Sulfonated Pentablock Copolymer Coating of Polypropylene Filters for Dye and Metal Ions Effective Removal by Integrated Adsorption and Filtration Process
Abstract
1. Introduction
2. Results
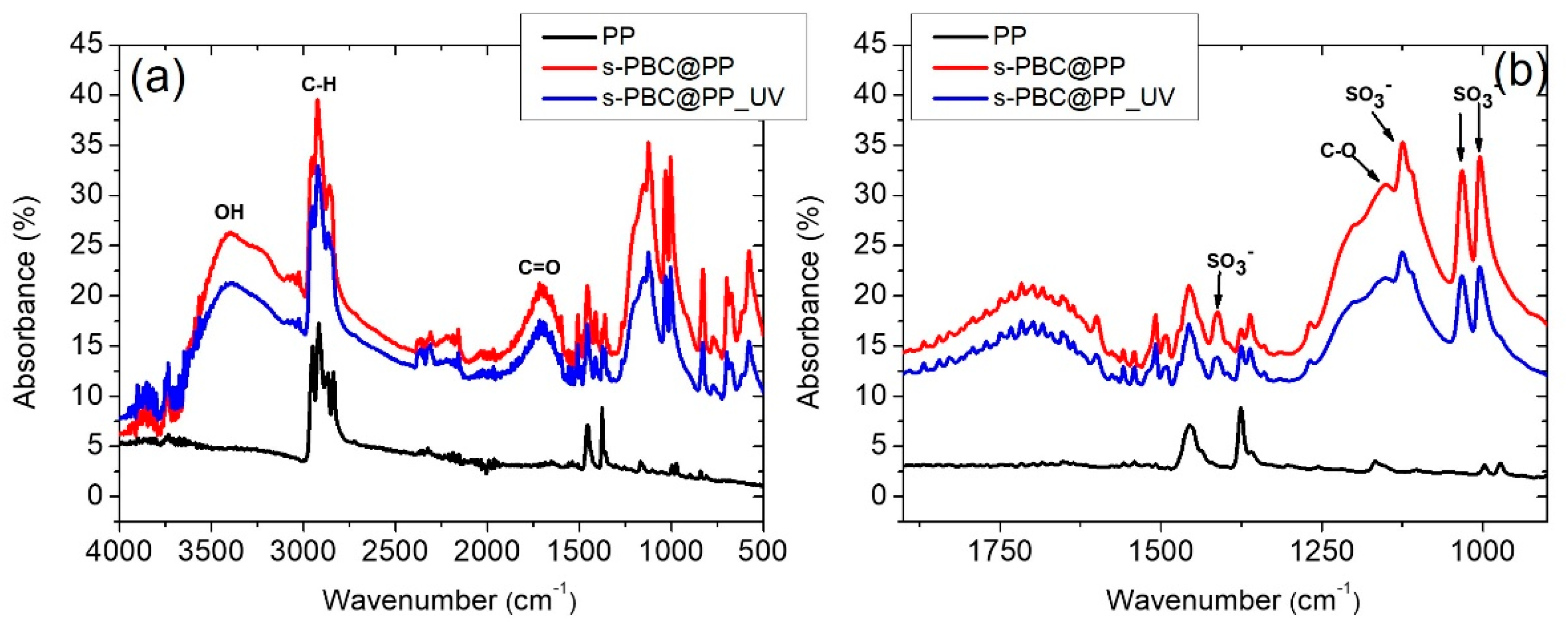
2.1. Samples Characterization
2.2. Adsorption and Filtration Tests
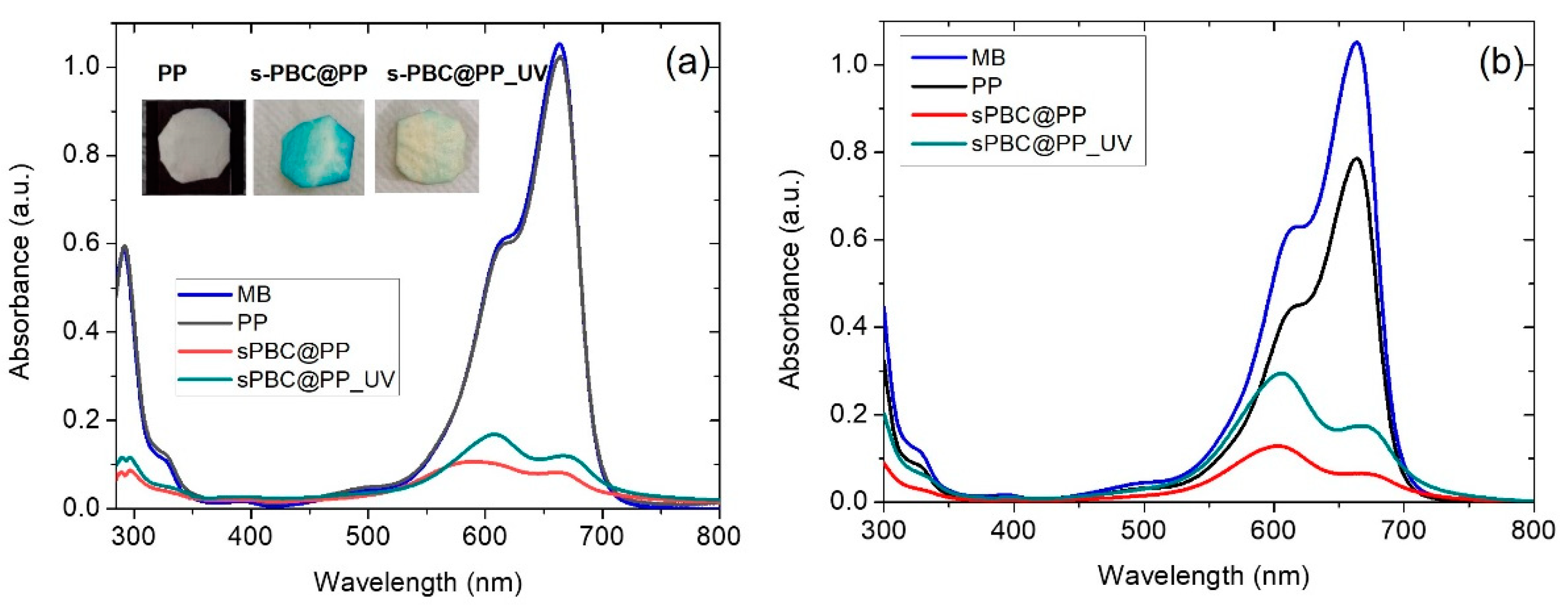
2.2.1. MB Adsorption and Filtration
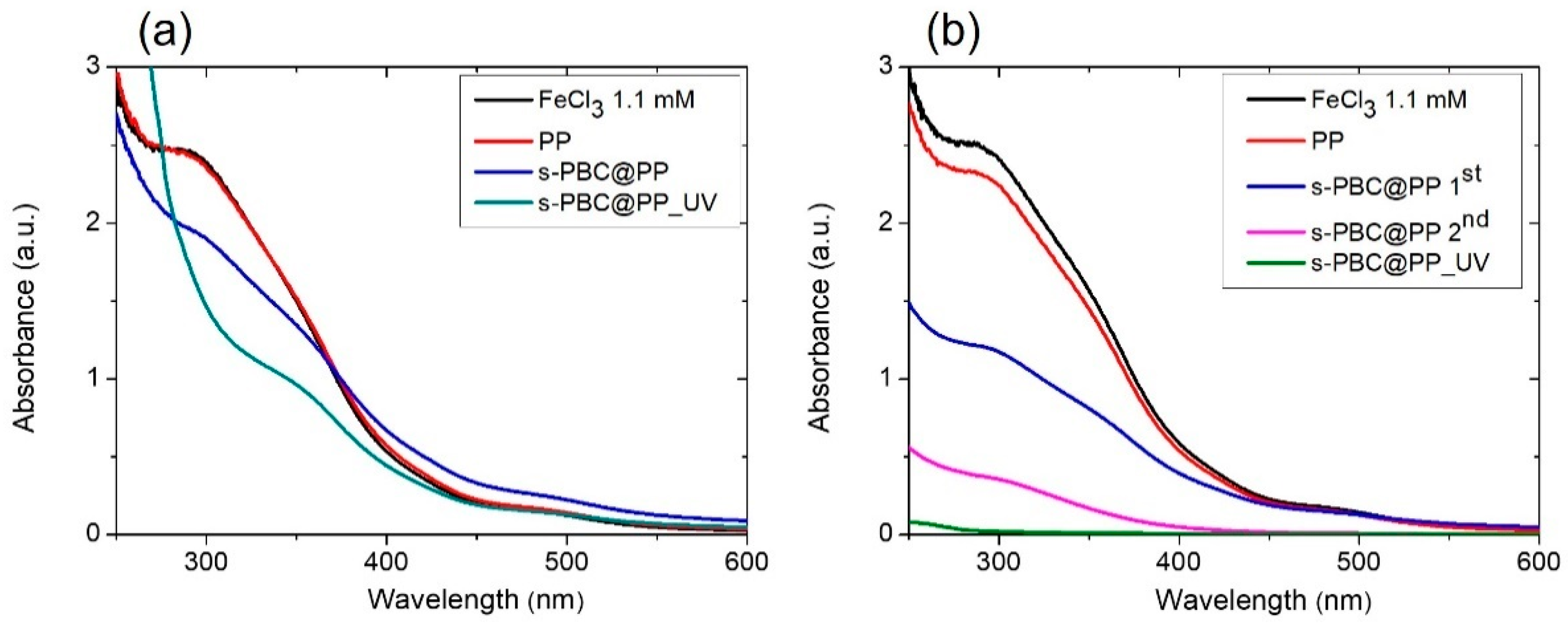
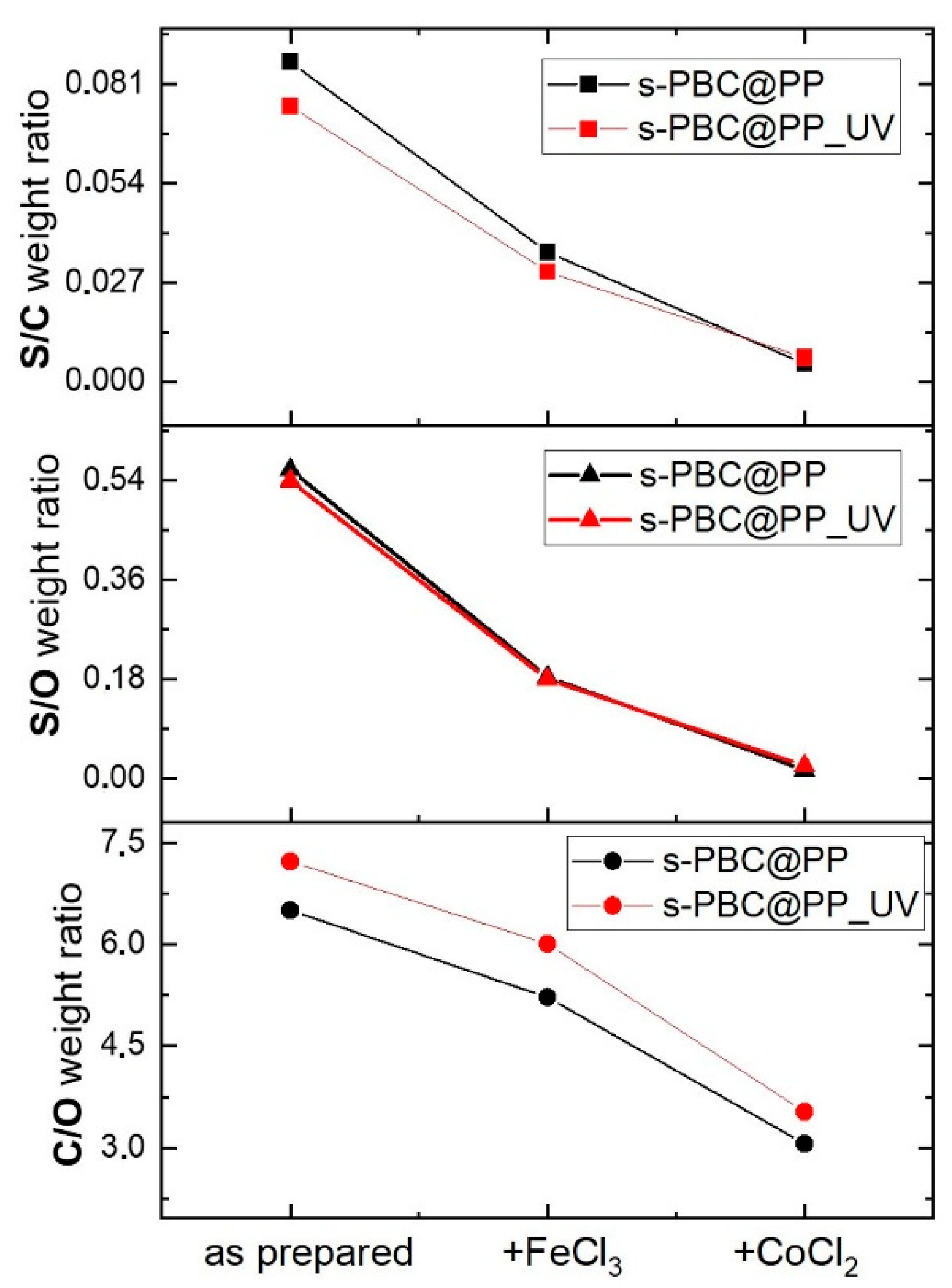
2.2.2. Fe3+ Adsorption and Filtration
2.2.3. Co2+ Adsorption and Filtration
2.3. Post-Process Filters Characterization
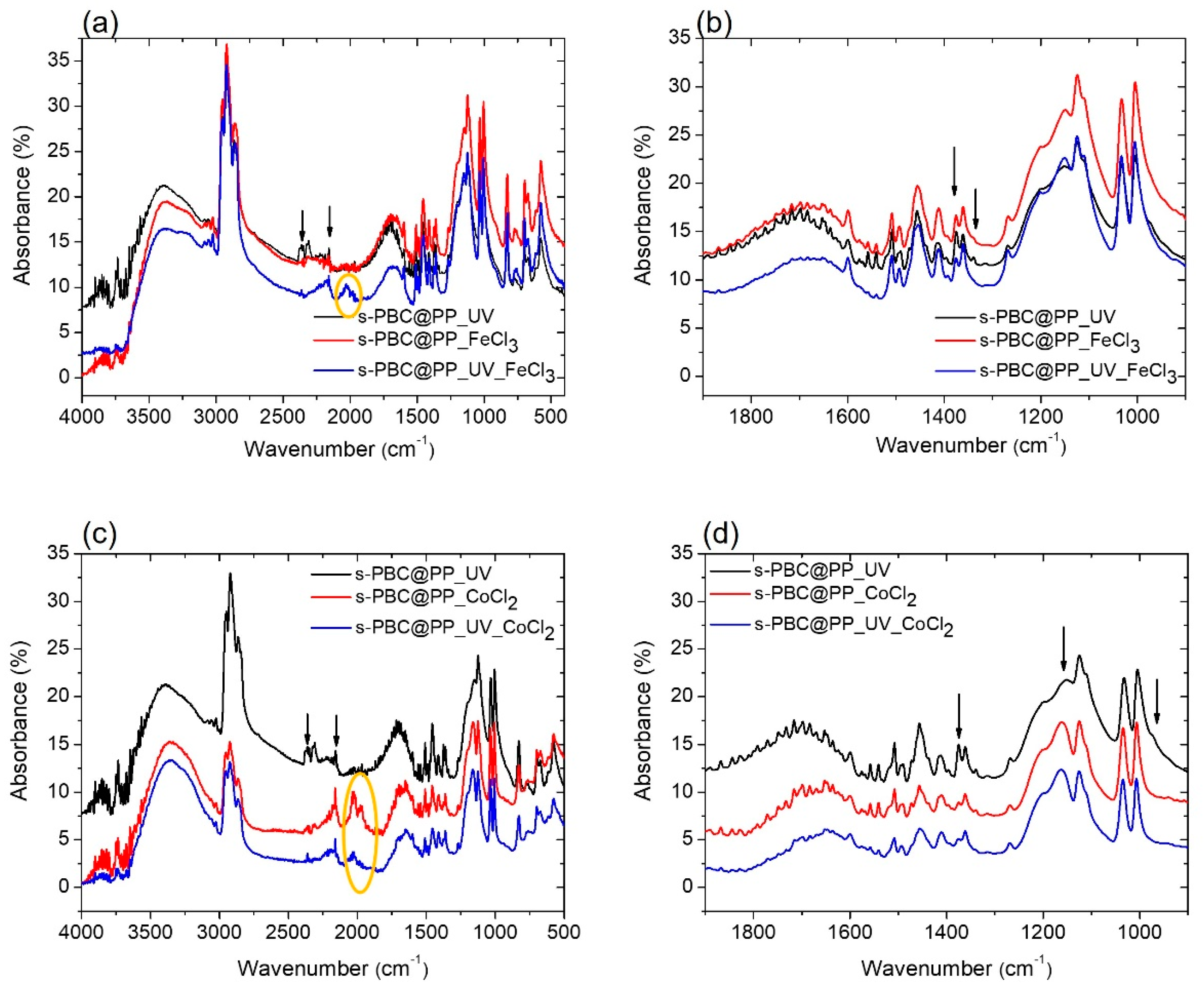
2.3.1. FT-IR Analysis
2.3.2. EDX Microanalysis
3. Materials and Methods
3.1. Chemicals
3.2. Samples Preparation
3.3. Samples Characterization
3.4. Adsorption and Filtration Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wu, H.; Zhao, S.; Zhang, W.; Tahir, M.; Wang, Z.; Wang, J. Interfacial polymerized and pore-variable covalent organic framework composite membrane for dye separation. Chem. Eng. J. 2020, 384, 123347. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, G.; Li, W.; Cui, Z.; Wu, J.; Akpinar, I.; Yu, L.; He, G.; Hu, J. Loofah activated carbon with hierarchical structures for high-efficiency adsorption of multi-level antibiotic pollutants. Appl. Surf. Sci. 2021, 550, 149313. [Google Scholar] [CrossRef]
- Bedia, J.; Muelas-Ramos, V.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodríguez, J.J.; Belver, C. A Review on the Synthesis and Characterization of Metal Organic Frameworks for Photocatalytic Water Purification. Catalysts 2019, 9, 52. [Google Scholar] [CrossRef]
- Chankhanittha, T.; Komchoo, N.; Senasu, T.; Piriyanon, J.; Youngme, S.; Hemavibool, K.; Nanan, S. Silver decorated ZnO photocatalyst for effective removal of reactive red azo dye and ofloxacin antibiotic under solar light irradiation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127034. [Google Scholar] [CrossRef]
- Scuderi, V.; Amiard, G.; Sanz, R.; Boninelli, S.; Impellizzeri, G.; Privitera, V. TiO2 coated CuO nanowire array: Ultrathin p–n heterojunction to modulate cationic/anionic dye photo-degradation in water. Appl. Surf. Sci. 2017, 416, 885–890. [Google Scholar] [CrossRef]
- Senasu, T.; Nijpanich, S.; Juabrum, S.; Chanlek, N.; Nanan, S. CdS/BiOBr heterojunction photocatalyst with high performance for solar-light-driven degradation of ciprofloxacin and norfloxacin antibiotics. Appl. Surf. Sci. 2021, 567, 150850. [Google Scholar] [CrossRef]
- Piriyanon, J.; Takhai, P.; Patta, S.; Chankhanittha, T.; Senasu, T.; Nijpanich, S.; Juabrum, S.; Chanlek, N.; Nanan, S. Performance of sunlight responsive WO3/AgBr heterojunction photocatalyst toward degradation of Rhodamine B dye and ofloxacin antibiotic. Opt. Mater. 2021, 121, 111573. [Google Scholar] [CrossRef]
- Mezher, T.; Fath, H.; Abbas, Z.; Khaled, A. Techno-economic assessment and environmental impacts of desalination technologies. J. Des. 2011, 266, 263–273. [Google Scholar] [CrossRef]
- Yuan, S.; Li, X.; Zhu, J.; Zhang, G.; Van Puyvelde, P.; Van der Bruggen, B. Covalent organic frameworks for membrane separation. Chem. Soc. Rev. 2019, 48, 2665–26815. [Google Scholar] [CrossRef]
- Caro, J. Hierarchy in inorganic membranes. Chem. Soc. Rev. 2016, 45, 3468–3478. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Gao, P.; Fang, R.; Cai, J.; Zhang, L.D.; He, Q.Y.; Zhou, Z.H.; Sun, S.P.; Cao, X.L. Constructing positively charged acid-resistant nanofiltration membranes via surface postgrafting for efficient removal of metal ions from electroplating rinse wastewater. Sep. Purif. Technol. 2022, 297, 121500. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, W.; Liu, J.; Li, X.; Zhang, S. A review on 2D porous organic polymers for membrane-based separations: Processing and engineering of transport channels. Adv. Membr. 2021, 1, 100014. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Z.; Liu, L.; Chen, Y. Construction of high performance thin-film nanocomposite nanofiltration membrane by incorporation of hydrophobic MOF-derived nanocages. Appl. Surf. Sci. 2021, 570, 151093. [Google Scholar] [CrossRef]
- Filice, S.; Urzì, G.; Milazzo, R.G.; Privitera, S.M.S.; Lombardo, S.A.; Compagnini, G.; Scalese, S. Applicability of a New Sulfonated Pentablock Copolymer Membrane and Modified Gas Diffusion Layers for Low-Cost Water Splitting Processes. Energies 2019, 12, 2064. [Google Scholar] [CrossRef]
- Filice, S.; Mazurkiewicz-Pawlicka, M.; Malolepszy, A.; Stobinski, L.; Kwiatkowski, R.; Boczkowska, A.; Gradon, L.; Scalese, S. Sulfonated Pentablock Copolymer Membranes and Graphene Oxide Addition for Efficient Removal of Metal Ions from Water. Nanomaterials 2020, 10, 1157. [Google Scholar] [CrossRef]
- Filice, S.; D’Angelo, D.; Scarangella, A.; Iannazzo, D.; Compagnini, G.; Scalese, S. Highly effective and reusable sulfonated pentablock copolymer nanocomposites for water purification applications. RSC Adv. 2017, 7, 45521–45534. [Google Scholar] [CrossRef]
- D’Angelo, D.; Filice, S.; Scarangella, A.; Iannazzo, D.; Compagnini, G.; Scalese, S. Bi2O3/Nexar® polymer nanocomposite membranes for azo dyes removal by UV–vis or visible light irradiation. Catal. Today 2019, 321, 158–163. [Google Scholar] [CrossRef]
- Sciuto, E.L.; Filice, S.; Coniglio, M.A.; Faro, G.; Gradon, L.; Galati, C.; Spinella, N.; Libertino, S.; Scalese, S. Antimicrobial s-PBC Coatings for Innovative Multifunctional Water Filters. Molecules 2020, 25, 5196. [Google Scholar] [CrossRef]
- Filice, S.; Sciuto, E.L.; Scalese, S.; Faro, G.; Libertino, S.; Corso, D.; Timpanaro, R.M.; Laganà, P.; Coniglio, M.A. Innovative Antibiofilm Smart Surface against Legionella for Water Systems. Microorganisms 2022, 10, 870. [Google Scholar] [CrossRef]
- Dai, Z.; Ansaloni, L.; Ryan, J.J.; Spontak, R.J.; Deng, L. Incorporation of an ionic liquid into a midblock-sulfonated multiblock polymer for CO2 capture. J. Membr. Sci. 2019, 588, 117193. [Google Scholar] [CrossRef]
- Available online: https://www.sigmaaldrich.com/IT/it/technical-documents/technical-article/analytical-chemistry/photometry-and-reflectometry/ir-spectrum-table (accessed on 29 August 2022).
- Chen, H.; Chang, K.; Men, X.; Sun, K.; Fang, X.; Ma, C.; Zhao, Y.; Yin, S.; Qin, W.; Wu, C. Covalent Patterning and Rapid Visualization of Latent Fingerprints with Photo-Cross-Linkable Semiconductor Polymer Dots. ACS Appl. Mater. Interfaces 2015, 7, 14477–14484. [Google Scholar] [CrossRef] [PubMed]
- Leea, S.-K.; Mills, A. Novel photochemistry of leuco-Methylene Blue. Chem. Commun. 2003, 18, 2366–2367. [Google Scholar] [CrossRef] [PubMed]
- Harito, C.; Bavykin, D.V.; Yuliarto, B.; Dipojono, H.K.; Walsh, F.C. Inhibition of Polyimide Photodegradation by Incorporation of Titanate Nanotubes into a Composite. J. Polym. Environ. 2019, 27, 1505–1515. [Google Scholar] [CrossRef]
- Le, T.G.; Nguyen, N.T.; Nguyen, Q.T.; De Laat, J.; Dao, H.Y. Effect of Chloride and Sulfate Ions on the Photoreduction Rate of Ferric Ion in UV Reactor Equipped with a Low Pressure Mercury Lamp. J. Adv. Oxid. Technol. 2014, 17, 305–330. [Google Scholar] [CrossRef]
- Sikorska, E.; Gradoń, L. Biofouling reduction for improvement of depth Water filtration. Filter production and testing. Chem. Process Eng. 2016, 37, 319–330. [Google Scholar] [CrossRef]
- Mineart, K.P.; Jiang, X.; Jinnai, H.; Takahara, A.; Spontak, R.J. Morphological Investigation of Midblock- Sulfonated Ionomers Prepared from Solvents Differing in Polarity. Macromol. Rapid Commun. 2015, 36, 432–438. [Google Scholar] [CrossRef]


| Sample | Water Uptake (%) | Average Contact Angle (Deg) |
|---|---|---|
| PP | 3 | 129.6 |
| s-PBC@PP | 262 | 103.1 |
| s-PBC@PP_UV | 308 | 85.6 |
| Sample | MB Removal by Adsorption (%) | Qt_ads (mg/g) | MB Removal by Filtration (%) | Qt_filt (mg/g) |
|---|---|---|---|---|
| PP | 5 | - | 20 | - |
| s-PBC@PP | 90 | 0.58 | 80 | 0.45 |
| s-PBC@PP-UV | 80 | 0.56 | 70 | 0.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filice, S.; Scuderi, V.; Libertino, S.; Zimbone, M.; Galati, C.; Spinella, N.; Gradon, L.; Falqui, L.; Scalese, S. Sulfonated Pentablock Copolymer Coating of Polypropylene Filters for Dye and Metal Ions Effective Removal by Integrated Adsorption and Filtration Process. Int. J. Mol. Sci. 2022, 23, 11777. https://doi.org/10.3390/ijms231911777
Filice S, Scuderi V, Libertino S, Zimbone M, Galati C, Spinella N, Gradon L, Falqui L, Scalese S. Sulfonated Pentablock Copolymer Coating of Polypropylene Filters for Dye and Metal Ions Effective Removal by Integrated Adsorption and Filtration Process. International Journal of Molecular Sciences. 2022; 23(19):11777. https://doi.org/10.3390/ijms231911777
Chicago/Turabian StyleFilice, Simona, Viviana Scuderi, Sebania Libertino, Massimo Zimbone, Clelia Galati, Natalia Spinella, Leon Gradon, Luciano Falqui, and Silvia Scalese. 2022. "Sulfonated Pentablock Copolymer Coating of Polypropylene Filters for Dye and Metal Ions Effective Removal by Integrated Adsorption and Filtration Process" International Journal of Molecular Sciences 23, no. 19: 11777. https://doi.org/10.3390/ijms231911777
APA StyleFilice, S., Scuderi, V., Libertino, S., Zimbone, M., Galati, C., Spinella, N., Gradon, L., Falqui, L., & Scalese, S. (2022). Sulfonated Pentablock Copolymer Coating of Polypropylene Filters for Dye and Metal Ions Effective Removal by Integrated Adsorption and Filtration Process. International Journal of Molecular Sciences, 23(19), 11777. https://doi.org/10.3390/ijms231911777









