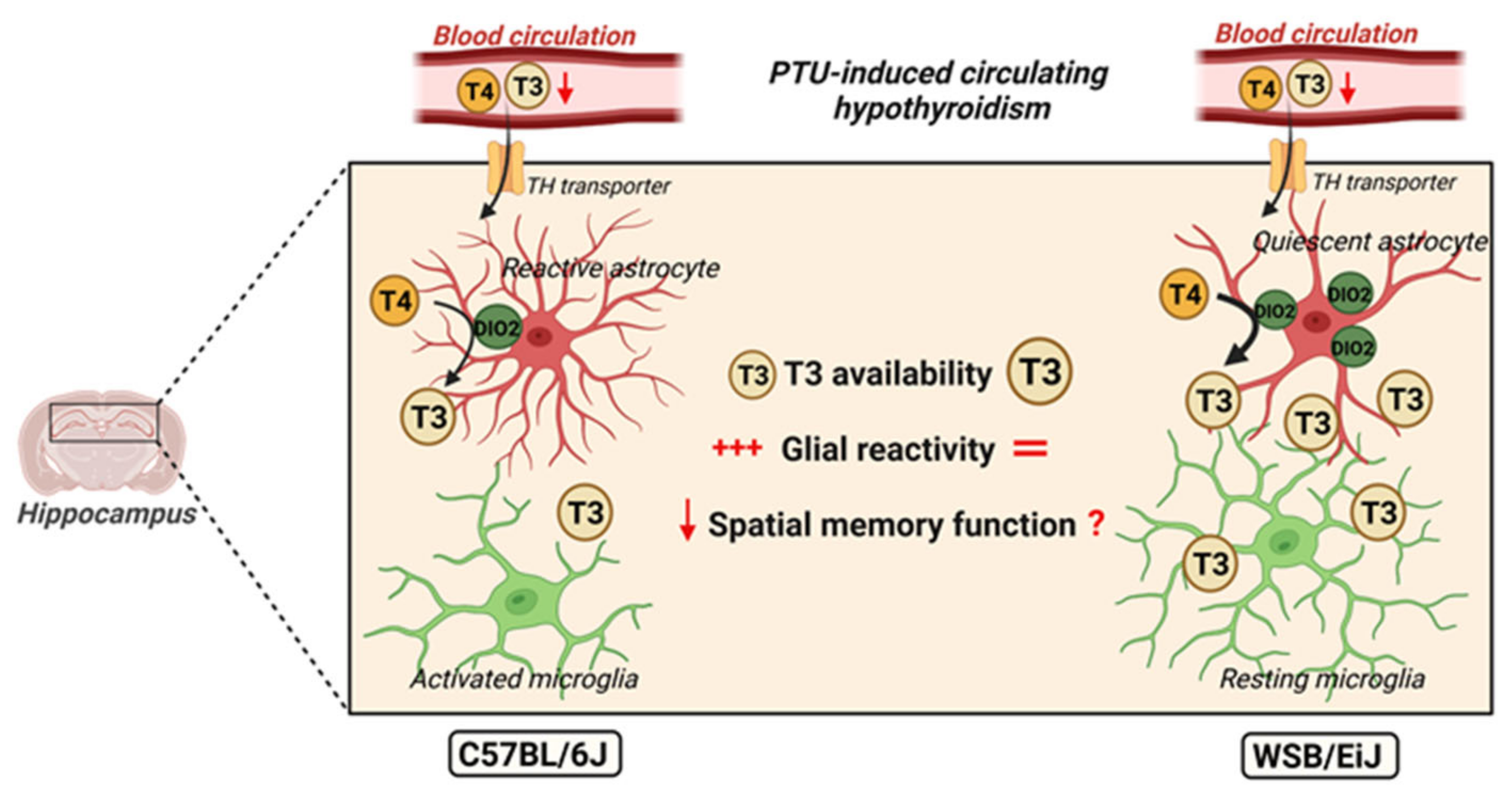
3. Discussion
4. Materials and Methods
4.1. Animals, Treatment, and Sample Collections
4.2. Circulating Thyroid Hormone Levels
4.3. Reverse-Transcription qPCR
4.4. Immunohistochemistry
4.5. Western Blot Analysis
4.6. Behavioural Test
4.6.1. Open Field
4.6.2. Barnes Maze
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raymaekers, S.R.; Darras, V.M. Thyroid hormones and learning-associated neuroplasticity. Gen. Comp. Endocrinol. 2017, 247, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Accorroni, A.; Chiellini, G.; Origlia, N. Effects of Thyroid Hormones and their Metabolites on Learning and Memory in Normal and Pathological Conditions. Curr. Drug Metab. 2017, 18, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, A.C.; Privalsky, M.L. Thyroid hormones, T3 and T4, in the brain. Front. Endocrinol. 2014, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Noda, M. Possible role of glial cells in the relationship between thyroid dysfunction and mental disorders. Front. Cell. Neurosci. 2015, 9, 1–7. [Google Scholar] [CrossRef]
- Bianco, A.C.; Kim, B.W. Deiodinases: Implications of the local control of thyroid hormone action. J. Clin. Invest. 2006, 116, 2571–2579. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.; Quignodon, L.; Martinez, M.E.; Flamant, F.; St. Germain, D.L. Type 3 deiodinase deficiency causes spatial and temporal alterations in brain T3 signaling that are dissociated from serum thyroid hormone levels. Endocrinology 2010, 151, 5550–5558. [Google Scholar] [CrossRef] [PubMed]
- Brent, G.A. Mechanisms of thyroid hormone action. J. Clin. Invest. 2012, 122, 3035–3043. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, C. Antonio C. Bianco, MD, PhD: Is T4 enough for patients with hypothyroid dysfunction? Integr. Med. 2014, 13, 20–22. [Google Scholar]
- McAninch, E.A.; Bianco, A.C. The history and future of treatment of hypothyroidism. Ann. Intern. Med. 2016, 164, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Biondi, B.; Cooper, D.S. Thyroid hormone therapy for hypothyroidism. Endocrine 2019, 66, 18–26. [Google Scholar] [CrossRef]
- Bernal, J. Thyroid hormone receptors in brain development and function. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Samuels, M.H. Psychiatric and cognitive manifestations of hypothyroidism. Curr. Opin. Endocrinol. Diabetes. Obes. 2014, 21, 377–383. [Google Scholar] [CrossRef]
- Alzoubi, K.H.; Alkadhi, K.A. Levothyroxin replacement therapy restores hypothyroidism induced impairment of L-LTP induction: Critical role of CREB. Brain Res. Bull. 2014, 100, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Noda, M.; Ifuku, M.; Hossain, M.S.; Katafuchi, T. Glial activation and expression of the serotonin transporter in chronic fatigue syndrome. Front. Psychiatry 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Tomonaga, D.; Kalashnikova, A.; Furuya, F.; Akimoto, N.; Ifuku, M.; Okuno, Y.; Beppu, K.; Fujita, K.; Katafuchi, T.; et al. Effects of 3,3’,5-triiodothyronine on microglial functions. Glia 2015, 63, 906–920. [Google Scholar] [CrossRef] [PubMed]
- De Vito, P.; Incerpi, S.; Pedersen, J.Z.; Luly, P.; Davis, F.B.; Davis, P.J. Thyroid hormones as modulators of immune activities at the cellular level. Thyroid 2011, 21, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Cortés, C.; Eugenin, E.; Aliaga, E.; Carreño, L.J.; Bueno, S.M.; Gonzalez, P.A.; Gayol, S.; Naranjo, D.; Noches, V.; Marassi, M.P.; et al. Hypothyroidism in the adult rat causes incremental changes in brain-derived neurotrophic factor, neuronal and astrocyte apoptosis, gliosis, and deterioration of postsynaptic density. Thyroid 2012, 22, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.M.; Kim, J.W.; Yoo, D.Y.; Jung, H.Y.; Chung, J.Y.; Kim, D.W.; Hwang, I.K.; Yoon, Y.S. Hypothyroidism increases cyclooxygenase-2 levels and pro-inflammatory response and decreases cell proliferation and neuroblast differentiation in the hippocampus. Mol. Med. Rep. 2018, 17, 5782–5788. [Google Scholar] [CrossRef]
- Cooke, G.E.; Mullally, S.; Correia, N.; O’Mara, S.M.; Gibney, J. Hippocampal volume is decreased in adults with hypothyroidism. Thyroid 2014, 24, 433–440. [Google Scholar] [CrossRef]
- Gilbert, M.E.; Paczkowski, C. Propylthiouracil (PTU)-induced hypothyroidism in the developing rat impairs synaptic transmission and plasticity in the dentate gyrus of the adult hippocampus. Dev. Brain Res. 2003, 145, 19–29. [Google Scholar] [CrossRef]
- Artis, A.S.; Bitiktas, S.; Taşkin, E.; Dolu, N.; Liman, N.; Suer, C. Experimental Hypothyroidism Delays Field Excitatory Post-Synaptic Potentials and Disrupts Hippocampal Long-term Potentiation in the Dentate Gyrus of Hippocampal Formation and Y-Maze Performance in Adult Rats. J. Neuroendocrinol. 2012, 24, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Chaalal, A.; Poirier, R.; Blum, D.; Gillet, B.; Le Blanc, P.; Basquin, M.; Buée, L.; Laroche, S.; Enderlin, V. PTU-induced hypothyroidism in rats leads to several early neuropathological signs of Alzheimer’s disease in the hippocampus and spatial memory impairments. Hippocampus 2014, 24, 1381–1393. [Google Scholar] [CrossRef] [PubMed]
- Alzoubi, K.H.; Gerges, N.Z.; Aleisa, A.M.; Alkadhi, K.A. Levothyroxin restores hypothyroidism-induced impairment of hippocampus-dependent learning and memory: Behavioral, electrophysiological, and molecular studies. Hippocampus 2009, 19, 66–78. [Google Scholar] [CrossRef]
- Chaalal, A.; Poirier, R.; Blum, D.; Laroche, S.; Enderlin, V. Thyroid Hormone Supplementation Restores Spatial Memory, Hippocampal Markers of Neuroinflammation, Plasticity-Related Signaling Molecules, and β-Amyloid Peptide Load in Hypothyroid Rats. Mol. Neurobiol. 2019, 56, 722–735. [Google Scholar] [CrossRef] [PubMed]
- Yirmiya, R.; Goshen, I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain. Behav. Immun. 2011, 25, 181–213. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Thangavel, R.; Selvakumar, G.P.; Zaheer, S.; Ahmed, M.E.; Raikwar, S.P.; Zahoor, H.; Saeed, D.; Natteru, P.A.; Iyer, S.; et al. Brain and peripheral atypical inflammatory mediators potentiate neuroinflammation and neurodegeneration. Front. Cell. Neurosci. 2017, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Delpech, J.C.; Thomazeau, A.; Madore, C.; Bosch-Bouju, C.; Larrieu, T.; Lacabanne, C.; Remus-Borel, J.; Aubert, A.; Joffre, C.; Nadjar, A.; et al. Dietary n-3 PUFAs deficiency increases vulnerability to inflammation-induced spatial memory impairment. Neuropsychopharmacology 2015, 40, 2774–2787. [Google Scholar] [CrossRef]
- Keane, T.M.; Goodstadt, L.; Danecek, P.; White, M.A.; Wong, K.; Yalcin, B.; Heger, A.; Agam, A.; Slater, G.; Goodson, M.; et al. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature 2011, 477, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Terrien, J.; Seugnet, I.; Seffou, B.; Herrero, M.J.; Bowers, J.; Chamas, L.; Decherf, S.; Duvernois-Berthet, E.; Djediat, C.; Ducos, B.; et al. Reduced central and peripheral inflammatory responses and increased mitochondrial activity contribute to diet-induced obesity resistance in WSB/EiJ mice. Sci. Rep. 2019, 9, 19696. [Google Scholar] [CrossRef]
- Guillemot-legris, O.; Muccioli, G.G. Obesity-Induced Neuroinflammation: Beyond the Hypothalamus. Trends Neurosci. 2017, 40, 237–253. [Google Scholar] [CrossRef]
- Salazar, P.; Cisternas, P.; Martinez, M.; Inestrosa, N.C. Hypothyroidism and Cognitive Disorders during Development and Adulthood: Implications in the Central Nervous System. Mol. Neurobiol. 2019, 56, 2952–2963. [Google Scholar] [CrossRef] [PubMed]
- Laurberg, P.; Knudsen, N.; Andersen, S.; Carlé, A.; Pedersen, I.B.; Karmisholt, J. Thyroid Function and Obesity. Eur. Thyroid J. 2012, 1, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Muthu, P.R.; Bobby, Z.; Sankar, P.; Vickneshwaran, V.; Jacob, S.E. Amla (Emblica officinalis) improves hepatic and renal oxidative stress and the inflammatory response in hypothyroid female wistar rats fed with a high-fat diet. J. Basic Clin. Physiol. Pharmacol. 2018, 29, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Iniguez, M.A. Thyroid hormone regulation of RC3, a brain-specific gene encoding a protein kinase-C substrate. Endocrinology 1993, 133, 467–473. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Denver, R.J.; Williamson, K.E. Identification of a thyroid hormone response element in the mouse Krüppel-like factor 9 gene to explain its postnatal expression in the brain. Endocrinology 2009, 150, 3935–3943. [Google Scholar] [CrossRef]
- Piosik, P.A.; Van Groenigen, M.; Baas, F. Effect of thyroid hormone deficiency on RC3/neurogranin mRNA expression in the prenatal and adult caprine brain. Mol. Brain Res. 1996, 42, 227–235. [Google Scholar] [CrossRef]
- Sadeghian, A.; Fathollahi, Y.; Javan, M.; Shojaei, A.; Kosarmadar, N.; Rezaei, M.; Mirnajafi-Zadeh, J. Research paper: Spatial learning and memory in Barnes maze test and synaptic potentiation in Schaffer collateral-CA1 synapses of dorsal hippocampus in freely moving rats. Basic Clin. Neurosci. 2019, 10, 461–468. [Google Scholar] [CrossRef]
- Şener, G.; Şehirli, Ö.; Velioǧlu-Öǧünç, A.; Ercan, F.; Erkanli, G.; Gedik, N.; Yeǧen, B.Ç. Propylthiouracil (PTU)-induced hypothyroidism alleviates burn-induced multiple organ injury. Burns 2006, 32, 728–736. [Google Scholar] [CrossRef]
- Vallortigara, J.; Alfos, S.; Micheau, J.; Higueret, P.; Enderlin, V. T3 administration in adult hypothyroid mice modulates expression of proteins involved in striatal synaptic plasticity and improves motor behavior. Neurobiol. Dis. 2008, 31, 378–385. [Google Scholar] [CrossRef]
- Chen, L.; Shi, T.; Wang, Y.T.; He, J.; Zhao, X.; Wang, Y.K.; Giesy, J.P.; Chen, F.; Chen, Y.; Tuo, X.; et al. Effects of acute exposure to microcystins on hypothalamic-pituitary-adrenal (HPA), -gonad (HPG) and -thyroid (HPT) axes of female rats. Sci. Total Environ. 2021, 778, 145196. [Google Scholar] [CrossRef]
- De Castro, J.P.W.; Fonseca, T.L.; Ueta, C.B.; McAninch, E.A.; Abdalla, S.; Wittmann, G.; Lechan, R.M.; Gereben, B.; Bianco, A.C. Differences in hypothalamic type 2 deiodinase ubiquitination explain localized sensitivity to thyroxine. J. Clin. Invest. 2015, 125, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Morte, B.; Bernal, J. Thyroid hormone action: Astrocyte-neuron communication. Front. Endocrinol. 2014, 5, 82. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Martinsen, A.; Layé, S. Neuroinflammatory processes in cognitive disorders: Is there a role for flavonoids and n-3 polyunsaturated fatty acids in counteracting their detrimental effects? Neurochem. Int. 2015, 89, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Haim, L.B.; Ceyzériat, K.; de Sauvage, M.A.C.; Aubry, F.; Auregan, G.; Guillermier, M.; Ruiz, M.; Petit, F.; Houitte, D.; Faivre, E.; et al. The JAK/STAT3 pathway is a common inducer of astrocyte reactivity in Alzheimer’s and Huntington’s diseases. J. Neurosci. 2015, 35, 2817–2829. [Google Scholar] [CrossRef] [PubMed]
- Ceyzériat, K.; Abjean, L.; Carrillo-de Sauvage, M.A.; Ben Haim, L.; Escartin, C. The complex STATes of astrocyte reactivity: How are they controlled by the JAK-STAT3 pathway? Neuroscience 2016, 330, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, F.; Quintana, F.J. The Role of Astrocytes in CNS Inflammation. Trends Immunol. 2020, 41, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Villarino, A.V.; Kanno, Y.; Ferdinand, J.R.; O’Shea, J.J. Mechanisms of Jak/STAT Signaling in Immunity and Disease. J. Immunol. 2015, 194, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Millot, P.; San, C.; Bennana, E.; Porte, B.; Vignal, N.; Hugon, J.; Paquet, C.; Hosten, B.; Mouton-Liger, F. STAT3 inhibition protects against neuroinflammation and BACE1 upregulation induced by systemic inflammation. Immunol. Lett. 2020, 228, 129–134. [Google Scholar] [CrossRef]
- Tramontina, F.; Leite, M.C.; Cereser, K.; de Souza, D.F.; Tramontina, A.C.; Nardin, P.; Andreazza, A.C.; Gottfried, C.; Kapczinski, F.; Gonçalves, C.A. Immunoassay for glial fibrillary acidic protein: Antigen recognition is affected by its phosphorylation state. J. Neurosci. Methods 2007, 162, 282–286. [Google Scholar] [CrossRef]
- Rodríguez-Arellano, J.J.; Parpura, V.; Zorec, R.; Verkhratsky, A. Astrocytes in physiological aging and Alzheimer’s disease. Neuroscience 2016, 323, 170–182. [Google Scholar] [CrossRef]
- Hol, E.M.; Pekny, M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr. Opin. Cell Biol. 2015, 32, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Karperien, A.; Ahammer, H.; Jelinek, H.F. Quantitating the subtleties of microglial morphology with fractal analysis. Front. Cell. Neurosci. 2013, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Calcia, M.A.; Bonsall, D.R.; Bloomfield, P.S.; Selvaraj, S.; Barichello, T.; Howes, O.D. Stress and neuroinflammation: A systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology 2016, 233, 1637–1650. [Google Scholar] [CrossRef] [PubMed]
- Ito, D.; Imai, Y.; Ohsawa, K.; Nakajima, K.; Fukuuchi, Y.; Kohsaka, S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Mol. Brain Res. 1998, 57, 1–9. [Google Scholar] [CrossRef]
- Marshall, S.A.; McClain, J.A.; Kelso, M.L.; Hopkins, D.M.; Pauly, J.R.; Nixon, K. Microglial activation is not equivalent to neuroinflammation in alcohol-induced neurodegeneration: The importance of microglia phenotype. Neurobiol. Dis. 2013, 54, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Mallat, M.; Lima, F.R.S.; Gervais, A.; Colin, C.; Moura Neto, V. New insights into the role of thyroid hormone in the cns: The microglial track. Mol. Psychiatry 2002, 7, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Alzoubi, K.H.; Aleisa, A.M.; Gerges, N.Z.; Alkadhi, K.A. Nicotine reverses adult-onset hypothyroidism-induced impairment of learning and memory: Behavioral and electrophysiological studies. J. Neurosci. Res. 2006, 84, 944–953. [Google Scholar] [CrossRef]
- Gerges, N.Z.; Alkadhi, K.A. Hypothyroidism impairs late LTP in CA1 region but not in dentate gyrus of the intact rat hippocampus: MAPK involvement. Hippocampus 2004, 14, 40–45. [Google Scholar] [CrossRef]
- Bach, M.E.; Hawkins, R.D.; Osman, M.; Kandel, E.R.; Mayford, M. Impairment of spatial but not contextual memory in CaMKII mutant mice with a selective loss of hippocampal ltp in the range of the θ frequency. Cell 1995, 81, 905–915. [Google Scholar] [CrossRef]
- O’Leary, T.P.; Brown, R.E. Visuo-spatial learning and memory deficits on the Barnes maze in the 16-month-old APPswe/PS1dE9 mouse model of Alzheimer’s disease. Behav. Brain Res. 2009, 201, 120–127. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Zheng, X.; Fang, T.; Yang, X.; Luo, X.; Guo, A.; Newell, K.A.; Huang, X.F.; Yu, Y. Galantamine improves cognition, hippocampal inflammation, and synaptic plasticity impairments induced by lipopolysaccharide in mice. J. Neuroinflammation 2018, 15, 112. [Google Scholar] [CrossRef] [PubMed]
- Marambaud, P.; Dreses-Werringloer, U.; Vingtdeux, V. Calcium signaling in neurodegeneration. Mol. Neurodegener. 2009, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Etchamendy, N.; Enderlin, V.; Marighetto, A.; Vouimba, R.M.; Pallet, V.; Jaffard, R.; Higueret, P. Alleviation of a selective age-related relational memory deficit in mice by pharmacologically induced normalization of brain retinoid signaling. J. Neurosci. 2001, 21, 6423–6429. [Google Scholar] [CrossRef] [PubMed]
- Díez-Guerra, F.J. Neurogranin, a link between calcium/calmodulin and protein kinase C signaling in synaptic plasticity. IUBMB Life 2010, 62, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.; Vishwakarma, J.; Malik, R.; Gupta, K.; Pandey, R.; Maurya, S.K.; Garg, A.; Shukla, M.; Chattopadhyay, N.; Bandyopadhyay, S. Hypothyroidism Induces Interleukin-1-Dependent Autophagy Mechanism as a Key Mediator of Hippocampal Neuronal Apoptosis and Cognitive Decline in Postnatal Rats. Mol. Neurobiol. 2021, 58, 1196–1211. [Google Scholar] [CrossRef]
- Onos, K.D.; Uyar, A.; Keezer, K.J.; Jackson, H.M.; Preuss, C.; Acklin, C.J.; O’Rourke, R.; Buchanan, R.; Cossette, T.L.; Sukoff Rizzo, S.J.; et al. Enhancing face validity of mouse models of Alzheimer’s disease with natural genetic variation. PLoS Genet. 2019, 15, e1008155. [Google Scholar] [CrossRef]
- Bocco, B.M.L.C.; Werneck-De-castro, J.P.; Oliveira, K.C.; Fernandes, G.W.; Fonseca, T.L.; Nascimento, B.P.P.; Mcaninch, E.A.; Ricci, E.; Kvárta-Papp, Z.; Fekete, C.; et al. Type 2 deiodinase disruption in astrocytes results in anxiety-depressive-like behavior in male mice. Endocrinology 2016, 157, 3682–3695. [Google Scholar] [CrossRef]
- Markova, N.; Chernopiatko, A.; Schroeter, C.A.; Malin, D.; Kubatiev, A.; Bachurin, S.; Costa-Nunes, J.; Steinbusch, H.M.W.; Strekalova, T. Hippocampal gene expression of deiodinases 2 and 3 and effects of 3,5-diiodo-L-thyronine T2 in mouse depression paradigms. Biomed Res. Int. 2013, 2013, 565218. [Google Scholar] [CrossRef]
- Bianco, A.C.; Salvatore, D.; Gereben, B.; Berry, M.J.; Larsen, P.R. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr. Rev. 2002, 23, 38–89. [Google Scholar] [CrossRef]
- Davey, J.C.; Schneider, M.J.; Becker, K.B.; Galton, V.A. Cloning of a 5.8 kb cDNA for a mouse type 2 deiodinase. Endocrinology 1999, 140, 1022–1025. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, 34. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gilda, J.E.; Gomes, A.V. Western blotting using in-gel protein labeling as a normalization control: Stain-free technology. Methods Mol. Biol. 2014, 1295, 381–391. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamas, L.; Seugnet, I.; Poirier, R.; Clerget-Froidevaux, M.-S.; Enderlin, V. A Fine Regulation of the Hippocampal Thyroid Signalling Protects Hypothyroid Mice against Glial Cell Activation. Int. J. Mol. Sci. 2022, 23, 11938. https://doi.org/10.3390/ijms231911938
Chamas L, Seugnet I, Poirier R, Clerget-Froidevaux M-S, Enderlin V. A Fine Regulation of the Hippocampal Thyroid Signalling Protects Hypothyroid Mice against Glial Cell Activation. International Journal of Molecular Sciences. 2022; 23(19):11938. https://doi.org/10.3390/ijms231911938
Chicago/Turabian StyleChamas, Lamis, Isabelle Seugnet, Roseline Poirier, Marie-Stéphanie Clerget-Froidevaux, and Valérie Enderlin. 2022. "A Fine Regulation of the Hippocampal Thyroid Signalling Protects Hypothyroid Mice against Glial Cell Activation" International Journal of Molecular Sciences 23, no. 19: 11938. https://doi.org/10.3390/ijms231911938
APA StyleChamas, L., Seugnet, I., Poirier, R., Clerget-Froidevaux, M.-S., & Enderlin, V. (2022). A Fine Regulation of the Hippocampal Thyroid Signalling Protects Hypothyroid Mice against Glial Cell Activation. International Journal of Molecular Sciences, 23(19), 11938. https://doi.org/10.3390/ijms231911938





