Diversified Effects of COVID-19 as a Consequence of the Differential Metabolism of Phospholipids and Lipid Peroxidation Evaluated in the Plasma of Survivors and Deceased Patients upon Admission to the Hospital
Abstract
1. Introduction
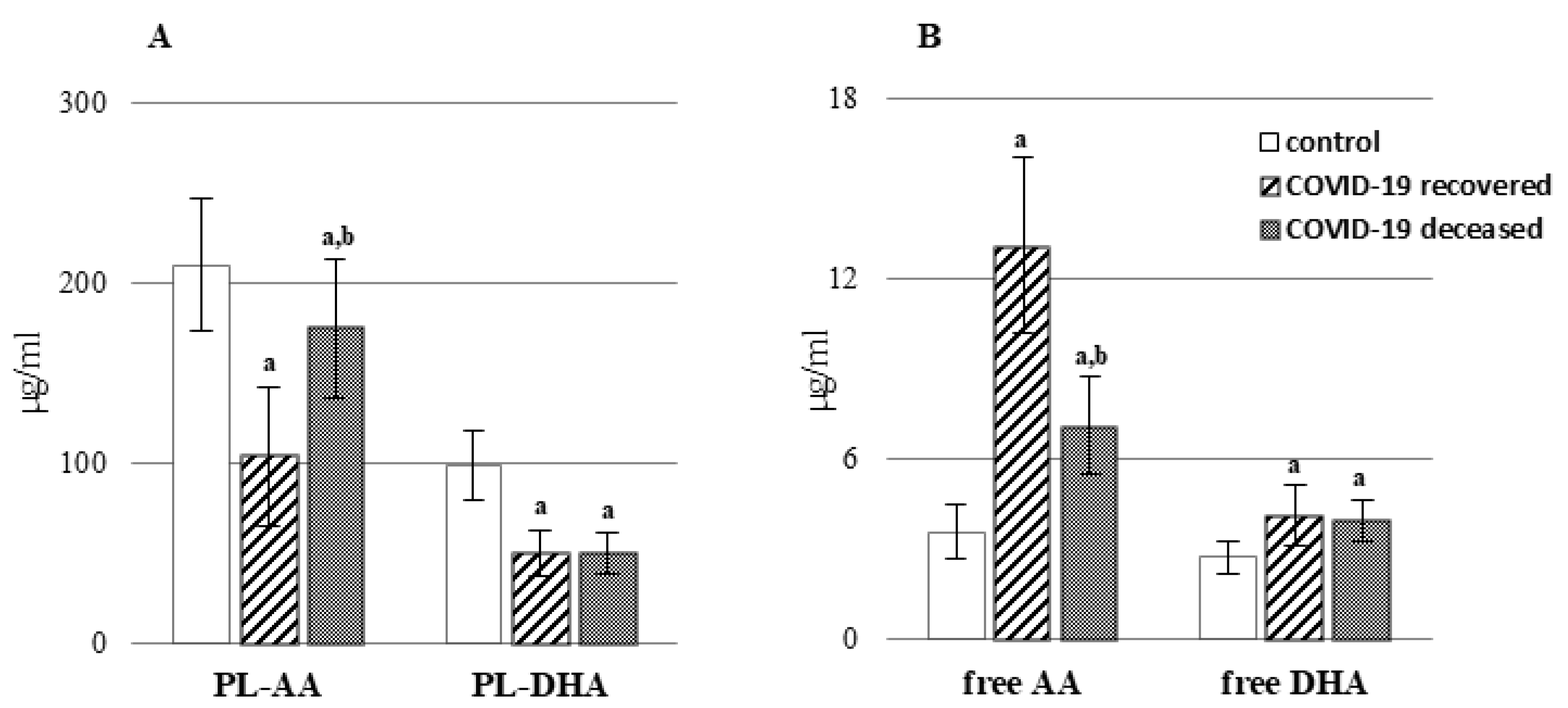
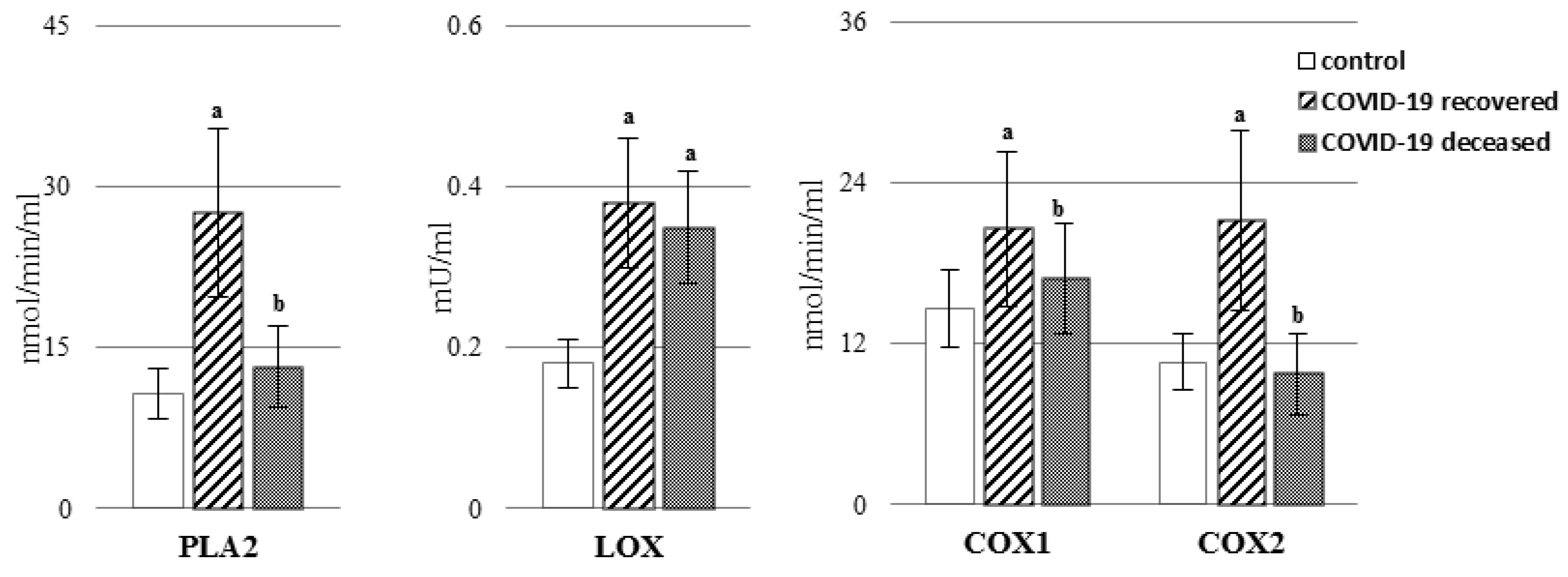
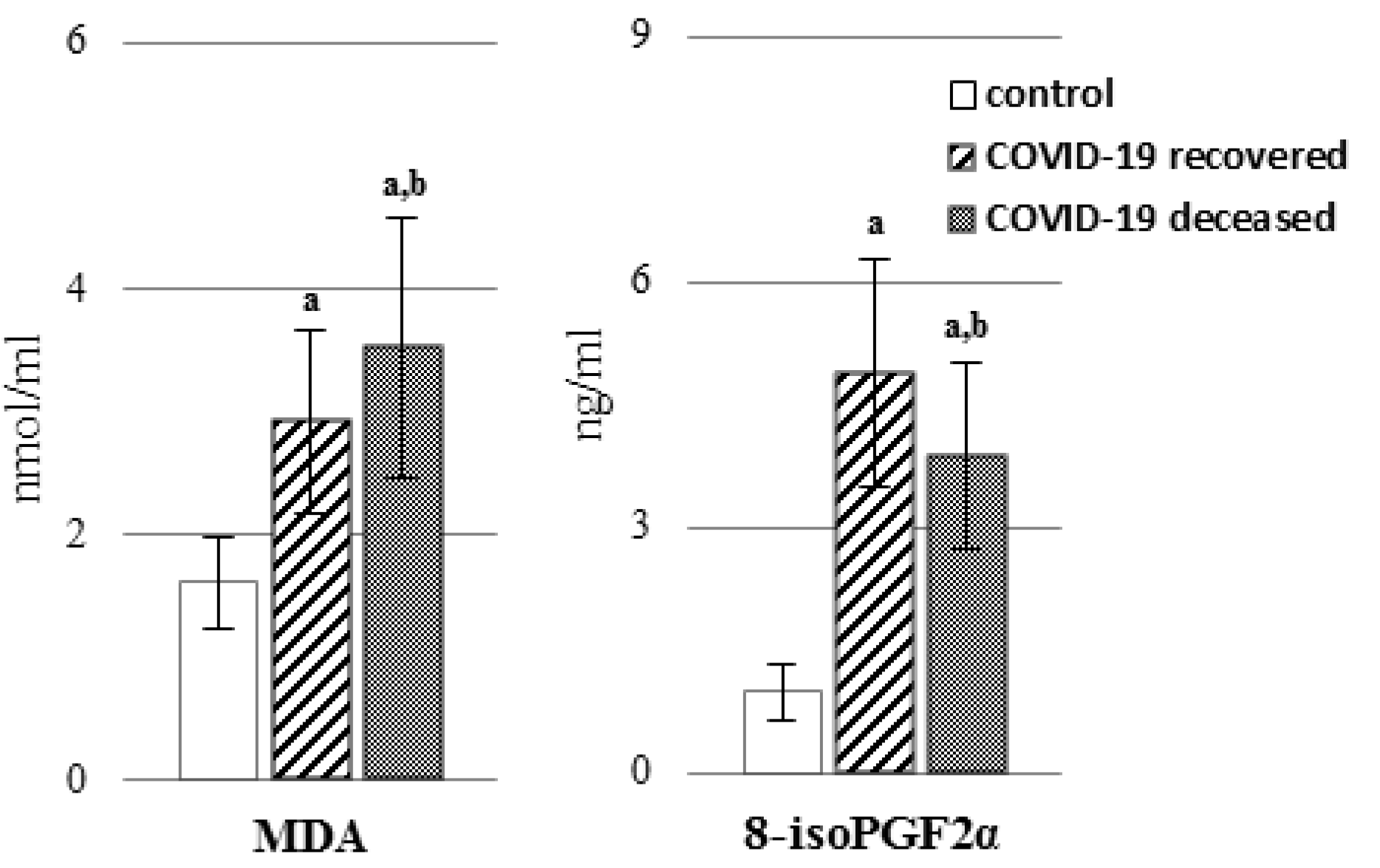
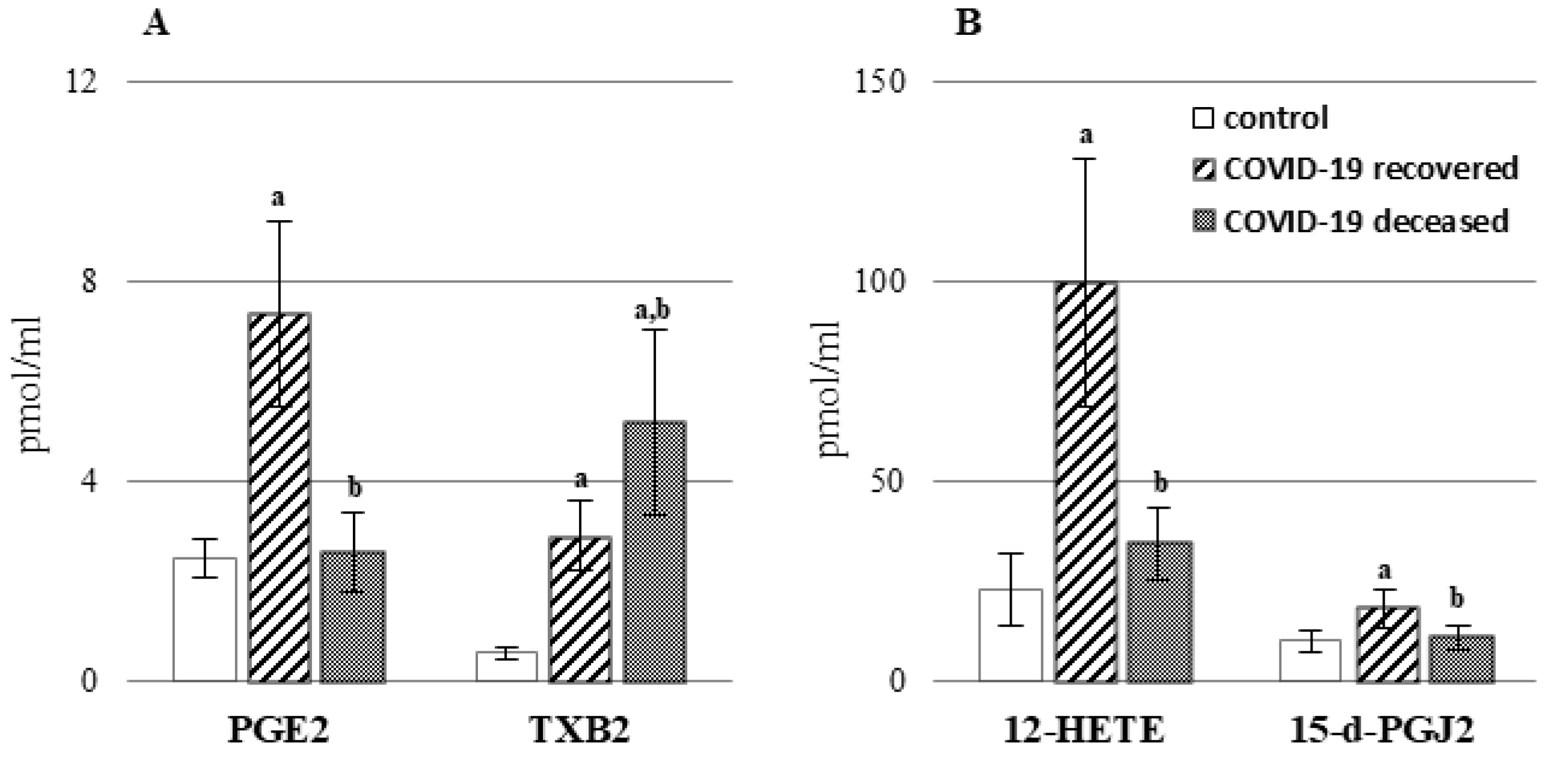
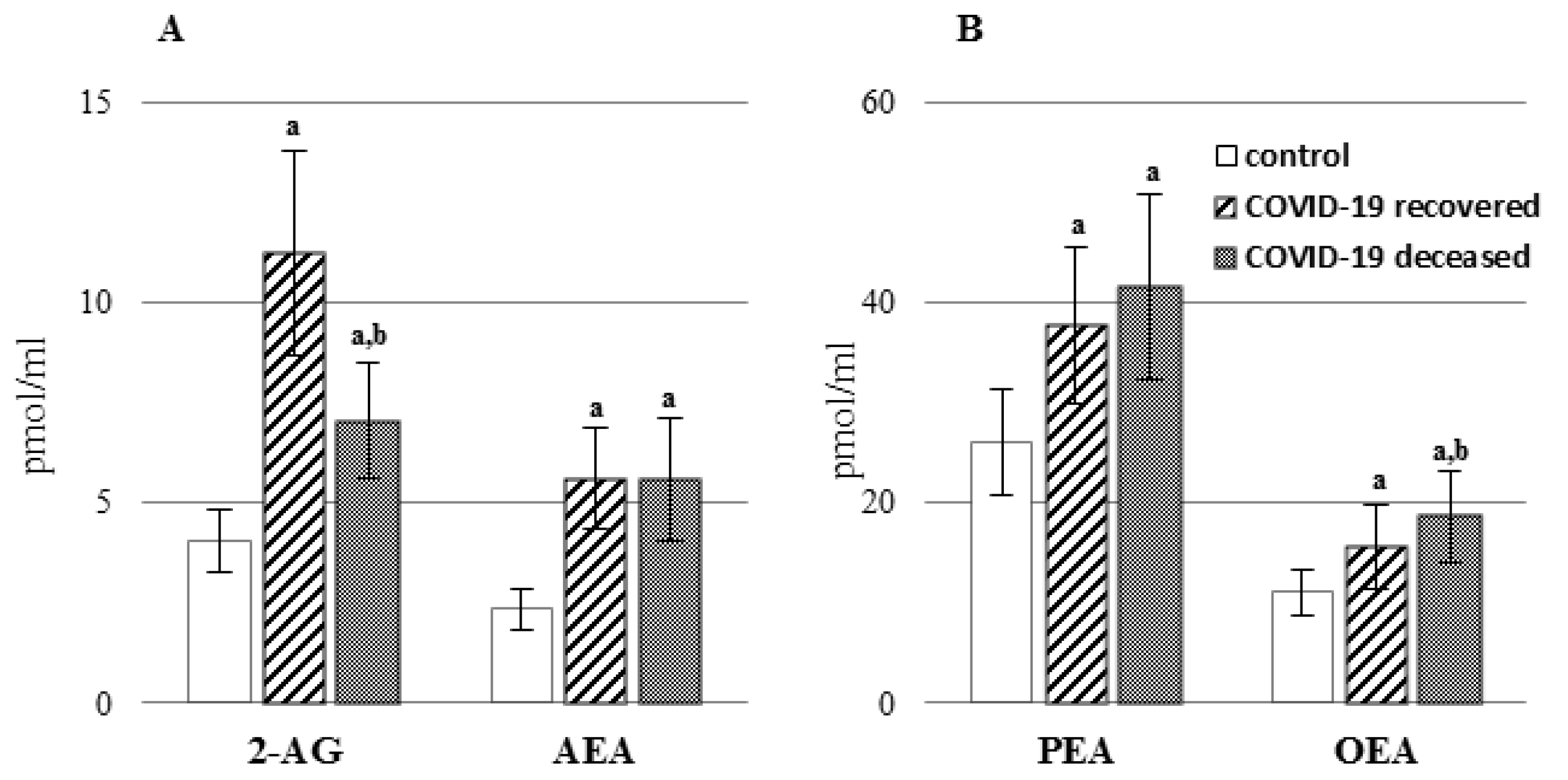
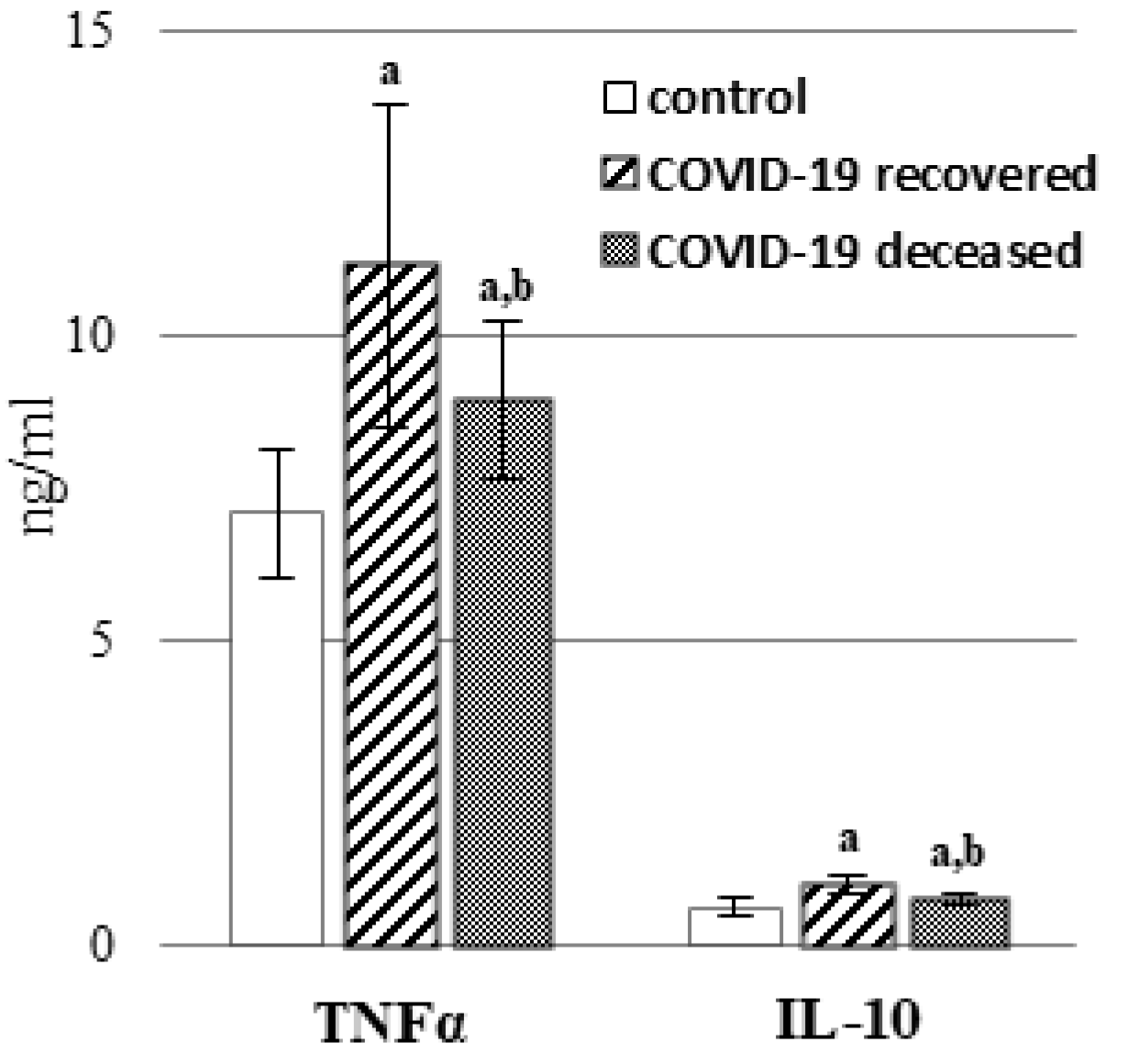
2. Results
3. Discussion
4. Materials and Methods
4.1. Samples Collection
4.2. Methods
4.2.1. Determination of Phospholipid Metabolism
4.2.2. Determination of the Level of Lipid Peroxidation Products
4.2.3. Determination of the Level of Lipids Mediators (Endocannabinoids and Eicosanoids)
4.2.4. Determination of the Level of TNFα and IL-10
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, A.; Tiwari, S.; Deb, M.K.; Marty, J.L. Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2): A Global Pandemic and Treatment Strategies. Int. J. Antimicrob. Agents 2020, 56, 106054. [Google Scholar] [CrossRef] [PubMed]
- Zaim, S.; Chong, J.H.; Sankaranarayanan, V.; Harky, A. COVID-19 and Multiorgan Response. Curr. Probl. Cardiol. 2020, 45, 100618. [Google Scholar] [CrossRef] [PubMed]
- De Las Heras, N.; Martín Giménez, V.M.; Ferder, L.; Manucha, W.; Lahera, V. Implications of Oxidative Stress and Potential Role of Mitochondrial Dysfunction in COVID-19: Therapeutic Effects of Vitamin D. Antioxidants 2020, 9, 897. [Google Scholar] [CrossRef] [PubMed]
- Chernyak, B.V.; Popova, E.N.; Prikhodko, A.S.; Grebenchikov, O.A.; Zinovkina, L.A.; Zinovkin, R.A. COVID-19 and Oxidative Stress. Biochemistry 2020, 85, 1543–1553. [Google Scholar] [CrossRef]
- Soto, M.E.; Guarner-Lans, V.; Díaz-Díaz, E.; Manzano-Pech, L.; Palacios-Chavarría, A.; Valdez-Vázquez, R.R.; Aisa-Álvarez, A.; Saucedo-Orozco, H.; Pérez-Torres, I. Hyperglycemia and Loss of Redox Homeostasis in COVID-19 Patients. Cells 2022, 11, 932. [Google Scholar] [CrossRef] [PubMed]
- Žarković, N.; Orehovec, B.; Milković, L.; Baršić, B.; Tatzber, F.; Wonisch, W.; Tarle, M.; Kmet, M.; Mataić, A.; Jakovčević, A.; et al. Preliminary Findings on the Association of the Lipid Peroxidation Product 4-Hydroxynonenal with the Lethal Outcome of Aggressive COVID-19. Antioxidants 2021, 10, 1341. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, B.; Sharma, L.; Roberts, L.; Peng, X.; Bermejo, S.; Leighton, I.; Casanovas-Massana, A.; Minasyan, M.; Farhadian, S.; Ko, A.I.; et al. Cutting Edge: Severe SARS-CoV-2 Infection in Humans Is Defined by a Shift in the Serum Lipidome, Resulting in Dysregulation of Eicosanoid Immune Mediators. J. Immunol. 2021, 206, 329–334. [Google Scholar] [CrossRef]
- Martín-Fernández, M.; Aller, R.; Heredia-Rodríguez, M.; Gómez-Sánchez, E.; Martínez-Paz, P.; Gonzalo-Benito, H.; Sánchez-de Prada, L.; Gorgojo, Ó.; Carnicero-Frutos, I.; Tamayo, E.; et al. Lipid Peroxidation as a Hallmark of Severity in COVID-19 Patients. Redox Biol. 2021, 48, 102181. [Google Scholar] [CrossRef]
- Zarkovic, N.; Jakovcevic, A.; Mataic, A.; Jaganjac, M.; Vukovic, T.; Waeg, G.; Zarkovic, K. Post-Mortem Findings of Inflammatory Cells and the Association of 4-Hydroxynonenal with Systemic Vascular and Oxidative Stress in Lethal COVID-19. Cells 2022, 11, 444. [Google Scholar] [CrossRef]
- Nagoor Meeran, M.F.; Sharma, C.; Goyal, S.N.; Kumar, S.; Ojha, S. CB2 Receptor-Selective Agonists as Candidates for Targeting Infection, Inflammation, and Immunity in SARS-CoV-2 Infections. Drug Dev. Res. 2021, 82, 7–11. [Google Scholar] [CrossRef]
- Cinar, R.; Iyer, M.R.; Kunos, G. Dual Inhibition of CB1 Receptors and INOS, as a Potential Novel Approach to the Pharmacological Management of Acute and Long COVID-19. Br. J. Pharmacol. 2022, 179, 2121–2127. [Google Scholar] [CrossRef] [PubMed]
- Sultan, M.; Alghetaa, H.; Mohammed, A.; Abdulla, O.A.; Wisniewski, P.J.; Singh, N.; Nagarkatti, P.; Nagarkatti, M. The Endocannabinoid Anandamide Attenuates Acute Respiratory Distress Syndrome by Downregulating MiRNA That Target Inflammatory Pathways. Front. Pharmacol. 2021, 12, 644281. [Google Scholar] [CrossRef]
- Snider, J.M.; You, J.K.; Wang, X.; Snider, A.J.; Hallmark, B.; Zec, M.M.; Seeds, M.C.; Sergeant, S.; Johnstone, L.; Wang, Q.; et al. Group IIA Secreted Phospholipase A2 Is Associated with the Pathobiology Leading to COVID-19 Mortality. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef] [PubMed]
- Palmas, F.; Clarke, J.; Colas, R.A.; Gomez, E.A.; Keogh, A.; Boylan, M.; McEvoy, N.; McElvaney, O.J.; McElvaney, O.; Alalqam, R.; et al. Dysregulated Plasma Lipid Mediator Profiles in Critically Ill COVID-19 Patients. PLoS ONE 2021, 16, e0256226. [Google Scholar] [CrossRef] [PubMed]
- Dalli, J.; Colas, R.A.; Serhan, C.N. Novel N-3 Immunoresolvents: Structures and Actions. Sci. Rep. 2013, 3, 1940. [Google Scholar] [CrossRef]
- Pistorius, K.; Souza, P.R.; De Matteis, R.; Austin-Williams, S.; Primdahl, K.G.; Vik, A.; Mazzacuva, F.; Colas, R.A.; Marques, R.M.; Hansen, T.V.; et al. PDn-3 DPA Pathway Regulates Human Monocyte Differentiation and Macrophage Function. Cell Chem. Biol. 2018, 25, 749–760.e9. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Dalli, J. New Pro-Resolving n-3 Mediators Bridge Resolution of Infectious Inflammation to Tissue Regeneration. Mol. Asp. Med. 2018, 64, 1–17. [Google Scholar] [CrossRef]
- Dennis, E.A.; Norris, P.C. Eicosanoid Storm in Infection and Inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef]
- Sahanic, S.; Löffler-Ragg, J.; Tymoszuk, P.; Hilbe, R.; Demetz, E.; Masanetz, R.K.; Theurl, M.; Holfeld, J.; Gollmann-Tepeköylü, C.; Tzankov, A.; et al. The Role of Innate Immunity and Bioactive Lipid Mediators in COVID-19 and Influenza. Front. Physiol 2021, 12, 688946. [Google Scholar] [CrossRef]
- Vijay, R.; Hua, X.; Meyerholz, D.K.; Miki, Y.; Yamamoto, K.; Gelb, M.; Murakami, M.; Perlman, S. Critical Role of Phospholipase A2 Group IID in Age-Related Susceptibility to Severe Acute Respiratory Syndrome-CoV Infection. J. Exp. Med. 2015, 212, 1851–1868. [Google Scholar] [CrossRef]
- Rajasagi, N.K.; Reddy, P.B.J.; Mulik, S.; Gjorstrup, P.; Rouse, B.T. Neuroprotectin D1 Reduces the Severity of Herpes Simplex Virus-Induced Corneal Immunopathology. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6269–6279. [Google Scholar] [CrossRef] [PubMed]
- Koenis, D.S.; Beegun, I.; Jouvene, C.C.; Aguirre, G.A.; Souza, P.R.; Gonzalez-Nunez, M.; Ly, L.; Pistorius, K.; Kocher, H.M.; Ricketts, W.; et al. Disrupted Resolution Mechanisms Favor Altered Phagocyte Responses in COVID-19. Circ. Res. 2021, 129, e54–e71. [Google Scholar] [CrossRef] [PubMed]
- McElvaney, O.J.; McEvoy, N.L.; McElvaney, O.F.; Carroll, T.P.; Murphy, M.P.; Dunlea, D.M.; Ní Choileáin, O.; Clarke, J.; O’Connor, E.; Hogan, G.; et al. Characterization of the Inflammatory Response to Severe COVID-19 Illness. Am. J. Respir. Crit. Care Med. 2020, 202, 812–821. [Google Scholar] [CrossRef]
- Kessler, B.; Rinchai, D.; Kewcharoenwong, C.; Nithichanon, A.; Biggart, R.; Hawrylowicz, C.M.; Bancroft, G.J.; Lertmemongkolchai, G. Interleukin 10 Inhibits Pro-Inflammatory Cytokine Responses and Killing of Burkholderia Pseudomallei. Sci. Rep. 2017, 7, 42791. [Google Scholar] [CrossRef] [PubMed]
- Hammock, B.D.; Wang, W.; Gilligan, M.M.; Panigrahy, D. Eicosanoids: The Overlooked Storm in Coronavirus Disease 2019 (COVID-19)? Am. J. Pathol. 2020, 190, 1782–1788. [Google Scholar] [CrossRef] [PubMed]
- Casari, I.; Manfredi, M.; Metharom, P.; Falasca, M. Dissecting Lipid Metabolism Alterations in SARS-CoV-2. Prog. Lipid Res. 2021, 82, 101092. [Google Scholar] [CrossRef]
- Gallo, C.G.; Fiorino, S.; Posabella, G.; Antonacci, D.; Tropeano, A.; Pausini, E.; Pausini, C.; Guarniero, T.; Hong, W.; Giampieri, E.; et al. The Function of Specialized Pro-Resolving Endogenous Lipid Mediators, Vitamins, and Other Micronutrients in the Control of the Inflammatory Processes: Possible Role in Patients with SARS-CoV-2 Related Infection. Prostaglandins Other Lipid Mediat. 2022, 159, 106619. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Escrivá, C.; Dromant, M.; Borrás, C.; Viña, J. Lipid Peroxidation as Measured by Chromatographic Determination of Malondialdehyde. Human Plasma Reference Values in Health and Disease. Arch. Biochem. Biophys. 2021, 709, 108941. [Google Scholar] [CrossRef]
- Komaravelli, N.; Casola, A. Respiratory Viral Infections and Subversion of Cellular Antioxidant Defenses. J. Pharm. Pharm. 2014, 5, 1000141. [Google Scholar] [CrossRef]
- Finn, D.P.; Haroutounian, S.; Hohmann, A.G.; Krane, E.; Soliman, N.; Rice, A.S.C. Cannabinoids, the Endocannabinoid System, and Pain: A Review of Preclinical Studies. Pain 2021, 162, S5–S25. [Google Scholar] [CrossRef]
- Rahman, S.M.K.; Uyama, T.; Hussain, Z.; Ueda, N. Roles of Endocannabinoids and Endocannabinoid-Like Molecules in Energy Homeostasis and Metabolic Regulation: A Nutritional Perspective. Annu. Rev. Nutr. 2021, 41, 177–202. [Google Scholar] [CrossRef] [PubMed]
- Sultan, M.; Wilson, K.; Abdulla, O.A.; Busbee, P.B.; Hall, A.; Carter, T.; Singh, N.; Chatterjee, S.; Nagarkatti, P.; Nagarkatti, M. Endocannabinoid Anandamide Attenuates Acute Respiratory Distress Syndrome through Modulation of Microbiome in the Gut-Lung Axis. Cells 2021, 10, 3305. [Google Scholar] [CrossRef] [PubMed]
- Gubernatorova, E.O.; Gorshkova, E.A.; Polinova, A.I.; Drutskaya, M.S. IL-6: Relevance for Immunopathology of SARS-CoV-2. Cytokine Growth Factor Rev. 2020, 53, 13–24. [Google Scholar] [CrossRef] [PubMed]
- McGonagle, D.; Sharif, K.; O’Regan, A.; Bridgewood, C. The Role of Cytokines Including Interleukin-6 in COVID-19 Induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun. Rev. 2020, 19, 102537. [Google Scholar] [CrossRef]
- Hernández-Cervantes, R.; Méndez-Díaz, M.; Prospéro-García, Ó.; Morales-Montor, J. Immunoregulatory Role of Cannabinoids during Infectious Disease. NIM 2017, 24, 183–199. [Google Scholar] [CrossRef]
- Krishnan, G.; Chatterjee, N. Endocannabinoids Affect Innate Immunity of Muller Glia during HIV-1 Tat Cytotoxicity. Mol. Cell. Neurosci. 2014, 59, 10–23. [Google Scholar] [CrossRef]
- Mestre, L.; Iñigo, P.M.; Mecha, M.; Correa, F.G.; Hernangómez-Herrero, M.; Loría, F.; Docagne, F.; Borrell, J.; Guaza, C. Anandamide Inhibits Theiler’s Virus Induced VCAM-1 in Brain Endothelial Cells and Reduces Leukocyte Transmigration in a Model of Blood Brain Barrier by Activation of CB1receptors. J. Neuroinflamm. 2011, 8, 102. [Google Scholar] [CrossRef]
- Turcotte, C.; Blanchet, M.-R.; Laviolette, M.; Flamand, N. The CB2 Receptor and Its Role as a Regulator of Inflammation. Cell. Mol. Life Sci. 2016, 73, 4449–4470. [Google Scholar] [CrossRef]
- Ghaffari, S.; Roshanravan, N.; Tutunchi, H.; Ostadrahimi, A.; Pouraghaei, M.; Kafil, B. Oleoylethanolamide, A Bioactive Lipid Amide, as A Promising Treatment Strategy for Coronavirus/COVID-19. Arch. Med. Res. 2020, 51, 464–467. [Google Scholar] [CrossRef]
- Schönrich, G.; Raftery, M.J.; Samstag, Y. Devilishly Radical NETwork in COVID-19: Oxidative Stress, Neutrophil Extracellular Traps (NETs), and T Cell Suppression. Adv. Biol. Regul. 2020, 77, 100741. [Google Scholar] [CrossRef]
- Jensen, M.D.; Sheng, W.; Simonyi, A.; Johnson, G.S.; Sun, A.Y.; Sun, G.Y. Involvement of Oxidative Pathways in Cytokine-Induced Secretory Phospholipase A2-IIA in Astrocytes. Neurochem. Int. 2009, 55, 362–368. [Google Scholar] [CrossRef]
- Mazidimoradi, A.; Alemzadeh, E.; Alemzadeh, E.; Salehiniya, H. The Effect of Polyunsaturated Fatty Acids on the Severity and Mortality of COVID Patients: A Systematic Review. Life Sci. 2022, 299, 120489. [Google Scholar] [CrossRef] [PubMed]
- Biagini, D.; Franzini, M.; Oliveri, P.; Lomonaco, T.; Ghimenti, S.; Bonini, A.; Vivaldi, F.; Macera, L.; Balas, L.; Durand, T.; et al. MS-Based Targeted Profiling of Oxylipins in COVID-19: A New Insight into Inflammation Regulation. Free Radic. Biol. Med. 2022, 180, 236–243. [Google Scholar] [CrossRef]
- Savard, M.; Bélanger, C.; Tremblay, M.J.; Dumais, N.; Flamand, L.; Borgeat, P.; Gosselin, J. EBV Suppresses Prostaglandin E2 Biosynthesis in Human Monocytes. J. Immunol. 2000, 164, 6467–6473. [Google Scholar] [CrossRef] [PubMed]
- Honda, T.; Segi-Nishida, E.; Miyachi, Y.; Narumiya, S. Prostacyclin-IP Signaling and Prostaglandin E2-EP2/EP4 Signaling Both Mediate Joint Inflammation in Mouse Collagen-Induced Arthritis. J. Exp. Med. 2006, 203, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Muramoto, K.; Masaaki, N.; Ding, Y.; Yang, H.; Mackey, M.; Li, W.; Inoue, Y.; Ackermann, K.; Shirota, H.; et al. A Novel Antagonist of the Prostaglandin E(2) EP(4) Receptor Inhibits Th1 Differentiation and Th17 Expansion and Is Orally Active in Arthritis Models. Br. J. Pharmacol. 2010, 160, 292–310. [Google Scholar] [CrossRef]
- Aoki, T.; Narumiya, S. Prostaglandins and Chronic Inflammation. Trends Pharmacol. Sci. 2012, 33, 304–311. [Google Scholar] [CrossRef]
- Wu, D.; Mura, C.; Beharka, A.A.; Han, S.N.; Paulson, K.E.; Hwang, D.; Meydani, S.N. Age-Associated Increase in PGE2 Synthesis and COX Activity in Murine Macrophages Is Reversed by Vitamin E. Am. J. Physiol. 1998, 275, C661–C668. [Google Scholar] [CrossRef]
- Duffin, R.; O’Connor, R.A.; Crittenden, S.; Forster, T.; Yu, C.; Zheng, X.; Smyth, D.; Robb, C.T.; Rossi, F.; Skouras, C.; et al. Prostaglandin E2 Constrains Systemic Inflammation through an Innate Lymphoid Cell-IL-22 Axis. Science 2016, 351, 1333–1338. [Google Scholar] [CrossRef]
- FitzGerald, G.A. BIOMEDICINE. Bringing PGE2 in from the Cold. Science 2015, 348, 1208–1209. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving Inflammation: Dual Anti-Inflammatory and pro-Resolution Lipid Mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.R.; Koster, K.M.; Murphy, P.S. Efferocytosis Signaling in the Regulation of Macrophage Inflammatory Responses. J. Immunol. 2017, 198, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Canzano, P.; Brambilla, M.; Porro, B.; Cosentino, N.; Tortorici, E.; Vicini, S.; Poggio, P.; Cascella, A.; Pengo, M.F.; Veglia, F.; et al. Platelet and Endothelial Activation as Potential Mechanisms Behind the Thrombotic Complications of COVID-19 Patients. JACC Basic Transl. Sci. 2021, 6, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Perico, L.; Benigni, A.; Casiraghi, F.; Ng, L.F.P.; Renia, L.; Remuzzi, G. Immunity, Endothelial Injury and Complement-Induced Coagulopathy in COVID-19. Nat. Rev. Nephrol. 2021, 17, 46–64. [Google Scholar] [CrossRef]
- Tantry, U.S.; Bliden, K.P.; Cho, A.; Walia, N.; Dahlen, J.R.; Ens, G.; Traianova, M.; Jerjian, C.; Usman, A.; Gurbel, P.A. First Experience Addressing the Prognostic Utility of Novel Urinary Biomarkers in Patients With COVID-19. Open Forum Infect. Dis. 2021, 8, ofab274. [Google Scholar] [CrossRef]
- Korbecki, J.; Bobiński, R.; Dutka, M. Self-Regulation of the Inflammatory Response by Peroxisome Proliferator-Activated Receptors. Inflamm. Res. 2019, 68, 443–458. [Google Scholar] [CrossRef]
- Schwager, J.; Gagno, L.; Richard, N.; Simon, W.; Weber, P.; Bendik, I. Z-Ligustilide and Anti-Inflammatory Prostaglandins Have Common Biological Properties in Macrophages and Leukocytes. Nutr. Metab. 2018, 15, 4. [Google Scholar] [CrossRef]
- Lee, C. Collaborative Power of Nrf2 and PPARγ Activators against Metabolic and Drug-Induced Oxidative Injury. Oxid. Med. Cell. Longev. 2017, 2017, 1378175. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, W.; Giroux, C.; Cai, Y.; Ekambaram, P.; Dilly, A.-K.; Hsu, A.; Zhou, S.; Maddipati, K.R.; Liu, J.; et al. Identification of the Orphan G Protein-Coupled Receptor GPR31 as a Receptor for 12-(S)-Hydroxyeicosatetraenoic Acid. J. Biol. Chem. 2011, 286, 33832–33840. [Google Scholar] [CrossRef]
- Porro, B.; Songia, P.; Squellerio, I.; Tremoli, E.; Cavalca, V. Analysis, Physiological and Clinical Significance of 12-HETE: A Neglected Platelet-Derived 12-Lipoxygenase Product. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 964, 26–40. [Google Scholar] [CrossRef]
- Archambault, A.-S.; Zaid, Y.; Rakotoarivelo, V.; Doré, É.; Dubuc, I.; Martin, C.; Amar, Y.; Cheikh, A.; Fares, H.; Hassani, A.E.; et al. Lipid Storm within the Lungs of Severe COVID-19 Patients: Extensive Levels of Cyclooxygenase and Lipoxygenase-Derived Inflammatory Metabolites. medRxiv 2020. [Google Scholar] [CrossRef]
- Morita, M.; Kuba, K.; Ichikawa, A.; Nakayama, M.; Katahira, J.; Iwamoto, R.; Watanebe, T.; Sakabe, S.; Daidoji, T.; Nakamura, S.; et al. The Lipid Mediator Protectin D1 Inhibits Influenza Virus Replication and Improves Severe Influenza. Cell 2013, 153, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Torrinhas, R.S.; Calder, P.C.; Lemos, G.O.; Waitzberg, D.L. Parenteral Fish Oil: An Adjuvant Pharmacotherapy for Coronavirus Disease 2019? Nutrition 2021, 81, 110900. [Google Scholar] [CrossRef] [PubMed]
- Weill, P.; Plissonneau, C.; Legrand, P.; Rioux, V.; Thibault, R. May Omega-3 Fatty Acid Dietary Supplementation Help Reduce Severe Complications in Covid-19 Patients? Biochimie 2020, 179, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Hu, K.; Li, Y.; Lu, C.; Ling, K.; Cai, C.; Wang, W.; Ye, D. Targeting TNF-α for COVID-19: Recent Advanced and Controversies. Front. Public Health 2022, 10, 833967. [Google Scholar] [CrossRef]
- Zhao, Y.; Qin, L.; Zhang, P.; Li, K.; Liang, L.; Sun, J.; Xu, B.; Dai, Y.; Li, X.; Zhang, C.; et al. Longitudinal COVID-19 Profiling Associates IL-1RA and IL-10 with Disease Severity and RANTES with Mild Disease. JCI Insight 2020, 5, 139834. [Google Scholar] [CrossRef]
- Christie, W.W.; Esterification, B.A. Preparation of Ester Derivatives of Fatty Acids for Chromatographic Analysis. In Advances in Lipid Methodology-Two; The Oily Press LTD: Calgary, AB, Canada, 1993. [Google Scholar]
- Reynolds, L.J.; Hughes, L.L.; Yu, L.; Dennis, E.A. 1-Hexadecyl-2-Arachidonoylthio-2-Deoxy-Sn-Glycero-3-Phosphorylcholine as a Substrate for the Microtiterplate Assay of Human Cytosolic Phospholipase A2. Anal. Biochem. 1994, 217, 25–32. [Google Scholar] [CrossRef]
- Kulmacz, R.J.; Wang, L.-H. Comparison of Hydroperoxide Initiator Requirements for the Cyclooxygenase Activities of Prostaglandin H Synthase-1 and −2 (∗). J. Biol. Chem. 1995, 270, 24019–24023. [Google Scholar] [CrossRef]
- Luo, X.P.; Yazdanpanah, M.; Bhooi, N.; Lehotay, D.C. Determination of Aldehydes and Other Lipid Peroxidation Products in Biological Samples by Gas Chromatography-Mass Spectrometry. Anal. Biochem. 1995, 228, 294–298. [Google Scholar] [CrossRef]
- Coolen, S.A.J.; van Buuren, B.; Duchateau, G.; Upritchard, J.; Verhagen, H. Kinetics of Biomarkers: Biological and Technical Validity of Isoprostanes in Plasma. Amino Acids 2005, 29, 429–436. [Google Scholar] [CrossRef]
- Luque-Córdoba, D.; Calderón-Santiago, M.; Luque de Castro, M.D.; Priego-Capote, F. Study of Sample Preparation for Determination of Endocannabinoids and Analogous Compounds in Human Serum by LC-MS/MS in MRM Mode. Talanta 2018, 185, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Watkins, B.A.; Kim, J.; Kenny, A.; Pedersen, T.L.; Pappan, K.L.; Newman, J.W. Circulating Levels of Endocannabinoids and Oxylipins Altered by Dietary Lipids in Older Women Are Likely Associated with Previously Identified Gene Targets. Biochim. Biophys. Acta 2016, 1861, 1693–1704. [Google Scholar] [CrossRef] [PubMed]
- Hnasko, R.; Lin, A.; McGarvey, J.A.; Stanker, L.H. A Rapid Method to Improve Protein Detection by Indirect ELISA. Biochem. Biophys. Res. Commun. 2011, 410, 726–731. [Google Scholar] [CrossRef] [PubMed]
| Normal Range | COVID-19 Recovered | COVID-19 Deceased | |
|---|---|---|---|
| WBC [103/μL] | 4.00–10.00 | 11.17 ± 3.44 | 11.43 ± 3.52 |
| Neutrophils [%] | 40.0–72.0 | 80.67 ± 5.54 | 86.60 ± 4.62 * |
| Platelets [103/μL] | 150–400 | 292.50 ± 105.14 | 226.61 ± 57.37 |
| Blood oxygen saturation [%] | >95% | 91.17 ± 5.65 | 90.67 ± 5.33 |
| Ferritin [μg/L] | 11–336 | 913 ± 475 | 943 ± 436 |
| PCT [ng/mL] | <0.1 | 0.38 ± 0.35 | 1.04 ± 1.09 |
| LDH [U/L] | 140–280 | 420 ± 130 | 358 ± 140 |
| CRP [mg/L] | 0.00–5.00 | 139.01 ± 74.08 | 185.14 ± 59.37 |
| IL-6 [pg/mL] | 0–43.5 | 87 ± 45 | 185 ± 104 * |
| Healthy Subjects | COVID-19 Recovered | COVID-19 Deceased | |
|---|---|---|---|
| Age (years) | 45 ± 12.6 | 58.9 ± 9.1 a | 72.3 ± 6.9 a,b |
| Sex | 24F 9M | 25F 41M | 13F 9M |
| Body Mass Index | 25.9 ± 4.7 | 32.5 ± 5.8 a | 28.5 ± 3.3 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Žarković, N.; Łuczaj, W.; Jarocka-Karpowicz, I.; Orehovec, B.; Baršić, B.; Tarle, M.; Kmet, M.; Lukšić, I.; Biernacki, M.; Skrzydlewska, E. Diversified Effects of COVID-19 as a Consequence of the Differential Metabolism of Phospholipids and Lipid Peroxidation Evaluated in the Plasma of Survivors and Deceased Patients upon Admission to the Hospital. Int. J. Mol. Sci. 2022, 23, 11810. https://doi.org/10.3390/ijms231911810
Žarković N, Łuczaj W, Jarocka-Karpowicz I, Orehovec B, Baršić B, Tarle M, Kmet M, Lukšić I, Biernacki M, Skrzydlewska E. Diversified Effects of COVID-19 as a Consequence of the Differential Metabolism of Phospholipids and Lipid Peroxidation Evaluated in the Plasma of Survivors and Deceased Patients upon Admission to the Hospital. International Journal of Molecular Sciences. 2022; 23(19):11810. https://doi.org/10.3390/ijms231911810
Chicago/Turabian StyleŽarković, Neven, Wojciech Łuczaj, Iwona Jarocka-Karpowicz, Biserka Orehovec, Bruno Baršić, Marko Tarle, Marta Kmet, Ivica Lukšić, Michał Biernacki, and Elżbieta Skrzydlewska. 2022. "Diversified Effects of COVID-19 as a Consequence of the Differential Metabolism of Phospholipids and Lipid Peroxidation Evaluated in the Plasma of Survivors and Deceased Patients upon Admission to the Hospital" International Journal of Molecular Sciences 23, no. 19: 11810. https://doi.org/10.3390/ijms231911810
APA StyleŽarković, N., Łuczaj, W., Jarocka-Karpowicz, I., Orehovec, B., Baršić, B., Tarle, M., Kmet, M., Lukšić, I., Biernacki, M., & Skrzydlewska, E. (2022). Diversified Effects of COVID-19 as a Consequence of the Differential Metabolism of Phospholipids and Lipid Peroxidation Evaluated in the Plasma of Survivors and Deceased Patients upon Admission to the Hospital. International Journal of Molecular Sciences, 23(19), 11810. https://doi.org/10.3390/ijms231911810











