Epigenetic and Neuronal Activity Markers Suggest the Recruitment of the Prefrontal Cortex and Hippocampus in the Three-Hit Model of Depression in Male PACAP Heterozygous Mice
Abstract
1. Introduction
2. Results
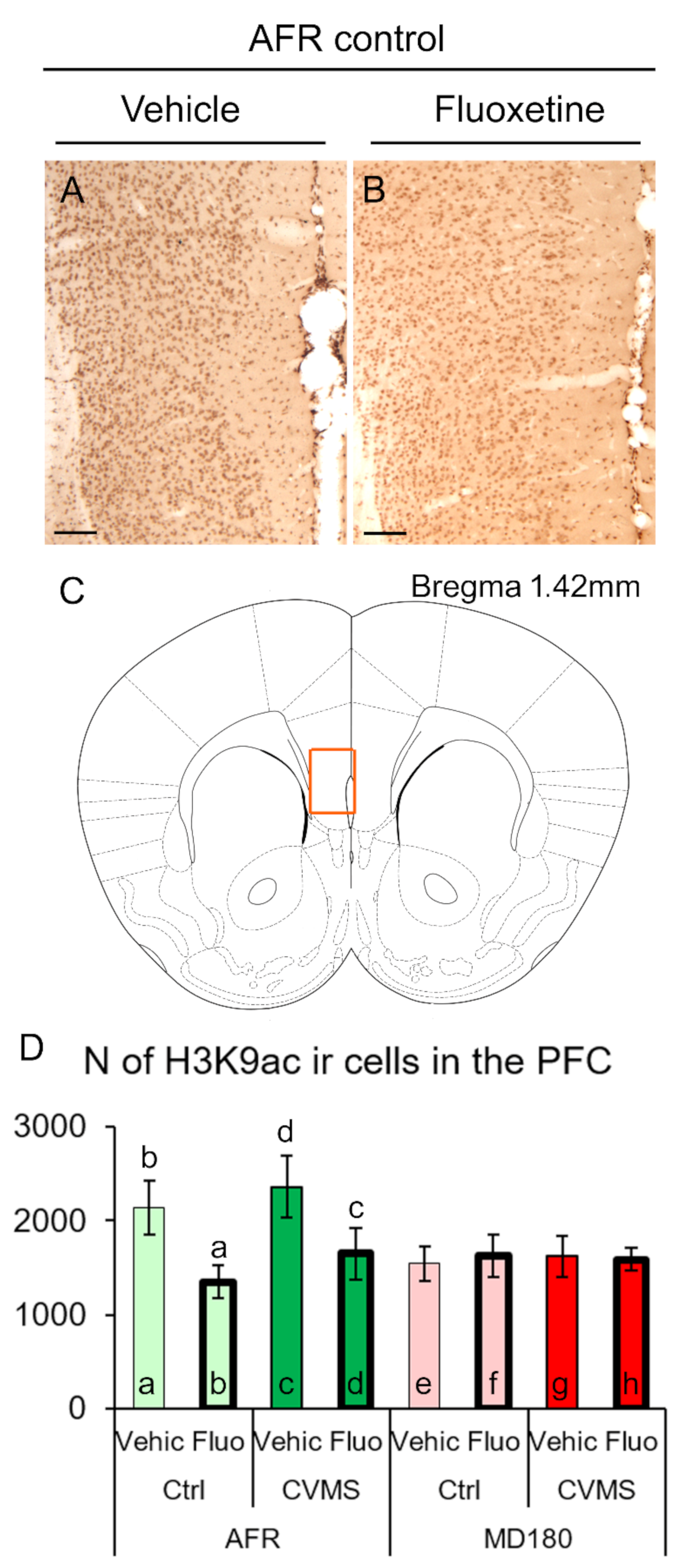
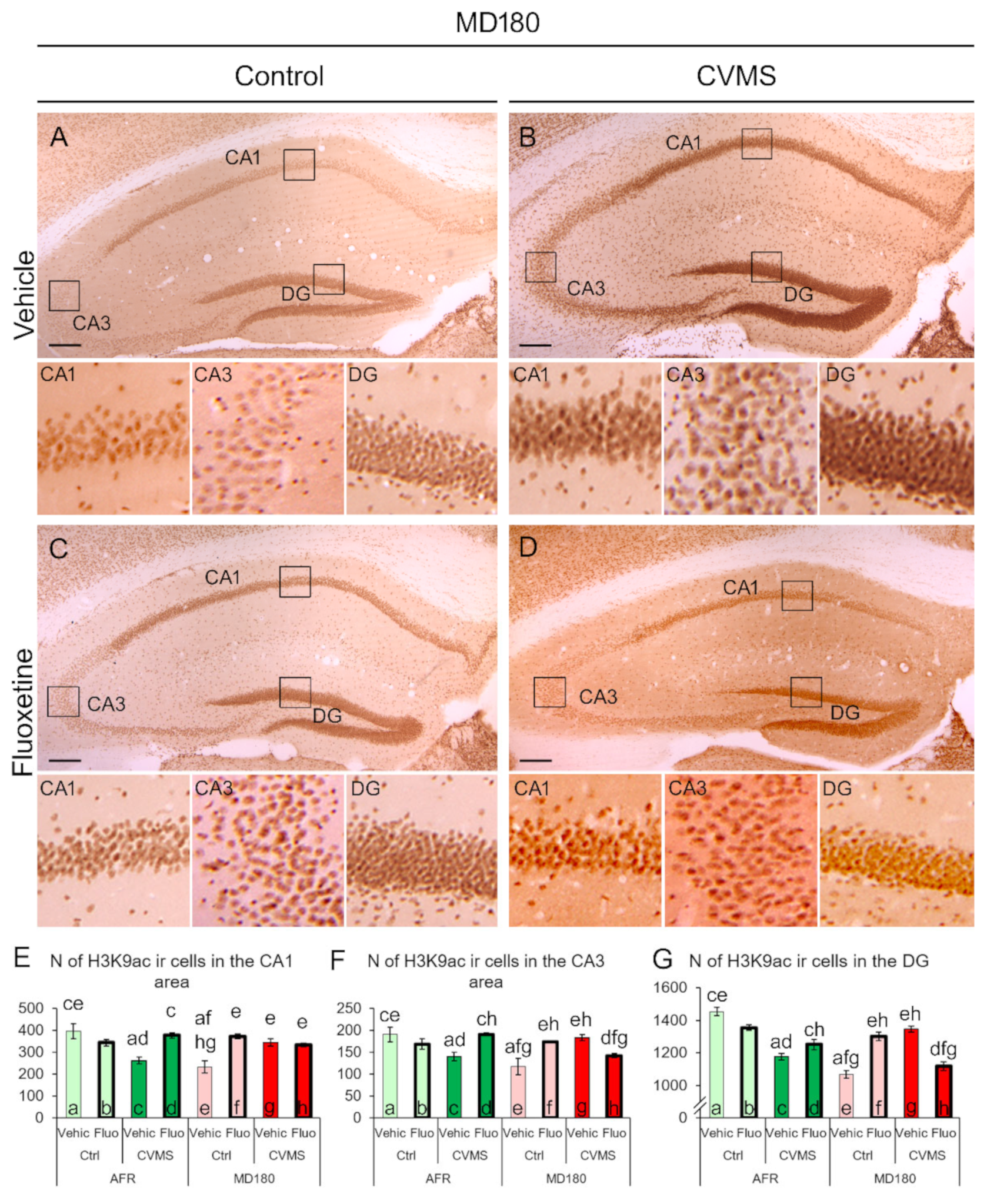
2.1. H3K9ac Immunoreactivity
2.1.1. Prefrontal Cortex
2.1.2. Hippocampus
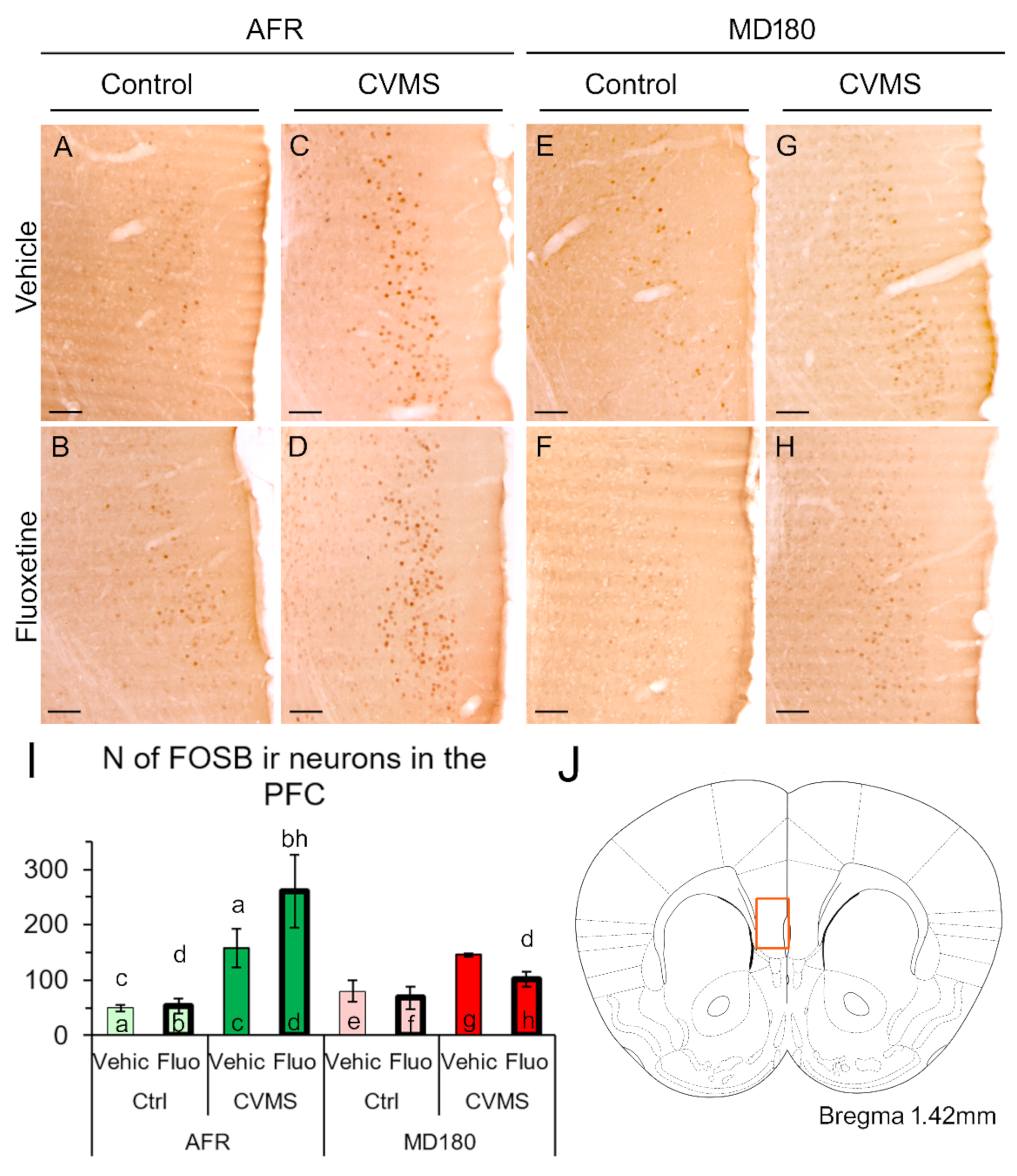
2.2. FOSB Immunoreactivity
2.2.1. Prefrontal Cortex
2.2.2. Hippocampus
3. Discussion
3.1. The H3K9ac-ir Is Affected by Both Maternal Deprivation and Fluoxetine Therapy
3.2. FOSB Reactivity to CVMS Is Influenced Both by Maternal Care Quality and SSRI Treatment
3.3. Limitations
3.4. Conclusions
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Perfusion and Sample Preparation
4.3. Free-Floating Immunocytochemistry for H3K9ac and FOSB by Diaminobenzidine
4.4. Microscopy, Digital Imaging, and Morphometry
4.5. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 1 October 2022).
- Park, C.; Rosenblat, J.D.; Brietzke, E.; Pan, Z.; Lee, Y.; Cao, B.; Zuckerman, H.; Kalantarova, A.; McIntyre, R.S. Stress, epigenetics and depression: A systematic review. Neurosci. Biobehav. Rev. 2019, 102, 139–152. [Google Scholar] [CrossRef] [PubMed]
- De Kloet, E.R.; DeRijk, R.H.; Meijer, O.C. Therapy insight: Is there an imbalanced response of mineralocorticoid and glucocorticoid receptors in depression? Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Daskalakis, N.P.; Bagot, R.C.; Parker, K.J.; Vinkers, C.H.; de Kloet, E.R. The three-hit concept of vulnerability and resilience: Toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology 2013, 38, 1858–1873. [Google Scholar] [CrossRef]
- Farkas, J.; Kovacs, L.Á.; Gaszner, T.; Gaszner, B. Using PACAP heterozygous mice as models of the three hit theory of depression. In Pituitary Adenylate Cyclase Activating Polypeptide—PACAP; Reglődi, D., Tamás, A., Eds.; Springer: Cham, Switzerland, 2016; pp. 731–741. [Google Scholar]
- Farkas, J.; Kovács, L.; Gáspár, L.; Nafz, A.; Gaszner, T.; Ujvári, B.; Kormos, V.; Csernus, V.; Hashimoto, H.; Reglődi, D.; et al. Construct and face validity of a new model for the three-hit theory of depression using PACAP mutant mice on CD1 background. Neuroscience 2017, 354, 11–29. [Google Scholar] [CrossRef]
- Hashimoto, H.; Shintani, N.; Tanida, M.; Hayata, A.; Hashimoto, R.; Baba, A. PACAP is Implicated in the Stress Axes. Curr. Pharm. Des. 2011, 17, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Gaszner, B.; Kormos, V.; Kozicz, T.; Hashimoto, H.; Reglodi, D.; Helyes, Z. The behavioral phenotype of pituitary adenylate-cyclase activating polypeptide-deficient mice in anxiety and depression tests is accompanied by blunted c-Fos expression in the bed nucleus of the stria terminalis, central projecting Edinger–Westphal nucleus, ventral lateral septum, and dorsal raphe nucleus. Neuroscience 2012, 202, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Kormos, V.; Gáspár, L.; Kovács, L.; Farkas, J.; Gaszner, T.; Csernus, V.; Balogh, A.; Hashimoto, H.; Reglődi, D.; Helyes, Z.; et al. Reduced response to chronic mild stress in PACAP mutant mice is associated with blunted FosB expression in limbic forebrain and brainstem centers. Neuroscience 2016, 330, 335–358. [Google Scholar] [CrossRef]
- Agarwal, A.; Halvorson, L.M.; Legradi, G. Pituitary adenylate cyclase-activating polypeptide (PACAP) mimics neuroendocrine and behavioral manifestations of stress: Evidence for PKA-mediated expression of the corticotropin-releasing hormone (CRH) gene. Mol. Brain Res. 2005, 138, 45–57. [Google Scholar] [CrossRef]
- Stroth, N.; Eiden, L. Stress hormone synthesis in mouse hypothalamus and adrenal gland triggered by restraint is dependent on pituitary adenylate cyclase-activating polypeptide signaling. Neuroscience 2010, 165, 1025–1030. [Google Scholar] [CrossRef]
- Stroth, N.; Holighaus, Y.; Ait-Ali, D.; Eiden, L.E. PACAP: A master regulator of neuroendocrine stress circuits and the cellular stress response. Ann. N. Y. Acad. Sci. 2011, 1220, 49–59. [Google Scholar] [CrossRef]
- Tsukiyama, N.; Saida, Y.; Kakuda, M.; Shintani, N.; Hayata, A.; Morita, Y.; Tanida, M.; Tajiri, M.; Hazama, K.; Ogata, K.; et al. PACAP centrally mediates emotional stress-induced corticosterone responses in mice. Stress 2011, 14, 368–375. [Google Scholar] [CrossRef]
- Pinhasov, A.; Nesher, E.; Gross, M.; Turgeman, G.; Kreinin, A.; Yadid, G. The Role of the PACAP Signaling System in Depression. Curr. Pharm. Des. 2011, 17, 990–1001. [Google Scholar] [CrossRef] [PubMed]
- Reglodi, D.; Kiss, P.; Lubics, A.; Tamas, A. Review on the Protective Effects of PACAP in Models of Neurodegenerative Diseases In Vitro and In Vivo. Curr. Pharm. Des. 2011, 17, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Reglodi, D.; Kiss, P.; Szabadfi, K.; Atlasz, T.; Gabriel, R.; Horvath, G.; Szakaly, P.; Sandor, B.; Lubics, A.; Laszlo, E.; et al. PACAP is an Endogenous Protective Factor—Insights from PACAP-Deficient Mice. J. Mol. Neurosci. 2012, 48, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Kormos, V.; Gaszner, B. Role of neuropeptides in anxiety, stress, and depression: From animals to humans. Neuropeptides 2013, 47, 401–419. [Google Scholar] [CrossRef]
- Hammack, S.E.; May, V. Pituitary Adenylate Cyclase Activating Polypeptide in Stress-Related Disorders: Data Convergence from Animal and Human Studies. Biol. Psychiatry 2015, 78, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Lutfy, K.; Shankar, G. Emerging evidence for the role of pituitary adenylate cyclase-activating peptide in neuropsychiatric disorders. Prog. Mol. Biol. Transl. Sci. 2019, 167, 143–157. [Google Scholar] [CrossRef]
- Boucher, M.N.; May, V.; Braas, K.M.; Hammack, S.E. PACAP orchestration of stress-related responses in neural circuits. Peptides 2021, 142, 170554. [Google Scholar] [CrossRef]
- Gaszner, T.; Farkas, J.; Kun, D.; Ujvári, B.; Berta, G.; Csernus, V.; Füredi, N.; Kovács, L.; Hashimoto, H.; Reglődi, D.; et al. Fluoxetine treatment supports predictive validity of the three hit model of depression in male PACAP heterozygous mice and underpins the impact of early life adversity on therapeutic efficacy. Front. Endocrinol. 2022, 13, 995900. [Google Scholar] [CrossRef]
- Lange, U.C.; Schneider, R. What an epigenome remembers. BioEssays 2010, 32, 659–668. [Google Scholar] [CrossRef]
- Ng, R.K.; Gurdon, J.B. Epigenetic inheritance of cell differentiation status. Cell Cycle 2008, 7, 1173–1177. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J. Stress makes its molecular mark. Nature 2012, 490, 171–172. [Google Scholar] [CrossRef]
- Raabe, F.J.; Spengler, D. Epigenetic Risk Factors in PTSD and Depression. Front. Psychiatry 2013, 4, 80. [Google Scholar] [CrossRef]
- Heim, C.; Binder, E.B. Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene–environment interactions, and epigenetics. Exp. Neurol. 2012, 233, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Willner, P. The chronic mild stress (CMS) model of depression: History, evaluation and usage. Neurobiol. Stress 2017, 6, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Willner, P. The validity of animal models of depression. Psychopharmacology 1984, 83, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ge, T.; Leng, Y.; Pan, Z.; Fan, J.; Yang, W.; Cui, R. The Role of Neural Plasticity in Depression: From Hippocampus to Prefrontal Cortex. Neural Plast. 2017, 2017, 6871089. [Google Scholar] [CrossRef]
- Belleau, E.L.; Treadway, M.T.; Pizzagalli, D.A. The Impact of Stress and Major Depressive Disorder on Hippocampal and Medial Prefrontal Cortex Morphology. Biol. Psychiatry 2019, 85, 443–453. [Google Scholar] [CrossRef]
- Treadway, M.T.; Waskom, M.L.; Dillon, D.G.; Holmes, A.; Park, M.T.; Chakravarty, M.M.; Dutra, S.J.; Polli, F.E.; Iosifescu, D.V.; Fava, M.; et al. Illness Progression, Recent Stress, and Morphometry of Hippocampal Subfields and Medial Prefrontal Cortex in Major Depression. Biol. Psychiatry 2015, 77, 285–294. [Google Scholar] [CrossRef]
- George, M.S.; Ketter, T.A.; Post, R.M. Prefrontal cortex dysfunction in clinical depression. Depression 1994, 2, 59–72. [Google Scholar] [CrossRef]
- Pizzagalli, D.A.; Roberts, A.C. Prefrontal cortex and depression. Neuropsychopharmacology 2022, 47, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Mayberg, H.S.; Liotti, M.; Brannan, S.K.; McGinnis, S.; Mahurin, R.K.; Jerabek, P.A.; Silva, J.A.; Tekell, J.L.; Martin, C.C.; Lancaster, J.L.; et al. Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. Am. J. Psychiatry 1999, 156, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Ellenbogen, J.M.; Hurford, M.O.; Liebeskind, D.S.; Neimark, G.B.; Weiss, D. Ventromedial frontal lobe trauma. Neurology 2005, 64, 757. [Google Scholar] [CrossRef] [PubMed]
- Mayberg, H.S.; Lozano, A.M.; Voon, V.; McNeely, H.E.; Seminowicz, D.; Hamani, C.; Schwalb, J.M.; Kennedy, S.H. Deep brain stimulation for treatment-resistant depression. Neuron 2005, 45, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Koenigs, M.; Grafman, J. The functional neuroanatomy of depression: Distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav. Brain Res. 2009, 201, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Albert, P.R.; Vahid-Ansari, F.; Luckhart, C. Serotonin-prefrontal cortical circuitry in anxiety and depression phenotypes: Pivotal role of pre- and post-synaptic 5-HT1A receptor expression. Front. Behav. Neurosci. 2014, 8, 199. [Google Scholar] [CrossRef]
- Ghosal, S.; Duman, C.H.; Liu, R.-J.; Wu, M.; Terwilliger, R.; Girgenti, M.J.; Wohleb, E.; Fogaca, M.V.; Teichman, E.M.; Hare, B.; et al. Ketamine rapidly reverses stress-induced impairments in GABAergic transmission in the prefrontal cortex in male rodents. Neurobiol. Dis. 2020, 134, 104669. [Google Scholar] [CrossRef]
- Veeraiah, P.; Noronha, J.M.; Maitra, S.; Bagga, P.; Khandelwal, N.; Chakravarty, S.; Kumar, A.; Patel, A.B. Dysfunctional Glutamatergic and γ-Aminobutyric Acidergic Activities in Prefrontal Cortex of Mice in Social Defeat Model of Depression. Biol. Psychiatry 2014, 76, 231–238. [Google Scholar] [CrossRef]
- Blendy, J.A. The role of CREB in depression and antidepressant treatment. Biol. Psychiatry 2006, 59, 1144–1150. [Google Scholar] [CrossRef]
- Yan, L.; Xu, X.; He, Z.; Wang, S.; Zhao, L.; Qiu, J.; Wang, D.; Gong, Z.; Qiu, X.; Huang, H. Antidepressant-Like Effects and Cognitive Enhancement of Coadministration of Chaihu Shugan San and Fluoxetine: Dependent on the BDNF-ERK-CREB Signaling Pathway in the Hippocampus and Frontal Cortex. BioMed Res. Int. 2020, 2020, 2794263. [Google Scholar] [CrossRef]
- Dionisie, V.; Ciobanu, A.M.; Toma, V.A.; Manea, M.C.; Baldea, I.; Olteanu, D.; Sevastre-Berghian, A.; Clichici, S.; Manea, M.; Riga, S.; et al. Escitalopram Targets Oxidative Stress, Caspase-3, BDNF and MeCP2 in the Hippocampus and Frontal Cortex of a Rat Model of Depression Induced by Chronic Unpredictable Mild Stress. Int. J. Mol. Sci. 2021, 22, 7483. [Google Scholar] [CrossRef] [PubMed]
- Baudin, A.; Blot, K.; Verney, C.; Estevez, L.; Santamaria, J.; Gressens, P.; Giros, B.; Otani, S.; Daugé, V.; Naudon, L. Maternal deprivation induces deficits in temporal memory and cognitive flexibility and exaggerates synaptic plasticity in the rat medial prefrontal cortex. Neurobiol. Learn. Mem. 2012, 98, 207–214. [Google Scholar] [CrossRef]
- Hinwood, M.; Morandini, J.; Day, T.A.; Walker, F.R. Evidence that Microglia Mediate the Neurobiological Effects of Chronic Psychological Stress on the Medial Prefrontal Cortex. Cereb. Cortex 2012, 22, 1442–1454. [Google Scholar] [CrossRef] [PubMed]
- Kovács, K.J. Measurement of immediate-early gene activation-c-fos and beyond. J. Neuroendocrinol. 2008, 20, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J. ∆FosB: A transcriptional regulator of stress and antidepressant responses. Eur. J. Pharmacol. 2015, 753, 66–72. [Google Scholar] [CrossRef]
- Sampath, D.; Sathyanesan, M.; Newton, S.S. Cognitive dysfunction in major depression and Alzheimer’s disease is associated with hippocampal–prefrontal cortex dysconnectivity. Neuropsychiatr. Dis. Treat. 2017, 13, 1509. [Google Scholar] [CrossRef]
- Campbell, S.; MacQueen, G. The role of the hippocampus in the pathophysiology of major depression. J. Psychiatry Neurosci. 2004, 29, 417–426. [Google Scholar]
- Xu, L.; Anwyl, R.; Rowan, M.J. Behavioural stress facilitates the induction of long-term depression in the hippocampus. Nature 1997, 387, 497–500. [Google Scholar] [CrossRef]
- Pittenger, C.; Duman, R.S. Stress, Depression, and Neuroplasticity: A Convergence of Mechanisms. Neuropsychopharmacology 2008, 33, 88–109. [Google Scholar] [CrossRef]
- Czéh, B.; Michaelis, T.; Watanabe, T.; Frahm, J.; de Biurrun, G.; van Kampen, M.; Bartolomucci, A.; Fuchs, E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc. Natl. Acad. Sci. USA 2001, 98, 12796–12801. [Google Scholar] [CrossRef]
- Czéh, B.; Lucassen, P.J. What causes the hippocampal volume decrease in depression? Eur. Arch. Psychiatry Clin. Neurosci. 2007, 257, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Masi, G.; Brovedani, P. The Hippocampus, Neurotrophic Factors and Depression. CNS Drugs 2011, 25, 913–931. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, E.; Czéh, B.; Kole, M.; Michaelis, T.; Lucassen, P.J. Alterations of neuroplasticity in depression: The hippocampus and beyond. Eur. Neuropsychopharmacol. 2004, 14, S481–S490. [Google Scholar] [CrossRef]
- MacQueen, G.M.; Ramakrishnan, K.; Ratnasingan, R.; Chen, B.; Young, L.T. Desipramine treatment reduces the long-term behavioural and neurochemical sequelae of early-life maternal separation. Int. J. Neuropsychopharmacol. 2003, 6, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Rong, J.; Zhu, C.; Liang, M.; Li, Y.; Zhou, R. Epigenetic Modifications of GABAergic Interneurons Contribute to Deficits in Adult Hippocampus Neurogenesis and Depression-Like Behavior in Prenatally Stressed Mice. Int. J. Neuropsychopharmacol. 2020, 23, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, X.; Kong, Y.; Gou, L.; Lian, B.; Wang, Y.; Jiang, L.; Li, Q.; Sun, H.; Sun, L. Maternal Separation-Induced Histone Acetylation Correlates with BDNF-Programmed Synaptic Changes in an Animal Model of PTSD with Sex Differences. Mol. Neurobiol. 2021, 58, 1738–1754. [Google Scholar] [CrossRef]
- Ferland, C.; Schrader, L. Regulation of histone acetylation in the hippocampus of chronically stressed rats: A potential role of sirtuins. Neuroscience 2011, 174, 104–114. [Google Scholar] [CrossRef]
- Hunter, R.G.; McCarthy, K.J.; Milne, T.A.; Pfaff, D.W.; McEwen, B.S. Regulation of hippocampal H3 histone methylation by acute and chronic stress. Proc. Natl. Acad. Sci. USA 2009, 106, 20912–20917. [Google Scholar] [CrossRef]
- Perrotti, L.I.; Hadeishi, Y.; Ulery, P.G.; Barrot, M.; Monteggia, L.; Duman, R.S.; Nestler, E.J. Induction of ΔFosB in reward-related brain structures after chronic stress. J. Neurosci. 2004, 24, 10594–10602. [Google Scholar] [CrossRef]
- Paxinos, G.; Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates, 2nd ed.; Academic Press: San Diego, CA, USA, 2001. [Google Scholar]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef]
- Kuo, M.H.; Allis, C.D. Roles of histone acetyltransferases and deacetylases in gene regulation. Bioessays 1998, 20, 615–626. [Google Scholar] [CrossRef]
- Levine, A.; Worrell, T.R.; Zimnisky, R.; Schmauss, C. Early life stress triggers sustained changes in histone deacetylase expression and histone H4 modifications that alter responsiveness to adolescent antidepressant treatment. Neurobiol. Dis. 2012, 45, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Robison, A.; Vialou, V.; Sun, H.-S.; LaBonte, B.; Golden, S.; Dias, C.; Turecki, G.; Tamminga, C.A.; Russo, S.; Mazei-Robison, M.; et al. Fluoxetine Epigenetically Alters the CaMKIIα Promoter in Nucleus Accumbens to Regulate ΔFosB Binding and Antidepressant Effects. Neuropsychopharmacology 2014, 39, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Füredi, N.; Nagy, Á.; Mikó, A.; Berta, G.; Kozicz, T.; Pétervári, E.; Balaskó, M.; Gaszner, B. Melanocortin 4 receptor ligands modulate energy homeostasis through urocortin 1 neurons of the centrally projecting Edinger-Westphal nucleus. Neuropharmacology 2017, 118, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Kovács, L.; Berta, G.; Csernus, V.; Ujvári, B.; Füredi, N.; Gaszner, B. Corticotropin-Releasing Factor-Producing Cells in the Paraventricular Nucleus of the Hypothalamus and Extended Amygdala Show Age-Dependent FOS and FOSB/DeltaFOSB Immunoreactivity in Acute and Chronic Stress Models in the Rat. Front. Aging Neurosci. 2019, 11, 274. [Google Scholar] [CrossRef]
- Kovács, L.Á.; Füredi, N.; Ujvári, B.; Golgol, A.; Gaszner, B. Age-Dependent FOSB/ΔFOSB Response to Acute and Chronic Stress in the Extended Amygdala, Hypothalamic Paraventricular, Habenular, Centrally-Projecting Edinger-Westphal, and Dorsal Raphe Nuclei in Male Rats. Front. Aging Neurosci. 2022, 14, 862098. [Google Scholar] [CrossRef] [PubMed]
- Sterrenburg, L.; Gaszner, B.; Boerrigter, J.; Santbergen, L.; Bramini, M.; Elliott, E.; Chen, A.; Peeters, B.W.M.M.; Roubos, E.W.; Kozicz, T. Chronic Stress Induces Sex-Specific Alterations in Methylation and Expression of Corticotropin-Releasing Factor Gene in the Rat. PLoS ONE 2011, 6, e28128. [Google Scholar] [CrossRef]
- Perrotti, L.; Weaver, R.; Robison, B.; Renthal, W.; Maze, I.; Yazdani, S.; Elmore, R.; Knapp, D.; Selley, D.; Martin, B.; et al. Distinct patterns of ΔFosB induction in brain by drugs of abuse. Synapse 2008, 62, 358–369. [Google Scholar] [CrossRef]
- Lehmann, M.L.; Herkenham, M. Environmental Enrichment Confers Stress Resiliency to Social Defeat through an Infralimbic Cortex-Dependent Neuroanatomical Pathway. J. Neurosci. 2011, 31, 6159–6173. [Google Scholar] [CrossRef]
- Laine, M.; Sokolowska, E.; Dudek, M.; Callan, S.-A.; Hyytiä, P.; Hovatta, I. Brain activation induced by chronic psychosocial stress in mice. Sci. Rep. 2017, 7, 15061. [Google Scholar] [CrossRef]
- Vialou, V.; Thibault, M.; Kaska, S.; Cooper, S.; Gajewski, P.; Eagle, A.; Mazei-Robison, M.; Nestler, E.J.; Robison, A. Differential induction of FosB isoforms throughout the brain by fluoxetine and chronic stress. Neuropharmacology 2015, 99, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Shansky, R.M.; Hamo, C.; Hof, P.R.; Lou, W.; McEwen, B.S.; Morrison, J.H. Estrogen Promotes Stress Sensitivity in a Prefrontal Cortex-Amygdala Pathway. Cereb. Cortex 2010, 20, 2560–2567. [Google Scholar] [CrossRef]
- Spencer, J.L.; Waters, E.M.; Romeo, R.D.; Wood, G.E.; Milner, T.A.; McEwen, B.S. Uncovering the mechanisms of estrogen effects on hippocampal function. Front. Neuroendocr. 2008, 29, 219–237. [Google Scholar] [CrossRef]
- Dragunow, M.; Faull, R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J. Neurosci. Methods 1989, 29, 261–265. [Google Scholar] [CrossRef]
- Hoffman, G.E.; Le, W.-W.; Abbud, R.; Lee, W.-S.; Smith, M.S. Use of Fos-related antigens (FRAs) as markers of neuronal activity: FRA changes in dopamine neurons during proestrus, pregnancy and lactation. Brain Res. 1994, 654, 207–215. [Google Scholar] [CrossRef]
- Bowers, G.; Cullinan, W.E.; Herman, J. Region-Specific Regulation of Glutamic Acid Decarboxylase (GAD) mRNA Expression in Central Stress Circuits. J. Neurosci. 1998, 18, 5938–5947. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Choi, D.C.; Furay, A.R.; Evanson, N.K.; Ostrander, M.M.; Ulrich-Lai, Y.M.; Herman, J.P. Bed Nucleus of the Stria Terminalis Subregions Differentially Regulate Hypothalamic-Pituitary-Adrenal Axis Activity: Implications for the Integration of Limbic Inputs. J. Neurosci. 2007, 27, 2025–2034. [Google Scholar] [CrossRef]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef]
- Dwivedi, Y. Brain-derived neurotrophic factor: Role in depression and suicide. Neuropsychiatr. Dis. Treat. 2009, 5, 433–449. [Google Scholar] [CrossRef]
- Snyder, J.S.; Soumier, A.; Brewer, M.; Pickel, J.; Cameron, H.A. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 2011, 476, 458–461. [Google Scholar] [CrossRef]
- Saavedra, K.; Molina-Márquez, A.M.; Saavedra, N.; Zambrano, T.; Salazar, L.A. Epigenetic Modifications of Major Depressive Disorder. Int. J. Mol. Sci. 2016, 17, 1279. [Google Scholar] [CrossRef] [PubMed]
- Szabó, T.; Kormos, V.; Rékási, Z.; Gaszner, B. Epineural Methylene Blue Injection May Aid Localization of Digital Nerves in Dupuytren’s Surgery. Eur. Surg. Res. 2021, 63, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 1st ed.; Iowa State University Press: Ames, IA, USA, 1989. [Google Scholar]

| AFR | MD180 | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | CVMS | Control | CVMS | |||||
| Vehicle | Fluoxetine | Vehicle | Fluoxetine | Vehicle | Fluoxetine | Vehicle | Fluoxetine | |
| group | a | b | c | d | e | f | g | h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaszner, T.; Farkas, J.; Kun, D.; Ujvári, B.; Füredi, N.; Kovács, L.Á.; Hashimoto, H.; Reglődi, D.; Kormos, V.; Gaszner, B. Epigenetic and Neuronal Activity Markers Suggest the Recruitment of the Prefrontal Cortex and Hippocampus in the Three-Hit Model of Depression in Male PACAP Heterozygous Mice. Int. J. Mol. Sci. 2022, 23, 11739. https://doi.org/10.3390/ijms231911739
Gaszner T, Farkas J, Kun D, Ujvári B, Füredi N, Kovács LÁ, Hashimoto H, Reglődi D, Kormos V, Gaszner B. Epigenetic and Neuronal Activity Markers Suggest the Recruitment of the Prefrontal Cortex and Hippocampus in the Three-Hit Model of Depression in Male PACAP Heterozygous Mice. International Journal of Molecular Sciences. 2022; 23(19):11739. https://doi.org/10.3390/ijms231911739
Chicago/Turabian StyleGaszner, Tamás, József Farkas, Dániel Kun, Balázs Ujvári, Nóra Füredi, László Ákos Kovács, Hitoshi Hashimoto, Dóra Reglődi, Viktória Kormos, and Balázs Gaszner. 2022. "Epigenetic and Neuronal Activity Markers Suggest the Recruitment of the Prefrontal Cortex and Hippocampus in the Three-Hit Model of Depression in Male PACAP Heterozygous Mice" International Journal of Molecular Sciences 23, no. 19: 11739. https://doi.org/10.3390/ijms231911739
APA StyleGaszner, T., Farkas, J., Kun, D., Ujvári, B., Füredi, N., Kovács, L. Á., Hashimoto, H., Reglődi, D., Kormos, V., & Gaszner, B. (2022). Epigenetic and Neuronal Activity Markers Suggest the Recruitment of the Prefrontal Cortex and Hippocampus in the Three-Hit Model of Depression in Male PACAP Heterozygous Mice. International Journal of Molecular Sciences, 23(19), 11739. https://doi.org/10.3390/ijms231911739







