Mini Review: Molecular Interpretation of the IGF/IGF-1R Axis in Cancer Treatment and Stem Cells-Based Therapy in Regenerative Medicine
Abstract
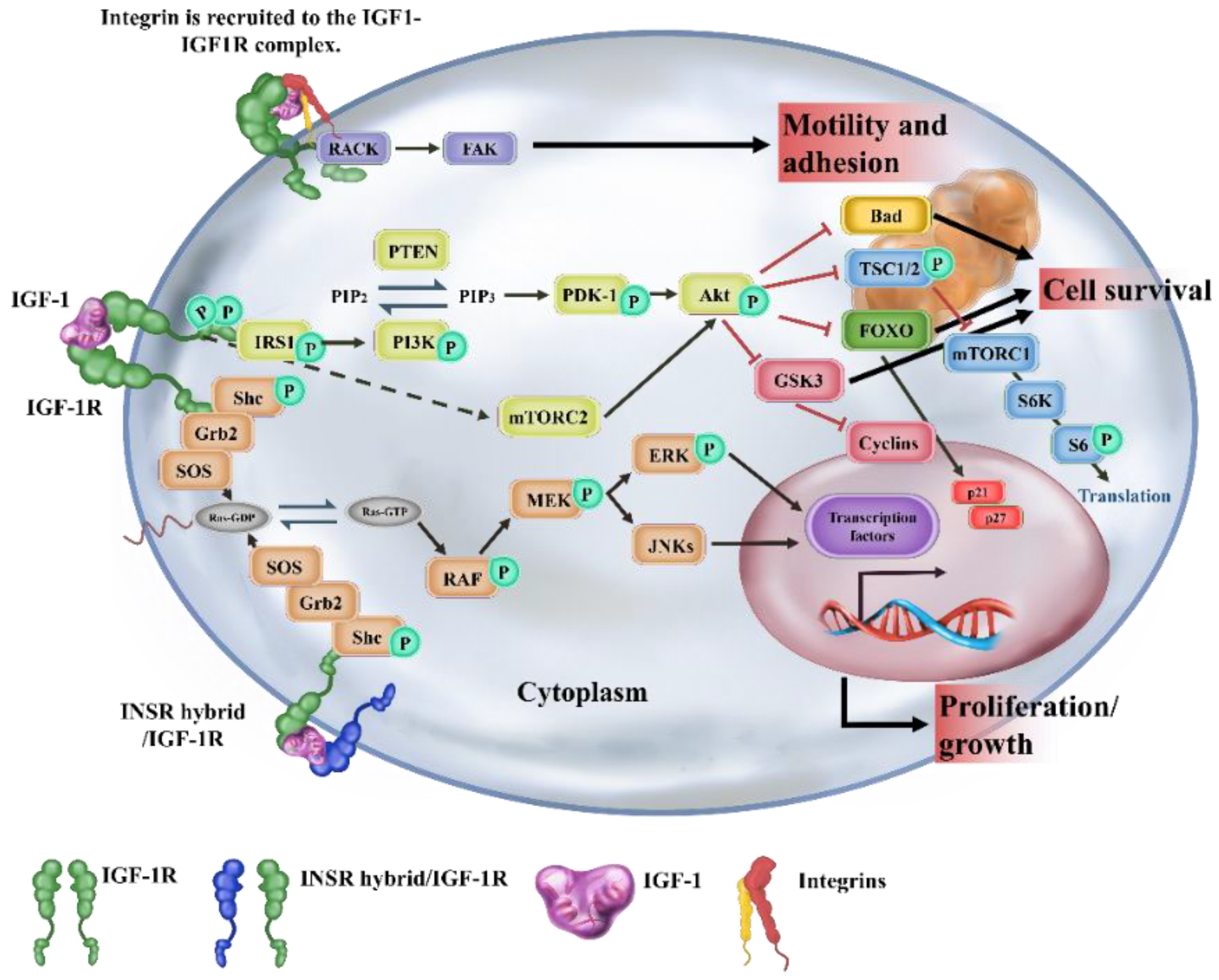
1. Introduction
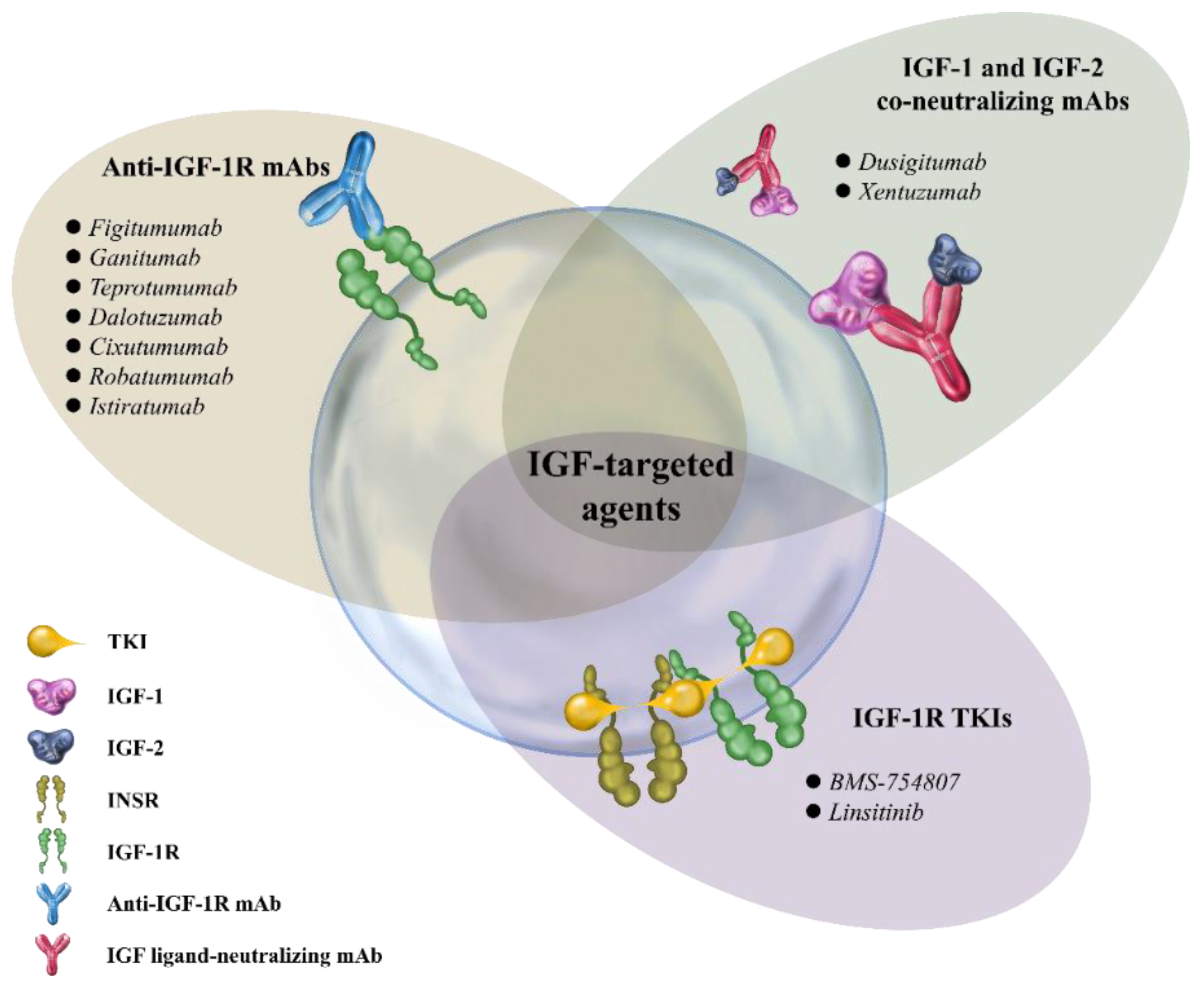
2. Therapeutic Strategies Targeting IGF/IGF-1R Axis in Cancer
2.1. Anti-IGF-1R mAbs
2.2. IGF-1R TKIs
2.3. IGF-Neutralizing Antibodies
3. Activation of IGF/IGF-1R Signaling Improves Stem Cell-Based Therapeutic Strategies in Regenerative Medicine
3.1. Human Embryonic Stem Cells (hESCs)
3.2. Human Neural Stem Cells (hNSCs)
3.3. Cardiac Stem Cells (CSCs)
3.4. Mesenchymal Stem Cells (MSCs)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kasprzak, A.; Kwasniewski, W.; Adamek, A.; Gozdzicka-Jozefiak, A. Insulin-like growth factor (IGF) axis in cancerogenesis. Mutat. Res. Rev. Mutat. Res. 2017, 772, 78–104. [Google Scholar] [CrossRef] [PubMed]
- LeRoith, D.; Roberts, C.T., Jr. The insulin-like growth factor system and cancer. Cancer Lett. 2003, 195, 127–137. [Google Scholar] [CrossRef]
- De Meyts, P.; Whittaker, J. Structural biology of insulin and IGF1 receptors: Implications for drug design. Nat. Rev. Drug Discov. 2002, 1, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Toretsky, J.A.; Scher, D.; Helman, L.J. The role of IGF-1R in pediatric malignancies. Oncologist 2009, 14, 83–91. [Google Scholar] [CrossRef]
- Baxter, R.C. IGF binding proteins in cancer: Mechanistic and clinical insights. Nat. Rev. Cancer 2014, 14, 329–341. [Google Scholar] [CrossRef]
- Adams, T.E.; Epa, V.C.; Garrett, T.P.; Ward, C.W. Structure and function of the type 1 insulin-like growth factor receptor. Cell Mol. Life Sci. 2000, 57, 1050–1093. [Google Scholar] [CrossRef]
- Xu, Y.; Kong, G.K.; Menting, J.G.; Margetts, M.B.; Delaine, C.A.; Jenkin, L.M.; Kiselyov, V.V.; De Meyts, P.; Forbes, B.E.; Lawrence, M.C. How ligand binds to the type 1 insulin-like growth factor receptor. Nat. Commun. 2018, 9, 821. [Google Scholar] [CrossRef]
- Kavran, J.M.; McCabe, J.M.; Byrne, P.O.; Connacher, M.K.; Wang, Z.; Ramek, A.; Sarabipour, S.; Shan, Y.; Shaw, D.E.; Hristova, K.; et al. How IGF-1 activates its receptor. Elife 2014, 3, 3772. [Google Scholar] [CrossRef]
- Dearth, R.K.; Cui, X.; Kim, H.J.; Hadsell, D.L.; Lee, A.V. Oncogenic transformation by the signaling adaptor proteins insulin receptor substrate (IRS)-1 and IRS-2. Cell Cycle 2007, 6, 705–713. [Google Scholar] [CrossRef]
- Limesand, K.H.; Chibly, A.M.; Fribley, A. Impact of targeting insulin-like growth factor signaling in head and neck cancers. Growth Horm. IGF Res. 2013, 23, 135–140. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Z.; Tang, H.; Jiang, Z.; You, L.; Liao, Y. Crosstalk between IGF-1R and other tumor promoting pathways. Curr. Pharm. Des. 2014, 20, 2912–2921. [Google Scholar] [CrossRef]
- Tang, R.; Chen, J.; Tang, M.; Liao, Z.; Zhou, L.; Jiang, J.; Hu, Y.; Liao, Q.; Xiong, W.; Tang, Y.; et al. LncRNA SLCO4A1-AS1 predicts poor prognosis and promotes proliferation and metastasis via the EGFR/MAPK pathway in colorectal cancer. Int. J. Biol. Sci. 2019, 15, 2885–2896. [Google Scholar] [CrossRef]
- Brader, S.; Eccles, S.A. Phosphoinositide 3-kinase signalling pathways in tumor progression, invasion and angiogenesis. Tumori 2004, 90, 2–8. [Google Scholar] [CrossRef]
- Owusu, B.Y.; Galemmo, R.; Janetka, J.; Klampfer, L. Hepatocyte Growth Factor, a Key Tumor-Promoting Factor in the Tumor Microenvironment. Cancers 2017, 9, 35. [Google Scholar] [CrossRef]
- Dallas, N.A.; Xia, L.; Fan, F.; Gray, M.J.; Gaur, P.; van Buren, G., 2nd; Samuel, S.; Kim, M.P.; Lim, S.J.; Ellis, L.M. Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition. Cancer Res. 2009, 69, 1951–1957. [Google Scholar] [CrossRef]
- Chang, W.W.; Lin, R.J.; Yu, J.; Chang, W.Y.; Fu, C.H.; Lai, A.; Yu, J.C.; Yu, A.L. The expression and significance of insulin-like growth factor-1 receptor and its pathway on breast cancer stem/progenitors. Breast Cancer Res. 2013, 15, R39. [Google Scholar] [CrossRef]
- Chang, T.S.; Chen, C.L.; Wu, Y.C.; Liu, J.J.; Kuo, Y.C.; Lee, K.F.; Lin, S.Y.; Lin, S.E.; Tung, S.Y.; Kuo, L.M.; et al. Inflammation Promotes Expression of Stemness-Related Properties in HBV-Related Hepatocellular Carcinoma. PLoS ONE 2016, 11, e0149897. [Google Scholar] [CrossRef]
- Xu, C.; Xie, D.; Yu, S.C.; Yang, X.J.; He, L.R.; Yang, J.; Ping, Y.F.; Wang, B.; Yang, L.; Xu, S.L.; et al. beta-Catenin/POU5F1/SOX2 transcription factor complex mediates IGF-I receptor signaling and predicts poor prognosis in lung adenocarcinoma. Cancer Res. 2013, 73, 3181–3189. [Google Scholar] [CrossRef]
- Hart, L.S.; Dolloff, N.G.; Dicker, D.T.; Koumenis, C.; Christensen, J.G.; Grimberg, A.; El-Deiry, W.S. Human colon cancer stem cells are enriched by insulin-like growth factor-1 and are sensitive to figitumumab. Cell Cycle 2011, 10, 2331–2338. [Google Scholar] [CrossRef]
- Ojo, D.; Wei, F.; Liu, Y.; Wang, E.; Zhang, H.; Lin, X.; Wong, N.; Bane, A.; Tang, D. Factors Promoting Tamoxifen Resistance in Breast Cancer via Stimulating Breast Cancer Stem Cell Expansion. Curr. Med. Chem. 2015, 22, 2360–2374. [Google Scholar] [CrossRef]
- Urtasun, N.; Vidal-Pla, A.; Perez-Torras, S.; Mazo, A. Human pancreatic cancer stem cells are sensitive to dual inhibition of IGF-IR and ErbB receptors. BMC Cancer 2015, 15, 223. [Google Scholar] [CrossRef]
- Osher, E.; Macaulay, V.M. Therapeutic Targeting of the IGF Axis. Cells 2019, 8, 895. [Google Scholar] [CrossRef]
- Huang, X.; Park, H.; Greene, J.; Pao, J.; Mulvey, E.; Zhou, S.X.; Albert, C.M.; Moy, F.; Sachdev, D.; Yee, D.; et al. IGF1R- and ROR1-Specific CAR T Cells as a Potential Therapy for High Risk Sarcomas. PLoS ONE 2015, 10, e0133152. [Google Scholar] [CrossRef]
- Suman, S.; Domingues, A.; Ratajczak, J.; Ratajczak, M.Z. Potential Clinical Applications of Stem Cells in Regenerative Medicine. Adv. Exp. Med. Biol. 2019, 1201, 1–22. [Google Scholar] [CrossRef]
- Xi, J.; Zhang, S.C. Stem cells in development of therapeutics for Parkinson’s disease: A perspective. J. Cell Biochem. 2008, 105, 1153–1160. [Google Scholar] [CrossRef]
- Magga, J.; Savchenko, E.; Malm, T.; Rolova, T.; Pollari, E.; Valonen, P.; Lehtonen, S.; Jantunen, E.; Aarnio, J.; Lehenkari, P.; et al. Production of monocytic cells from bone marrow stem cells: Therapeutic usage in Alzheimer’s disease. J. Cell Mol. Med. 2012, 16, 1060–1073. [Google Scholar] [CrossRef]
- Perin, E.C.; Silva, G.V.; Zheng, Y.; Gahremanpour, A.; Canales, J.; Patel, D.; Fernandes, M.R.; Keller, L.H.; Quan, X.; Coulter, S.A.; et al. Randomized, double-blind pilot study of transendocardial injection of autologous aldehyde dehydrogenase-bright stem cells in patients with ischemic heart failure. Am. Heart J. 2012, 163, 415–421.e1. [Google Scholar] [CrossRef]
- Cerletti, M.; Jurga, S.; Witczak, C.A.; Hirshman, M.F.; Shadrach, J.L.; Goodyear, L.J.; Wagers, A.J. Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell 2008, 134, 37–47. [Google Scholar] [CrossRef]
- Weiss, D.J.; Bertoncello, I.; Borok, Z.; Kim, C.; Panoskaltsis-Mortari, A.; Reynolds, S.; Rojas, M.; Stripp, B.; Warburton, D.; Prockop, D.J. Stem cells and cell therapies in lung biology and lung diseases. Proc. Am. Thorac Soc. 2011, 8, 223–272. [Google Scholar] [CrossRef]
- Rashid, S.T.; Corbineau, S.; Hannan, N.; Marciniak, S.J.; Miranda, E.; Alexander, G.; Huang-Doran, I.; Griffin, J.; Ahrlund-Richter, L.; Skepper, J.; et al. Modeling inherited metabolic disorders of the liver using human induced pluripotent stem cells. J. Clin. Invest. 2010, 120, 3127–3136. [Google Scholar] [CrossRef]
- Wang, L.; Schulz, T.C.; Sherrer, E.S.; Dauphin, D.S.; Shin, S.; Nelson, A.M.; Ware, C.B.; Zhan, M.; Song, C.Z.; Chen, X.; et al. Self-renewal of human embryonic stem cells requires insulin-like growth factor-1 receptor and ERBB2 receptor signaling. Blood 2007, 110, 4111–4119. [Google Scholar] [CrossRef] [PubMed]
- Bendall, S.C.; Stewart, M.H.; Menendez, P.; George, D.; Vijayaragavan, K.; Werbowetski-Ogilvie, T.; Ramos-Mejia, V.; Rouleau, A.; Yang, J.; Bosse, M.; et al. IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature 2007, 448, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Magner, N.L.; Jung, Y.; Wu, J.; Nolta, J.A.; Zern, M.A.; Zhou, P. Insulin and IGFs enhance hepatocyte differentiation from human embryonic stem cells via the PI3K/AKT pathway. Stem Cells 2013, 31, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- Waraky, A.; Aleem, E.; Larsson, O. Downregulation of IGF-1 receptor occurs after hepatic linage commitment during hepatocyte differentiation from human embryonic stem cells. Biochem. Biophys. Res. Commun. 2016, 478, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Haider, H.; Jiang, S.; Idris, N.M.; Ashraf, M. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. Circ. Res. 2008, 103, 1300–1308. [Google Scholar] [CrossRef]
- Smith, T.J.; Janssen, J. Insulin-like Growth Factor-I Receptor and Thyroid-Associated Ophthalmopathy. Endocr. Rev. 2019, 40, 236–267. [Google Scholar] [CrossRef]
- Loughran, G.; Huigsloot, M.; Kiely, P.A.; Smith, L.M.; Floyd, S.; Ayllon, V.; O’Connor, R. Gene expression profiles in cells transformed by overexpression of the IGF-I receptor. Oncogene 2005, 24, 6185–6193. [Google Scholar] [CrossRef][Green Version]
- Jones, R.A.; Campbell, C.I.; Wood, G.A.; Petrik, J.J.; Moorehead, R.A. Reversibility and recurrence of IGF-IR-induced mammary tumors. Oncogene 2009, 28, 2152–2162. [Google Scholar] [CrossRef]
- Taunk, N.K.; Goyal, S.; Moran, M.S.; Yang, Q.; Parikh, R.; Haffty, B.G. Prognostic significance of IGF-1R expression in patients treated with breast-conserving surgery and radiation therapy. Radiother. Oncol. 2010, 96, 204–208. [Google Scholar] [CrossRef]
- King, H.; Aleksic, T.; Haluska, P.; Macaulay, V.M. Can we unlock the potential of IGF-1R inhibition in cancer therapy? Cancer Treat. Rev. 2014, 40, 1096–1105. [Google Scholar] [CrossRef]
- Sachdev, D.; Li, S.L.; Hartell, J.S.; Fujita-Yamaguchi, Y.; Miller, J.S.; Yee, D. A chimeric humanized single-chain antibody against the type I insulin-like growth factor (IGF) receptor renders breast cancer cells refractory to the mitogenic effects of IGF-I. Cancer Res. 2003, 63, 627–635. [Google Scholar]
- Pavlicek, A.; Lira, M.E.; Lee, N.V.; Ching, K.A.; Ye, J.; Cao, J.; Garza, S.J.; Hook, K.E.; Ozeck, M.; Shi, S.T.; et al. Molecular predictors of sensitivity to the insulin-like growth factor 1 receptor inhibitor Figitumumab (CP-751,871). Mol. Cancer Ther. 2013, 12, 2929–2939. [Google Scholar] [CrossRef]
- de Bono, J.S.; Piulats, J.M.; Pandha, H.S.; Petrylak, D.P.; Saad, F.; Aparicio, L.M.; Sandhu, S.K.; Fong, P.; Gillessen, S.; Hudes, G.R.; et al. Phase II randomized study of figitumumab plus docetaxel and docetaxel alone with crossover for metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2014, 20, 1925–1934. [Google Scholar] [CrossRef]
- Calvo, E.; Soria, J.C.; Ma, W.W.; Wang, T.; Bahleda, R.; Tolcher, A.W.; Gernhardt, D.; O’Connell, J.; Millham, R.; Giri, N.; et al. A Phase I Clinical Trial and Independent Patient-Derived Xenograft Study of Combined Targeted Treatment with Dacomitinib and Figitumumab in Advanced Solid Tumors. Clin. Cancer Res. 2017, 23, 1177–1185. [Google Scholar] [CrossRef]
- Beltran, P.J.; Chung, Y.A.; Moody, G.; Mitchell, P.; Cajulis, E.; Vonderfecht, S.; Kendall, R.; Radinsky, R.; Calzone, F.J. Efficacy of ganitumab (AMG 479), alone and in combination with rapamycin, in Ewing’s and osteogenic sarcoma models. J. Pharmacol. Exp. Ther. 2011, 337, 644–654. [Google Scholar] [CrossRef]
- Glisson, B.; Besse, B.; Dols, M.C.; Dubey, S.; Schupp, M.; Jain, R.; Jiang, Y.; Menon, H.; Nackaerts, K.; Orlov, S.; et al. A Randomized, Placebo-Controlled, Phase 1b/2 Study of Rilotumumab or Ganitumab in Combination With Platinum-Based Chemotherapy as First-Line Treatment for Extensive-Stage Small-Cell Lung Cancer. Clin. Lung Cancer 2017, 18, 615–625.e8. [Google Scholar] [CrossRef]
- Beltran, P.J.; Mitchell, P.; Chung, Y.A.; Cajulis, E.; Lu, J.; Belmontes, B.; Ho, J.; Tsai, M.M.; Zhu, M.; Vonderfecht, S.; et al. AMG 479, a fully human anti-insulin-like growth factor receptor type I monoclonal antibody, inhibits the growth and survival of pancreatic carcinoma cells. Mol. Cancer Ther. 2009, 8, 1095–1105. [Google Scholar] [CrossRef]
- Brana, I.; Berger, R.; Golan, T.; Haluska, P.; Edenfield, J.; Fiorica, J.; Stephenson, J.; Martin, L.P.; Westin, S.; Hanjani, P.; et al. A parallel-arm phase I trial of the humanised anti-IGF-1R antibody dalotuzumab in combination with the AKT inhibitor MK-2206, the mTOR inhibitor ridaforolimus, or the NOTCH inhibitor MK-0752, in patients with advanced solid tumours. Br. J. Cancer 2014, 111, 1932–1944. [Google Scholar] [CrossRef]
- Sclafani, F.; Kim, T.Y.; Cunningham, D.; Kim, T.W.; Tabernero, J.; Schmoll, H.J.; Roh, J.K.; Kim, S.Y.; Park, Y.S.; Guren, T.K.; et al. A Randomized Phase II/III Study of Dalotuzumab in Combination With Cetuximab and Irinotecan in Chemorefractory, KRAS Wild-Type, Metastatic Colorectal Cancer. J. Natl. Cancer Inst. 2015, 107, djv258. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Spigel, D.R.; Chen, D.; Steins, M.B.; Engelman, J.A.; Schneider, C.P.; Novello, S.; Eberhardt, W.E.; Crino, L.; Habben, K.; et al. Randomized phase II study of erlotinib in combination with placebo or R1507, a monoclonal antibody to insulin-like growth factor-1 receptor, for advanced-stage non-small-cell lung cancer. J. Clin. Oncol. 2011, 29, 4574–4580. [Google Scholar] [CrossRef]
- Pappo, A.S.; Vassal, G.; Crowley, J.J.; Bolejack, V.; Hogendoorn, P.C.; Chugh, R.; Ladanyi, M.; Grippo, J.F.; Dall, G.; Staddon, A.P.; et al. A phase 2 trial of R1507, a monoclonal antibody to the insulin-like growth factor-1 receptor (IGF-1R), in patients with recurrent or refractory rhabdomyosarcoma, osteosarcoma, synovial sarcoma, and other soft tissue sarcomas: Results of a Sarcoma Alliance for Research through Collaboration study. Cancer 2014, 120, 2448–2456. [Google Scholar] [CrossRef]
- Malempati, S.; Weigel, B.; Ingle, A.M.; Ahern, C.H.; Carroll, J.M.; Roberts, C.T.; Reid, J.M.; Schmechel, S.; Voss, S.D.; Cho, S.Y.; et al. Phase I/II trial and pharmacokinetic study of cixutumumab in pediatric patients with refractory solid tumors and Ewing sarcoma: A report from the Children’s Oncology Group. J. Clin. Oncol. 2012, 30, 256–262. [Google Scholar] [CrossRef]
- Attias-Geva, Z.; Bentov, I.; Ludwig, D.L.; Fishman, A.; Bruchim, I.; Werner, H. Insulin-like growth factor-I receptor (IGF-IR) targeting with monoclonal antibody cixutumumab (IMC-A12) inhibits IGF-I action in endometrial cancer cells. Eur. J. Cancer 2011, 47, 1717–1726. [Google Scholar] [CrossRef]
- Wang, Y.; Hailey, J.; Williams, D.; Wang, Y.; Lipari, P.; Malkowski, M.; Wang, X.; Xie, L.; Li, G.; Saha, D.; et al. Inhibition of insulin-like growth factor-I receptor (IGF-IR) signaling and tumor cell growth by a fully human neutralizing anti-IGF-IR antibody. Mol. Cancer Ther. 2005, 4, 1214–1221. [Google Scholar] [CrossRef]
- Anderson, P.M.; Bielack, S.S.; Gorlick, R.G.; Skubitz, K.; Daw, N.C.; Herzog, C.E.; Monge, O.R.; Lassaletta, A.; Boldrini, E.; Papai, Z.; et al. A phase II study of clinical activity of SCH 717454 (robatumumab) in patients with relapsed osteosarcoma and Ewing sarcoma. Pediatr. Blood Cancer 2016, 63, 1761–1770. [Google Scholar] [CrossRef]
- von Mehren, M.; Britten, C.D.; Pieslor, P.; Saville, W.; Vassos, A.; Harris, S.; Galluppi, G.R.; Darif, M.; Wainberg, Z.A.; Cohen, R.B.; et al. A phase 1, open-label, dose-escalation study of BIIB022 (anti-IGF-1R monoclonal antibody) in subjects with relapsed or refractory solid tumors. Invest. New Drugs 2014, 32, 518–525. [Google Scholar] [CrossRef]
- Fitzgerald, J.B.; Johnson, B.W.; Baum, J.; Adams, S.; Iadevaia, S.; Tang, J.; Rimkunas, V.; Xu, L.; Kohli, N.; Rennard, R.; et al. MM-141, an IGF-IR- and ErbB3-directed bispecific antibody, overcomes network adaptations that limit activity of IGF-IR inhibitors. Mol. Cancer Ther. 2014, 13, 410–425. [Google Scholar] [CrossRef]
- Molina-Arcas, M.; Hancock, D.C.; Sheridan, C.; Kumar, M.S.; Downward, J. Coordinate direct input of both KRAS and IGF1 receptor to activation of PI3 kinase in KRAS-mutant lung cancer. Cancer Discov. 2013, 3, 548–563. [Google Scholar] [CrossRef]
- Cohn, A.L.; Tabernero, J.; Maurel, J.; Nowara, E.; Sastre, J.; Chuah, B.Y.S.; Kopp, M.V.; Sakaeva, D.D.; Mitchell, E.P.; Dubey, S.; et al. A randomized, placebo-controlled phase 2 study of ganitumab or conatumumab in combination with FOLFIRI for second-line treatment of mutant KRAS metastatic colorectal cancer. Ann. Oncol. 2013, 24, 1777–1785. [Google Scholar] [CrossRef]
- Smith, T.J.; Kahaly, G.J.; Ezra, D.G.; Fleming, J.C.; Dailey, R.A.; Tang, R.A.; Harris, G.J.; Antonelli, A.; Salvi, M.; Goldberg, R.A.; et al. Teprotumumab for Thyroid-Associated Ophthalmopathy. N. Engl. J. Med. 2017, 376, 1748–1761. [Google Scholar] [CrossRef]
- Slentz, D.H.; Nelson, C.C.; Smith, T.J. Teprotumumab: A novel therapeutic monoclonal antibody for thyroid-associated ophthalmopathy. Expert Opin. Investig. Drugs 2020, 29, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Markham, A. Teprotumumab: First Approval. Drugs 2020, 80, 509–512. [Google Scholar] [CrossRef]
- Favelyukis, S.; Till, J.H.; Hubbard, S.R.; Miller, W.T. Structure and autoregulation of the insulin-like growth factor 1 receptor kinase. Nat. Struct. Biol. 2001, 8, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Mulvihill, M.J.; Cooke, A.; Rosenfeld-Franklin, M.; Buck, E.; Foreman, K.; Landfair, D.; O’Connor, M.; Pirritt, C.; Sun, Y.; Yao, Y.; et al. Discovery of OSI-906: A selective and orally efficacious dual inhibitor of the IGF-1 receptor and insulin receptor. Future Med. Chem. 2009, 1, 1153–1171. [Google Scholar] [CrossRef]
- Carboni, J.M.; Wittman, M.; Yang, Z.; Lee, F.; Greer, A.; Hurlburt, W.; Hillerman, S.; Cao, C.; Cantor, G.H.; Dell-John, J.; et al. BMS-754807, a small molecule inhibitor of insulin-like growth factor-1R/IR. Mol. Cancer Ther. 2009, 8, 3341–3349. [Google Scholar] [CrossRef] [PubMed]
- Buck, E.; Gokhale, P.C.; Koujak, S.; Brown, E.; Eyzaguirre, A.; Tao, N.; Rosenfeld-Franklin, M.; Lerner, L.; Chiu, M.I.; Wild, R.; et al. Compensatory insulin receptor (IR) activation on inhibition of insulin-like growth factor-1 receptor (IGF-1R): Rationale for cotargeting IGF-1R and IR in cancer. Mol. Cancer Ther. 2010, 9, 2652–2664. [Google Scholar] [CrossRef]
- Creighton, C.J.; Casa, A.; Lazard, Z.; Huang, S.; Tsimelzon, A.; Hilsenbeck, S.G.; Osborne, C.K.; Lee, A.V. Insulin-like growth factor-I activates gene transcription programs strongly associated with poor breast cancer prognosis. J. Clin. Oncol. 2008, 26, 4078–4085. [Google Scholar] [CrossRef] [PubMed]
- Litzenburger, B.C.; Creighton, C.J.; Tsimelzon, A.; Chan, B.T.; Hilsenbeck, S.G.; Wang, T.; Carboni, J.M.; Gottardis, M.M.; Huang, F.; Chang, J.C.; et al. High IGF-IR activity in triple-negative breast cancer cell lines and tumorgrafts correlates with sensitivity to anti-IGF-IR therapy. Clin. Cancer Res. 2011, 17, 2314–2327. [Google Scholar] [CrossRef] [PubMed]
- Piao, W.; Wang, Y.; Adachi, Y.; Yamamoto, H.; Li, R.; Imsumran, A.; Li, H.; Maehata, T.; Ii, M.; Arimura, Y.; et al. Insulin-like growth factor-I receptor blockade by a specific tyrosine kinase inhibitor for human gastrointestinal carcinomas. Mol. Cancer Ther. 2008, 7, 1483–1493. [Google Scholar] [CrossRef][Green Version]
- Chitnis, M.M.; Lodhia, K.A.; Aleksic, T.; Gao, S.; Protheroe, A.S.; Macaulay, V.M. IGF-1R inhibition enhances radiosensitivity and delays double-strand break repair by both non-homologous end-joining and homologous recombination. Oncogene 2014, 33, 5262–5273. [Google Scholar] [CrossRef]
- Litzenburger, B.C.; Kim, H.J.; Kuiatse, I.; Carboni, J.M.; Attar, R.M.; Gottardis, M.M.; Fairchild, C.R.; Lee, A.V. BMS-536924 reverses IGF-IR-induced transformation of mammary epithelial cells and causes growth inhibition and polarization of MCF7 cells. Clin. Cancer Res. 2009, 15, 226–237. [Google Scholar] [CrossRef]
- Vasilcanu, R.; Vasilcanu, D.; Rosengren, L.; Natalishvili, N.; Sehat, B.; Yin, S.; Girnita, A.; Axelson, M.; Girnita, L.; Larsson, O. Picropodophyllin induces downregulation of the insulin-like growth factor 1 receptor: Potential mechanistic involvement of Mdm2 and beta-arrestin1. Oncogene 2008, 27, 1629–1638. [Google Scholar] [CrossRef]
- Girnita, A.; Girnita, L.; del Prete, F.; Bartolazzi, A.; Larsson, O.; Axelson, M. Cyclolignans as inhibitors of the insulin-like growth factor-1 receptor and malignant cell growth. Cancer Res. 2004, 64, 236–242. [Google Scholar] [CrossRef]
- Waraky, A.; Akopyan, K.; Parrow, V.; Stromberg, T.; Axelson, M.; Abrahmsen, L.; Lindqvist, A.; Larsson, O.; Aleem, E. Picropodophyllin causes mitotic arrest and catastrophe by depolymerizing microtubules via insulin-like growth factor-1 receptor-independent mechanism. Oncotarget 2014, 5, 8379–8392. [Google Scholar] [CrossRef]
- Ekman, S.; Harmenberg, J.; Frodin, J.E.; Bergstrom, S.; Wassberg, C.; Eksborg, S.; Larsson, O.; Axelson, M.; Jerling, M.; Abrahmsen, L.; et al. A novel oral insulin-like growth factor-1 receptor pathway modulator and its implications for patients with non-small cell lung carcinoma: A phase I clinical trial. Acta Oncol. 2016, 55, 140–148. [Google Scholar] [CrossRef]
- Friedlander, T.W.; Weinberg, V.K.; Huang, Y.; Mi, J.T.; Formaker, C.G.; Small, E.J.; Harzstark, A.L.; Lin, A.M.; Fong, L.; Ryan, C.J. A phase II study of insulin-like growth factor receptor inhibition with nordihydroguaiaretic acid in men with non-metastatic hormone-sensitive prostate cancer. Oncol. Rep. 2012, 27, 3–9. [Google Scholar] [CrossRef][Green Version]
- Gao, J.; Chesebrough, J.W.; Cartlidge, S.A.; Ricketts, S.A.; Incognito, L.; Veldman-Jones, M.; Blakey, D.C.; Tabrizi, M.; Jallal, B.; Trail, P.A.; et al. Dual IGF-I/II-neutralizing antibody MEDI-573 potently inhibits IGF signaling and tumor growth. Cancer Res. 2011, 71, 1029–1040. [Google Scholar] [CrossRef]
- Haluska, P.; Menefee, M.; Plimack, E.R.; Rosenberg, J.; Northfelt, D.; LaVallee, T.; Shi, L.; Yu, X.Q.; Burke, P.; Huang, J.; et al. Phase I dose-escalation study of MEDI-573, a bispecific, antiligand monoclonal antibody against IGFI and IGFII, in patients with advanced solid tumors. Clin. Cancer Res. 2014, 20, 4747–4757. [Google Scholar] [CrossRef]
- Friedbichler, K.; Hofmann, M.H.; Kroez, M.; Ostermann, E.; Lamche, H.R.; Koessl, C.; Borges, E.; Pollak, M.N.; Adolf, G.; Adam, P.J. Pharmacodynamic and antineoplastic activity of BI 836845, a fully human IGF ligand-neutralizing antibody, and mechanistic rationale for combination with rapamycin. Mol. Cancer Ther. 2014, 13, 399–409. [Google Scholar] [CrossRef]
- Goya, M.; Miyamoto, S.; Nagai, K.; Ohki, Y.; Nakamura, K.; Shitara, K.; Maeda, H.; Sangai, T.; Kodama, K.; Endoh, Y.; et al. Growth inhibition of human prostate cancer cells in human adult bone implanted into nonobese diabetic/severe combined immunodeficient mice by a ligand-specific antibody to human insulin-like growth factors. Cancer Res. 2004, 64, 6252–6258. [Google Scholar] [CrossRef]
- Dransfield, D.T.; Cohen, E.H.; Chang, Q.; Sparrow, L.G.; Bentley, J.D.; Dolezal, O.; Xiao, X.; Peat, T.S.; Newman, J.; Pilling, P.A.; et al. A human monoclonal antibody against insulin-like growth factor-II blocks the growth of human hepatocellular carcinoma cell lines in vitro and in vivo. Mol. Cancer Ther. 2010, 9, 1809–1819. [Google Scholar] [CrossRef]
- Xu, C.; Rosler, E.; Jiang, J.; Lebkowski, J.S.; Gold, J.D.; O’Sullivan, C.; Delavan-Boorsma, K.; Mok, M.; Bronstein, A.; Carpenter, M.K. Basic fibroblast growth factor supports undifferentiated human embryonic stem cell growth without conditioned medium. Stem Cells 2005, 23, 315–323. [Google Scholar] [CrossRef]
- Mullen, A.C.; Wrana, J.L. TGF-beta Family Signaling in Embryonic and Somatic Stem-Cell Renewal and Differentiation. Cold Spring Harb. Perspect. Biol. 2017, 9, a022186. [Google Scholar] [CrossRef]
- Williams, R.L.; Hilton, D.J.; Pease, S.; Willson, T.A.; Stewart, C.L.; Gearing, D.P.; Wagner, E.F.; Metcalf, D.; Nicola, N.A.; Gough, N.M. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature 1988, 336, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Ying, Q.L.; Nichols, J.; Chambers, I.; Smith, A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell 2003, 115, 281–292. [Google Scholar] [CrossRef]
- Dravid, G.; Ye, Z.; Hammond, H.; Chen, G.; Pyle, A.; Donovan, P.; Yu, X.; Cheng, L. Defining the role of Wnt/beta-catenin signaling in the survival, proliferation, and self-renewal of human embryonic stem cells. Stem Cells 2005, 23, 1489–1501. [Google Scholar] [CrossRef] [PubMed]
- Ying, Q.L.; Wray, J.; Nichols, J.; Batlle-Morera, L.; Doble, B.; Woodgett, J.; Cohen, P.; Smith, A. The ground state of embryonic stem cell self-renewal. Nature 2008, 453, 519–523. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, T.C.; Laflamme, M.A.; Murry, C.E. Proliferation of cardiomyocytes derived from human embryonic stem cells is mediated via the IGF/PI 3-kinase/Akt signaling pathway. J. Mol. Cell Cardiol. 2005, 39, 865–873. [Google Scholar] [CrossRef]
- Park, S.B.; Yu, K.R.; Jung, J.W.; Lee, S.R.; Roh, K.H.; Seo, M.S.; Park, J.R.; Kang, S.K.; Lee, Y.S.; Kang, K.S. bFGF enhances the IGFs-mediated pluripotent and differentiation potentials in multipotent stem cells. Growth Factors 2009, 27, 425–437. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, J.; Karner, C.M.; Long, F. Hedgehog signaling activates a positive feedback mechanism involving insulin-like growth factors to induce osteoblast differentiation. Proc. Natl. Acad. Sci. USA 2015, 112, 4678–4683. [Google Scholar] [CrossRef]
- Youssef, A.; Han, V.K. Low Oxygen Tension Modulates the Insulin-Like Growth Factor-1 or -2 Signaling via Both Insulin-Like Growth Factor-1 Receptor and Insulin Receptor to Maintain Stem Cell Identity in Placental Mesenchymal Stem Cells. Endocrinology 2016, 157, 1163–1174. [Google Scholar] [CrossRef]
- Youssef, A.; Iosef, C.; Han, V.K. Low-oxygen tension and IGF-I promote proliferation and multipotency of placental mesenchymal stem cells (PMSCs) from different gestations via distinct signaling pathways. Endocrinology 2014, 155, 1386–1397. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.; Li, Y.; Chen, J.; Yang, M.; Katakowski, M.; Lu, M.; Chopp, M. Expression of insulin-like growth factor 1 and receptor in ischemic rats treated with human marrow stromal cells. Brain Res. 2004, 1030, 19–27. [Google Scholar] [CrossRef]
- Lee, H.T.; Chang, H.T.; Lee, S.; Lin, C.H.; Fan, J.R.; Lin, S.Z.; Hsu, C.Y.; Hsieh, C.H.; Shyu, W.C. Role of IGF1R(+) MSCs in modulating neuroplasticity via CXCR4 cross-interaction. Sci. Rep. 2016, 6, 32595. [Google Scholar] [CrossRef]
- Lunn, J.S.; Sakowski, S.A.; McGinley, L.M.; Pacut, C.; Hazel, T.G.; Johe, K.; Feldman, E.L. Autocrine production of IGF-I increases stem cell-mediated neuroprotection. Stem Cells 2015, 33, 1480–1489. [Google Scholar] [CrossRef]
- Troncoso, R.; Ibarra, C.; Vicencio, J.M.; Jaimovich, E.; Lavandero, S. New insights into IGF-1 signaling in the heart. Trends Endocrinol. Metab. 2014, 25, 128–137. [Google Scholar] [CrossRef]
- Johnson, A.M.; Kartha, C.C. Proliferation of murine c-kit(pos) cardiac stem cells stimulated with IGF-1 is associated with Akt-1 mediated phosphorylation and nuclear export of FoxO3a and its effect on downstream cell cycle regulators. Growth Factors 2014, 32, 53–62. [Google Scholar] [CrossRef]
- Klimanskaya, I.; Kimbrel, E.A.; Lanza, R. Embryonic Stem Cells. In Principles of Tissue Engineering; Academic Press: London, UK, 2014; pp. 565–579. [Google Scholar] [CrossRef]
- Mossahebi-Mohammadi, M.; Quan, M.; Zhang, J.S.; Li, X. FGF Signaling Pathway: A Key Regulator of Stem Cell Pluripotency. Front. Cell Dev. Biol. 2020, 8, 79. [Google Scholar] [CrossRef]
- Amit, M.; Shariki, C.; Margulets, V.; Itskovitz-Eldor, J. Feeder layer- and serum-free culture of human embryonic stem cells. Biol. Reprod. 2004, 70, 837–845. [Google Scholar] [CrossRef]
- Beattie, G.M.; Lopez, A.D.; Bucay, N.; Hinton, A.; Firpo, M.T.; King, C.C.; Hayek, A. Activin A maintains pluripotency of human embryonic stem cells in the absence of feeder layers. Stem Cells 2005, 23, 489–495. [Google Scholar] [CrossRef]
- Pyle, A.D.; Lock, L.F.; Donovan, P.J. Neurotrophins mediate human embryonic stem cell survival. Nat. Biotechnol. 2006, 24, 344–350. [Google Scholar] [CrossRef]
- Pebay, A.; Wong, R.C.; Pitson, S.M.; Wolvetang, E.J.; Peh, G.S.; Filipczyk, A.; Koh, K.L.; Tellis, I.; Nguyen, L.T.; Pera, M.F. Essential roles of sphingosine-1-phosphate and platelet-derived growth factor in the maintenance of human embryonic stem cells. Stem Cells 2005, 23, 1541–1548. [Google Scholar] [CrossRef]
- Yu, Y.H.; Zhang, L.; Wu, D.S.; Zhang, Z.; Huang, F.F.; Zhang, J.; Chen, X.P.; Liang, D.S.; Zeng, H.; Chen, F.P. MiR-223 regulates human embryonic stem cell differentiation by targeting the IGF-1R/Akt signaling pathway. PLoS ONE 2013, 8, e78769. [Google Scholar] [CrossRef]
- Feldman, E.L.; Boulis, N.M.; Hur, J.; Johe, K.; Rutkove, S.B.; Federici, T.; Polak, M.; Bordeau, J.; Sakowski, S.A.; Glass, J.D. Intraspinal neural stem cell transplantation in amyotrophic lateral sclerosis: Phase 1 trial outcomes. Ann. Neurol. 2014, 75, 363–373. [Google Scholar] [CrossRef]
- Glass, J.D.; Hertzberg, V.S.; Boulis, N.M.; Riley, J.; Federici, T.; Polak, M.; Bordeau, J.; Fournier, C.; Johe, K.; Hazel, T.; et al. Transplantation of spinal cord-derived neural stem cells for ALS: Analysis of phase 1 and 2 trials. Neurology 2016, 87, 392–400. [Google Scholar] [CrossRef]
- Zarei, S.; Carr, K.; Reiley, L.; Diaz, K.; Guerra, O.; Altamirano, P.F.; Pagani, W.; Lodin, D.; Orozco, G.; Chinea, A. A comprehensive review of amyotrophic lateral sclerosis. Surg. Neurol. Int. 2015, 6, 171. [Google Scholar] [CrossRef]
- Koutsoudaki, P.N.; Papastefanaki, F.; Stamatakis, A.; Kouroupi, G.; Xingi, E.; Stylianopoulou, F.; Matsas, R. Neural stem/progenitor cells differentiate into oligodendrocytes, reduce inflammation, and ameliorate learning deficits after transplantation in a mouse model of traumatic brain injury. Glia 2016, 64, 763–779. [Google Scholar] [CrossRef]
- Lewis, F.C.; Kumar, S.D.; Ellison-Hughes, G.M. Non-invasive strategies for stimulating endogenous repair and regenerative mechanisms in the damaged heart. Pharmacol. Res. 2018, 127, 33–40. [Google Scholar] [CrossRef]
- Kawaguchi, N.; Smith, A.J.; Waring, C.D.; Hasan, M.K.; Miyamoto, S.; Matsuoka, R.; Ellison, G.M. c-kitpos GATA-4 high rat cardiac stem cells foster adult cardiomyocyte survival through IGF-1 paracrine signalling. PLoS ONE 2010, 5, e14297. [Google Scholar] [CrossRef]
- Jackson, R.; Tilokee, E.L.; Latham, N.; Mount, S.; Rafatian, G.; Strydhorst, J.; Ye, B.; Boodhwani, M.; Chan, V.; Ruel, M.; et al. Paracrine Engineering of Human Cardiac Stem Cells With Insulin-Like Growth Factor 1 Enhances Myocardial Repair. J. Am. Heart Assoc. 2015, 4, e002104. [Google Scholar] [CrossRef]
- Andrade, D.; Oliveira, G.; Menezes, L.; Nascimento, A.L.; Carvalho, S.; Stumbo, A.C.; Thole, A.; Garcia-Souza, E.; Moura, A.; Carvalho, L.; et al. Insulin-like growth factor-1 short-period therapy improves cardiomyopathy stimulating cardiac progenitor cells survival in obese mice. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, R.; Jimenez-Luna, C.; Perales-Adan, J.; Perazzoli, G.; Melguizo, C.; Prados, J. Differentiation of Human Mesenchymal Stem Cells towards Neuronal Lineage: Clinical Trials in Nervous System Disorders. Biomol. Ther. 2020, 28, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, N.; Hong, H.; Qi, J.; Zhang, S.; Wang, J. Mesenchymal Stem Cells: Therapeutic Mechanisms for Stroke. Int. J. Mol. Sci. 2022, 23, 2550. [Google Scholar] [CrossRef]
- Jackson, W.M.; Nesti, L.J.; Tuan, R.S. Concise review: Clinical translation of wound healing therapies based on mesenchymal stem cells. Stem Cells Transl. Med. 2012, 1, 44–50. [Google Scholar] [CrossRef]
- Scavo, L.M.; Karas, M.; Murray, M.; Leroith, D. Insulin-like growth factor-I stimulates both cell growth and lipogenesis during differentiation of human mesenchymal stem cells into adipocytes. J. Clin. Endocrinol. Metab. 2004, 89, 3543–3553. [Google Scholar] [CrossRef]
- Peng, X.D.; Xu, P.Z.; Chen, M.L.; Hahn-Windgassen, A.; Skeen, J.; Jacobs, J.; Sundararajan, D.; Chen, W.S.; Crawford, S.E.; Coleman, K.G.; et al. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003, 17, 1352–1365. [Google Scholar] [CrossRef]
- Chen, J.; Yuan, K.; Mao, X.; Miano, J.M.; Wu, H.; Chen, Y. Serum response factor regulates bone formation via IGF-1 and Runx2 signals. J. Bone Miner. Res. 2012, 27, 1659–1668. [Google Scholar] [CrossRef]
- Gomez-Mauricio, G.; Moscoso, I.; Martin-Cancho, M.F.; Crisostomo, V.; Prat-Vidal, C.; Baez-Diaz, C.; Sanchez-Margallo, F.M.; Bernad, A. Combined administration of mesenchymal stem cells overexpressing IGF-1 and HGF enhances neovascularization but moderately improves cardiac regeneration in a porcine model. Stem Cell Res. Ther. 2016, 7, 94. [Google Scholar] [CrossRef]
- Pumberger, M.; Qazi, T.H.; Ehrentraut, M.C.; Textor, M.; Kueper, J.; Stoltenburg-Didinger, G.; Winkler, T.; von Roth, P.; Reinke, S.; Borselli, C.; et al. Synthetic niche to modulate regenerative potential of MSCs and enhance skeletal muscle regeneration. Biomaterials 2016, 99, 95–108. [Google Scholar] [CrossRef]
- Guo, J.; Zheng, D.; Li, W.F.; Li, H.R.; Zhang, A.D.; Li, Z.C. Insulin-like growth factor 1 treatment of MSCs attenuates inflammation and cardiac dysfunction following MI. Inflammation 2014, 37, 2156–2163. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Dang, H.; Liu, P.; Zhang, B.O.; Xu, F. Insulin-like growth factor 2 regulates the proliferation and differentiation of rat adipose-derived stromal cells via IGF-1R and IR. Cytotherapy 2019, 21, 619–630. [Google Scholar] [CrossRef]
- Tufail, M.; Wu, C. Targeting the IGF-1R in prostate and colorectal cancer: Reasons behind trial failure and future directions. Ther. Deliv. 2022, 13, 167–186. [Google Scholar] [CrossRef]
- Ahearn, T.U.; Peisch, S.; Pettersson, A.; Ebot, E.M.; Zhou, C.K.; Graff, R.E.; Sinnott, J.A.; Fazli, L.; Judson, G.L.; Bismar, T.A.; et al. Expression of IGF/insulin receptor in prostate cancer tissue and progression to lethal disease. Carcinogenesis 2018, 39, 1431–1437. [Google Scholar] [CrossRef]
- Lero, M.W.; Shaw, L.M. Diversity of insulin and IGF signaling in breast cancer: Implications for therapy. Mol. Cell Endocrinol. 2021, 527, 111213. [Google Scholar] [CrossRef]
- Wolpin, B.M.; Meyerhardt, J.A.; Chan, A.T.; Ng, K.; Chan, J.A.; Wu, K.; Pollak, M.N.; Giovannucci, E.L.; Fuchs, C.S. Insulin, the insulin-like growth factor axis, and mortality in patients with nonmetastatic colorectal cancer. J. Clin. Oncol. 2009, 27, 176–185. [Google Scholar] [CrossRef]
- Robertson, J.F.; Ferrero, J.M.; Bourgeois, H.; Kennecke, H.; de Boer, R.H.; Jacot, W.; McGreivy, J.; Suzuki, S.; Zhu, M.; McCaffery, I.; et al. Ganitumab with either exemestane or fulvestrant for postmenopausal women with advanced, hormone-receptor-positive breast cancer: A randomised, controlled, double-blind, phase 2 trial. Lancet Oncol. 2013, 14, 228–235. [Google Scholar] [CrossRef]
- Hartog, H.; Horlings, H.M.; van der Vegt, B.; Kreike, B.; Ajouaou, A.; van de Vijver, M.J.; Marike Boezen, H.; de Bock, G.H.; van der Graaf, W.T.; Wesseling, J. Divergent effects of insulin-like growth factor-1 receptor expression on prognosis of estrogen receptor positive versus triple negative invasive ductal breast carcinoma. Breast Cancer Res. Treat. 2011, 129, 725–736. [Google Scholar] [CrossRef][Green Version]
- Davison, Z.; de Blacquiere, G.E.; Westley, B.R.; May, F.E. Insulin-like growth factor-dependent proliferation and survival of triple-negative breast cancer cells: Implications for therapy. Neoplasia 2011, 13, 504–515. [Google Scholar] [CrossRef]
| Overexpression of IGF/IGF-1R in Stem Cells | Function | Pathway | Disease | Ref. |
|---|---|---|---|---|
| hESCs | Promoting self-renewal and survival | Self-renewal through HRG/ERBB2, and anti-apoptosis through PI3K/AKT | - | [31,88] |
| UMSCs | Adipogenic differentiation | bFGF induces IGF and FGF receptor | - | [89] |
| BMSCs | Osteogenic differentiation | Hedgehog pathway | - | [90] |
| PMSCs | Increasing cell proliferation and maintaining multipotency | Induce OCT4 expression | - | [91,92] |
| BMSCs | Promoting cell survival and neural progenitor cell recruitment | - | Ischemic stroke | [93] |
| DPSCs | Promoting neuroplasticity | Crosstalk between IGF-1/IGF-1R and CXCL12/CXCR4 pathway | Ischemic stroke | [94] |
| hNSCs | Promoting neuroprotection | - | Amyotrophic lateral sclerosis (ALS) | [95] |
| CSCs | Promoting cardiomyocyte survival and myocardial regeneration | FOXO3/p27/p51 pathway | Myocardial infarction | [96,97] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-L.; Lin, C.-Y.; Lee, W.; Teng, C.-F.; Shyu, W.-C.; Jeng, L.-B. Mini Review: Molecular Interpretation of the IGF/IGF-1R Axis in Cancer Treatment and Stem Cells-Based Therapy in Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 11781. https://doi.org/10.3390/ijms231911781
Lin S-L, Lin C-Y, Lee W, Teng C-F, Shyu W-C, Jeng L-B. Mini Review: Molecular Interpretation of the IGF/IGF-1R Axis in Cancer Treatment and Stem Cells-Based Therapy in Regenerative Medicine. International Journal of Molecular Sciences. 2022; 23(19):11781. https://doi.org/10.3390/ijms231911781
Chicago/Turabian StyleLin, Syuan-Ling, Chih-Yang Lin, Wei Lee, Chiao-Fang Teng, Woei-Cherng Shyu, and Long-Bin Jeng. 2022. "Mini Review: Molecular Interpretation of the IGF/IGF-1R Axis in Cancer Treatment and Stem Cells-Based Therapy in Regenerative Medicine" International Journal of Molecular Sciences 23, no. 19: 11781. https://doi.org/10.3390/ijms231911781
APA StyleLin, S.-L., Lin, C.-Y., Lee, W., Teng, C.-F., Shyu, W.-C., & Jeng, L.-B. (2022). Mini Review: Molecular Interpretation of the IGF/IGF-1R Axis in Cancer Treatment and Stem Cells-Based Therapy in Regenerative Medicine. International Journal of Molecular Sciences, 23(19), 11781. https://doi.org/10.3390/ijms231911781








