Effects of Maca on Muscle Hypertrophy in C2C12 Skeletal Muscle Cells
Abstract
:1. Introduction
2. Results
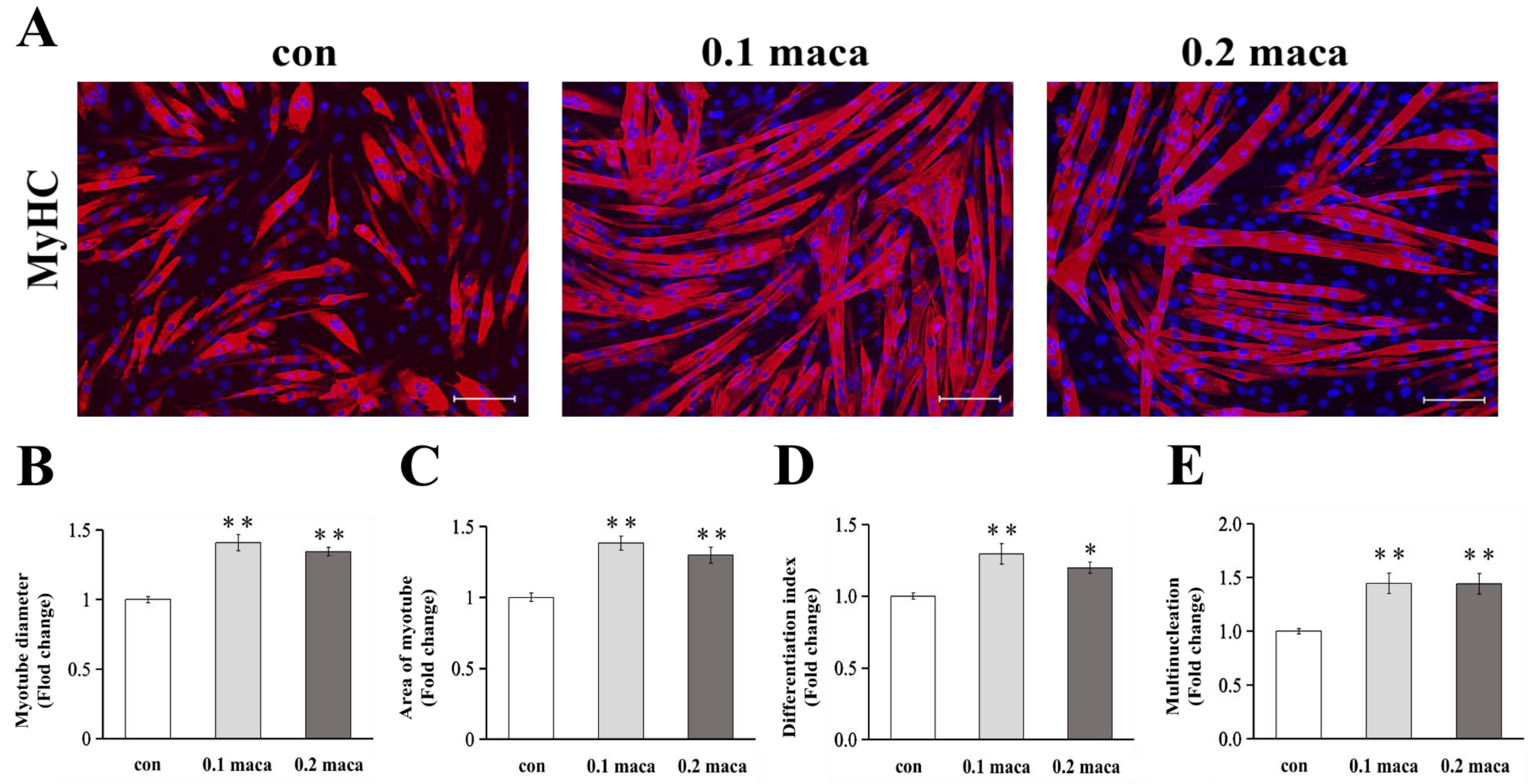
2.1. Adding Maca Promoted Skeletal Muscle Hypertrophy, Differentiation, and Maturation
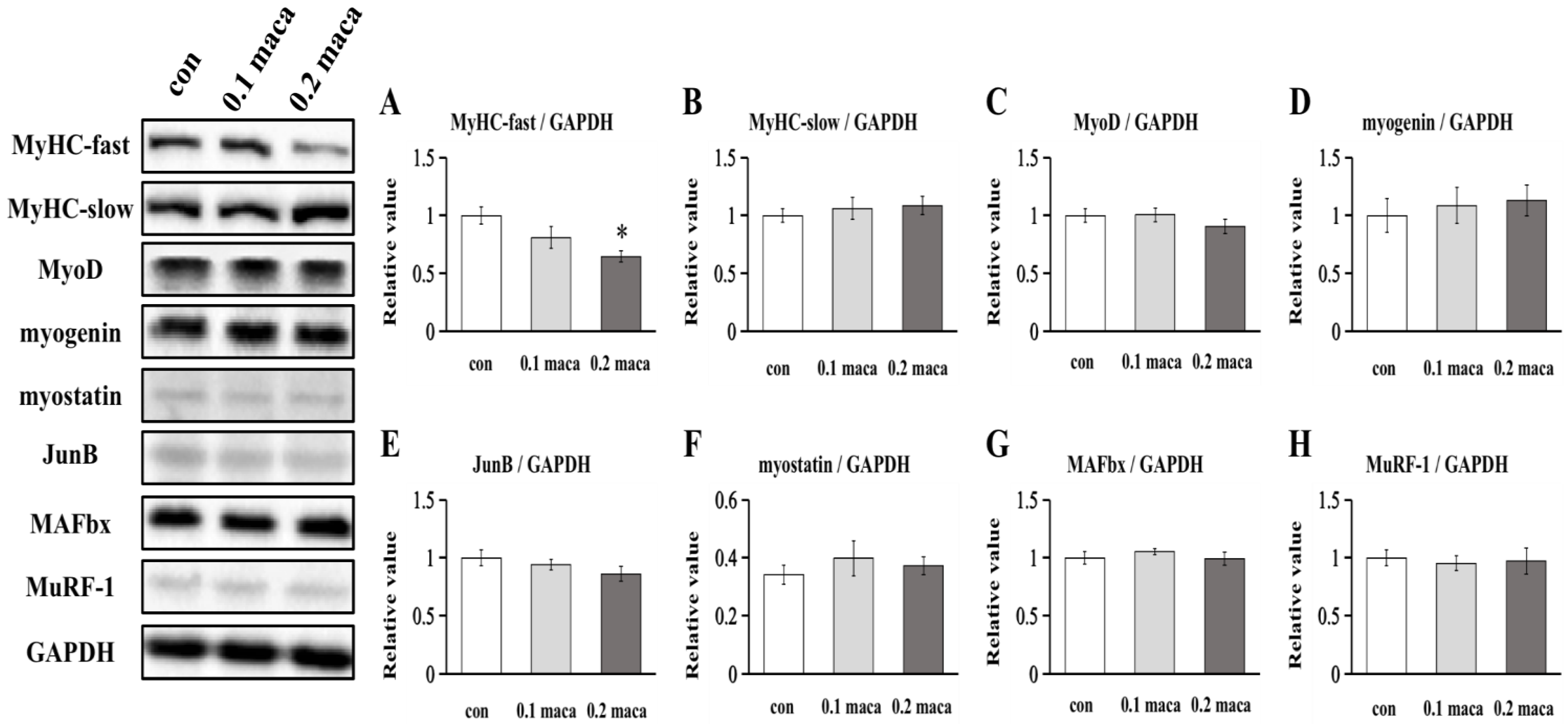
2.2. Effect of Maca on Protein Expression Associated with Muscle Hypertrophy, Differentiation, and Maturation
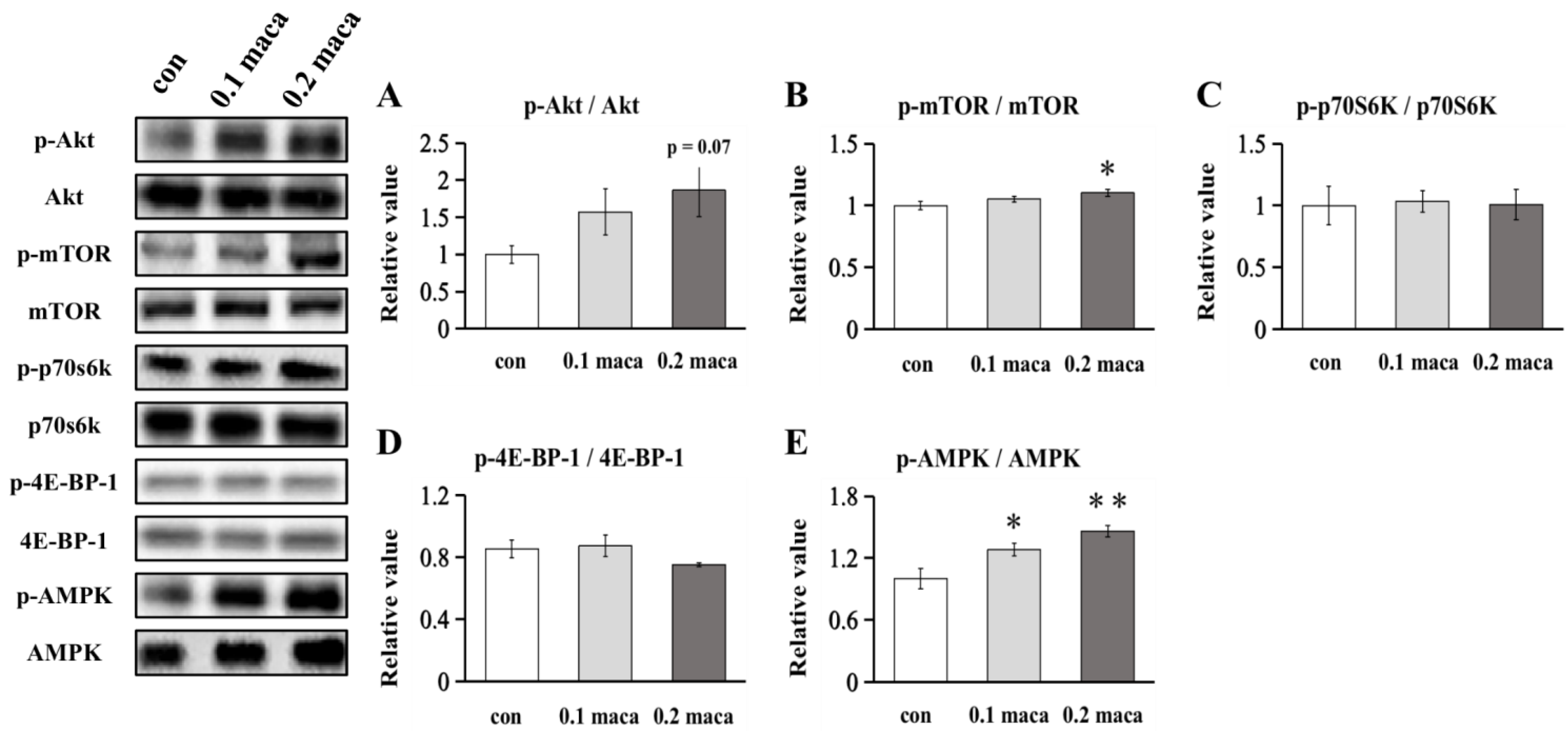
2.3. Effect of Maca on Protein Expression Related to Muscle Protein Synthesis and Metabolism
3. Discussion
4. Materials and Methods
4.1. Cell Culture
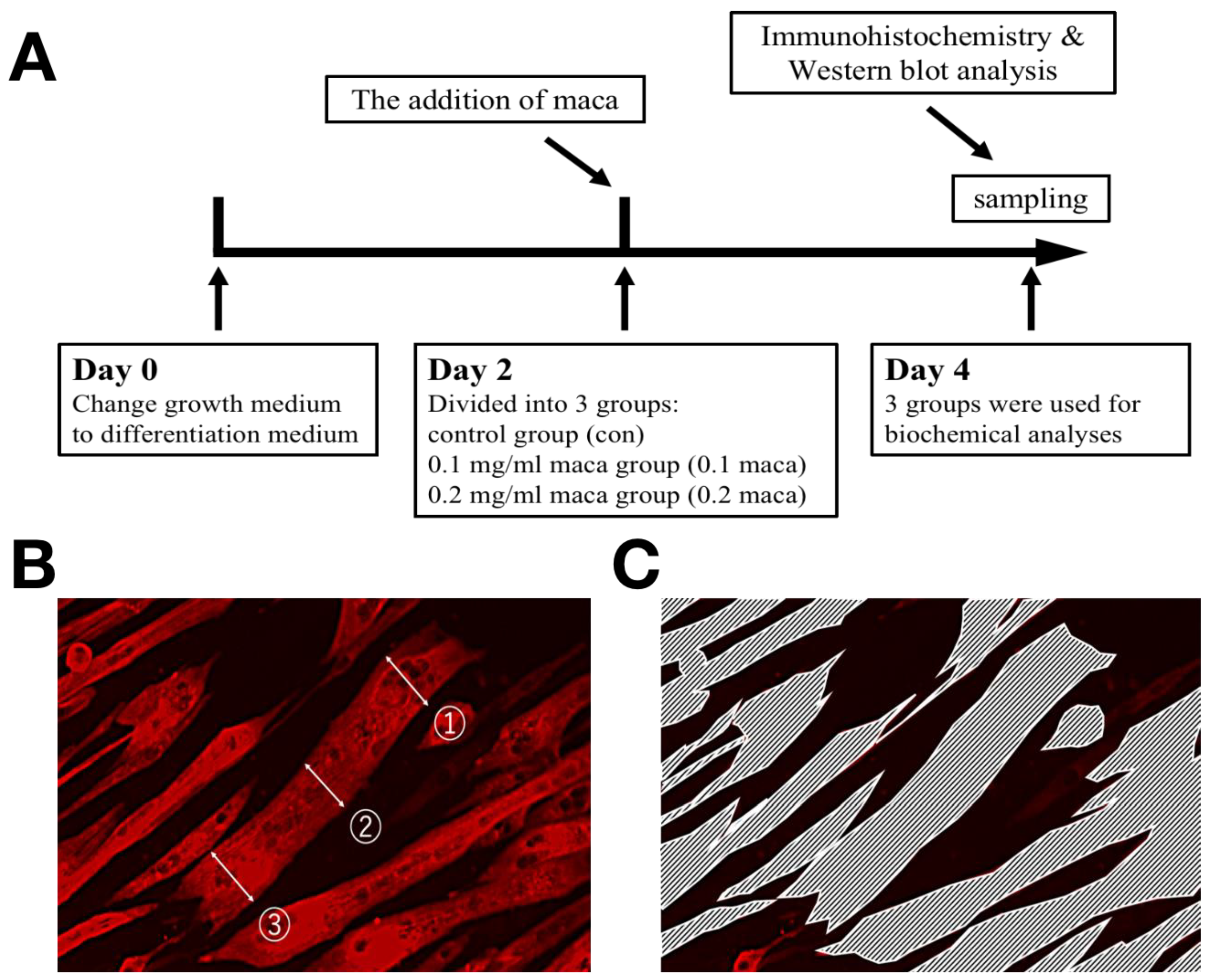
4.2. Experimental Design
4.3. Immunohistochemistry
4.4. Western Blot Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimby, G.; Saltin, B. The Ageing Muscle. Clin. Physiol. 1983, 3, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, E.; Napierała, P.; Podfigurna, A.; Męczekalski, B.; Smolarczyk, R.; Grymowicz, M. The World Health Organization (WHO) Approach to Healthy Ageing. Maturitas 2020, 139, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Rennie, M.J.R.J. Anabolic Resistance: The Effects of Aging, Sexual Dimorphism, and Immobilization on Human Muscle Protein Turnover This Paper Is One of a Selection of Papers Published in This Special Issue, Entitled 14th International Biochemistry of Exercise Conference—Muscles as Molecular and Metabolic Machines, and Has Undergone the Journal’s Usual Peer Review Process. Appl. Physiol. Nutr. Metab. 2009, 34, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Wall, B.T.; Gorissen, S.H.; Pennings, B.; Koopman, R.; Groen, B.B.L.; Verdijk, L.B.; van Loon, L.J.C. Aging Is Accompanied by a Blunted Muscle Protein Synthetic Response to Protein Ingestion. PLoS ONE 2015, 10, e0140903. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhu, F. Chemical Composition and Health Effects of Maca (Lepidium meyenii). Food Chem. 2019, 288, 422–443. [Google Scholar] [CrossRef]
- Lee, M.S.; Shin, B.-C.; Yang, E.J.; Lim, H.-J.; Ernst, E. Maca (Lepidium meyenii) for Treatment of Menopausal Symptoms: A Systematic Review. Maturitas 2011, 70, 227–233. [Google Scholar] [CrossRef]
- Gonzales-Arimborgo, C.; Yupanqui, I.; Montero, E.; Alarcón-Yaquetto, D.E.; Zevallos-Concha, A.; Caballero, L.; Gasco, M.; Zhao, J.; Khan, I.A.; Gonzales, G.F. Acceptability, Safety, and Efficacy of Oral Administration of Extracts of Black or Red Maca (Lepidium meyenii) in Adult Human Subjects: A Randomized, Double-Blind, Placebo-Controlled Study. Pharmaceuticals 2016, 9, 49. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Jin, W.; Lv, X.; Dai, P.; Ao, Y.; Wu, M.; Deng, W.; Yu, L. Effects of Macamides on Endurance Capacity and Anti-Fatigue Property in Prolonged Swimming Mice. Pharm. Biol. 2016, 54, 827–834. [Google Scholar] [CrossRef]
- Rubio, J.; Caldas, M.; Dávila, S.; Gasco, M.; Gonzales, G.F. Effect of Three Different Cultivars of Lepidium meyenii (Maca) on Learning and Depression in Ovariectomized Mice. BMC Complement. Altern. Med. 2006, 6, 23. [Google Scholar] [CrossRef] [Green Version]
- Takamura, Y.; Nomura, M.; Uchiyama, A.; Fujita, S. Effects of Aerobic Exercise Combined with Panaxatriol Derived from Ginseng on Insulin Resistance and Skeletal Muscle Mass in Type 2 Diabetic Mice. J. Nutr. Sci. Vitaminol. 2017, 63, 339–348. [Google Scholar] [CrossRef] [Green Version]
- Kunkel, S.D.; Elmore, C.J.; Bongers, K.S.; Ebert, S.M.; Fox, D.K.; Dyle, M.C.; Bullard, S.A.; Adams, C.M. Ursolic Acid Increases Skeletal Muscle and Brown Fat and Decreases Diet-Induced Obesity, Glucose Intolerance and Fatty Liver Disease. PLoS ONE 2012, 7, e39332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, R.; Chen, J.; Xu, J.; Cao, J.; Wang, Y.; Thomas, S.S.; Hu, Z. Suppression of Muscle Wasting by the Plant-derived Compound Ursolic Acid in a Model of Chronic Kidney Disease. J Cachexia Sarcopenia Muscle 2017, 8, 327–341. [Google Scholar] [CrossRef] [Green Version]
- Bakhtiari, N.; Moslemee-Jalalvand, E.; Kazemi, J. Ursolic Acid: A Versatile Triterpenoid Compound in Regulating the Aging. Physiol. Pharmacol. 2017, 21, 15–24. [Google Scholar]
- Lee, Y.-K.; Chang, Y.H. Physicochemical and Antioxidant Properties of Methanol Extract from Maca (Lepidium meyenii Walp.) Leaves and Roots. Food Sci. Technol. 2019, 39, 278–286. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Li, J.; Fan, L. The Nutritional Composition of Maca in Hypocotyls (Lepidium meyenii Walp.) Cultivated in Different Regions of China. J. Food Qual. 2017, 2017, e3749627. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.H.; Gil, J.H.; Quan, H.; Viet, D.H.; Kim, C.K. Effect of 8-week Leucine Supplementation and Resistance Exercise Training on Muscle Hypertrophy and Satellite Cell Activation in Rats. Physiol. Rep. 2018, 6, e13725. [Google Scholar] [CrossRef]
- Pasiakos, S.M.; McClung, J.P. Supplemental Dietary Leucine and the Skeletal Muscle Anabolic Response to Essential Amino Acids. Nutr. Rev. 2011, 69, 550–557. [Google Scholar] [CrossRef]
- Tan, B.; Yin, Y.; Liu, Z.; Li, X.; Xu, H.; Kong, X.; Huang, R.; Tang, W.; Shinzato, I.; Smith, S.B.; et al. Dietary L-Arginine Supplementation Increases Muscle Gain and Reduces Body Fat Mass in Growing-Finishing Pigs. Amino Acids 2009, 37, 169–175. [Google Scholar] [CrossRef]
- Kim, M.; Sung, B.; Kang, Y.J.; Kim, D.H.; Lee, Y.; Hwang, S.Y.; Yoon, J.-H.; Yoo, M.-A.; Kim, C.M.; Chung, H.Y.; et al. The Combination of Ursolic Acid and Leucine Potentiates the Differentiation of C2C12 Murine Myoblasts through the MTOR Signaling Pathway. Int. J. Mol. Med. 2015, 35, 755–762. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.C.; Kang, Y.S.; Noh, E.B.; Seo, B.W.; Seo, D.Y.; Park, G.D.; Kim, S.H. Concurrent Treatment with Ursolic Acid and Low-Intensity Treadmill Exercise Improves Muscle Atrophy and Related Outcomes in Rats. Korean J. Physiol. Pharm. 2018, 22, 427–436. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, Y.; Ferdousi, F.; Fukumitsu, S.; Isoda, H. Maslinic Acid Attenuates Denervation-Induced Loss of Skeletal Muscle Mass and Strength. Nutrients 2021, 13, 2950. [Google Scholar] [CrossRef] [PubMed]
- Shirai, T.; Uemichi, K.; Kubota, K.; Yamauchi, Y.; Takemasa, T. Maslinic Acid Promotes Hypertrophy Induced by Functional Overload in Mouse Skeletal Muscle. J. Nutr. Sci. Vitaminol. 2021, 67, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Rommel, C.; Bodine, S.C.; Clarke, B.A.; Rossman, R.; Nunez, L.; Stitt, T.N.; Yancopoulos, G.D.; Glass, D.J. Mediation of IGF-1-Induced Skeletal Myotube Hypertrophy by PI(3)K/Akt/MTOR and PI(3)K/Akt/GSK3 Pathways. Nat. Cell. Biol. 2001, 3, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Raffaello, A.; Milan, G.; Masiero, E.; Carnio, S.; Lee, D.; Lanfranchi, G.; Goldberg, A.L.; Sandri, M.; Jun, B. Transcription Factor Maintains Skeletal Muscle Mass and Promotes Hypertrophy. J. Cell. Biol. 2010, 191, 101–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Liang, D.; Huang, Z.; Jia, G.; Zhao, H.; Liu, G. Quercetin Regulates Skeletal Muscle Fiber Type Switching via Adiponectin Signaling. Food Funct. 2021, 12, 2693–2702. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-J.; Dabrowski, K.; Sandoval, M.; Miller, M.J.S. Activity-Guided Fractionation of Phytochemicals of Maca Meal, Their Antioxidant Activities and Effects on Growth, Feed Utilization, and Survival in Rainbow Trout (Oncorhynchus Mykiss) Juveniles. Aquaculture 2005, 244, 293–301. [Google Scholar] [CrossRef]
- Rom, O.; Reznick, A.Z. The Role of E3 Ubiquitin-Ligases MuRF-1 and MAFbx in Loss of Skeletal Muscle Mass. Free. Radic. Biol. Med. 2016, 98, 218–230. [Google Scholar] [CrossRef]
- Chu, X.; He, X.; Shi, Z.; Li, C.; Guo, F.; Li, S.; Li, Y.; Na, L.; Sun, C. Ursolic Acid Increases Energy Expenditure through Enhancing Free Fatty Acid Uptake and β-Oxidation via an UCP3/AMPK-Dependent Pathway in Skeletal Muscle. Mol. Nutr. Food Res. 2015, 59, 1491–1503. [Google Scholar] [CrossRef]
- Kunkel, S.D.; Suneja, M.; Ebert, S.M.; Bongers, K.S.; Fox, D.K.; Malmberg, S.E.; Alipour, F.; Shields, R.K.; Adams, C.M. MRNA Expression Signatures of Human Skeletal Muscle Atrophy Identify a Natural Compound That Increases Muscle Mass. Cell Metab. 2011, 13, 627–638. [Google Scholar] [CrossRef] [Green Version]
- Bakhtiari, N.; Hosseinkhani, S.; Tashakor, A.; Hemmati, R. Ursolic Acid Ameliorates Aging-Metabolic Phenotype through Promoting of Skeletal Muscle Rejuvenation. Med. Hypotheses 2015, 85, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Thomson, D.M. The Role of AMPK in the Regulation of Skeletal Muscle Size, Hypertrophy, and Regeneration. Int. J. Mol. Sci. 2018, 19, 3125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolster, D.R.; Crozier, S.J.; Kimball, S.R.; Jefferson, L.S. AMP-Activated Protein Kinase Suppresses Protein Synthesis in Rat Skeletal Muscle through Down-Regulated Mammalian Target of Rapamycin (MTOR) Signaling. J. Biol. Chem. 2002, 277, 23977–23980. [Google Scholar] [CrossRef] [Green Version]
- Egawa, T.; Ohno, Y.; Goto, A.; Ikuta, A.; Suzuki, M.; Ohira, T.; Yokoyama, S.; Sugiura, T.; Ohira, Y.; Yoshioka, T.; et al. AICAR-Induced Activation of AMPK Negatively Regulates Myotube Hypertrophy through the HSP72-Mediated Pathway in C2C12 Skeletal Muscle Cells. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E344–E354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakushima, K.; Yoshikawa, M.; Osaki, T.; Miyamoto, N.; Hashimoto, T. Moderate Hypoxia Promotes Skeletal Muscle Cell Growth and Hypertrophy in C2C12 Cells. Biochem. Biophys. Res. Commun. 2020, 525, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Hosokawa, M.; Miyashita, K.; Fujita, T.; Nishino, H.; Hashimoto, T. Fucoxanthinol Attenuates Oxidative Stress-Induced Atrophy and Loss in Myotubes and Reduces the Triacylglycerol Content in Mature Adipocytes. Mol. Biol. Rep. 2020, 47, 2703–2711. [Google Scholar] [CrossRef]
- Vavvas, D.; Apazidis, A.; Saha, A.K.; Gamble, J.; Patel, A.; Kemp, B.E.; Witters, L.A.; Ruderman, N.B. Contraction-Induced Changes in Acetyl-CoA Carboxylase and 5′-AMP-Activated Kinase in Skeletal Muscle. J. Biol. Chem. 1997, 272, 13255–13261. [Google Scholar] [CrossRef] [Green Version]
- Lai, Y.-C.; Kviklyte, S.; Vertommen, D.; Lantier, L.; Foretz, M.; Viollet, B.; Hallén, S.; Rider, M.H. A Small-Molecule Benzimidazole Derivative That Potently Activates AMPK to Increase Glucose Transport in Skeletal Muscle: Comparison with Effects of Contraction and Other AMPK Activators. Biochem. J. 2014, 460, 363–375. [Google Scholar] [CrossRef]
- Gonzales, C.; Rubio, J.; Gasco, M.; Nieto, J.; Yucra, S.; Gonzales, G.F. Effect of Short-Term and Long-Term Treatments with Three Ecotypes of Lepidium meyenii (MACA) on Spermatogenesis in Rats. J. Ethnopharmacol. 2006, 103, 448–454. [Google Scholar] [CrossRef]
- Yu, Z.; Jin, W.; Dong, X.; Ao, M.; Liu, H.; Yu, L. Safety Evaluation and Protective Effects of Ethanolic Extract from Maca (Lepidium meyenii Walp.) against Corticosterone and H2O2 Induced Neurotoxicity. Regul. Toxicol. Pharmacol. 2020, 111, 104570. [Google Scholar] [CrossRef]
- Zevallos-Concha, A.; Nuñez, D.; Gasco, M.; Vasquez, C.; Quispe, M.; Gonzales, G.F. Effect of Gamma Irradiation on Phenol Content, Antioxidant Activity and Biological Activity of Black Maca and Red Maca Extracts (Lepidium meyenii Walp). Toxicol. Mech. Methods 2016, 26, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Jin, L.; Xie, L.; Huang, J.; Wang, N.; Chu, B.; Dai, X.; Liu, Y.; Wang, R.; Zhang, Y. Structural Characterization and Antifatigue Effect In Vivo of Maca (Lepidium meyenii Walp) Polysaccharide. J. Food Sci. 2017, 82, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wei, J.; Gao, Y.; Chen, R. Antioxidant and Antitumoral Activities of Isolated Macamide and Macaene Fractions from Lepidium meyenii (Maca). Talanta 2021, 221, 121635. [Google Scholar] [CrossRef] [PubMed]
- Gencoglu, H. Maca Modulates Fat and Liver Energy Metabolism Markers Insulin, IRS1, Leptin, and SIRT1 in Rats Fed Normal and High-Fat Diets. Arch. Physiol. Biochem. 2020, 1–7. [Google Scholar] [CrossRef]
- Rubio, J.; Qiong, W.; Liu, X.; Jiang, Z.; Dang, H.; Chen, S.-L.; Gonzales, G.F. Aqueous Extract of Black Maca (Lepidium meyenii) on Memory Impairment Induced by Ovariectomy in Mice. Evid. Based Complementary Altern. Med. 2011, 2011, enen063. [Google Scholar] [CrossRef] [Green Version]
- Fei, W.; Hou, Y.; Yue, N.; Zhou, X.; Wang, Y.; Wang, L.; Li, A.; Zhang, J. The Effects of Aqueous Extract of Maca on Energy Metabolism and Immunoregulation. Eur. J. Med. Res. 2020, 25, 24. [Google Scholar] [CrossRef]
- Oishi, Y.; Tsukamoto, H.; Yokokawa, T.; Hirotsu, K.; Shimazu, M.; Uchida, K.; Tomi, H.; Higashida, K.; Iwanaka, N.; Hashimoto, T. Mixed Lactate and Caffeine Compound Increases Satellite Cell Activity and Anabolic Signals for Muscle Hypertrophy. J. Appl. Physiol. 2015, 118, 742–749. [Google Scholar] [CrossRef] [Green Version]
- Horsley, V.; Friday, B.B.; Matteson, S.; Kegley, K.M.; Gephart, J.; Pavlath, G.K. Regulation of the Growth of Multinucleated Muscle Cells by an Nfatc2-Dependent Pathway. J. Cell. Biol. 2001, 153, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Oyama, A.; Kaneko, H.; Egawa, T.; Yokoyama, S.; Sugiura, T.; Ohira, Y.; Yoshioka, T.; Goto, K. Lactate Increases Myotube Diameter via Activation of MEK/ERK Pathway in C2C12 Cells. Acta Physiol. 2018, 223, e13042. [Google Scholar] [CrossRef]
- Hashimoto, T.; Okada, Y.; Yamanaka, A.; Ono, N.; Uryu, K.; Maru, I. The Effect of Eleutherococcus Senticosus on Metabolism-Associated Protein Expression in 3T3-L1 and C2C12 Cells. Phys. Act. Nutr. 2020, 24, 13–18. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, D.; Yoshikawa, M.; Sugimoto, T.; Tomoo, K.; Okada, Y.; Hashimoto, T. Effects of Maca on Muscle Hypertrophy in C2C12 Skeletal Muscle Cells. Int. J. Mol. Sci. 2022, 23, 6825. https://doi.org/10.3390/ijms23126825
Yi D, Yoshikawa M, Sugimoto T, Tomoo K, Okada Y, Hashimoto T. Effects of Maca on Muscle Hypertrophy in C2C12 Skeletal Muscle Cells. International Journal of Molecular Sciences. 2022; 23(12):6825. https://doi.org/10.3390/ijms23126825
Chicago/Turabian StyleYi, Dong, Maki Yoshikawa, Takeshi Sugimoto, Keigo Tomoo, Yoko Okada, and Takeshi Hashimoto. 2022. "Effects of Maca on Muscle Hypertrophy in C2C12 Skeletal Muscle Cells" International Journal of Molecular Sciences 23, no. 12: 6825. https://doi.org/10.3390/ijms23126825
APA StyleYi, D., Yoshikawa, M., Sugimoto, T., Tomoo, K., Okada, Y., & Hashimoto, T. (2022). Effects of Maca on Muscle Hypertrophy in C2C12 Skeletal Muscle Cells. International Journal of Molecular Sciences, 23(12), 6825. https://doi.org/10.3390/ijms23126825





