First Report on the Streptococcus gallolyticus (S. bovis Biotype I) DSM 13808 Exopolysaccharide Structure
Abstract
1. Introduction
2. Results
2.1. Isolation of S. gallolyticus subsp. gallolyticus DSM 13808 Exopolysaccharide
2.2. Structure of the Exopolysaccharide
2.3. Mass Spectrometry Analysis of the EPS
3. Discussion
4. Materials and Methods
4.1. Bacteria
4.2. Exopolysaccharide
4.3. Partial Acid Hydrolysis
4.4. Analytical Methods
4.5. NMR Spectroscopy
4.6. Mass Spectrometry
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EPS | exopolysaccharide |
| TCA | trichloroacetic acid |
| MALDI-TOF | matrix-assisted laser-desorption/ionisation time-of-flight |
| MS | mass spectrometry |
| NMR | nuclear magnetic resonance |
| COSY | correlated spectroscopy |
| TOCSY | total correlation spectroscopy |
| NOESY | nuclear Overhauser effect spectroscopy |
| HMBC | heteronuclear multiple bond correlation |
| HSQC | heteronuclear single quantum coherence |
| DEPT | distortionless enhancement by polarisation transfer |
| CRC | colorectal cancer |
| DSMZ | German Collection of Microorganisms and Cell Cultures GmbH |
References
- Chaturvedi, A.K. Beyond Cervical Cancer: Burden of Other HPV-Related Cancers among Men and Women. J. Adolesc. Health 2010, 46 (Suppl. S4), S20–S26. [Google Scholar] [CrossRef]
- Suerbaum, S.; Michetti, P. Helicobacter Pylori Infection. N. Engl. J. Med. 2002, 347, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Xu, P. Global Colorectal Cancer Burden in 2020 and Projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef] [PubMed]
- Dumke, J.; Hinse, D.; Vollmer, T.; Knabbe, C.; Dreier, J. Development and Application of a Multilocus Sequence Typing Scheme for Streptococcus Gallolyticus Subsp. Gallolyticus. J. Clin. Microbiol. 2014, 52, 2472–2478. [Google Scholar] [CrossRef]
- Romero, B.; Morosini, M.-I.; Loza, E.; Rodríguez-Baños, M.; Navas, E.; Cantón, R.; Campo, R.D. Reidentification of Streptococcus Bovis Isolates Causing Bacteremia According to the New Taxonomy Criteria: Still an Issue? J. Clin. Microbiol. 2011, 49, 3228–3233. [Google Scholar] [CrossRef]
- Boleij, A.; Tjalsma, H. The Itinerary of Streptococcus Gallolyticus Infection in Patients with Colonic Malignant Disease. Lancet Infect. Dis. 2013, 13, 719–724. [Google Scholar] [CrossRef]
- Butt, J.; Romero-Hernández, B.; Pérez-Gómez, B.; Willhauck-Fleckenstein, M.; Holzinger, D.; Martin, V.; Moreno, V.; Linares, C.; Dierssen-Sotos, T.; Barricarte, A.; et al. Association of Streptococcus Gallolyticus Subspecies Gallolyticus with Colorectal Cancer: Serological Evidence. Int. J. Cancer 2016, 138, 1670–1679. [Google Scholar] [CrossRef]
- Kumar, R.; Herold, J.L.; Taylor, J.; Xu, J.; Xu, Y. Variations among Streptococcus Gallolyticus Subsp. Gallolyticus Strains in Connection with Colorectal Cancer. Sci. Rep. 2018, 8, 1514. [Google Scholar] [CrossRef]
- Isenring, J.; Köhler, J.; Nakata, M.; Frank, M.; Jans, C.; Renault, P.; Danne, C.; Dramsi, S.; Kreikemeyer, B.; Oehmcke-Hecht, S. Streptococcus Gallolyticus Subsp. Gallolyticus Endocarditis Isolate Interferes with Coagulation and Activates the Contact System. Virulence 2018, 9, 248–261. [Google Scholar] [CrossRef]
- Boleij, A.; Muytjens, C.M.J.; Bukhari, S.I.; Cayet, N.; Glaser, P.; Hermans, P.W.M.; Swinkels, D.W.; Bolhuis, A.; Tjalsma, H. Novel Clues on the Specific Association of Streptococcus Gallolyticus Subsp Gallolyticus with Colorectal Cancer. J. Infect. Dis. 2011, 203, 1101–1109. [Google Scholar] [CrossRef]
- Taylor, J.C.; Gao, X.; Xu, J.; Holder, M.; Petrosino, J.; Kumar, R.; Liu, W.; Höök, M.; Mackenzie, C.; Hillhouse, A.; et al. A Type VII Secretion System of Streptococcus Gallolyticus Subsp. Gallolyticus Contributes to Gut Colonization and the Development of Colon Tumors. PLoS Pathog. 2021, 17, e1009182. [Google Scholar] [CrossRef]
- Molinari, A.; Orefici, G.; Donelli, G.; Von Hunolstein, C.; Paradisi, S.; Arancia, G. Preservation of Capsular Material of Streptococcal Cells by Specific Lectins Determined by Immunoelectron Microscopy. Histochem. J. 1988, 20, 526–530. [Google Scholar] [CrossRef]
- Vincent, S.J.; Faber, E.J.; Neeser, J.R.; Stingele, F.; Kamerling, J.P. Structure and Properties of the Exopolysaccharide Produced by Streptococcus Macedonicus Sc136. Glycobiology 2001, 11, 131–139. [Google Scholar] [CrossRef]
- Górska, S.; Jachymek, W.; Rybka, J.; Strus, M.; Heczko, P.B.; Gamian, A. Structural and Immunochemical Studies of Neutral Exopolysaccharide Produced by Lactobacillus Johnsonii 142. Carbohydr. Res. 2010, 345, 108–114. [Google Scholar] [CrossRef]
- Jachymek, W.; Petersson, C.; Helander, A.; Kenne, L.; Lugowski, C.; Niedziela, T. Structural Studies of the O-Specific Chain and a Core Hexasaccharide of Hafnia Alvei Strain 1192 Lipopolysaccharide. Carbohydr. Res. 1995, 269, 125–138. [Google Scholar] [CrossRef]
- Li, C.; Duda, K.A.; Elverdal, P.L.; Skovsted, I.C.; Kjeldsen, C.; Duus, J.Ø. Structural, Biosynthetic, and Serological Cross-Reactive Elucidation of Capsular Polysaccharides from Streptococcus Pneumoniae Serogroup 16. J. Bacteriol. 2019, 201, e00453-19. [Google Scholar] [CrossRef]
- Domon, B.; Costello, C.E. A Systematic Nomenclature for Carbohydrate Fragmentations in FAB-MS/MS Spectra of Glycoconjugates. Glycoconj. J. 1988, 5, 397–409. [Google Scholar] [CrossRef]
- Verma, D.K.; Niamah, A.K.; Patel, A.R.; Thakur, M.; Singh Sandhu, K.; Chávez-González, M.L.; Shah, N.; Noe Aguilar, C. Chemistry and Microbial Sources of Curdlan with Potential Application and Safety Regulations as Prebiotic in Food and Health. Food Res. Int. Ott. Ont 2020, 133, 109136. [Google Scholar] [CrossRef]
- Wang, K.; Li, W.; Rui, X.; Chen, X.; Jiang, M.; Dong, M. Characterization of a Novel Exopolysaccharide with Antitumor Activity from Lactobacillus Plantarum 70810. Int. J. Biol. Macromol. 2014, 63, 133–139. [Google Scholar] [CrossRef]
- Alvarez, X. Biochemical Characterization of Nostoc Sp. Exopolysaccharides and Evaluation of Potential Use in Wound Healing. Carbohydr. Polym. 2020, 254, 117303. [Google Scholar] [CrossRef]
- Ciszek-Lenda, M.; Strus, M.; Górska, S.; Targosz-Korecka, M.; Śróttek, M.; Heczko, P.; Gamian, A.; Szymonski, M.; Marcinkiewicz, J. Strain Specific Immunostimulatory Potential of Lactobacilli-Derived Exopolysaccharides. Cent.-Eur. J. Immunol. 2011, 36, 121–129. [Google Scholar]
- Fanning, S.; Hall, L.J.; van Sinderen, D. Bifidobacterium Breve UCC2003 Surface Exopolysaccharide Production Is a Beneficial Trait Mediating Commensal-Host Interaction through Immune Modulation and Pathogen Protection. Gut Microbes 2012, 3, 420–425. [Google Scholar] [CrossRef]
- Górska, S.; Schwarzer, M.; Jachymek, W.; Srutkova, D.; Brzozowska, E.; Kozakova, H.; Gamian, A. Distinct Immunomodulation of Bone Marrow-Derived Dendritic Cell Responses to Lactobacillus Plantarum WCFS1 by Two Different Polysaccharides Isolated from Lactobacillus Rhamnosus LOCK 0900. Appl. Environ. Microbiol. 2014, 80, 6506–6516. [Google Scholar] [CrossRef]
- Laws, A.; Gu, Y.; Marshall, V. Biosynthesis, Characterisation, and Design of Bacterial Exopolysaccharides from Lactic Acid Bacteria. Biotechnol. Adv. 2001, 19, 597–625. [Google Scholar] [CrossRef]
- Niedziela, T.; Kenne, L.; Lugowski, C. Novel O-Antigen of Hafnia Alvei PCM 1195 Lipopolysaccharide with a Teichoic Acid-like Structure. Carbohydr. Res. 2010, 345, 270–274. [Google Scholar] [CrossRef]
- Adlam, C.; Knights, J.M.; Mugridge, A.; Lindon, J.C.; Williams, J.M.; Beesley, J.E.Y. 1986. Purification, Characterization and Immunological Properties of the Serotype-Specific Capsular Polysaccharide of Pasteurella Haemolytica Serotype A7 Organisms. Microbiology 1986, 132, 1079–1087. [Google Scholar] [CrossRef]
- Petersson, C.; Niedziela, T.; Jachymek, W.; Kenne, L.; Zarzecki, P.; Lugowski, C. Structural Studies of the O-Specific Polysaccharide of Hafnia Alvei Strain PCM 1206 Lipopolysaccharide Containing D-Allothreonine. Eur. J. Biochem. 1997, 244, 580–586. [Google Scholar] [CrossRef]
- Ciucanu, I.; Kerek, F. A Simple and Rapid Method for the Permethylation of Carbohydrates. Carbohydr. Res. 1984, 131, 209–217. [Google Scholar] [CrossRef]
- Gerwig, G.J.; Kamerling, J.P.; Vliegenthart, J.F.G. Determination of the d and l Configuration of Neutral Monosaccharides by High-Resolution Capillary g.l.c. Carbohydr. Res. 1978, 62, 349–357. [Google Scholar] [CrossRef]
- Gerwig, G.J.; Kamerling, J.P.; Vliegenthart, J.F. Determination of the Absolute Configuration of Mono-Saccharides in Complex Carbohydrates by Capillary G.L.C. Carbohydr. Res. 1979, 77, 10–17. [Google Scholar] [CrossRef]
- Lee, W.; Tonelli, M.; Markley, J.L. NMRFAM-SPARKY: Enhanced Software for Biomolecular NMR Spectroscopy. Bioinforma. Oxf. Engl. 2015, 31, 1325–1327. [Google Scholar] [CrossRef] [PubMed]
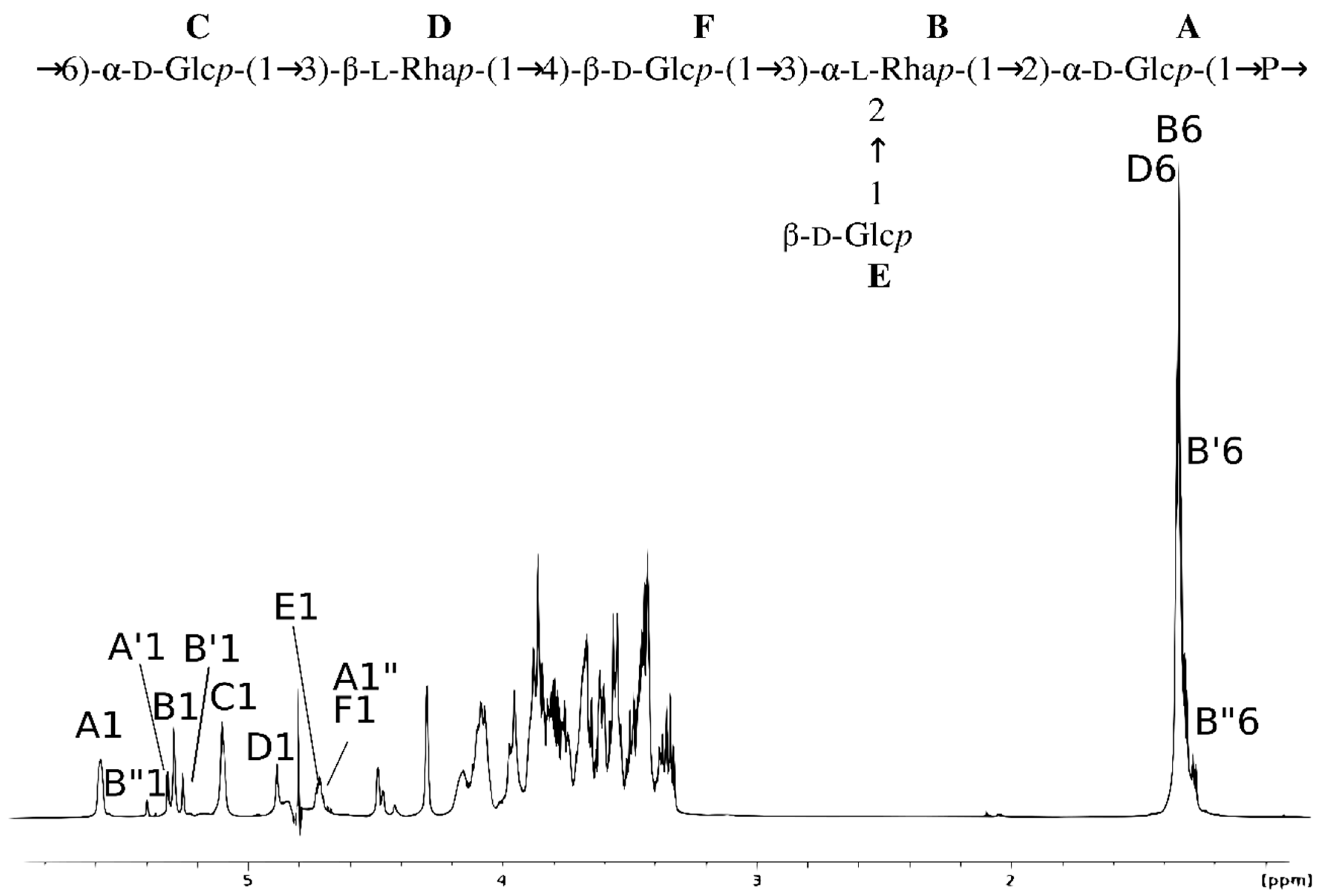
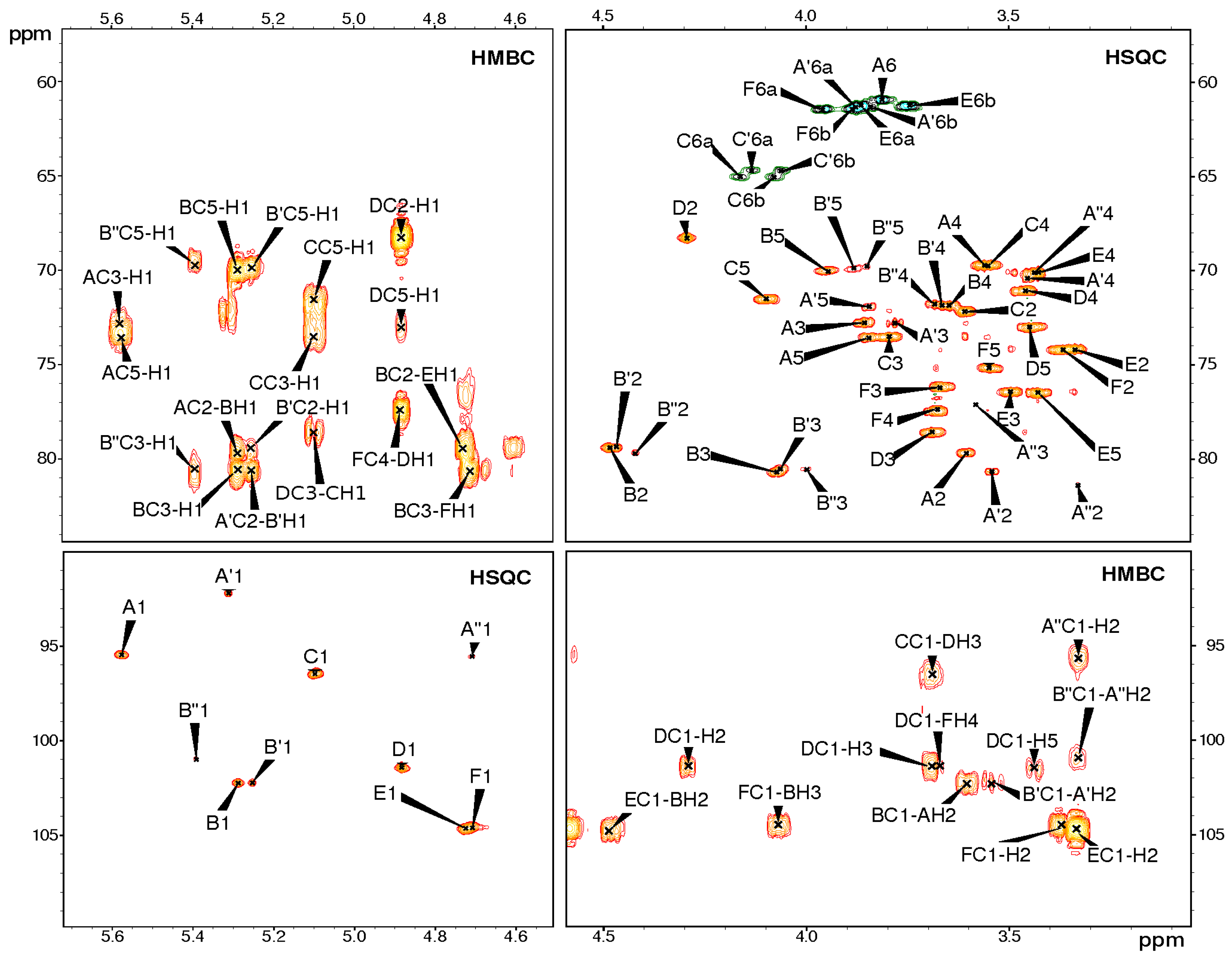
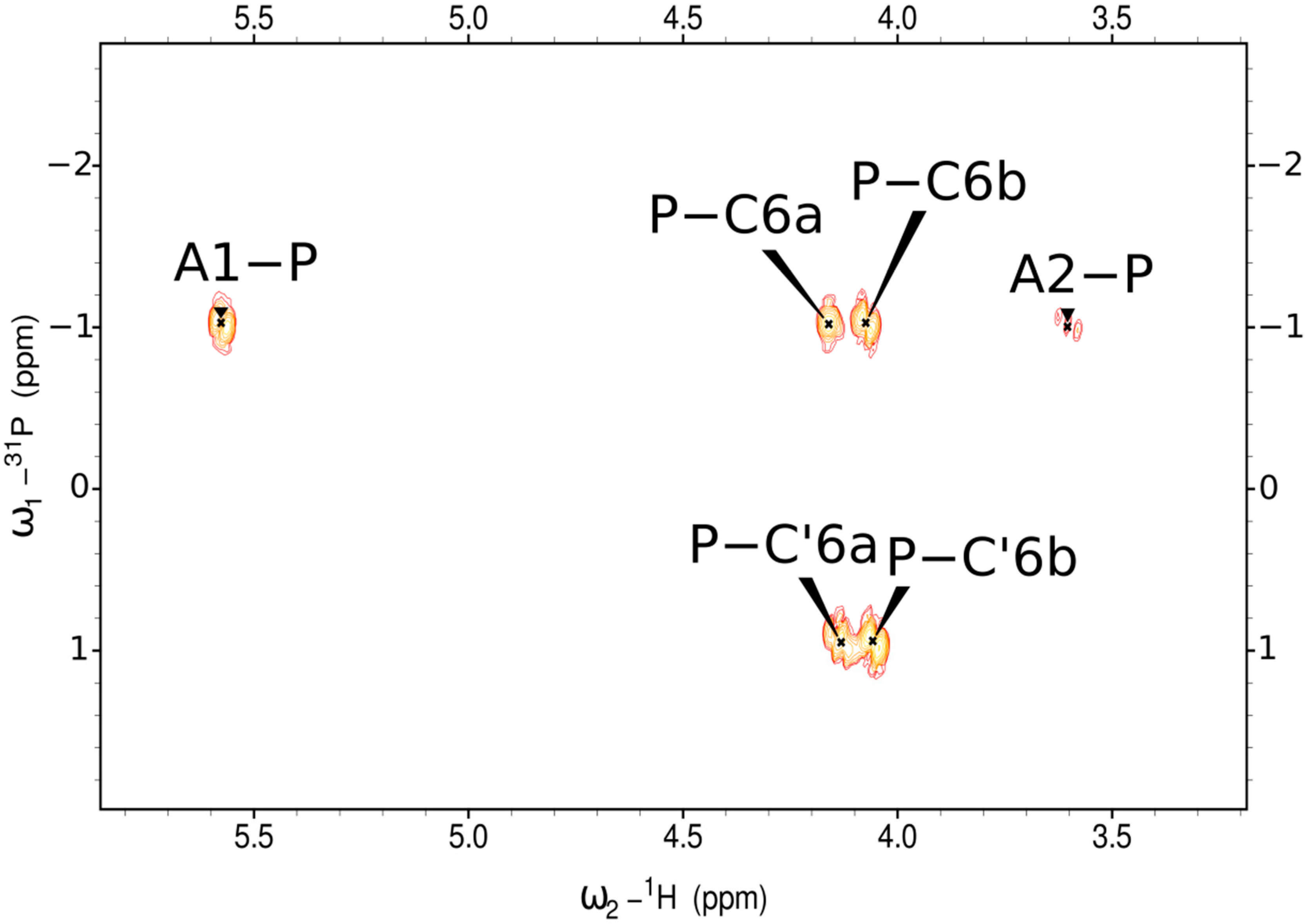
| Residue (a) | Chemical Shifts (ppm) | Selected Inter-Residue NOE and 3JH,C Connectivities | |||||||
|---|---|---|---|---|---|---|---|---|---|
| H1 C1 | H2, C2 | H3 C3 | H4 C4 | H5 C5 | H6a, H6b C6 | H1/C1 Connectivities to | Inter-Residue Atom/Residue | ||
| A | →2)-α-d-Glcp-(1→P (b) | 5.58 95.5 | 3.61 79.7 | 3.86 72.8 | 3.55 69.7 | 3.85 73.6 | 3.81 (c) 61.0 | - | - |
| A′ | →2)-α-d-Glcp-(1→ | 5.31 92.2 | 3.54 80.7 | 3.78 72.8 | 3.45 70.4 | 3.85 71.8 | 3.88, 3.84 61.3 | - | - |
| A″ | →2)-β-d-Glcp-(1→ | 4.71 95.6 | 3.33 81.3 | 3.58 77.1 | 3.44 70.1 | nd (d) | nd (d) | - | - |
| B | →2,3)-α-l-Rhap-(1→ | 5.29 102.2 | 4.49 79.4 | 4.07 80.7 | 3.65 71.9 | 3.95 70.0 | 1.33 17.7 | 3.61 79.7 | H2 of A |
| B′ | →2,3)-α-l-Rhap-(1→ | 5.26 102.2 | 4.47 79.4 | 4.07 80.5 | 3.67 71.8 | 3.88 69.9 | 1.31 17.4 | 3.54 80.7 | H2 of A′ |
| B″ | →2,3)-α-l-Rhap-(1→ | 5.39 101.0 | 4.42 79.6 | 4.00 80.5 | 3.68 71.8 | 3.85 69.7 | 1.28 17.4 | 3.33 81.3 | H2 of A″ |
| C | P→6)-α-d-Glcp-(1→ | 5.10 96.5 | 3.61 72.2 | 3.80 73.5 | 3.55 69.7 | 4.10 71.5 | 4.16, 4.08 64.9 | 3.69 78.6 | H3 of D |
| D | →3)-β-l-Rhap-(1→ | 4.88 101.4 | 4.30 68.3 | 3.69 78.6 | 3.46 71.1 | 3.45 73.0 | 1.34 17.6 | 3.68 77.4 | H4 of F |
| E | β-d-Glcp-(1→ | 4.72 104.7 | 3.34 74.2 | 3.50 76.4 | 3.43 70.1 | 3.43 76.5 | 3.87, 3.75 61.2 | 4.49 79.4 | H2 of B |
| F | →4)-β-d-Glcp-(1→ | 4.71 104.5 | 3.37 74.2 | 3.67 76.2 | 3.68 77.4 | 3.55 75.2 | 3.96, 3.89 61.4 | 4.07 80.7 | H3 of B |
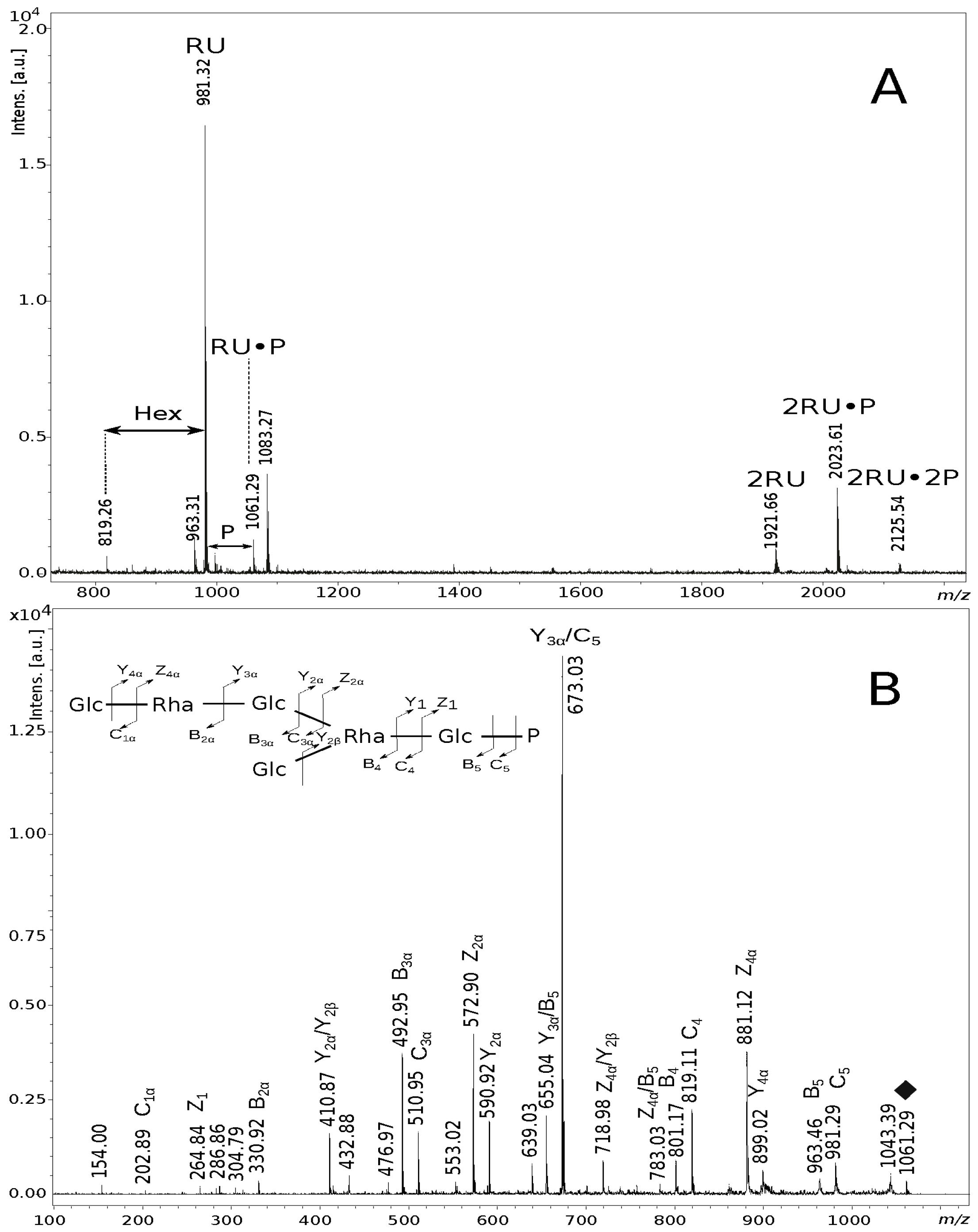
| Oligosaccharide Structure | Calculated Mass (Da) | Observed Ion (m/z) | Calculated Ion (m/z) | Interpretation of the Ion |
|---|---|---|---|---|
| Glc8·Rha4·P2 | 2058.60 | 2125.54 | 2125.55 | [M + H, 3Na]+ |
| Glc8·Rha4·P | 1978.63 | 2023.61 | 2023.60 | [M + H, 2Na]+ |
| Glc8·Rha4 | 1898.66 | 1921.66 | 1921.65 | [M + H, Na]+ |
| Glc4·Rha2·P | 1038.30 | 1083.27 | 1083.28 | [M + H, 2Na]+ |
| Glc4·Rha2·P | 1038.30 | 1061.29 | 1061.29 | [M + H, Na]+ |
| Glc4·Rha2·P | 1038.30 | 1043.39 | 1043.28 | [M–H2O + H, Na]+ |
| Glc4·Rha2 | 958.34 | 981.29 | 981.33 | [M + H, Na]+ |
| Glc4·Rha2 | 958.34 | 963.46 | 963.31 | [M–H2O + H, Na]+ |
| Glc3·Rha2·P | 876.25 | 899.02 | 899.24 | [M + H, Na]+ |
| Glc3·Rha2·P | 876.25 | 881.12 | 881.23 | [M–H2O + H, Na]+ |
| Glc3·Rha2 | 796.28 | 819.11 | 819.27 | [M + H, Na]+ |
| Glc3·Rha2 | 796.28 | 801.17 | 801.26 | [M–H2O + H, Na]+ |
| Glc3·Rha2 | 796.28 | 783.03 | 783.26 | [M–2H2O + H, Na]+ |
| Glc2·Rha2·P | 714.20 | 718.98 | 719.18 | [M–H2O + H, Na]+ |
| Glc3·Rha | 650.23 | 673.07 | 673.22 | [M + H, Na]+ |
| Glc3·Rha | 650.23 | 655.04 | 655.20 | [M–H2O + H, Na]+ |
| Glc2·Rha·P | 568.14 | 590.92 | 591.13 | [M + H, Na]+ |
| Glc2·Rha·P | 568.14 | 572.90 | 573.12 | [M–H2O + H, Na]+ |
| Glc2·Rha | 488.17 | 510.95 | 511.16 | [M + H, Na]+ |
| Glc2·Rha | 488.17 | 492.95 | 493.15 | [M–H2O + H, Na]+ |
| Glc·Rha·P | 406.09 | 432.88 | 433.05 | [M–H2O + H, 2Na]+ |
| Glc·Rha·P | 406.09 | 410.87 | 411.06 | [M–H2O + H, Na]+ |
| Glc·Rha | 309.12 | 330.92 | 331.11 | [M + H, Na]+ |
| Glc·P | 242.02 | 304.79 | 305.00 | [M + H, 2Na]+ |
| Glc·P | 242.02 | 286.86 | 287.01 | [M–H2O + H, 2Na]+ |
| Glc·P | 242.02 | 264.84 | 265.02 | [M + H, Na]+ |
| Glc | 180.06 | 202.97 | 203.05 | [M + H, Na]+ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maciejewska, A.; Lugowski, C.; Lukasiewicz, J. First Report on the Streptococcus gallolyticus (S. bovis Biotype I) DSM 13808 Exopolysaccharide Structure. Int. J. Mol. Sci. 2022, 23, 11797. https://doi.org/10.3390/ijms231911797
Maciejewska A, Lugowski C, Lukasiewicz J. First Report on the Streptococcus gallolyticus (S. bovis Biotype I) DSM 13808 Exopolysaccharide Structure. International Journal of Molecular Sciences. 2022; 23(19):11797. https://doi.org/10.3390/ijms231911797
Chicago/Turabian StyleMaciejewska, Anna, Czeslaw Lugowski, and Jolanta Lukasiewicz. 2022. "First Report on the Streptococcus gallolyticus (S. bovis Biotype I) DSM 13808 Exopolysaccharide Structure" International Journal of Molecular Sciences 23, no. 19: 11797. https://doi.org/10.3390/ijms231911797
APA StyleMaciejewska, A., Lugowski, C., & Lukasiewicz, J. (2022). First Report on the Streptococcus gallolyticus (S. bovis Biotype I) DSM 13808 Exopolysaccharide Structure. International Journal of Molecular Sciences, 23(19), 11797. https://doi.org/10.3390/ijms231911797






