The Importance of Toll-like Receptor 9 Expression on Monocytes and Dendritic Cells in the Context of Epstein–Barr Virus Infection in the Immunopathogenesis of Primary Glomerulonephritis
Abstract
1. Introduction
2. Results
2.1. Clinical Characteristic of the GN Patients and the Control Group
Detailed Analysis of Morphological and Biochemical Parameters of Patients, Taking into Account the Disease Subunit: IgAN and MPGN
2.2. Assessment of the Frequency of TLR9-Positive DCs and Monocytes
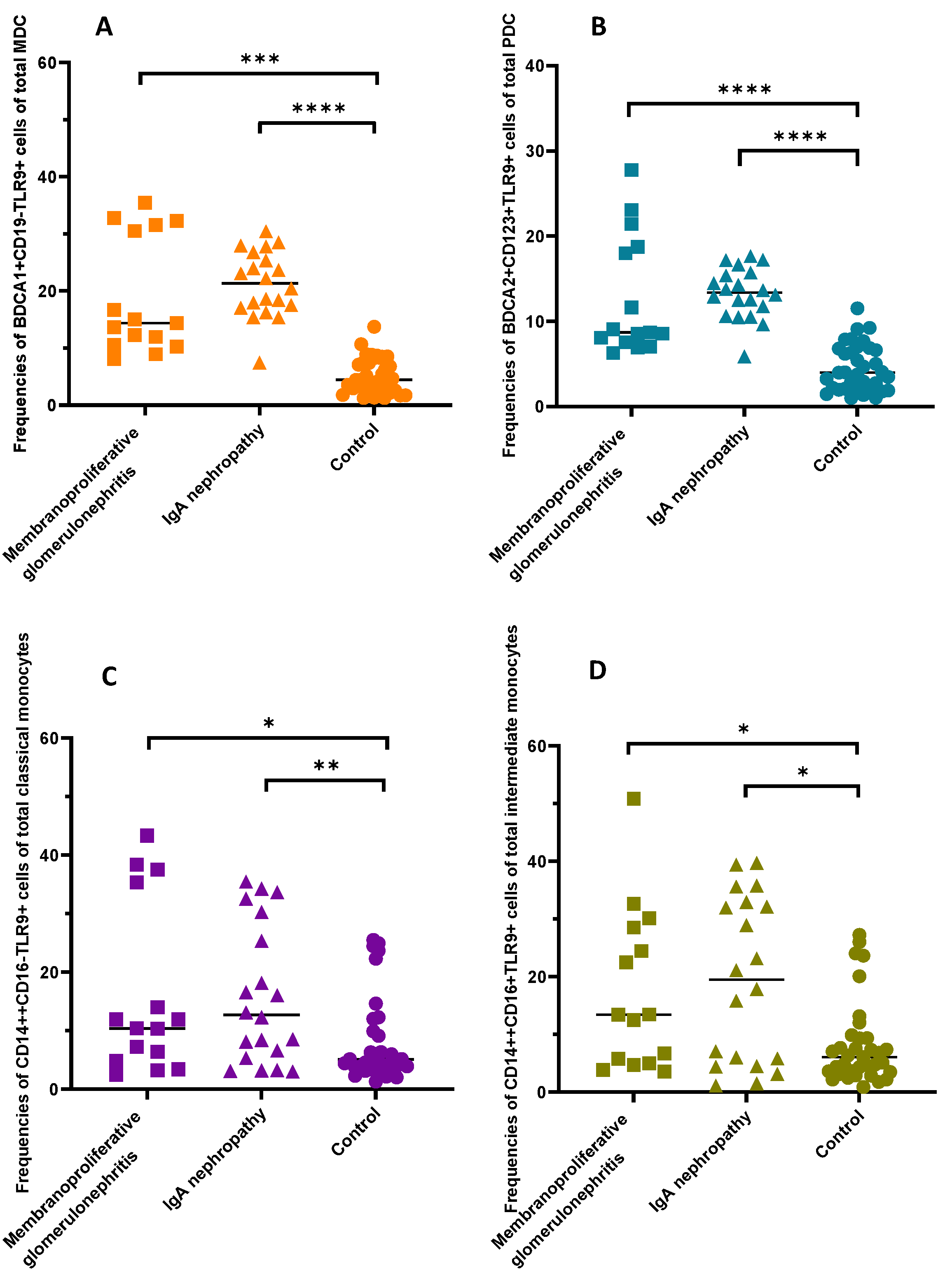
2.3. Detailed Analysis of the Frequencies of TLR9-Positive DCs and Monocytes of Patients TAking into Account the Disease Subunit (IgAN and MPGN)
2.4. Assessment of EBV Prevalence in Patients with GN and the Controls
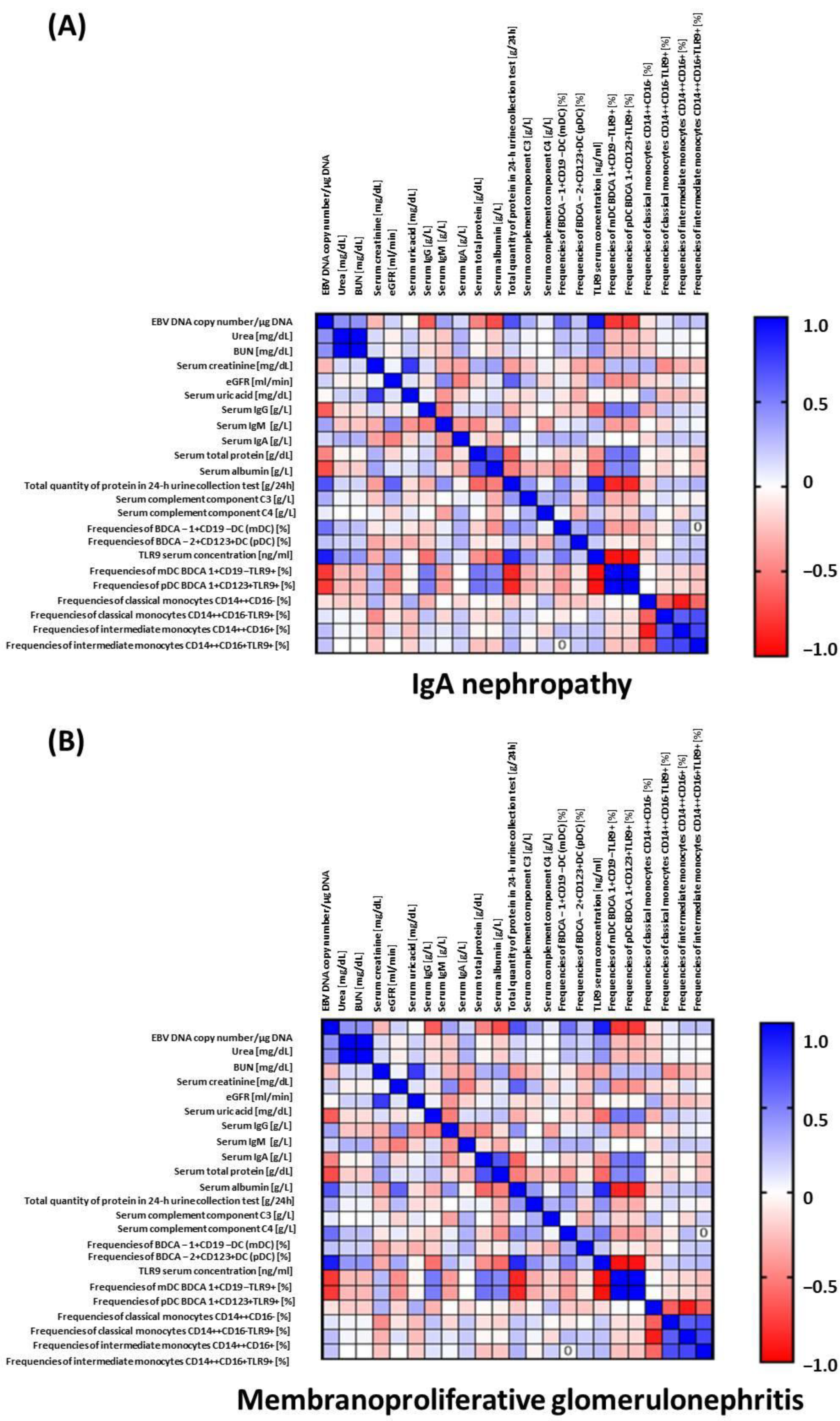
2.5. Analysis of the Correlation of TLR9 Concentration with Selected Biochemical Parameters, Immunophenotype and the Presence of EBV DNA
3. Discussion
4. Materials and Methods
4.1. Patients and Healthy Volunteers
4.2. Isolation of PB Mononuclear Cells
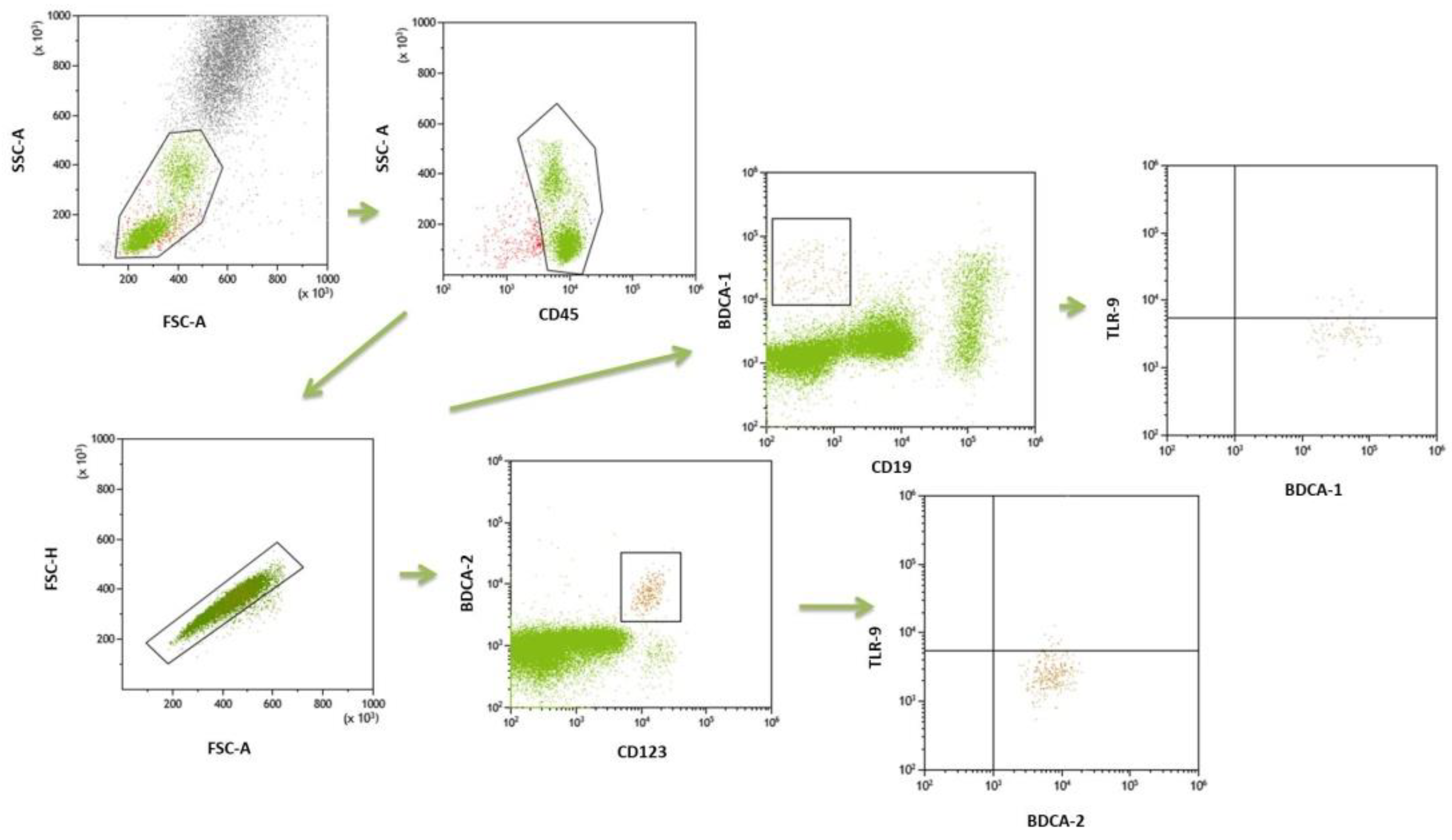
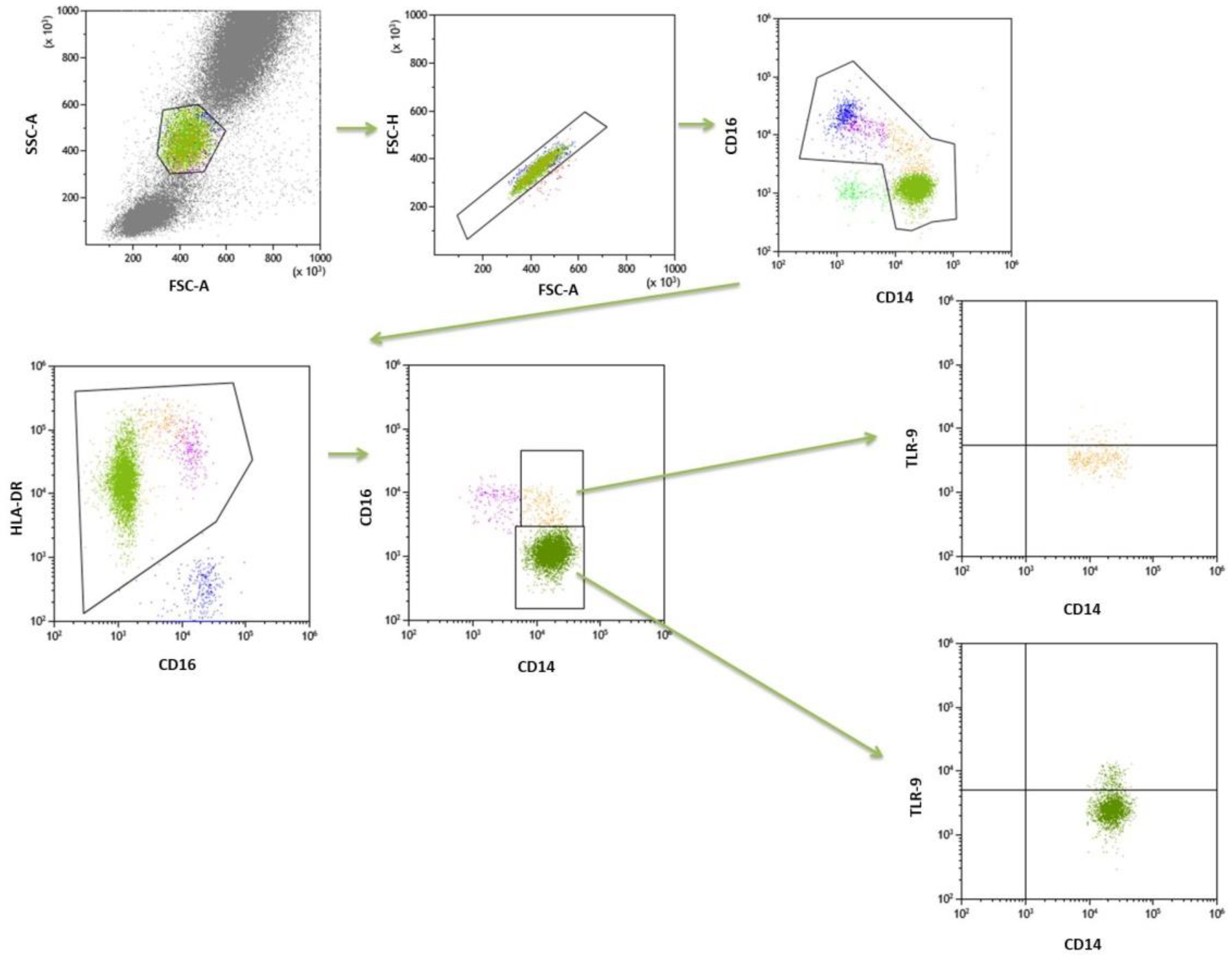
4.3. Flow Cytometry
4.4. Determination of TLR9 Concentration in the Patients Serum by ELISA Test
4.5. DNA Isolation and Calculation of EBV Load and Assessment of Anti-EBV Antibody Status
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pesce, F.; Stea, E.D.; Rossini, M.; Fiorentino, M.; Piancone, F.; Infante, B.; Stallone, G.; Castellano, G.; Gesualdo, L. Glomerulonephritis in AKI: From Pathogenesis to Therapeutic Intervention. Front. Med. 2021, 7, 983. [Google Scholar] [CrossRef] [PubMed]
- Khanna, R. Clinical Presentation & Management of Glomerular Diseases: Hematuria, Nephritic & Nephrotic Syndrome. Mo. Med. 2011, 108, 33–36. [Google Scholar] [PubMed]
- Smith, K.D. Toll-like Receptors in Kidney Disease. Curr. Opin. Nephrol. Hypertens. 2009, 18, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, I.; Giroux, N.; Olson, L.; Morrison, S.A.; Llanga, T.; Akinade, T.O.; Zhu, Y.; Zhong, Y.; Bose, S.; Arvai, S.; et al. DAMPs/PAMPs Induce Monocytic TLR Activation and Tolerance in COVID-19 Patients; Nucleic Acid Binding Scavengers Can Counteract Such TLR Agonists. Biomaterials 2022, 283, 121393. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Coyne, C.B.; Zeh, H.J.; Lotze, M.T. PAMPs and DAMPs: Signal 0s That Spur Autophagy and Immunity. Immunol. Rev. 2012, 249, 158–175. [Google Scholar] [CrossRef]
- TLR9 Protein Expression Summary—The Human Protein Atlas. Available online: http://www.proteinatlas.org/ENSG00000239732-TLR9 (accessed on 21 July 2022).
- Han, S.J.; Li, H.; Kim, M.; Shlomchik, M.J.; Lee, H.T. Kidney Proximal Tubular TLR9 Exacerbates Ischemic Acute Kidney Injury. J. Immunol. 2018, 201, 1073–1085. [Google Scholar] [CrossRef]
- Rogers, N.; Ferenbach, D.; Isenberg, J.; Thomson, A.; Hughes, J. Dendritic Cells and Macrophages in the Kidney: A Spectrum of Good and Evil. Nat. Rev. Nephrol. 2014, 10, 625–643. [Google Scholar] [CrossRef]
- Chen, T.; Cao, Q.; Wang, Y.; Harris, D. The Role of Dendritic Cells in Renal Inflammation. Curr. Pathobiol. Rep. 2014, 2, 225–234. [Google Scholar] [CrossRef][Green Version]
- Ferrario, F.; Castiglione, A.; Colasanti, G.; Barbiano di Belgioioso, G.; Bertoli, S.; D’Amico, G.; Nava, S. The Detection of Monocytes in Human Glomerulonephritis. Kidney Int. 1985, 28, 513–519. [Google Scholar] [CrossRef]
- Gulubova, M. Myeloid and Plasmacytoid Dendritic Cells and Cancer—New Insights. Maced. J. Med. Sci. 2019, 7, 3324–3340. [Google Scholar] [CrossRef]
- Lavine, N.; Ohayon, A.; Mahroum, N. Renal Autoimmunity: The Role of Bacterial and Viral Infections, an Extensive Review. Autoimmun. Rev. 2022, 21, 103073. [Google Scholar] [CrossRef] [PubMed]
- Martín-Gómez, M.A.; Caba Molina, M.; Cruz Caparros, G.; Guerrero Sánchez, E.; Caballero González, A.; Gómez Morales, M. Immuno Complex Mediated Acute Glomerulonephritis in a Patient with Infectious Mononucleosis. Nefrología 2017, 37, 439–441. [Google Scholar] [CrossRef]
- Sato, Y.; Furuyama, K.; Suzuki, T.; Tanaka, T.; Sato, A.; Iguchi, A.; Yoshita, K.; Ito, Y.; Imai, N.; Yamazaki, H.; et al. Acute Kidney Injury in an Adult Patient with IgA Nephropathy and Chronic Replicative Epstein–Barr Virus Infection. CEN Case Rep. 2019, 8, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Truszewska, A.; Wirkowska, A.; Gala, K.; Truszewski, P.; Krzemień-Ojak, Ł.; Mucha, K.; Pączek, L.; Foroncewicz, B. EBV Load Is Associated with CfDNA Fragmentation and Renal Damage in SLE Patients. Lupus 2021, 30, 1214–1225. [Google Scholar] [CrossRef] [PubMed]
- Grywalska, E.; Smarz-Widelska, I.; Korona-Głowniak, I.; Mertowski, S.; Gosik, K.; Hymos, A.; Ludian, J.; Niedźwiedzka-Rystwej, P.; Roliński, J.; Załuska, W. PD-1 and PD-L1 Expression on Circulating Lymphocytes as a Marker of Epstein-Barr Virus Reactivation-Associated Proliferative Glomerulonephritis. Int. J. Mol. Sci. 2020, 21, 8001. [Google Scholar] [CrossRef] [PubMed]
- Paschale, M.D.; Clerici, P. Serological Diagnosis of Epstein-Barr Virus Infection: Problems and Solutions. World J. Virol. 2012, 1, 31–43. [Google Scholar] [CrossRef]
- Gluba, A.; Banach, M.; Hannam, S.; Mikhailidis, D.P.; Sakowicz, A.; Rysz, J. The Role of Toll-like Receptors in Renal Diseases. Nat. Rev. Nephrol. 2010, 6, 224–235. [Google Scholar] [CrossRef]
- Mertowski, S.; Lipa, P.; Morawska, I.; Niedźwiedzka-Rystwej, P.; Bębnowska, D.; Hrynkiewicz, R.; Grywalska, E.; Roliński, J.; Załuska, W. Toll-Like Receptor as a PotentialBiomarker in RenalDiseases. Int. J. Mol. Sci. 2020, 21, 6712. [Google Scholar] [CrossRef]
- Liu, M.; Zen, K. Toll-Like Receptors Regulate the Development and Progression of Renal Diseases. Kidney Dis. 2021, 7, 14–23. [Google Scholar] [CrossRef]
- Wardle, E.N. Toll-Like Receptors and Glomerulonephritis. Saudi J. Kidney Dis. Transplant. 2007, 18, 159. [Google Scholar]
- Xia, H.; Bao, W.; Shi, S. Innate Immune Activity in Glomerular Podocytes. Front. Immunol. 2017, 8, 122. [Google Scholar] [CrossRef]
- Panzer, U.; Huber, T.B. Immune-Mediated Glomerular Diseases: New Basic Concepts and Clinical Implications. Cell Tissue Res. 2021, 385, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhang, Y.; Li, L.; Zhang, R.; Luo, Z.; Yang, Z.; Ye, Y.; He, J.; Sun, Q. Depletion of Toll-Like Receptor-9 Attenuates Renal Tubulointerstitial Fibrosis After Ischemia-Reperfusion Injury. Front. Cell Dev. Biol. 2021, 9, 641527. [Google Scholar] [CrossRef] [PubMed]
- Bossaller, L.; Christ, A.; Pelka, K.; Nündel, K.; Chiang, P.-I.; Pang, C.; Mishra, N.; Busto, P.; Bonegio, R.G.; Schmidt, R.E.; et al. TLR9 Deficiency Leads to Accelerated Renal Disease and Myeloid Lineage Abnormalities in Pristane-Induced Murine Lupus. J. Immunol. 2016, 197, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Dai, Z.; Li, Y.; Zhu, H.; Zhao, L. TLR9 Regulates NLRP3 Inflammasome Activation via the NF-KB Signaling Pathway in Diabetic Nephropathy. Diabetol. Metab. Syndr. 2022, 14, 26. [Google Scholar] [CrossRef]
- Makita, Y.; Suzuki, H.; Kano, T.; Takahata, A.; Julian, B.A.; Novak, J.; Suzuki, Y. TLR9 Activation Induces Aberrant IgA Glycosylation via APRIL- and IL-6–Mediated Pathways in IgA Nephropathy. Kidney Int. 2020, 97, 340–349. [Google Scholar] [CrossRef]
- Mohammadi, S.; Memarian, A.; Sedighi, S.; Behnampour, N.; Yazdani, Y. Immunoregulatory Effects of Indole-3-Carbinol on Monocyte-Derived Macrophages in Systemic Lupus Erythematosus: A Crucial Role for Aryl Hydrocarbon Receptor. Autoimmunity 2018, 51, 199–209. [Google Scholar] [CrossRef]
- Summers, S.A.; Steinmetz, O.M.; Ooi, J.D.; Gan, P.; O’Sullivan, K.M.; Visvanathan, K.; Akira, S.; Kitching, A.R.; Holdsworth, S.R. Toll-like Receptor 9 Enhances Nephritogenic Immunity and Glomerular Leukocyte Recruitment, Exacerbating Experimental Crescentic Glomerulonephritis. Am. J. Pathol. 2010, 177, 2234–2244. [Google Scholar] [CrossRef]
- Suzuki, H.; Suzuki, Y.; Narita, I.; Aizawa, M.; Kihara, M.; Yamanaka, T.; Kanou, T.; Tsukaguchi, H.; Novak, J.; Horikoshi, S.; et al. Toll-Like Receptor 9 Affects Severity of IgA Nephropathy. J. Am. Soc. Nephrol. 2008, 19, 2384–2395. [Google Scholar] [CrossRef]
- Myeloid Differentiation Primary Response Protein MyD88. Available online: https://www.Ebi.Ac.Uk/Interpro/Entry/InterPro/IPR017281 (accessed on 21 July 2022).
- Masum, M.A.; Ichii, O.; Hosny Ali Elewa, Y.; Nakamura, T.; Otani, Y.; Hosotani, M.; Kon, Y. Overexpression of Toll-like Receptor 9 Correlates with Podocyte Injury in a Murine Model of Autoimmune Membranoproliferative Glomerulonephritis. Autoimmunity 2018, 51, 386–398. [Google Scholar] [CrossRef]
- Lünemann, A.; Rowe, M.; Nadal, D. Innate Immune Recognition of EBV. In Epstein Barr Virus Volume 2: One Herpes Virus: Many Diseases; Münz, C., Ed.; Current Topics in Microbiology and Immunology; Springer International Publishing: Cham, Switzerland, 2015; pp. 265–287. ISBN 978-3-319-22834-1. [Google Scholar]
- Dworzanska, A.; Polz-Dacewicz, M. The Role of Toll-like Receptors (TLRs) in Virus-Related Cancers: A Mini Review. Curr. Issues Pharm. Med. Sci. 2020, 33, 225–227. [Google Scholar] [CrossRef]
- Hricik, D.E.; Chung-Park, M.; Sedor, J.R. Glomerulonephritis. N. Engl. J. Med. 1998, 339, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Kaartinen, K.; Safa, A.; Kotha, S.; Ratti, G.; Meri, S. Complement Dysregulation in Glomerulonephritis. Semin. Immunol. 2019, 45, 101331. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewski, M.; Grywalska, E.; Tomaszewski, A.; Błaszczak, P.; Kurzyna, M.; Roliński, J.; Kopeć, G. Overexpression of PD-1 on Peripheral Blood Lymphocytes in Patients with Idiopathic Pulmonary Arterial Hypertension and Its Association with High Viral Loads of Epstein-Barr Virus and Poor Clinical Parameters. J. Clin. Med. 2020, 9, 1966. [Google Scholar] [CrossRef] [PubMed]
| Parameter | GN Patients (n = 35) | Healthy Control Group (n = 35) | p Value | ||
|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | ||
| Age [years] | 37.1 ± 1.40 | 35.0 (26.0–45.0) | 37.1 ± 1.40 | 35.0 (26.0–45.0) | 1.0 |
| Male [%] | 21 (60) | 21 (60) | 1.0 | ||
| Glomerular hematuria (%) | 22 (62.9) | 0 (0) | <0.0001 * | ||
| Arterial hypertension (%) | 17 (48.6) | 0 (0) | <0.0001 * | ||
| WBC [103/mm3] | 7.0 ± 1.8 | 6.7 (5.3–8.6) | 6.71 ± 0.6 | 6.77 (6.4–7.2) | 0.96 |
| LYM [103/mm3] | 2.1 ± 0.8 | 1.98 (1.5–2.6) | 2.48 ± 0.8 | 2.44 (1.9–2.9) | 0.069 |
| RBC [106/mm3] | 4.5 ± 1.3 | 4.36 (4.1–4.7) | 4.88 ± 0.6 | 4.79 (4.4–5.4) | 0.00081 * |
| HGB [g/dL] | 13.1 ± 1.5 | 13.2 (12.2–14.0) | 14.17 ± 1.0 | 14.14 (13.4–15.1) | 0.00075 * |
| PLT [103/mm3] | 240.2 ± 57.4 | 223.0 (197.0–277.0) | 263.14 ± 55.7 | 263.0 (216.0–312.0) | 0.07 |
| Urea [mg/dL] | 51.8 ± 30.6 | 40.9 (26.6–78.0) | 30.8 ± 6.0 | 32.0 (28.0–35.8) | 0.0062 * |
| BUN [mg/dL] | 24.2 ± 14.3 | 19.1 (12.4–36.4) | 14.4 ± 2.8 | 14.95 (13.1–16.7) | 0.0062 * |
| Serum creatinine [mg/dL] | 1.2 ± 0.6 | 0.96 (0.8–1.8) | 0.95 ± 0.1 | 0.97 (0.85–1.1) | 0.28 |
| eGFR [mL/min] | 86.2 ± 35.2 | 81.5 (56.5–117.5) | 125.46 ± 9.7 | 122.36 (118.0–133.4) | <0.0001 * |
| Serum uric acid [mg/dL] | 7.1 ± 1.8 | 7.5 (5.4–8.2) | 5.87 ± 1.3 | 5.8 (4.6–7.3) | 0.0016 * |
| Serum IgG [g/L] | 6.2 ± 3.0 | 5.7 (4.2–6.9) | 12.48 ± 1.9 | 12.7 (11.4–13.9) | <0.0001 * |
| Serum IgM [g/L] | 1.7 ± 1.1 | 1.3 (0.8–2.9) | 1.58 ± 0.4 | 1.57 (1.3–1.8) | 0.63 |
| Serum IgA [g/L] | 2.4 ± 1.7 | 1.9 (1.0–3.4) | 2.39 ± 0.8 | 2.51 (1.8–3.0) | 0.29 |
| Serum total protein [g/dL] | 5.3 ± 0.9 | 5.3 (4.8–6.1) | 7.41 ± 0.5 | 7.6 (6.95–7.8) | <0.0001 * |
| Serum albumin [g/L] | 2.7 ± 0.9 | 2.8 (2.1–3.4) | 4.20 ± 0.4 | 4.29 (3.9–4.5) | <0.0001 * |
| Total quantity of protein in a 24-h urine collection test [g/24 h] | 3.8 ± 3.4 | 3.7 (0.3–7.0) | N/A | N/A | N/A |
| Serum complement component C3 [g/L] | 1.2 ± 0.4 | 1.2 (1.0–1.3) | 1.28 ± 0.2 | 1.25 (1.2–1.4) | 0.32 |
| Serum complement component C4 [g/L] | 0.30 ± 0.08 | 0.27 (0.25–0.35) | 0.27 ± 0.1 | 0.28 (0.2–0.3) | 0.42 |
| Parameter | IgAN (n = 20) | MPGN (n = 15) | Healthy Control group (n = 35) | p Value |
|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | ||
| Age [years] | 42.5 (31.5–55.5) | 27.0 (25.0–36.0) | 35.0 (26.0–45.0) | 0.030 * |
| Male [%] | 13 (65.0) | 8 (53.3) | 21 (60) | 0.78 |
| Glomerular hematuria (%) | 11 (55.0) | 11 (73.3) | 0 (0) | <0.0001 * |
| Arterial hypertension (%) | 8 (40.0) | 9 (60.0) | 0 (0) | <0.0001 * |
| WBC [103/mm3] | 6.75 (5.7–9.0) | 6.57 (5.1–8.4) | 6.77 (64–72) | 0.66 |
| LYM [103/mm3] | 2.43 (1.9–3.0) | 1.57 (1.3–1.98) | 2.44 (19–29) | 0.0005 * |
| RBC [106/mm3] | 4.35 (4.0–4.6) | 4.4 (4.1–4.9) | 4.79 (44–54) | 0.0027 * |
| HGB [g/dL] | 13.3 (12.5–14.2) | 13.0 (12.1–13.5) | 14.14 (134–151) | 0.0019 * |
| PLT [103/mm3] | 243.0 (209.5–272.5) | 212.0 (177.0–304.0) | 263.0 (2160–3120) | 0.11 |
| Urea [mg/dL] | 38.93 (26.8–75.5) | 49.5 (26.0–78.3) | 32.0 (280–358) | 0.023 * |
| BUN [mg/dL] | 18.19 (12.5–35.3) | 23.13 (12.1–36.6) | 14.95 (131–167) | 0.023 * |
| Serum creatinine [mg/dL] | 1.04 (0.8–2.2) | 0.96 (0.7–1.8) | 0.97 (085–11) | 0.50 |
| eGRF [ml/min] | 85.11 (66.2–120.4) | 81.54 (53.3–116.3) | 122.36 (1180–1334) | <0.0001 * |
| Serum uric acid [mg/dL] | 7.6 (5.8–8.3) | 7.1 (5.0–8.2) | 5.8 (46–73) | 0.0057 |
| Serum IgG [g/L] | 6.35 (5.2–8.5) | 4.24 (3.0–6.1) | 12.7 (114–139) | <0.0001 * |
| Serum IgM [g/L] | 1.37 (1.0–2.8) | 1.2 (0.5–3.1) | 1.57 (13–18) | 0.73 |
| Serum IgA [g/L] | 1.96 (1.4–4.1) | 1.39 (0.6–3.1) | 2.51 (18–30) | 0.26 |
| Serum total protein [g/dL] | 5.3 (4.9–6.2) | 5.4 (4.4–6.0) | 7.6 (695–78) | <0.0001 * |
| Serum albumin [g/L] | 2.9 (2.3–3.5) | 2.3 (1.9–3.1) | 4.29 (39–45) | <0.0001 * |
| Total quantity of protein in a 24-h urine collection test [g/24 h] | 0.78 (0.1–4.4) | 5.7 (3.0–8.0) | N/A | <0.0001 * |
| Serum complement component C3 [g/L] | 1.24 (1.1–1.4) | 1.18 (0.7–1.3) | 1.25 (12–14) | 0.14 |
| Serum complement component C4 [g/L] | 0.27 (0.2–0.4) | 0.27 (0.25–0.3) | 0.28 (02–03) | 0.63 |
| Parameter | GN Patients (n = 35) | Healthy Control Group (n = 35) | p Value | ||
|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | ||
| Frequencies of BDCA-1+CD19− DCs in the peripheral blood (mDC) [%] | 1.05 ± 0.4 | 1.0 (0.8–1.2) | 0.55 ± 0.3 | 0.50 (0.3–0.7) | <0.0001 * |
| Frequencies of BDCA-2+CD123+ DCs in the peripheral blood (pDC) [%] | 0.49 ± 0.4 | 0.42 (0.2–0.7) | 0.23 ± 0.2 | 0.17 (0.1–0.3) | 0.00022 * |
| mDC/pDC ratio | 4.87 ± 0.89 | 2.1 (1.8–5.1) | 8.73 ± 1.71 | 2.53 (1.5–6.6) | 0.61 |
| Frequencies of mDC BDCA1+CD19-TLR9+ of total mDC [%] | 20.24 ± 7.9 | 18.4 (14.4–27.8) | 5.26 ± 3.1 | 4.47 (2.6–8.2) | <0.0001 * |
| Frequencies of pDC BDCA2+CD123+TLR9+ of total pDC [%] | 13.07 ± 5.0 | 12.6 (8.7–16.7) | 4.52 ± 2.7 | 3.99 (2.0–6.8) | <0.0001 * |
| Frequencies of classical monocytes CD14++CD16- in the peripheral blood [%] | 85.82 ± 4.7 | 87.07 (80.9–89.1) | 90.63 ± 4.2 | 91.8 (88.3–93.9) | <0.0001 * |
| Frequencies of classical monocytes CD14++CD16-TLR9+ of total classical monocytes [%] | 15.92 ± 12.9 | 11.89 (5.3–30.3) | 7.99 ± 7.3 | 5.07 (3.4–9.9) | 0.0036 * |
| Frequencies of intermediate monocytes CD14++CD16+ in the peripheral blood [%] | 9.96 ± 4.9 | 8.49 (6.0–13.9) | 6.23 ± 3.1 | 5.68 (3.5–8.5) | 0.00089 * |
| Frequencies of intermediate monocytes CD14++CD16+TLR9+ of total [%] | 18.46 ± 1.39 | 15.83 (5.0–32.0) | 8.24 ± 7.3 | 6.04 (3.5–9.4) | 0.0024 * |
| TLR9 serum concentration [ng/mL] | 32.14 ± 1.31 | 30.4 (18.6–45.9) | 4.78 ± 2.8 | 4.29 (2.9–7.2) | <0.0001 * |
| Parameter | IgAN(n = 20) | MPGN (n = 15) | Healthy Control Group (n = 35) | p Value |
|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | ||
| Frequencies of BDCA-1+CD19− DC in the peripheral blood (mDC) [%] | 1.05 (0.8–1.2) | 0.9 (0.8–1.3) | 0.50 (03–07) | <0.0001 * |
| Frequencies of BDCA-2+CD123+ DC in the peripheral blood (pDC) [%] | 0.42 (0.2–0.6) | 0.4 (0.3–0.9) | 0.17 (01–03) | 0.0009 * |
| mDC/pDC ratio | 2.14 (1.6–5.2) | 2.1 (1.8–4.7) | 2.53 (15–66) | 0.82 |
| Frequencies of mDC BDCA1+CD19-TLR9+ of total mDC [%] | 21.34 (17.3–26.1) | 14.35 (10.5–31.5) | 4.47 (26–82) | <0.0001 * |
| Frequencies of pDC BDCA2+CD123+TLR9+ of total pDC [%] | 13.34 (11.2–15.6) | 8.7 (7.6–18.8) | 3.99 (20–68) | <0.0001 * |
| Frequencies of classical monocytes CD14++CD16- in the peripheral blood [%] | 86.98 (81.4–89.3) | 87.1 (80.9–89.1) | 91.8 (883–939) | 0.0002 * |
| Frequencies of classical monocytes CD14++CD16-TLR9+ of total classical monocytes [%] | 12.68 (5.9–27.8) | 10.4 (4.9–35.4) | 5.07 (34–99) | 0.014 * |
| Frequencies of intermediate monocytes CD14++CD16+ in the peripheral blood [%] | 8.53 (6.4–12.6) | 7.7 (6.0–16.6) | 5.68 (35–85) | 0.0038 * |
| Frequencies of intermediate monocytes CD14++CD16+TLR9+ of total intermediate [%] | 19.49 (5.2–32.6) | 13.4 (5.0–28.5) | 6.04 (35–94) | 0.0098 |
| TLR9 serum concentration [ng/mL] | 28.21 (22.1–34.3) | 45.9 (16.2–49.8) | 4.29 (29–72) | <0.0001 * |
| Parameter | TLR9 Serum Concentration [ng/mL] (n = 70) | ||
|---|---|---|---|
| Spearman R | t(N−2) | p Value | |
| EBV DNA copy number/µg DNA | 0.79 | 10.65 | <0.0001 * |
| Urea [mg/dL] | 0.30 | 2.62 | 0.011 * |
| BUN [mg/dL] | 0.30 | 2.62 | 0.011 * |
| Serum creatinine [mg/dL] | 0.07 | 0.60 | 0.55 |
| eGFR [ml/min] | −0.52 | −5.05 | <0.0001 * |
| Serum uric acid [mg/dL] | 0.35 | 3.06 | 0.0031 * |
| Serum IgG [g/L] | −0.73 | −8.83 | <0.0001 * |
| Serum IgM [g/L] | −0.23 | −1.94 | 0.057 |
| Serum IgA [g/L] | −0.12 | −1.00 | 0.32 |
| Serum total protein [g/dL] | −0.76 | −9.70 | <0.0001 * |
| Serum albumin [g/L] | −0.74 | −9.13 | <0.0001 * |
| Frequencies of BDCA-1+CD19− DC in the peripheral blood (mDC) [%] | 0.55 | 5.50 | <0.0001 * |
| Frequencies of BDCA-2+CD123+ DC in the peripheral blood (pDC) [%] | 0.41 | 3.75 | 0.0004 * |
| Parameter | TLR9 Serum Concentration [ng/mL] (n = 35) | ||
|---|---|---|---|
| Spearman R | t(N−2) | p Value | |
| EBV DNA copy number/µg DNA | 0.92 | 13.06 | <0.0001 * |
| Urea [mg/dL] | 0.10 | 0.59 | 0.56 |
| BUN [mg/dL] | 0.10 | 0.59 | 0.56 |
| Serum creatinine [mg/dL] | −0.17 | −1.01 | 0.32 |
| eGFR [ml/min] | −0.28 | −1.65 | 0.11 |
| Serum uric acid [mg/dL] | 0.19 | 1.10 | 0.28 |
| Serum IgG [g/L] | −0.40 | −2.49 | 0.018 * |
| Serum IgM [g/L] | −0.42 | −2.63 | 0.013 * |
| Serum IgA [g/L] | −0.22 | −1.27 | 0.21 |
| Serum total protein [g/dL] | −0.19 | −1.12 | 0.27 |
| Serum albumin [g/L] | −0.38 | −2.38 | 0.023 * |
| Total quantity of protein in a 24-h urine collection test [g/24 h] | 0.61 | 4.46 | <0.0001 * |
| Frequencies of BDCA-1+CD19− DC in the peripheral blood (mDC) [%] | 0.18 | 1.03 | 0.31 |
| Frequencies of BDCA-2+CD123+ DC in the peripheral blood (pDC) [%] | 0.41 | 2.59 | 0.014 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smarz-Widelska, I.; Mertowski, S.; Mertowska, P.; Korona-Głowniak, I.; Hymos, A.; Grywalska, E.; Załuska, W. The Importance of Toll-like Receptor 9 Expression on Monocytes and Dendritic Cells in the Context of Epstein–Barr Virus Infection in the Immunopathogenesis of Primary Glomerulonephritis. Int. J. Mol. Sci. 2022, 23, 11796. https://doi.org/10.3390/ijms231911796
Smarz-Widelska I, Mertowski S, Mertowska P, Korona-Głowniak I, Hymos A, Grywalska E, Załuska W. The Importance of Toll-like Receptor 9 Expression on Monocytes and Dendritic Cells in the Context of Epstein–Barr Virus Infection in the Immunopathogenesis of Primary Glomerulonephritis. International Journal of Molecular Sciences. 2022; 23(19):11796. https://doi.org/10.3390/ijms231911796
Chicago/Turabian StyleSmarz-Widelska, Iwona, Sebastian Mertowski, Paulina Mertowska, Izabela Korona-Głowniak, Anna Hymos, Ewelina Grywalska, and Wojciech Załuska. 2022. "The Importance of Toll-like Receptor 9 Expression on Monocytes and Dendritic Cells in the Context of Epstein–Barr Virus Infection in the Immunopathogenesis of Primary Glomerulonephritis" International Journal of Molecular Sciences 23, no. 19: 11796. https://doi.org/10.3390/ijms231911796
APA StyleSmarz-Widelska, I., Mertowski, S., Mertowska, P., Korona-Głowniak, I., Hymos, A., Grywalska, E., & Załuska, W. (2022). The Importance of Toll-like Receptor 9 Expression on Monocytes and Dendritic Cells in the Context of Epstein–Barr Virus Infection in the Immunopathogenesis of Primary Glomerulonephritis. International Journal of Molecular Sciences, 23(19), 11796. https://doi.org/10.3390/ijms231911796







