SERPINE1 DNA Methylation Levels Quantified in Blood Cells at Five Years of Age Are Associated with Adiposity and Plasma PAI-1 Levels at Five Years of Age
Abstract
1. Introduction
2. Results
2.1. Participant Characteristics
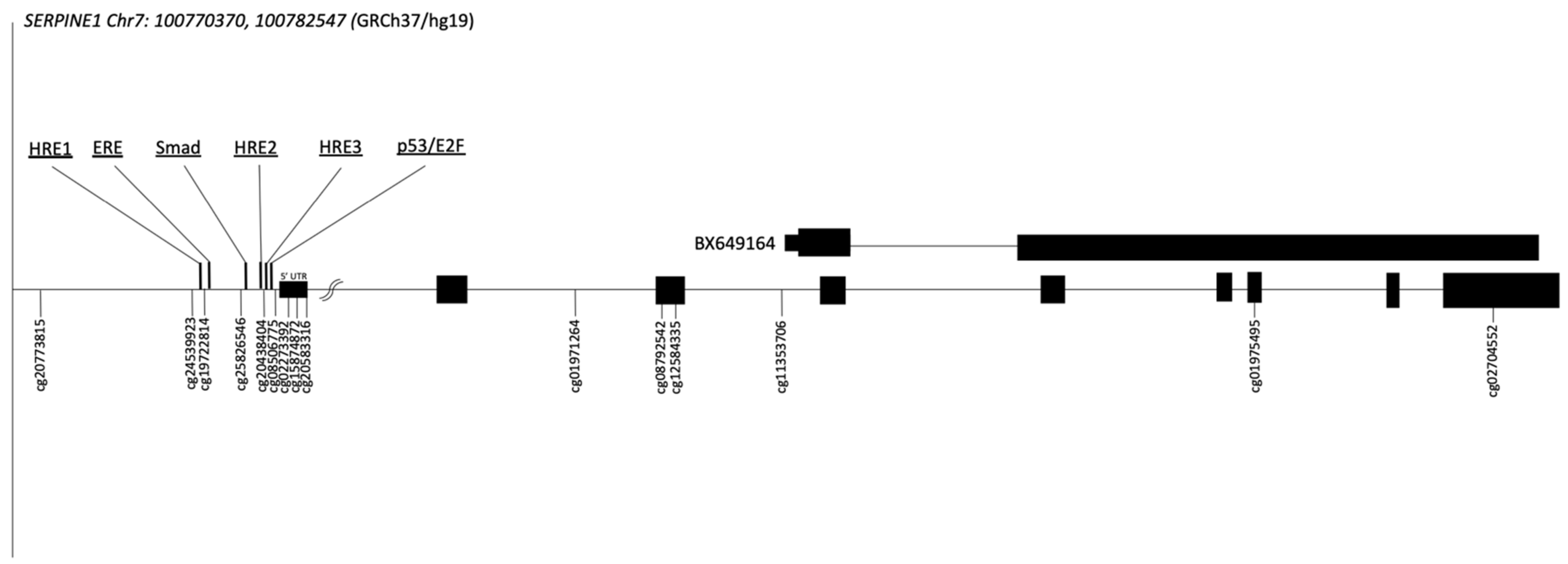
2.2. Genomic Context of the SERPINE1 Gene
2.3. Associations between Plasma PAI-1 Levels and Adiposity Levels at 5 Years of Age
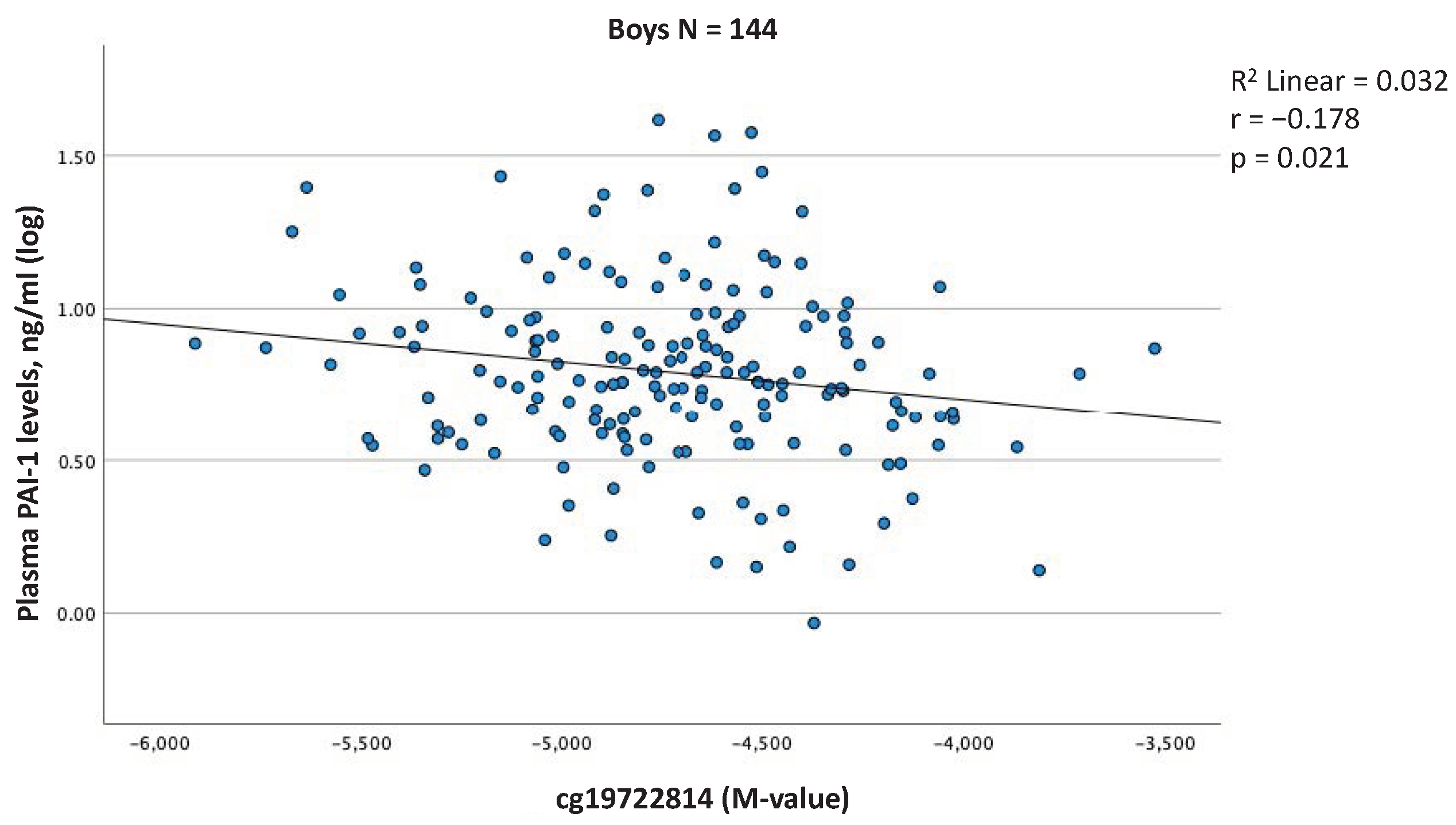
2.4. Associations between Blood Cell DNAm Levels at the SERPINE1 Gene Locus and Plasma PAI-1 Levels at 5 Years of Age
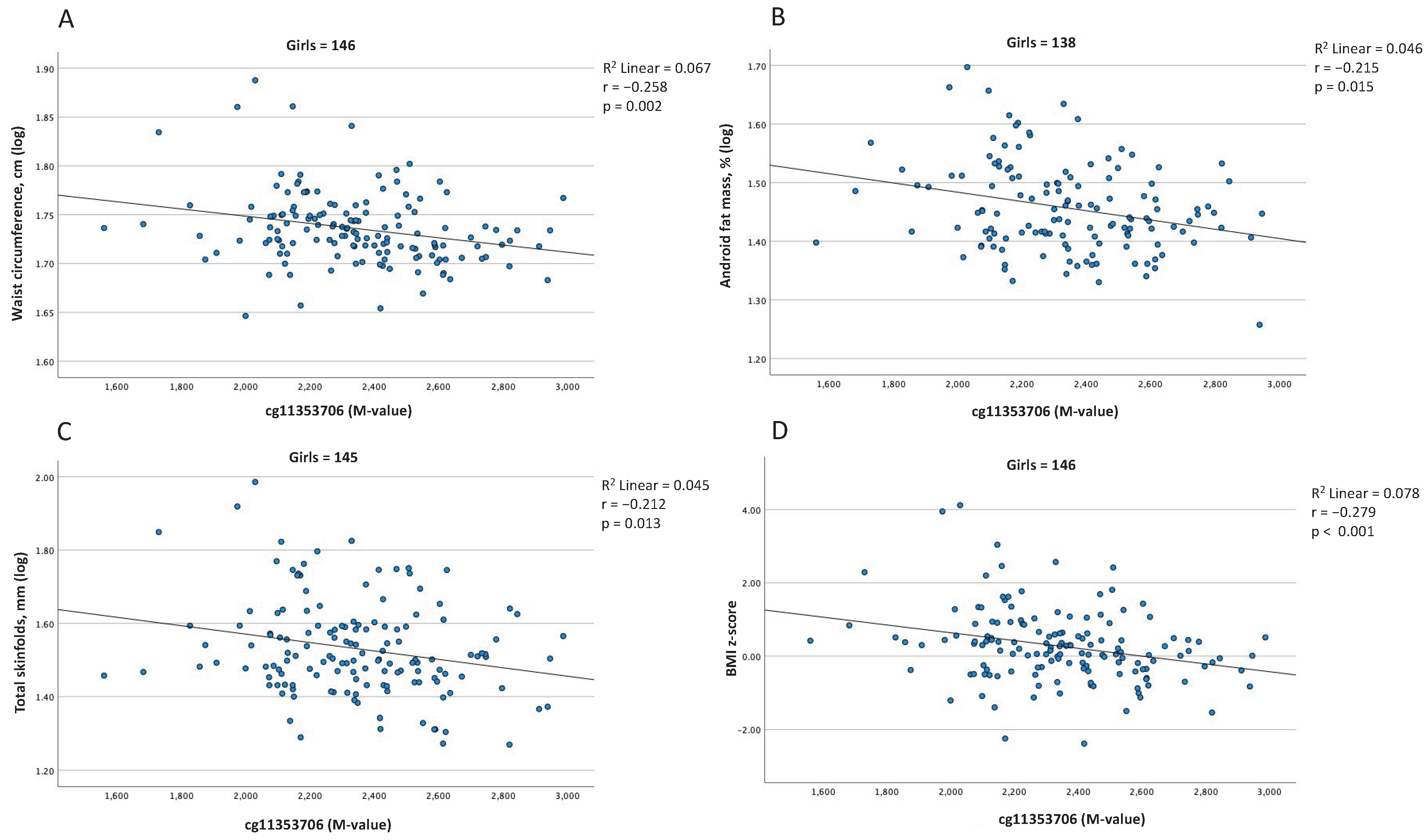
2.5. Associations between Blood Cell DNAm Levels at the SERPINE1 Gene Locus and Adiposity Levels at 5 Years of Age
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Participant’s Selection
5.2. Anthropometric Measurement
5.3. Plasmatic PAI-1 Levels Measurement
5.4. Measurements of DNAm
5.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 18 August 2022).
- Juneau, M. L’obésité Juvénile, Une Véritable Bombe à Retardement de Maladies Cardiométaboliques. Available online: https://observatoireprevention.org/2021/11/18/lobesite-juvenile-une-veritable-bombe-a-retardement-de-maladies-cardiometaboliques/ (accessed on 18 August 2022).
- Jackson-Leach, R.; Lobstein, T. Estimated Burden of Paediatric Obesity and Co-Morbidities in Europe. Part 1. The Increase in the Prevalence of Child Obesity in Europe Is Itself Increasing. Int. J. Pediatr. Obes. 2006, 1, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.S.; Mulder, C.; Twisk, J.W.R.; Van Mechelen, W.; Chinapaw, M.J.M. Tracking of Childhood Overweight into Adulthood: A Systematic Review of the Literature. Obes. Rev. 2008, 9, 474–488. [Google Scholar] [CrossRef] [PubMed]
- Altalhi, R.; Pechlivani, N.; Ajjan, R.A. PAI-1 in Diabetes: Pathophysiology and Role as a Therapeutic Target. Int. J. Mol. Sci. 2021, 22, 3170. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, C.H.; Brown, S.L.; Nordt, T.K.; Sobel, B.E.; Fujii, S. Elaboration of Type-1 Plasminogen Activator Inhibitor from Adipocytes. A Potential Pathogenetic Link between Obesity and Cardiovascular Disease. Circulation 1996, 93, 106–110. [Google Scholar] [CrossRef]
- Mavri, A.; Stegnar, M.; Krebs, M.; Sentocnik, J.T.; Geiger, M.; Binder, B.R. Impact of Adipose Tissue on Plasma Plasminogen Activator Inhibitor-1 in Dieting Obese Women. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1582–1587. [Google Scholar] [CrossRef][Green Version]
- Chen, R.; Yan, J.; Liu, P.; Wang, Z.; Wang, C. Plasminogen Activator Inhibitor Links Obesity and Thrombotic Cerebrovascular Diseases: The Roles of PAI-1 and Obesity on Stroke. Metab. Brain Dis. 2017, 32, 667–673. [Google Scholar] [CrossRef]
- Alessi, M.-C.; Juhan-Vague, I. PAI-1 and the Metabolic Syndrome: Links, Causes, and Consequences. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2200–2207. [Google Scholar] [CrossRef]
- Kohler, H.P.; Grant, P.J. Plasminogen-Activator Inhibitor Type 1 and Coronary Artery Disease. N. Engl. J. Med. 2000, 342, 1792–1801. [Google Scholar] [CrossRef]
- López-Alemany, R.; Redondo, J.M.; Nagamine, Y.; Muñoz-Cánoves, P. Plasminogen Activator Inhibitor Type-1 Inhibits Insulin Signaling by Competing with Avβ3 Integrin for Vitronectin Binding: PAI-1 Inhibition of Insulin/Vitronectin Signaling. Eur. J. Biochem. 2003, 270, 814–821. [Google Scholar] [CrossRef]
- Lijnen, H.R. Functional Role of the Fibrinolytic System in Development of Adipose Tissue. Verh.-K. Acad. Geneeskd. Belg. 2009, 71, 101–113. [Google Scholar]
- Ma, L.-J.; Mao, S.-L.; Taylor, K.L.; Kanjanabuch, T.; Guan, Y.; Zhang, Y.; Brown, N.J.; Swift, L.L.; McGuinness, O.P.; Wasserman, D.H.; et al. Prevention of Obesity and Insulin Resistance in Mice Lacking Plasminogen Activator Inhibitor 1. Diabetes 2004, 53, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Crandall, D.L.; Quinet, E.M.; El Ayachi, S.; Hreha, A.L.; Leik, C.E.; Savio, D.A.; Juhan-Vague, I.; Alessi, M.-C. Modulation of Adipose Tissue Development by Pharmacological Inhibition of PAI-1. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2209–2215. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, L.; Liu, Z.; Liu, Y.; Luo, M.; Chen, N.; Deng, X.; Luo, Y.; He, J.; Zhang, L.; et al. PAI-1 Exacerbates White Adipose Tissue Dysfunction and Metabolic Dysregulation in High Fat Diet-Induced Obesity. Front. Pharmacol. 2018, 9, 1087. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Luo, M.; Chen, N.; Deng, X.; He, J.; Zhang, L.; Luo, P.; Wu, J. Inhibition of PAI-1 Attenuates Perirenal Fat Inflammation and the Associated Nephropathy in High-Fat Diet-Induced Obese Mice. Am. J. Physiol.-Endocrinol. Metab. 2019, 316, E260–E267. [Google Scholar] [CrossRef] [PubMed]
- Lijnen, H.R.; Maquoi, E.; Morange, P.; Voros, G.; Van Hoef, B.; Kopp, F.; Collen, D.; Juhan-Vague, I.; Alessi, M.-C. Nutritionally Induced Obesity Is Attenuated in Transgenic Mice Overexpressing Plasminogen Activator Inhibitor-1. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 78–84. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lijnen, R. Effect of Plasminogen Activator Inhibitor-1 Deficiency on Nutritionally-Induced Obesity in Mice. Thromb. Haemost. 2005, 93, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Morange, P.E.; Lijnen, H.R.; Alessi, M.C.; Kopp, F.; Collen, D.; Juhan-Vague, I. Influence of PAI-1 on Adipose Tissue Growth and Metabolic Parameters in a Murine Model of Diet-Induced Obesity. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1150–1154. [Google Scholar] [CrossRef]
- Kinik, S.; Özbek, N.; Yuce, M.; Yazici, A.; Verdi, H.; Ataç, F.B. PAI-1 Gene 4G/5G Polymorphism, Cytokine Levels and Their Relations with Metabolic Parameters in Obese Children. Thromb. Haemost. 2008, 99, 352–356. [Google Scholar] [CrossRef]
- Lopez-Legarrea, P.; Mansego, M.L.; Zulet, M.A.; Martinez, J.A. SERPINE1, PAI-1 Protein Coding Gene, Methylation Levels and Epigenetic Relationships with Adiposity Changes in Obese Subjects with Metabolic Syndrome Features under Dietary Restriction. J. Clin. Biochem. Nutr. 2013, 53, 139–144. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Casanello, P.; Krause, B.J.; Castro-Rodriguez, J.A.; Uauy, R. Fetal programming of chronic diseases: Current concepts and epigenetics. Rev. Chil. Pediatr. 2015, 86, 135–137. [Google Scholar] [CrossRef]
- International Human Genome Sequencing Consortium. Initial Sequencing and Analysis of the Human Genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Skeldal, S.; Krogdahl, A.; Sørensen, J.A.; Andreasen, P.A. CpG Methylation of the PAI-1 Gene 5’-Flanking Region Is Inversely Correlated with PAI-1 MRNA Levels in Human Cell Lines. Thromb. Haemost. 2005, 94, 651–660. [Google Scholar] [PubMed]
- Park, Y.J.; Han, S.M.; Huh, J.Y.; Kim, J.B. Emerging Roles of Epigenetic Regulation in Obesity and Metabolic Disease. J. Biol. Chem. 2021, 297, 101296. [Google Scholar] [CrossRef] [PubMed]
- Deaton, A.M.; Bird, A. CpG Islands and the Regulation of Transcription. Genes Dev. 2011, 25, 1010–1022. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.O.; Baron, J.; Colli, M.J.; McDonnell, D.P.; Cutler, G.B. Estrogen Levels in Childhood Determined by an Ultrasensitive Recombinant Cell Bioassay. J. Clin. Investig. 1994, 94, 2475–2480. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A. Functions of DNA Methylation: Islands, Start Sites, Gene Bodies and Beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef]
- Maunakea, A.K.; Nagarajan, R.P.; Bilenky, M.; Ballinger, T.J.; D’Souza, C.; Fouse, S.D.; Johnson, B.E.; Hong, C.; Nielsen, C.; Zhao, Y.; et al. Conserved Role of Intragenic DNA Methylation in Regulating Alternative Promoters. Nature 2010, 466, 253–257. [Google Scholar] [CrossRef]
- Couture, F.; Sabbagh, R.; Kwiatkowska, A.; Desjardins, R.; Guay, S.-P.; Bouchard, L.; Day, R. PACE4 Undergoes an Oncogenic Alternative Splicing Switch in Cancer. Cancer Res. 2017, 77, 6863–6879. [Google Scholar] [CrossRef]
- Heindel, J.J.; Vandenberg, L.N. Developmental Origins of Health and Disease: A Paradigm for Understanding Disease Cause and Prevention. Curr. Opin. Pediatr. 2015, 27, 248–253. [Google Scholar] [CrossRef]
- Guillemette, L.; Allard, C.; Lacroix, M.; Patenaude, J.; Battista, M.-C.; Doyon, M.; Moreau, J.; Ménard, J.; Bouchard, L.; Ardilouze, J.-L.; et al. Genetics of Glucose Regulation in Gestation and Growth (Gen3G): A Prospective Prebirth Cohort of Mother–Child Pairs in Sherbrooke, Canada. BMJ Open 2016, 6, e010031. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Application Tools. Available online: https://www.who.int/growthref/tools/en/ (accessed on 18 August 2022).
- Aryee, M.J.; Jaffe, A.E.; Corrada-Bravo, H.; Ladd-Acosta, C.; Feinberg, A.P.; Hansen, K.D.; Irizarry, R.A. Minfi: A Flexible and Comprehensive Bioconductor Package for the Analysis of Infinium DNA Methylation Microarrays. Bioinformatics 2014, 30, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Fortin, J.-P.; Labbe, A.; Lemire, M.; Zanke, B.W.; Hudson, T.J.; Fertig, E.J.; Greenwood, C.M.; Hansen, K.D. Functional Normalization of 450k Methylation Array Data Improves Replication in Large Cancer Studies. Genome Biol. 2014, 15, 503. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.E.; Li, C.; Rabinovic, A. Adjusting Batch Effects in Microarray Expression Data Using Empirical Bayes Methods. Biostatistics 2007, 8, 118–127. [Google Scholar] [CrossRef]
- Du, P.; Zhang, X.; Huang, C.-C.; Jafari, N.; Kibbe, W.A.; Hou, L.; Lin, S.M. Comparison of Beta-Value and M-Value Methods for Quantifying Methylation Levels by Microarray Analysis. BMC Bioinform. 2010, 11, 587. [Google Scholar] [CrossRef]
| Characteristics | Girls | Boys | p * | ||||
|---|---|---|---|---|---|---|---|
| Nb | Mean ± SD | Range | Nb | Mean ± SD | Range | ||
| Age, months | 157 | 64.18 ± 3.83 | 58.07–79.47 | 184 | 64.17 ± 4.01 | 57.73–86.43 | 0.982 |
| PAI-1, pg/mL | 157 | 9.99 ± 12.29 | 1.32–111.87 | 184 | 7.84 ± 6.39 | 0.93–41.33 | 0.049 |
| Weight, kg | 157 | 19.22 ± 3.40 | 13.10–36.50 | 184 | 19.64 ± 2.79 | 13.00–32.80 | 0.212 |
| Height, cm | 157 | 109.9 ± 5.2 | 98.05–129.55 | 184 | 112.0 ± 4.9 | 101.0–126.1 | <0.001 |
| BMI, kg/m2 | 157 | 15.82 ± 1.77 | 12.36–24.15 | 184 | 15.59 ± 1.29 | 12.50–20.64 | 0.142 |
| BMI z-score | 157 | 0.279 ± 0.971 | −2.38–4.120 | 184 | 0.188 ± 0.932 | −2.57–3.22 | 0.382 |
| Total skinfold thickness a, mm | 155 | 35.47 ± 11.99 | 18.63–96.83 | 184 | 28.01 ± 7.77 | 15.67–76.42 | <0.001 |
| Waist circumference, cm | 157 | 54.61 ± 4.79 | 44.30–77.20 | 184 | 53.65 ± 3.44 | 45.85–72.50 | 0.038 |
| DXA trunk fat, % | 144 | 28.81 ± 4.60 | 18.90–45.80 | 164 | 23.89 ± 3.03 | 18.40–33.30 | <0.001 |
| DXA android fat, % | 144 | 29.03 ± 5.36 | 18.10–49.80 | 164 | 24.11 ± 3.26 | 17.90–34.80 | <0.001 |
| DXA total fat, % | 144 | 33.06 ± 4.33 | 21.90–47.00 | 164 | 28.46 ± 3.32 | 22.30–39.00 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taschereau, A.; Desgagné, V.; Faleschini, S.; Guérin, R.; Allard, C.; Perron, P.; Hivert, M.-F.; Bouchard, L. SERPINE1 DNA Methylation Levels Quantified in Blood Cells at Five Years of Age Are Associated with Adiposity and Plasma PAI-1 Levels at Five Years of Age. Int. J. Mol. Sci. 2022, 23, 11833. https://doi.org/10.3390/ijms231911833
Taschereau A, Desgagné V, Faleschini S, Guérin R, Allard C, Perron P, Hivert M-F, Bouchard L. SERPINE1 DNA Methylation Levels Quantified in Blood Cells at Five Years of Age Are Associated with Adiposity and Plasma PAI-1 Levels at Five Years of Age. International Journal of Molecular Sciences. 2022; 23(19):11833. https://doi.org/10.3390/ijms231911833
Chicago/Turabian StyleTaschereau, Amelie, Véronique Desgagné, Sabrina Faleschini, Renée Guérin, Catherine Allard, Patrice Perron, Marie-France Hivert, and Luigi Bouchard. 2022. "SERPINE1 DNA Methylation Levels Quantified in Blood Cells at Five Years of Age Are Associated with Adiposity and Plasma PAI-1 Levels at Five Years of Age" International Journal of Molecular Sciences 23, no. 19: 11833. https://doi.org/10.3390/ijms231911833
APA StyleTaschereau, A., Desgagné, V., Faleschini, S., Guérin, R., Allard, C., Perron, P., Hivert, M.-F., & Bouchard, L. (2022). SERPINE1 DNA Methylation Levels Quantified in Blood Cells at Five Years of Age Are Associated with Adiposity and Plasma PAI-1 Levels at Five Years of Age. International Journal of Molecular Sciences, 23(19), 11833. https://doi.org/10.3390/ijms231911833






