Short-Term Mild Hypoxia Modulates Na,K-ATPase to Maintain Membrane Electrogenesis in Rat Skeletal Muscle
Abstract
1. Introduction
2. Results
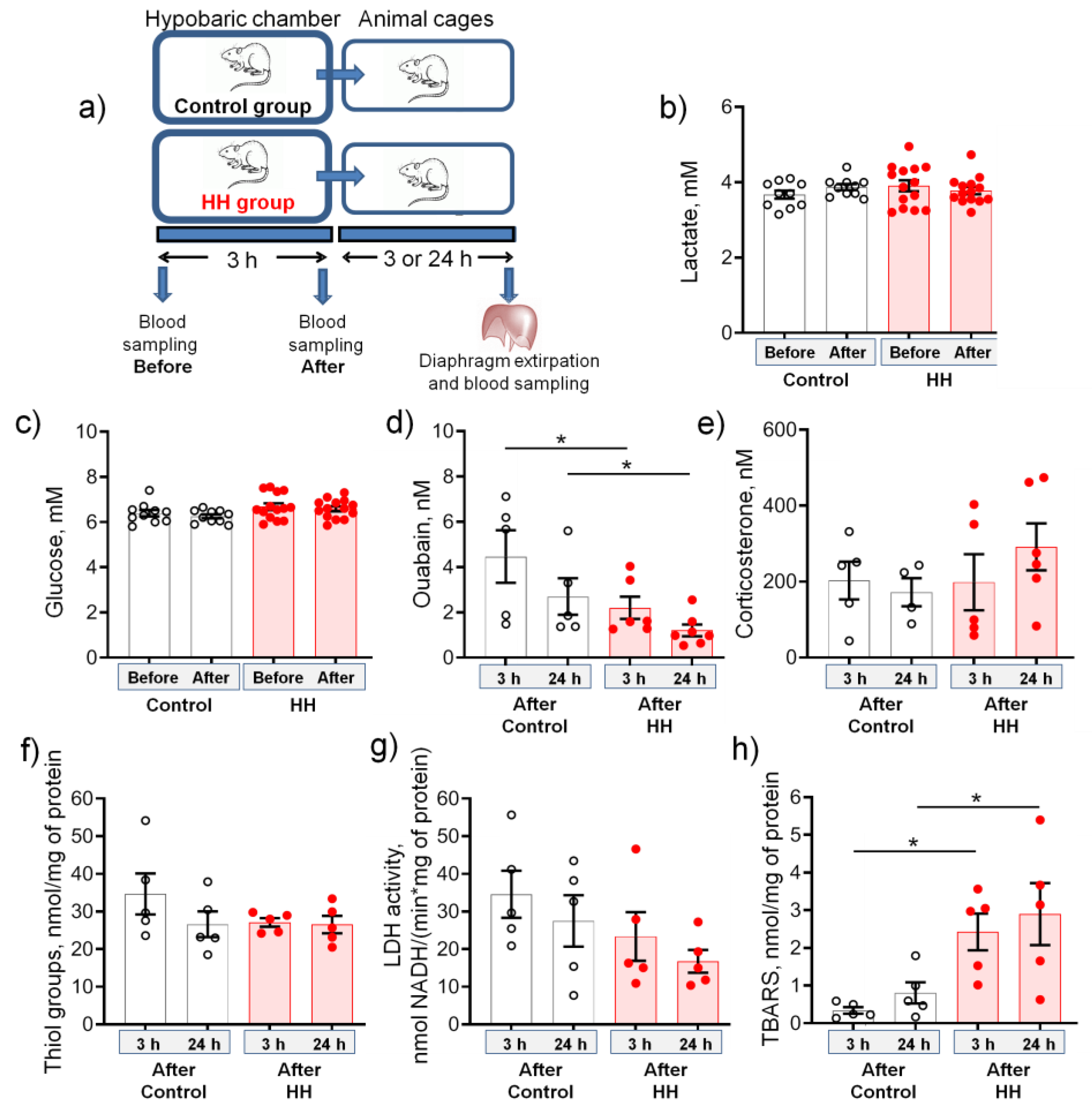
2.1. Hypobaric Hypoxia Decreases the Serum Level of Endogenous Ouabain and Increases the Production of TBARS
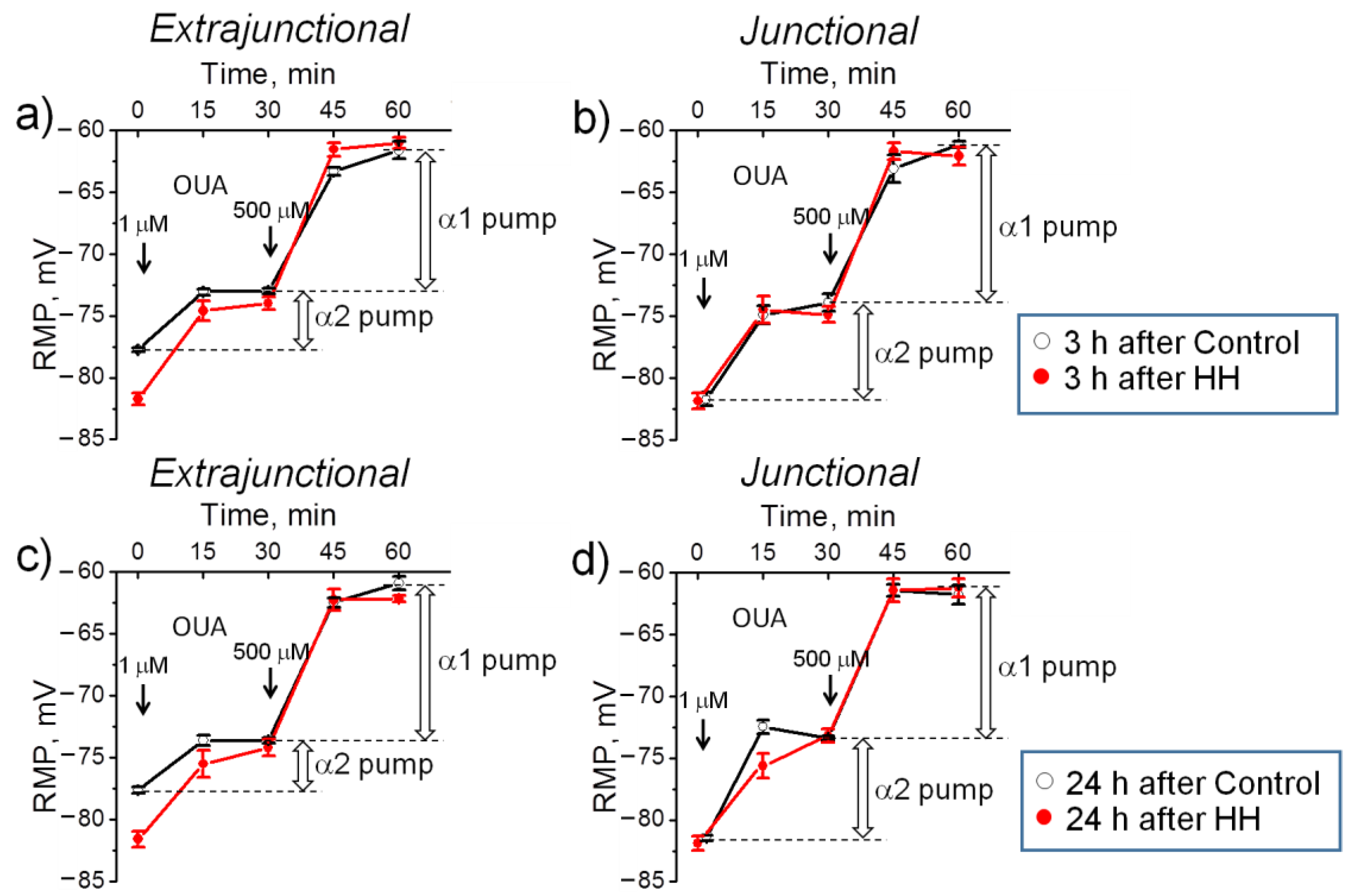
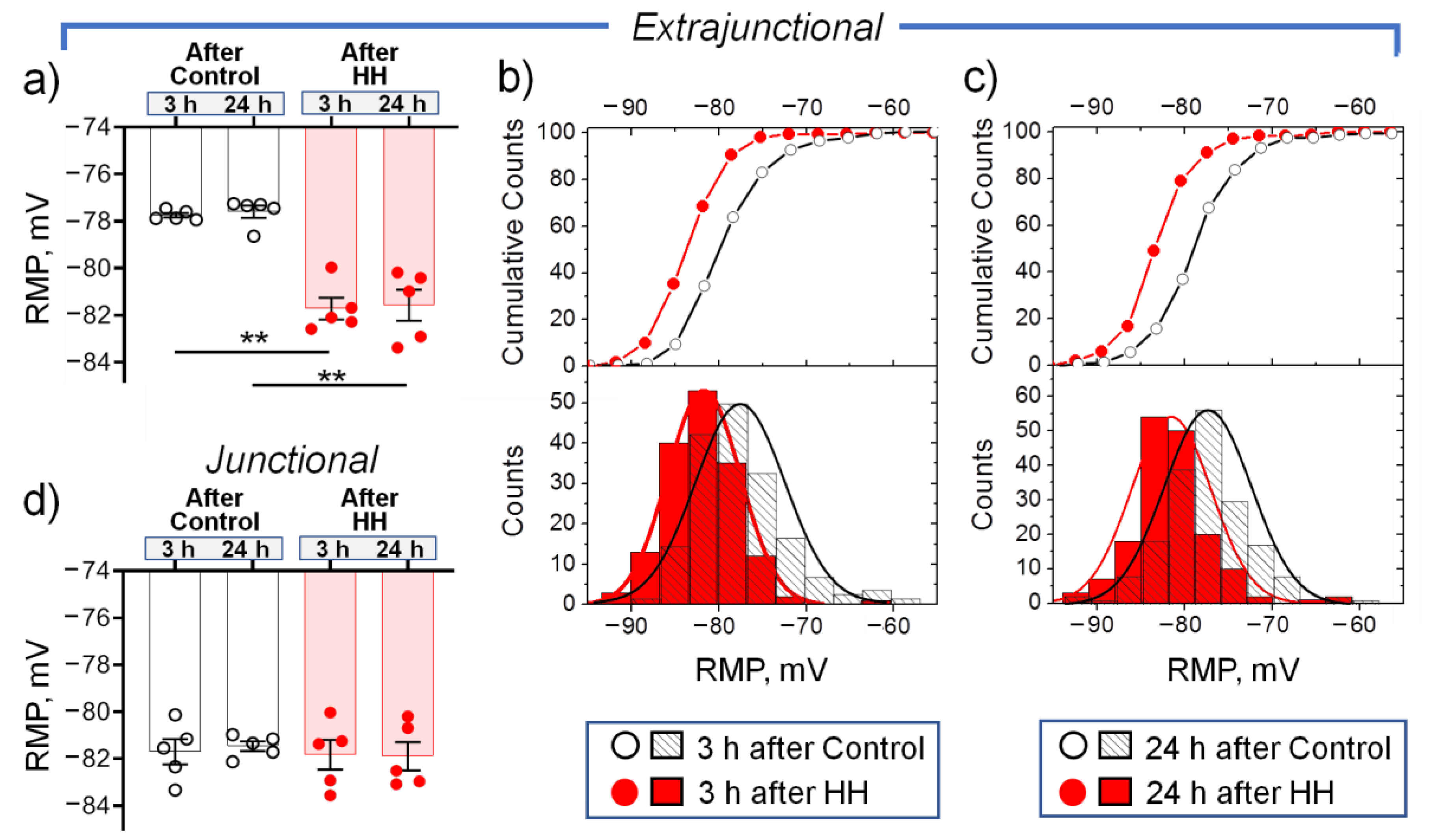
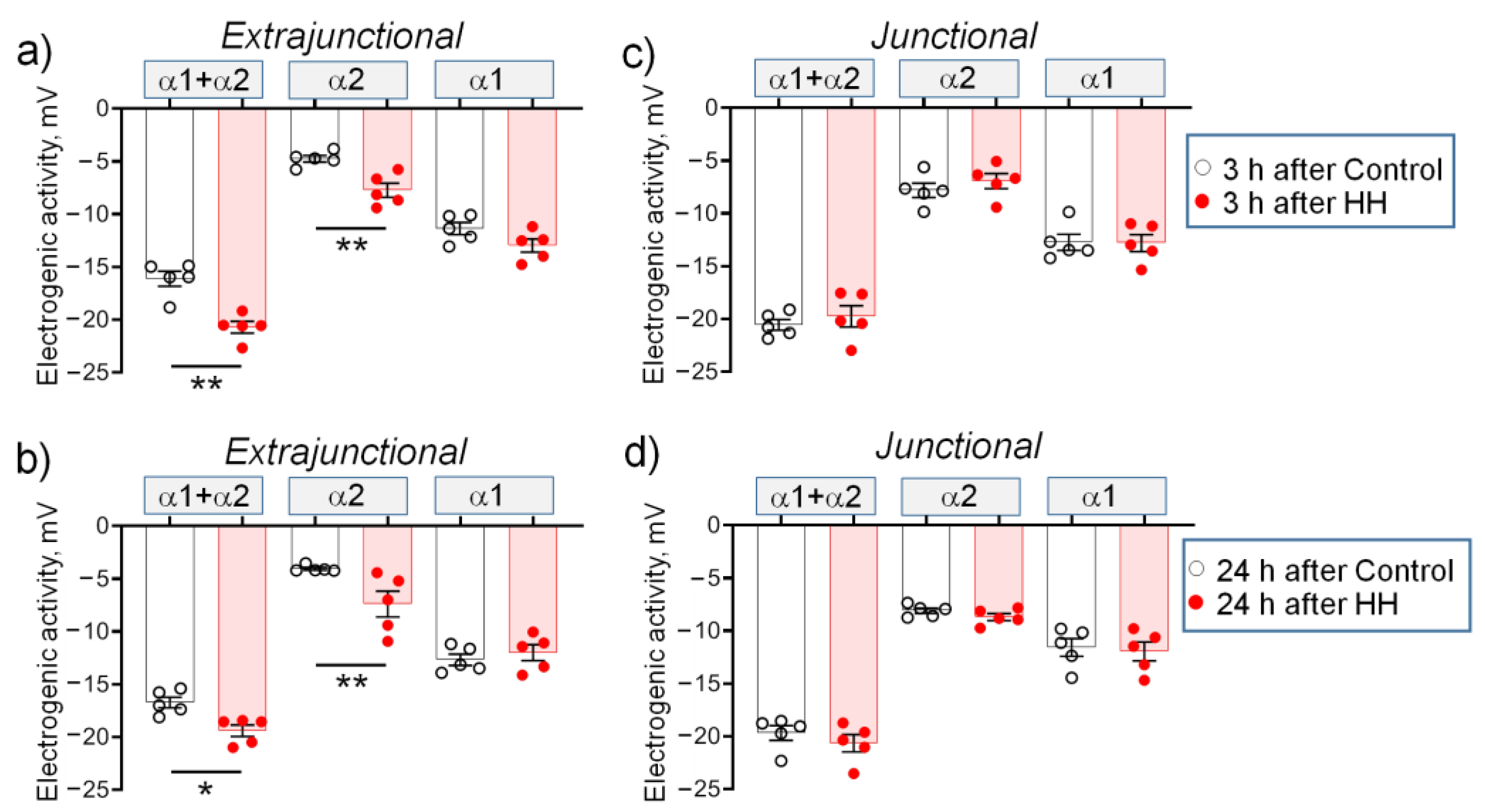
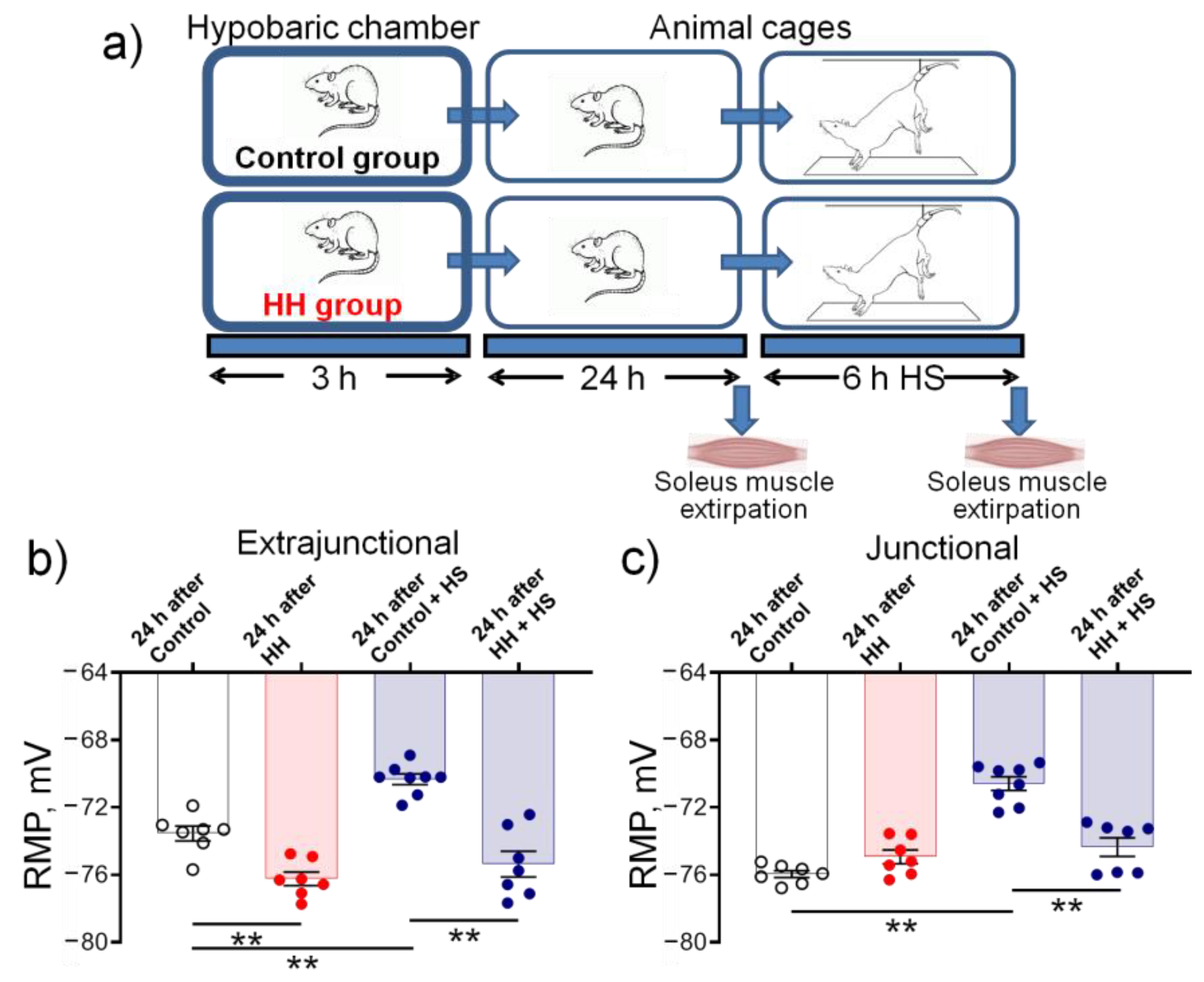
2.2. Hypobaric Hypoxia Hyperpolarizes Diaphragm Sarcolemma due to a Steady Increase in the α2 Na,K-ATPase Electrogenic Activity
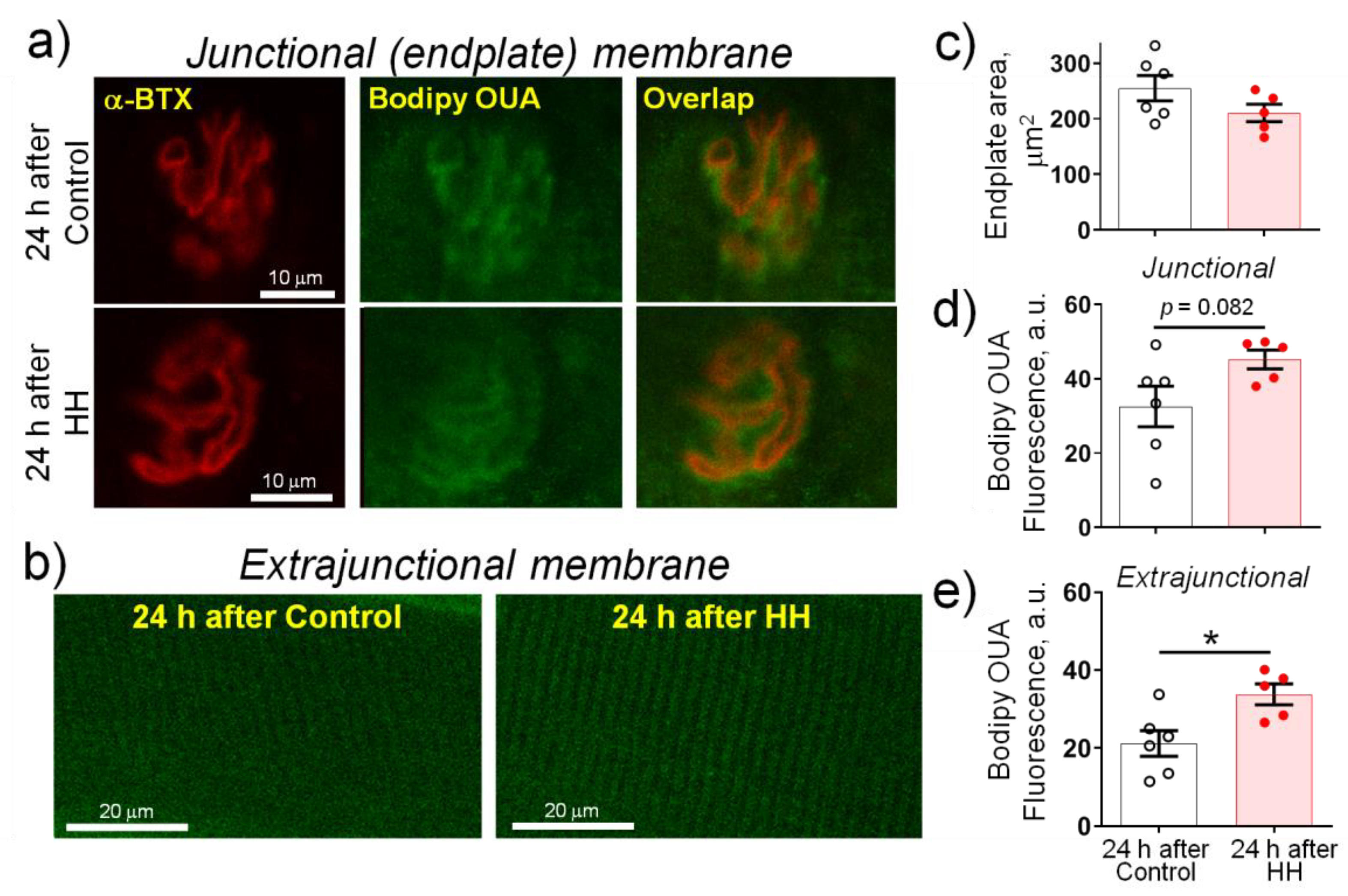
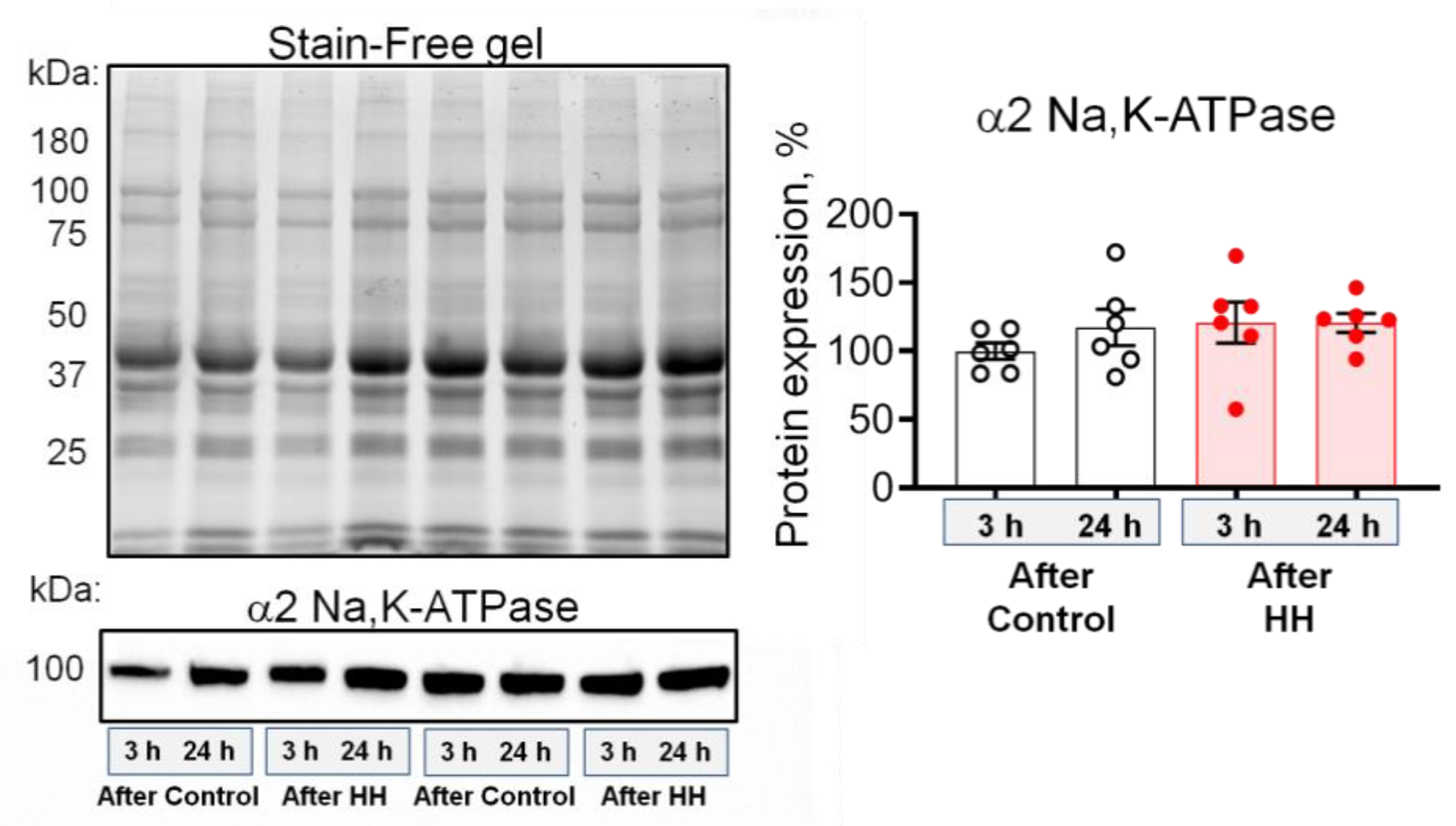
2.3. Hypobaric Hypoxia Increases the α2 Na,K-ATPase Membrane Abundance without Changing its Total Protein Content
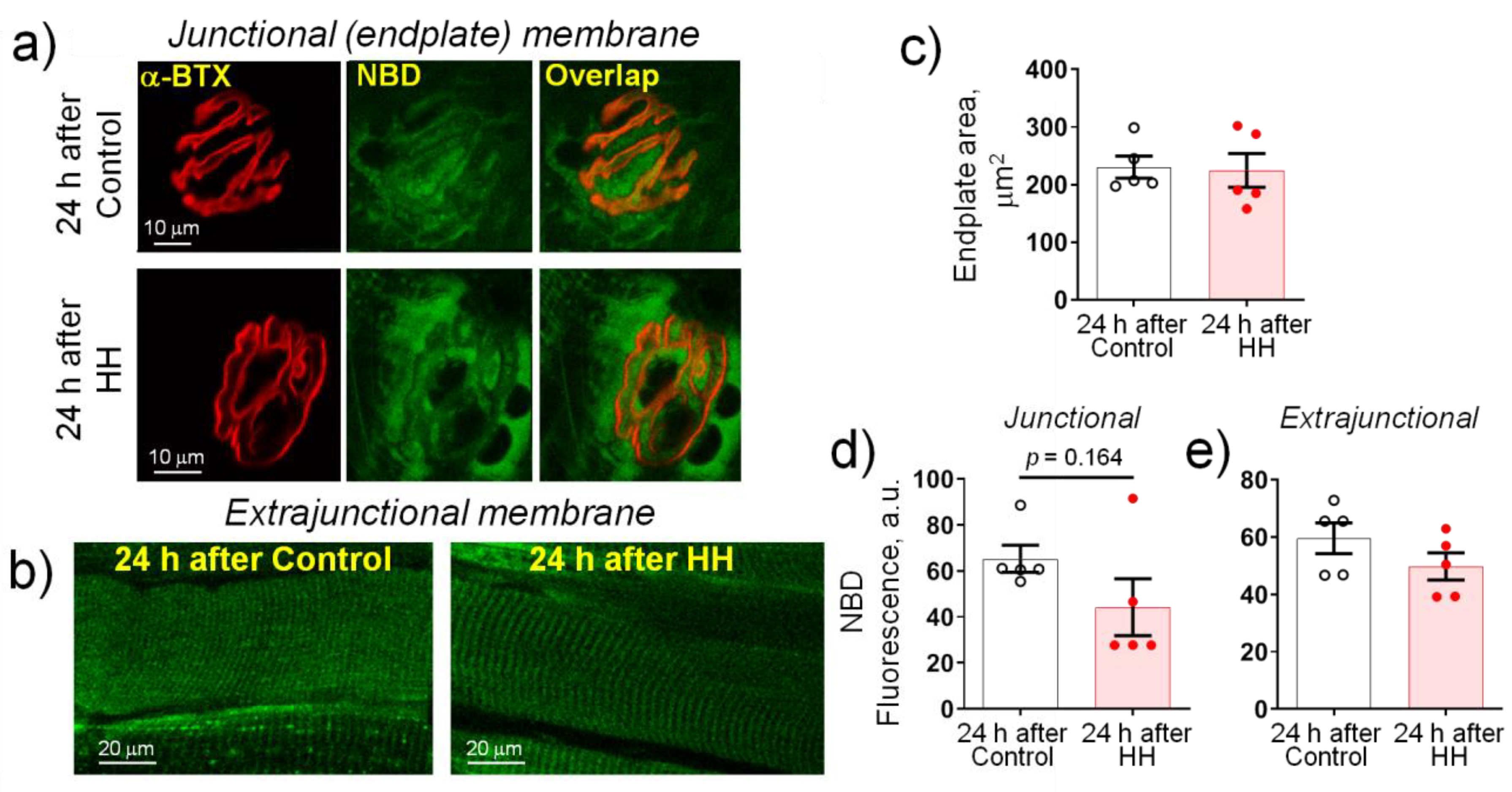
2.4. Hypobaric Hypoxia Does Not Affect the Plasma Membrane Lipid-Ordered Phase
2.5. Hypobaric Hypoxia Protects against Disuse-Induced Disruption of Soleus Muscle Electrogenesis
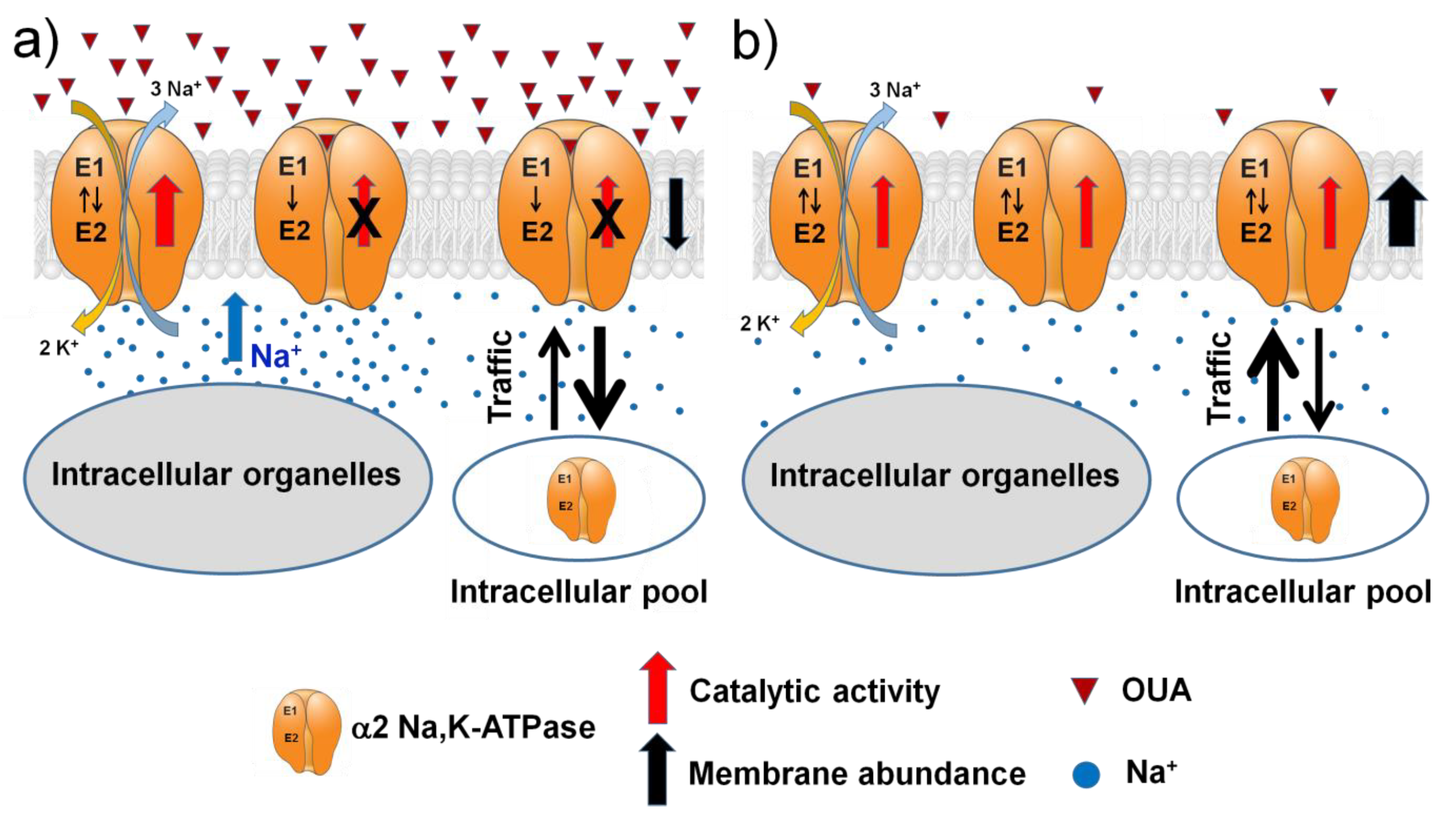
3. Discussion
- A single HH did not affect serum corticosterone levels, cellular redox status, and LDH activity, which emphasizes the mild nature of the hypoxic state.
- In the diaphragm muscle, HH stably increased the α2 Na,K-ATPase isozyme electrogenic activity and hyperpolarized the sarcolemma within 24 h.
- These changes were accompanied by a steady decrease in the serum level of endogenous ouabain, a specific ligand of the Na,K-ATPase, as well as an increase in the TBARS production.
- HH increased the α2 Na,K-ATPase membrane abundance without changing its total protein content and did not affect the plasma membrane lipid-ordered phase.
- Intact diaphragm muscles exposed to the hypoxic incubation solution for 1 h showed a similar but reversible membrane hyperpolarization.
- In the soleus muscle, HH protected against disuse-induced membrane depolarization.
4. Materials and Methods
4.1. Animals
4.2. Biochemical Analyses of Blood and Tissue Samples
4.3. Membrane Potential Recording
4.4. Measurement of the Na,K-ATPase Electrogenic Activity
4.5. Western Blot Assays
4.6. Confocal Microscopy Imaging
4.7. Materials
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baldwin, K.M.; Haddad, F.; Pandorf, C.E.; Roy, R.R.; Edgerton, V.R. Alterations in muscle mass and contractile phenotype in response to unloading models: Role of transcriptional/pretranslational mechanisms. Front. Physiol. 2013, 4, 284. [Google Scholar] [CrossRef]
- Bodine, S.C.; Edward, F. Adolph distinguished lecture. Skeletal muscle atrophy: Multiple pathways leading to a common outcome. J. Appl. Physiol. 2020, 129, 272–282. [Google Scholar] [CrossRef]
- Richalet, J.P. The invention of hypoxia. J. Appl. Physiol. 2021, 130, 1573–1582. [Google Scholar] [CrossRef]
- Lewis, P.; O’Halloran, K.D. Diaphragm Muscle Adaptation to Sustained Hypoxia: Lessons from Animal Models with Relevance to High Altitude and Chronic Respiratory Diseases. Front. Physiol. 2016, 7, 623. [Google Scholar] [CrossRef]
- Rathor, R.; Agrawal, A.; Kumar, R.; Suryakumar, G.; Singh, S.N. Ursolic acid ameliorates hypobaric hypoxia-induced skeletal muscle protein loss via upregulating Akt pathway: An experimental study using rat model. IUBMB Life 2021, 73, 375–389. [Google Scholar] [CrossRef]
- Agrawal, A.; Rathor, R.; Kumar, R.; Singh, S.N.; Kumar, B.; Suryakumar, G. Endogenous dipeptide-carnosine supplementation ameliorates hypobaric hypoxia-induced skeletal muscle loss via attenuating endoplasmic reticulum stress response and maintaining proteostasis. IUBMB Life 2022, 74, 101–116. [Google Scholar] [CrossRef]
- Lemieux, P.; Birot, O. Altitude, Exercise, and Skeletal Muscle Angio-Adaptive Responses to Hypoxia: A Complex Story. Front. Physiol. 2021, 12, 735557. [Google Scholar] [CrossRef]
- Debevec, T.; Ganse, B.; Mittag, U.; Eiken, O.; Mekjavic, I.B.; Rittweger, J. Hypoxia Aggravates Inactivity-Related Muscle Wasting. Front. Physiol. 2018, 9, 494. [Google Scholar] [CrossRef]
- Alayash, A.I. The Impact of COVID-19 Infection on Oxygen Homeostasis: A Molecular Perspective. Front. Physiol. 2021, 12, 711976. [Google Scholar] [CrossRef]
- Farr, E.; Wolfe, A.R.; Deshmukh, S.; Rydberg, L.; Soriano, R.; Walter, J.M.; Boon, A.J.; Wolfe, L.F.; Franz, C.K. Diaphragm dysfunction in severe COVID-19 as determined by neuromuscular ultrasound. Ann. Clin. Transl. Neurol. 2021, 8, 1745–1749. [Google Scholar] [CrossRef]
- FitzMaurice, T.S.; McCann, C.; Walshaw, M.; Greenwood, J. Unilateral diaphragm paralysis with COVID-19 infection. BMJ Case Rep. 2021, 14, e243115. [Google Scholar] [CrossRef]
- McMorrow, C.; Fredsted, A.; Carberry, J.; O’Connell, R.A.; Bradford, A.; Jones, J.F.; O’Halloran, K.D. Chronic hypoxia increases rat diaphragm muscle endurance and sodium-potassium ATPase pump content. Eur. Respir. J. 2011, 37, 1474–1481. [Google Scholar] [CrossRef]
- Zuo, L.; Re, A.T.; Roberts, W.J.; Zhou, T.; Hemmelgarn, B.T.; Pannell, B.K. Hypoxic preconditioning mitigates diaphragmatic skeletal muscle fatigue during reoxygenation via ROS and ERK signaling. Med. Sci. Sports Exerc. 2015, 47, 330. [Google Scholar] [CrossRef]
- Lewis, P.; Sheehan, D.; Soares, R.; Coelho, A.V.; O’Halloran, K.D. Redox Remodeling Is Pivotal in Murine Diaphragm Muscle Adaptation to Chronic Sustained Hypoxia. Am. J. Respir. Cell Mol. Biol. 2016, 55, 12–23. [Google Scholar] [CrossRef]
- Chuang, C.-C.; Zhou, T.; Olfert, I.M.; Zuo, L. Hypoxic Preconditioning Attenuates Reoxygenation-Induced Skeletal Muscle Dysfunction in Aged Pulmonary TNF-α Overexpressing Mice. Front. Physiol. 2018, 9, 1720. [Google Scholar] [CrossRef]
- Sejersted, O.M.; Sjogaard, G. Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol. Rev. 2000, 80, 1411–1481. [Google Scholar] [CrossRef]
- Clausen, T. Na+-K+ pump regulation and skeletal muscle contractility. Physiol. Rev. 2003, 83, 1269–1324. [Google Scholar] [CrossRef]
- Clausen, T. Quantification of Na+,K+ pumps and their transport rate in skeletal muscle: Functional significance. J. Gen. Physiol. 2013, 142, 327–345. [Google Scholar] [CrossRef]
- Bogdanova, A.; Petrushanko, I.Y.; Hernansanz-Agustín, P.; Martínez-Ruiz, A. “Oxygen sensing” by Na,K-ATPase: These miraculous thiols. Front. Physiol. 2016, 7, 314. [Google Scholar] [CrossRef]
- Blanco, G.; Mercer, R.W. Isozymes of the Na-K-ATPase: Heterogeneity in structure, diversity in function. Am. J. Physiol. 1998, 275, F633–F655. [Google Scholar] [CrossRef]
- Matchkov, V.V.; Krivoi, I.I. Specialized functional diversity and interactions of the Na,K-ATPase. Front Physiol. 2016, 7, 179. [Google Scholar] [CrossRef]
- Pirkmajer, S.; Chibalin, A.V. Na,K-ATPase regulation in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E1–E31. [Google Scholar] [CrossRef]
- Clausen, M.V.; Hilbers, F.; Poulsen, H. The structure and function of the Na,K-ATPase isoforms in health and disease. Front. Physiol. 2017, 8, 371. [Google Scholar] [CrossRef]
- Blaustein, M.P.; Hamlyn, J.M. Ouabain, endogenous ouabain and ouabain-like factors: The Na+ pump/ouabain receptor, its linkage to NCX, and its myriad functions. Cell Calcium 2020, 86, 102159. [Google Scholar] [CrossRef]
- Orlowski, J.; Lingrel, J.B. Tissue-Specific and developmental regulation of rat Na,K-ATPase catalytic α isoform and β subunit mRNAs. J. Biol. Chem. 1988, 263, 10436–10442. [Google Scholar] [CrossRef]
- He, S.; Shelly, D.A.; Moseley, A.E.; James, P.F.; James, J.H.; Paul, R.J.; Lingrel, J.B. The α1- and α2-isoforms of Na-K-ATPase play different roles in skeletal muscle contractility. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, R917–R925. [Google Scholar] [CrossRef]
- DiFranco, M.; Hakimjavadi, H.; Lingrel, J.B.; Heiny, J.A. Na,K-ATPase α2 activity in mammalian skeletal muscle T-tubules is acutely stimulated by extracellular K+. J. Gen. Physiol. 2015, 146, 281–294. [Google Scholar] [CrossRef]
- Heiny, J.A.; Kravtsova, V.V.; Mandel, F.; Radzyukevich, T.L.; Benziane, B.; Prokofiev, A.V.; Pedersen, S.E.; Chibalin, A.V.; Krivoi, I.I. The nicotinic acetylcholine receptor and the Na,K-ATPase α2 isoform interact to regulate membrane electrogenesis in skeletal muscle. J. Biol. Chem. 2010, 285, 28614–28626. [Google Scholar] [CrossRef]
- Blaustein, M.P. Physiological effects of endogenous ouabain: Control of intracellular Ca2+ stores and cell responsiveness. Am. J. Physiol. Cell Physiol. 1993, 264, C1367–C1387. [Google Scholar] [CrossRef]
- Schoner, W.; Scheiner-Bobis, G. Endogenous and exogenous cardiac glycosides and their mechanisms of action. Am. J. Cardiovasc. Drugs 2007, 7, 173–189. [Google Scholar] [CrossRef]
- Bagrov, A.Y.; Shapiro, J.I.; Fedorova, O.V. Endogenous cardiotonic steroids: Physiology, pharmacology, and novel therapeutic targets. Pharmacol. Rev. 2009, 61, 9–38. [Google Scholar] [CrossRef]
- Lingrel, J.B. The physiological significance of the cardiotonic steroid/ouabain-binding site of the Na,K-ATPase. Annu. Rev. Physiol. 2010, 72, 395–412. [Google Scholar] [CrossRef]
- Kravtsova, V.V.; Bouzinova, E.V.; Chibalin, A.V.; Matchkov, V.V.; Krivoi, I.I. Isoform-Specific Na,K-ATPase and membrane cholesterol remodeling in the motor endplates in distinct mouse models of myodystrophy. Am. J. Physiol. Cell Physiol. 2020, 318, C1030–C1041. [Google Scholar] [CrossRef]
- Kravtsova, V.V.; Matchkov, V.V.; Bouzinova, E.V.; Vasiliev, A.N.; Razgovorova, I.A.; Heiny, J.A.; Krivoi, I.I. Isoform-specific Na,K-ATPase alterations precede disuse-induced atrophy of rat soleus muscle. Biomed. Res. Int. 2015, 2015, 720172. [Google Scholar] [CrossRef]
- Kravtsova, V.V.; Petrov, A.M.; Matchkov, V.V.; Bouzinova, E.V.; Vasiliev, A.N.; Benziane, B.; Zefirov, A.L.; Chibalin, A.V.; Heiny, J.A.; Krivoi, I.I. Distinct α2 Na,K-ATPase membrane pools are differently involved in early skeletal muscle remodeling during disuse. J. Gen. Physiol. 2016, 147, 175–188. [Google Scholar] [CrossRef]
- Krivoi, I.I.; Kravtsova, V.V.; Altaeva, E.G.; Kubasov, I.V.; Prokofiev, A.V.; Drabkina, T.M.; Nikolsky, E.E.; Shenkman, B.S. A decrease in the electrogenic contribution of Na,K-ATPase and resting membrane potential as a possible mechanism of Ca2+ accumulation in musculus soleus of the rat at short-term gravity unloading. Biofizika 2008, 53, 1051–1057. [Google Scholar] [PubMed]
- Shenkman, B.S. How postural muscle senses disuse? Early signs and signals. Int. J. Mol. Sci. 2020, 21, 5037. [Google Scholar] [CrossRef]
- Canfora, I.; Tarantino, N.; Pierno, S. Metabolic Pathways and Ion Channels Involved in Skeletal Muscle Atrophy: A Starting Point for Potential Therapeutic Strategies. Cells 2022, 11, 2566. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, B.O.; Tan, Z.; Zhang, D.; Ma, H. Hypoxic preconditioning increases the protective effect of bone marrow mesenchymal stem cells on spinal cord ischemia/reperfusion injury. Mol. Med. Rep. 2016, 13, 1953–1960. [Google Scholar] [CrossRef]
- Torosyan, R.; Huang, S.; Bommi, P.V.; Tiwari, R.; An, S.Y.; Schonfeld, M.; Rajendran, G.; Kavanaugh, M.A.; Gibbs, B.; Truax, A.D.; et al. Hypoxic preconditioning protects against ischemic kidney injury through the IDO1/kynurenine pathway. Cell Rep. 2021, 36, 109547. [Google Scholar] [CrossRef]
- Filaretova, L.; Komkova, O.; Sudalina, M.; Yarushkina, N. Non-Invasive Remote Ischemic Preconditioning May Protect the Gastric Mucosa Against Ischemia-Reperfusion-Induced Injury Through Involvement of Glucocorticoids. Front. Pharmacol. 2021, 12, 682643. [Google Scholar] [CrossRef]
- De Angelis, C.; Haupert, G.T., Jr. Hypoxia triggers release of an endogenous inhibitor of Na+-K+-ATPase from midbrain and adrenal. Am. J. Physiol. 1998, 274, F182–F188. [Google Scholar] [CrossRef]
- Rybnikova, E.; Nalivaeva, N. Glucocorticoid-Dependent Mechanisms of Brain Tolerance to Hypoxia. Int. J. Mol. Sci. 2021, 22, 7982. [Google Scholar] [CrossRef]
- Kravtsova, V.V.; Bouzinova, E.V.; Matchkov, V.V.; Krivoi, I.I. Skeletal muscle Na,K-ATPase as a target for circulating ouabain. Int. J. Mol. Sci. 2020, 21, 2875. [Google Scholar] [CrossRef]
- Cornelius, F.; Habeck, M.; Kanai, R.; Toyoshima, C.; Karlish, S.J. General and specific lipid-protein interactions in Na,K-ATPase. Biochim. Biophys. Acta 2015, 1848, 1729–1743. [Google Scholar] [CrossRef]
- Habeck, M.; Haviv, H.; Katz, A.; Kapri-Pardes, E.; Ayciriex, S.; Shevchenko, A.; Ogawa, H.; Toyoshima, C.; Karlish, S.J. Stimulation, inhibition, or stabilization of Na,K-ATPase caused by specific lipid interactions at distinct sites. J. Biol. Chem. 2015, 290, 4829–4842. [Google Scholar] [CrossRef]
- Petrov, A.M.; Kravtsova, V.V.; Matchkov, V.V.; Vasiliev, A.N.; Zefirov, A.L.; Chibalin, A.V.; Heiny, J.A.; Krivoi, I.I. Membrane lipid rafts are disturbed in the response of rat skeletal muscle to short-term disuse. Am. J. Physiol. Cell Physiol. 2017, 312, C627–C637. [Google Scholar] [CrossRef]
- Loura, L.M.; Fedorov, A.; Prieto, M. Exclusion of a cholesterol analog from the cholesterol-rich phase in model membranes. Biochim. Biophys. Acta 2001, 1511, 236–243. [Google Scholar] [CrossRef]
- Ostašov, P.; Sýkora, J.; Brejchová, J.; Olżyńska, A.; Hof, M.; Svoboda, P. FLIM studies of 22- and 25-NBD-cholesterol in living HEK293 cells: Plasma membrane change induced by cholesterol depletion. Chem. Phys. Lipids 2013, 167–168, 62–69. [Google Scholar] [CrossRef]
- Filatov, G.N.; Pinter, M.J.; Rich, M.M. Resting potential-dependent regulation of the voltage sensitivity of sodium channel gating in rat skeletal muscle in vivo. J. Gen. Physiol. 2005, 126, 161–172. [Google Scholar] [CrossRef]
- Miles, M.T.; Cottey, E.; Cottey, A.; Stefanski, C.; Carlson, C.G. Reduced resting potentials in dystrophic (mdx) muscle fibers are secondary to NF-κB-dependent negative modulation of ouabain sensitive Na+-K+ pump activity. J. Neurosci. 2011, 303, 53–60. [Google Scholar] [CrossRef]
- Silva, L.F.; Hoffmann, M.S.; Rambo, L.M.; Ribeiro, L.R.; Lima, F.D.; Furian, A.F.; Oliveira, M.S.; Fighera, M.R.; Royes, L.F. The involvement of Na+,K+-ATPase activity and free radical generation in the susceptibility to pentylenetetrazol-induced seizures after experimental traumatic brain injury. J. Neurol. Sci. 2011, 308, 35–40. [Google Scholar] [CrossRef]
- Malfatti, C.R.; Burgos, L.T.; Rieger, A.; Rüdger, C.L.; Túrmina, J.A.; Pereira, R.A.; Pavlak, J.L.; Silva, L.A.; Osiecki, R. Decreased erythrocyte Na+,K+-ATPase activity and increased plasma TBARS in prehypertensive patients. Sci. World J. 2012, 2012, 348246. [Google Scholar] [CrossRef]
- Omotayo, T.I.; Akinyemi, G.S.; Omololu, P.A.; Ajayi, B.O.; Akindahunsi, A.A.; Rocha, J.B.; Kade, I.J. Possible involvement of membrane lipids peroxidation and oxidation of catalytically essential thiols of the cerebral transmembrane sodium pump as component mechanisms of iron-mediated oxidative stress-linked dysfunction of the pump’s activity. Redox Biol. 2015, 4, 234–241. [Google Scholar] [CrossRef]
- Catalá, A. Lipid peroxidation modifies the picture of membranes from the “Fluid Mosaic Model” to the “Lipid Whisker Model”. Biochimie 2012, 94, 101–109. [Google Scholar] [CrossRef]
- Li, W.; Duan, A.; Xing, Y.; Xu, L.; Yang, J. Transcription-Based Multidimensional Regulation of Fatty Acid Metabolism by HIF1α in Renal Tubules. Front. Cell Dev. Biol. 2021, 9, 690079. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, Q.; Zou, J.; Fan, J.; Li, Y.; Luo, Z. Chronic Intermittent Hypoxia Exposure Alternative to Exercise Alleviates High-Fat-Diet-Induced Obesity and Fatty Liver. Int. J. Mol. Sci. 2022, 23, 5209. [Google Scholar] [CrossRef]
- Li, Z.; Langhans, S.A. Transcriptional regulators of Na,K-ATPase subunits. Front. Cell Dev. Biol. 2015, 3, 66. [Google Scholar] [CrossRef]
- Thompson, C.B.; Dorup, I.; Ahn, J.; Leong, P.K.; McDonough, A.A. Glucocorticoids increase sodium pump α2- and β1-subunit abundance and mRNA in rat skeletal muscle. Am. J. Physiol. Cell Physiol. 2001, 280, C509–C516. [Google Scholar] [CrossRef]
- Lichtstein, D.; Ilani, A.; Rosen, H.; Horesh, N.; Singh, S.V.; Buzaglo, N.; Hodes, A. Na+,K+-ATPase signaling and bipolar disorder. Int. J. Mol. Sci. 2018, 19, 2314. [Google Scholar] [CrossRef]
- Singh, S.V.; Fedorova, O.V.; Wei, W.; Rosen, H.; Horesh, N.; Ilani, A.; Lichtstein, D. Na+,K+-ATPase α Isoforms and Endogenous Cardiac Steroids in Prefrontal Cortex of Bipolar Patients and Controls. Int. J. Mol. Sci. 2020, 21, 5912. [Google Scholar] [CrossRef]
- Khalaf, F.K.; Dube, P.; Mohamed, A.; Tian, J.; Malhotra, D.; Haller, S.T.; Kennedy, D.J. Cardiotonic steroids and the sodium trade balance: New insights into trade-off mechanisms mediated by the Na+/K+-ATPase. Int. J. Mol. Sci. 2018, 19, 2576. [Google Scholar] [CrossRef]
- Oshiro, N.; Dostanic-Larson, I.; Neumann, J.C.; Lingrel, J.B. The ouabain-binding site of the α2 isoform of Na,K-ATPase plays a role in blood pressure regulation during pregnancy. Am. J. Hypertens. 2010, 23, 1279–1285. [Google Scholar] [CrossRef]
- Dvela, M.; Rosen, H.; Ben-Ami, H.C.; Lichtstein, D. Endogenous ouabain regulates cell viability. Am. J. Physiol. Cell Physiol. 2012, 302, C442–C452. [Google Scholar] [CrossRef]
- Bauer, N.; Müller-Ehmsen, J.; Krämer, U.; Hambarchian, N.; Zobel, C.; Schwinger, R.H.; Neu, H.; Kirch, U.; Grünbaum, E.G.; Schoner, W. Ouabain-Like compound changes rapidly on physical exercise in humans and dogs: Effects of β-blockade and angiotensin-converting enzyme inhibition. Hypertension 2005, 45, 1024–1028. [Google Scholar] [CrossRef]
- Ferri, C.; Bellini, C.; Coassin, S.; De Angelis, C.; Perrone, A.; Santucci, A. Plasma endogenous digoxin-like substance levels are dependent on blood O2 in man. Clin. Sci. 1994, 87, 447–451. [Google Scholar] [CrossRef]
- Bagrov, A.Y.; Fedorova, O.V.; Austin-Lane, J.L.; Dmitrieva, R.I.; Anderson, D.E. Endogenous marinobufageninlike immunoreactive factor and Na+-K+-ATPase inhibition during voluntary hypoventilation. Hypertension 1995, 26, 781–788. [Google Scholar] [CrossRef]
- De Angelis, C.; Farrace, S.; Urbani, L.; Porcú, S.; Ferri, C.; D’Amelio, R.; Santucci, A.; Balsano, F. Effects of high altitude exposure on plasma and urinary digoxin-like immunoreactive substance. Am. J. Hypertens. 1992, 5, 600–607. [Google Scholar] [CrossRef]
- Green, H.; Roy, B.; Grant, S.; Burnett, M.; Tupling, R.; Otto, C.; Pipe, A.; McKenzie, D. Downregulation in muscle Na+-K+-ATPase following a 21-day expedition to 6,194 m. J. Appl. Physiol. 2000, 88, 634–640. [Google Scholar] [CrossRef][Green Version]
- Cherniavsky-Lev, M.; Golani, O.; Karlish, S.J.; Garty, H. Ouabain-induced internalization and lysosomal degradation of the Na+/K+-ATPase. J. Biol. Chem. 2014, 289, 1049–1059. [Google Scholar] [CrossRef]
- Xu, Y.; Marck, P.; Huang, M.; Xie, J.X.; Wang, T.; Shapiro, J.I.; Cai, L.; Feng, F.; Xie, Z. Biased Effect of Cardiotonic Steroids on Na/K-ATPase-Mediated Signal Transduction. Mol. Pharmacol. 2021, 99, 217–225. [Google Scholar] [CrossRef]
- Holthouser, K.A.; Mandal, A.; Merchant, M.L.; Schelling, J.R.; Delamere, N.A.; Valdes, R.R., Jr.; Tyagi, S.C.; Lederer, E.D.; Khundmiri, S.J. Ouabain stimulates Na-K-ATPase through a sodium/hydrogen exchanger-1 (NHE-1)-dependent mechanism in human kidney proximal tubule cells. Am. J. Physiol. Ren. Physiol. 2010, 299, F77–F90. [Google Scholar] [CrossRef]
- Kravtsova, V.V.; Paramonova, I.I.; Vilchinskaya, N.A.; Tishkova, M.V.; Matchkov, V.V.; Shenkman, B.S.; Krivoi, I.I. Chronic Ouabain Prevents Na,K-ATPase Dysfunction and Targets AMPK and IL-6 in Disused Rat Soleus Muscle. Int. J. Mol. Sci. 2021, 22, 3920. [Google Scholar] [CrossRef]
- Saunders, R.; Scheiner-Bobis, G. Ouabain stimulates endothelin release and expression in human endothelial cells without inhibiting the sodium pump. Eur. J. Biochem. 2004, 271, 1054–1062. [Google Scholar] [CrossRef]
- Klimanova, E.A.; Tverskoi, A.M.; Koltsova, S.V.; Sidorenko, S.V.; Lopina, O.D.; Tremblay, J.; Hamet, P.; Kapilevich, L.V.; Orlov, S.N. Time- and dose-dependent actions of cardiotonic steroids on transcriptome and intracellular content of Na+ and K+: A comparative analysis. Sci. Rep. 2017, 7, 45403. [Google Scholar] [CrossRef]
- Gao, J.; Wymore, R.S.; Wang, Y.; Gaudette, G.R.; Krukenkamp, I.B.; Cohen, I.S.; Mathias, R.T. Isoform-Specific stimulation of cardiac Na/K pumps by nanomolar concentrations of glycosides. J. Gen. Physiol. 2002, 119, 297–312. [Google Scholar] [CrossRef]
- Askari, A. Na+,K+-ATPase: On the number of the ATP sites of the functional unit. J. Bioenerg. Biomembr. 1987, 19, 359–374. [Google Scholar]
- Ketchem, C.J.; Conner, C.D.; Murray, R.D.; DuPlessis, M.; Lederer, E.D.; Wilkey, D.; Merchant, M.; Khundmiri, S.J. Low dose ouabain stimulates Na-K ATPase α1 subunit association with angiotensin II type 1 receptor in renal proximal tubule cells. Biochim. Biophys. Acta 2016, 1863, 2624–2636. [Google Scholar] [CrossRef]
- Launikonis, B.S.; Cully, T.R.; Csernoch, L.; Stephenson, D.G. NHE- and diffusion-dependent proton fluxes across the tubular system membranes of fast-twitch muscle fibers of the rat. J. Gen. Physiol. 2018, 150, 95–110. [Google Scholar] [CrossRef]
- Blaustein, M.P.; Chen, L.; Hamlyn, J.M.; Leenen, F.H.; Lingrel, J.B.; Wier, W.G.; Zhang, J. Pivotal role of α2 Na+ pumps and their high affinity ouabain binding site in cardiovascular health and disease. J. Physiol. 2016, 594, 6079–6103. [Google Scholar] [CrossRef]
- Rognant, S.; Kravtsova, V.V.; Bouzinova, E.V.; Melnikova, E.V.; Krivoi, I.I.; Pierre, S.V.; Aalkjaer, C.; Jepps, T.A.; Matchkov, V.V. The microtubule network enables Src kinase interaction with the Na,K-ATPase to generate Ca2+ flashes in smooth muscle cells. Front. Physiol. 2022, 13, 1007340. [Google Scholar] [CrossRef]
- Altamirano, F.; Eltit, J.M.; Robin, G.; Linares, N.; Ding, X.; Pessah, I.N.; Allen, P.D.; López, J.R. Ca2+ influx via the Na+/Ca2+ exchanger is enhanced in malignant hyperthermia skeletal muscle. J. Biol. Chem. 2014, 289, 19180–19190. [Google Scholar] [CrossRef]
- Katz, A.; Lifshitz, Y.; Bab-Dinitz, E.; Kapri-Pardes, E.; Goldshleger, R.; Tal, D.M.; Karlish, S.J. Selectivity of digitalis glycosides for isoforms of human Na,K-ATPase. J. Biol. Chem. 2010, 285, 19582–19592. [Google Scholar] [CrossRef]
- Cherniavsky Lev, M.; Karlish, S.J.; Garty, H. Cardiac glycosides induced toxicity in human cells expressing α1-, α2-, or α3-isoforms of Na-K-ATPase. Am. J. Physiol. Cell Physiol. 2015, 309, C126–C135. [Google Scholar] [CrossRef]
- Forsberg, A.M.; Bergström, J.; Lindholm, B.; Hultman, E. Resting membrane potential of skeletal muscle calculated from plasma and muscle electrolyte and water contents. Clin. Sci. 1997, 92, 391–396. [Google Scholar] [CrossRef]
- Wyckelsma, V.L.; McKenna, M.J. Effects of Age on Na+,K+-ATPase Expression in Human and Rodent Skeletal Muscle. Front. Physiol. 2016, 7, 316. [Google Scholar] [CrossRef]
- McBride, T. Increased depolarization, prolonged recovery and reduced adaptation of the resting membrane potential in aged rat skeletal muscles following eccentric contractions. Mech. Ageing Dev. 2000, 115, 127–138. [Google Scholar] [CrossRef]
- Nørgaard, A. Quantification of the Na,K-pumps in mammalian skeletal muscle. Acta Pharmacol. Toxicol. 1986, 58 (Suppl. 1), 1–34. [Google Scholar] [CrossRef]
- Murphy, K.T.; Aughey, R.J.; Petersen, A.C.; Clark, S.A.; Goodman, C.; Hawley, J.A.; Cameron-Smith, D.; Snow, R.J.; McKenna, M.J. Effects of endurance training status and sex differences on Na+,K+-pump mRNA expression, content and maximal activity in human skeletal muscle. Acta Physiol. 2007, 189, 259–269. [Google Scholar] [CrossRef]
- von Arnim, C.A.; Etrich, S.M.; Timmler, M.; Riepe, M.W. Gender-dependent hypoxic tolerance mediated via gender-specific mechanisms. J. Neurosci. Res. 2002, 68, 84–88. [Google Scholar] [CrossRef]
- Obradovic, M.; Stanimirovic, J.; Panic, A.; Bogdanovic, N.; Sudar-Milovanovic, E.; Cenic-Milosevic, D.; Isenovic, E.R. Regulation of Na+/K+-ATPase by Estradiol and IGF-1 in Cardio-Metabolic Diseases. Curr. Pharm. Des. 2017, 23, 1551–1561. [Google Scholar] [CrossRef]
- Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011; pp. 1–246.
- Morey-Holton, E.; Globus, R.K.; Kaplansky, A.; Durnova, G. The hindlimb unloading rat model: Literature overview, technique update and comparison with space flight data. Adv. Space Biol. Med. 2005, 10, 7–40. [Google Scholar] [CrossRef]
- Akerboom, T.; Sies, H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol. 1981, 77, 373–381. [Google Scholar] [CrossRef]
- Dasgupta, A.; Klein, K. Methods for measuring oxidative stress in the laboratory. In Antioxidants in Food, Vitamins and Supplements; Elsevier: Amsterdam, The Netherlands, 2014; pp. 19–40. [Google Scholar] [CrossRef]
- Bergmeyer, H.U.; Bernt, E.; Hess, B. Lactic dehydrogenase. In Methods of Enzymatic Analysis; Academic Press: Cambridge, MA, USA, 1965; pp. 736–743. [Google Scholar] [CrossRef]
- Krivoi, I.I.; Drabkina, T.M.; Kravtsova, V.V.; Vasiliev, A.N.; Eaton, M.J.; Skatchkov, S.N.; Mandel, F. On the functional interaction between nicotinic acetylcholine receptor and Na+,K+-ATPase. Pflug. Arch. 2006, 452, 756–765. [Google Scholar] [CrossRef]
- Krivoi, I.; Vasiliev, A.; Kravtsova, V.; Dobretsov, M.; Mandel, F. Porcine kidney extract contains factor(s) that inhibit the ouabain-sensitive isoform of Na,K-ATPase (α2) in rat skeletal muscle: A convenient electrophysiological assay. Ann. N. Y. Acad. Sci. 2003, 986, 639–641. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kravtsova, V.V.; Fedorova, A.A.; Tishkova, M.V.; Livanova, A.A.; Matytsin, V.O.; Ganapolsky, V.P.; Vetrovoy, O.V.; Krivoi, I.I. Short-Term Mild Hypoxia Modulates Na,K-ATPase to Maintain Membrane Electrogenesis in Rat Skeletal Muscle. Int. J. Mol. Sci. 2022, 23, 11869. https://doi.org/10.3390/ijms231911869
Kravtsova VV, Fedorova AA, Tishkova MV, Livanova AA, Matytsin VO, Ganapolsky VP, Vetrovoy OV, Krivoi II. Short-Term Mild Hypoxia Modulates Na,K-ATPase to Maintain Membrane Electrogenesis in Rat Skeletal Muscle. International Journal of Molecular Sciences. 2022; 23(19):11869. https://doi.org/10.3390/ijms231911869
Chicago/Turabian StyleKravtsova, Violetta V., Arina A. Fedorova, Maria V. Tishkova, Alexandra A. Livanova, Viacheslav O. Matytsin, Viacheslav P. Ganapolsky, Oleg V. Vetrovoy, and Igor I. Krivoi. 2022. "Short-Term Mild Hypoxia Modulates Na,K-ATPase to Maintain Membrane Electrogenesis in Rat Skeletal Muscle" International Journal of Molecular Sciences 23, no. 19: 11869. https://doi.org/10.3390/ijms231911869
APA StyleKravtsova, V. V., Fedorova, A. A., Tishkova, M. V., Livanova, A. A., Matytsin, V. O., Ganapolsky, V. P., Vetrovoy, O. V., & Krivoi, I. I. (2022). Short-Term Mild Hypoxia Modulates Na,K-ATPase to Maintain Membrane Electrogenesis in Rat Skeletal Muscle. International Journal of Molecular Sciences, 23(19), 11869. https://doi.org/10.3390/ijms231911869





