Investigation of Anxiety- and Depressive-like Symptoms in 4- and 8-Month-Old Male Triple Transgenic Mouse Models of Alzheimer’s Disease
Abstract
1. Introduction
2. Results
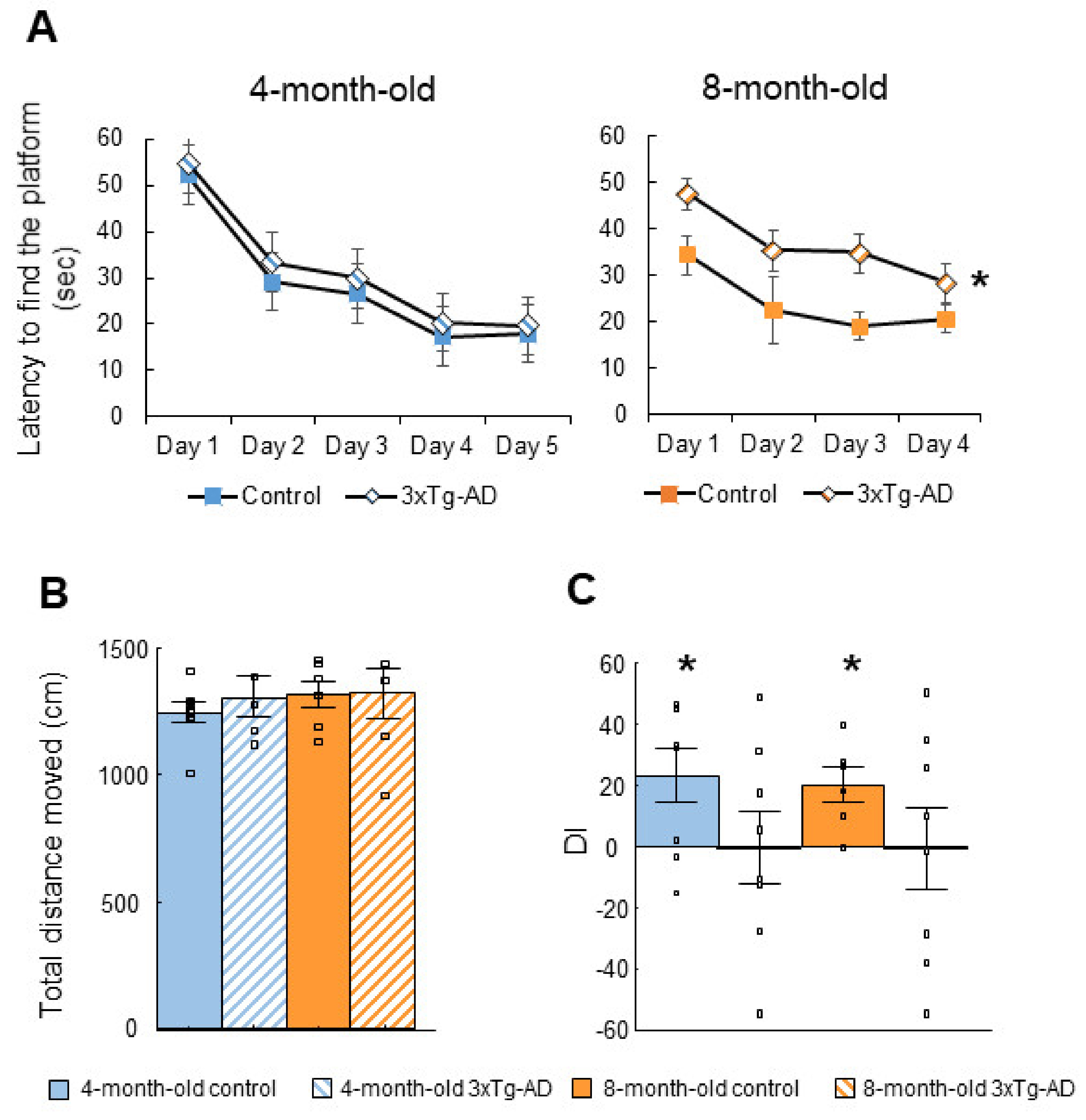
2.1. Cognitive Tests
2.1.1. Morris Water Maze (MWM) Test
2.1.2. Social Discrimination (SD) Test
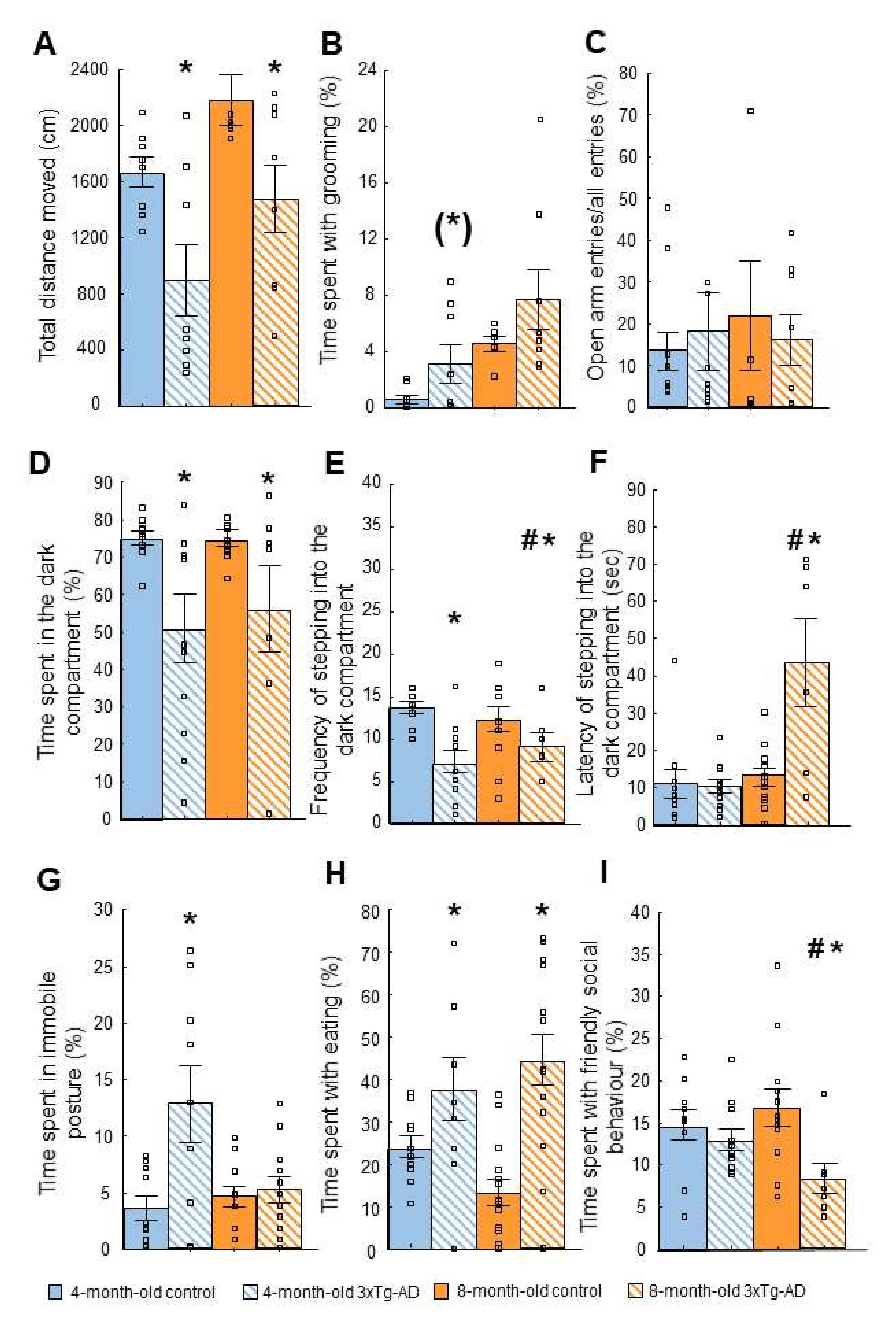
2.2. Tests of Anxiety-Like Behavior
2.2.1. Open Field (OF) Test
2.2.2. Elevated Plus Maze (EPM) Test
2.2.3. Light-Dark (LD) Test
2.2.4. Novelty Suppressed Feeding (NSF) Test
2.2.5. Social Interaction (SI) Test
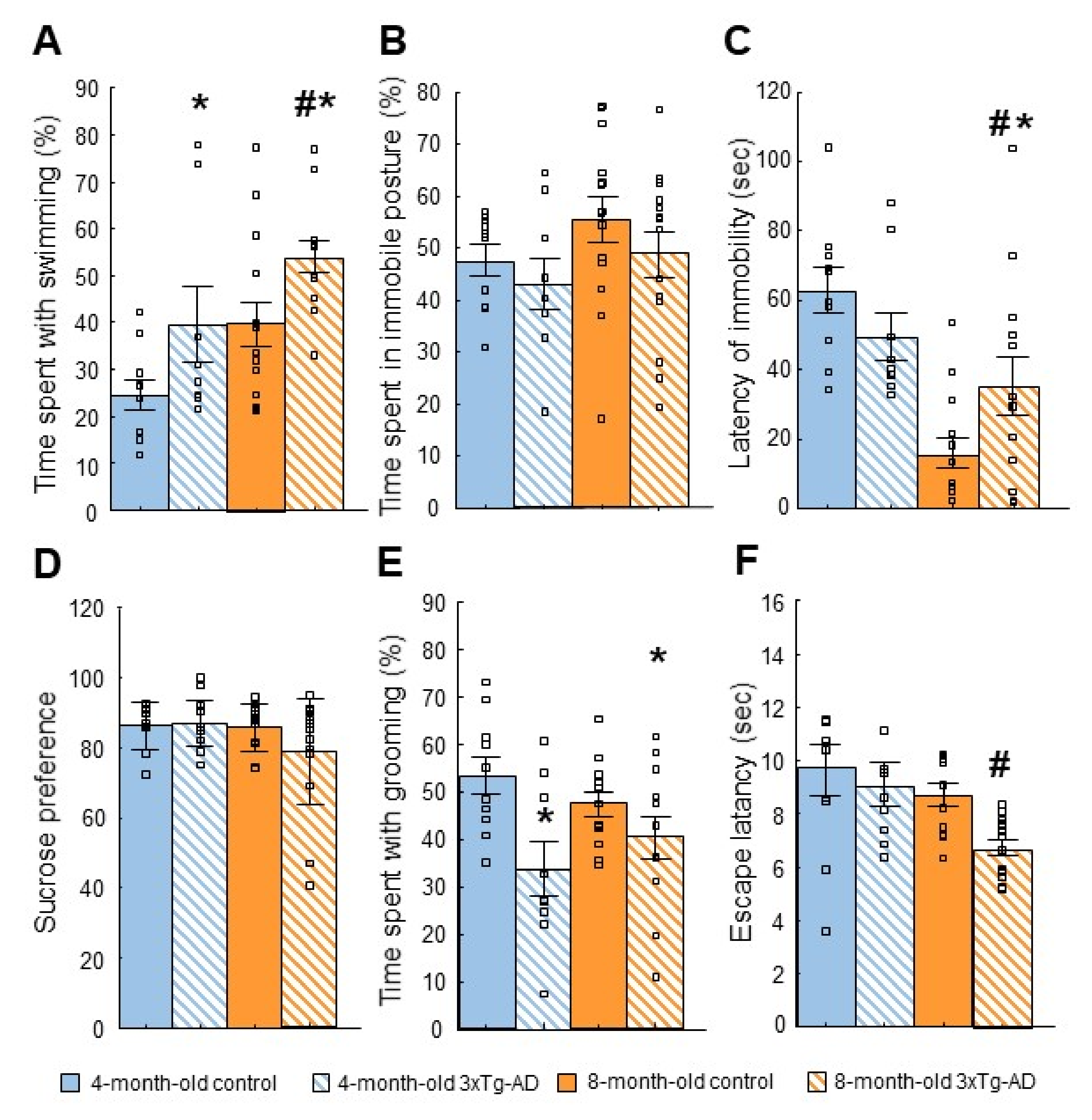
2.3. Tests of Depressive-like Behavior
2.3.1. Forced Swim Test (FST)
2.3.2. Tail Suspension Test (TST)
2.3.3. Sucrose Preference Test (SPT)
2.3.4. Splash Test
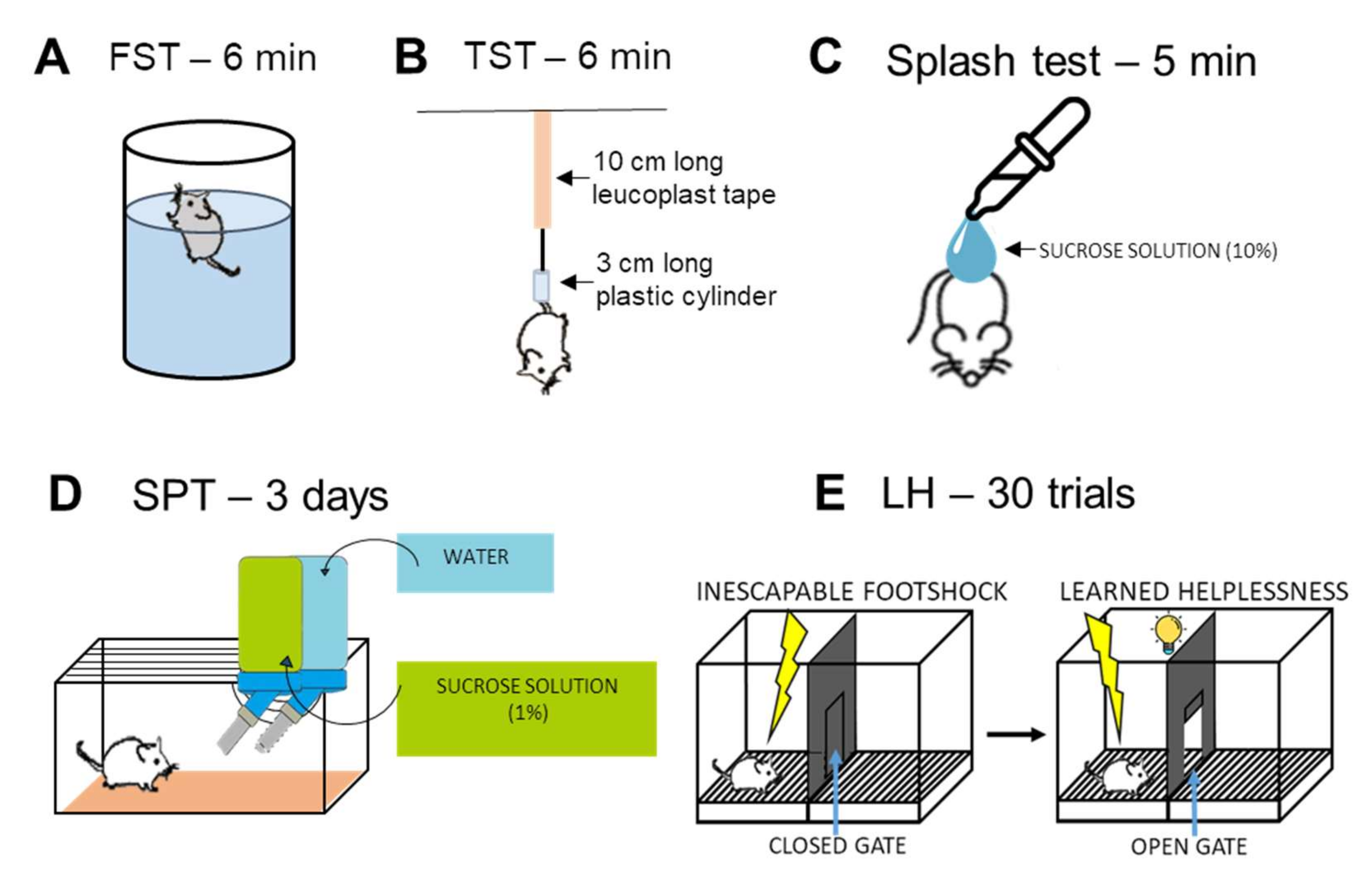
2.3.5. Learned Helplessness (LH) Test
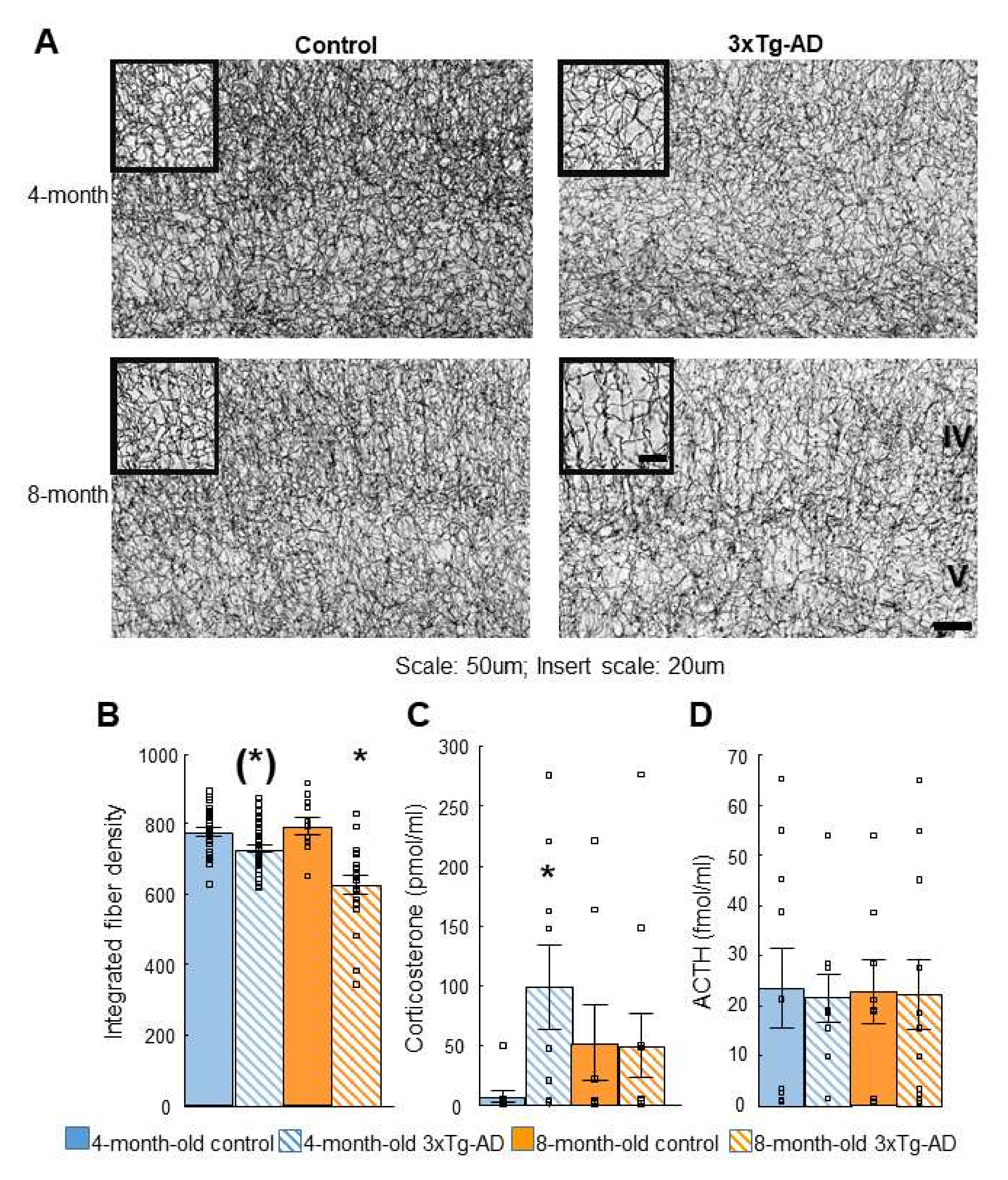
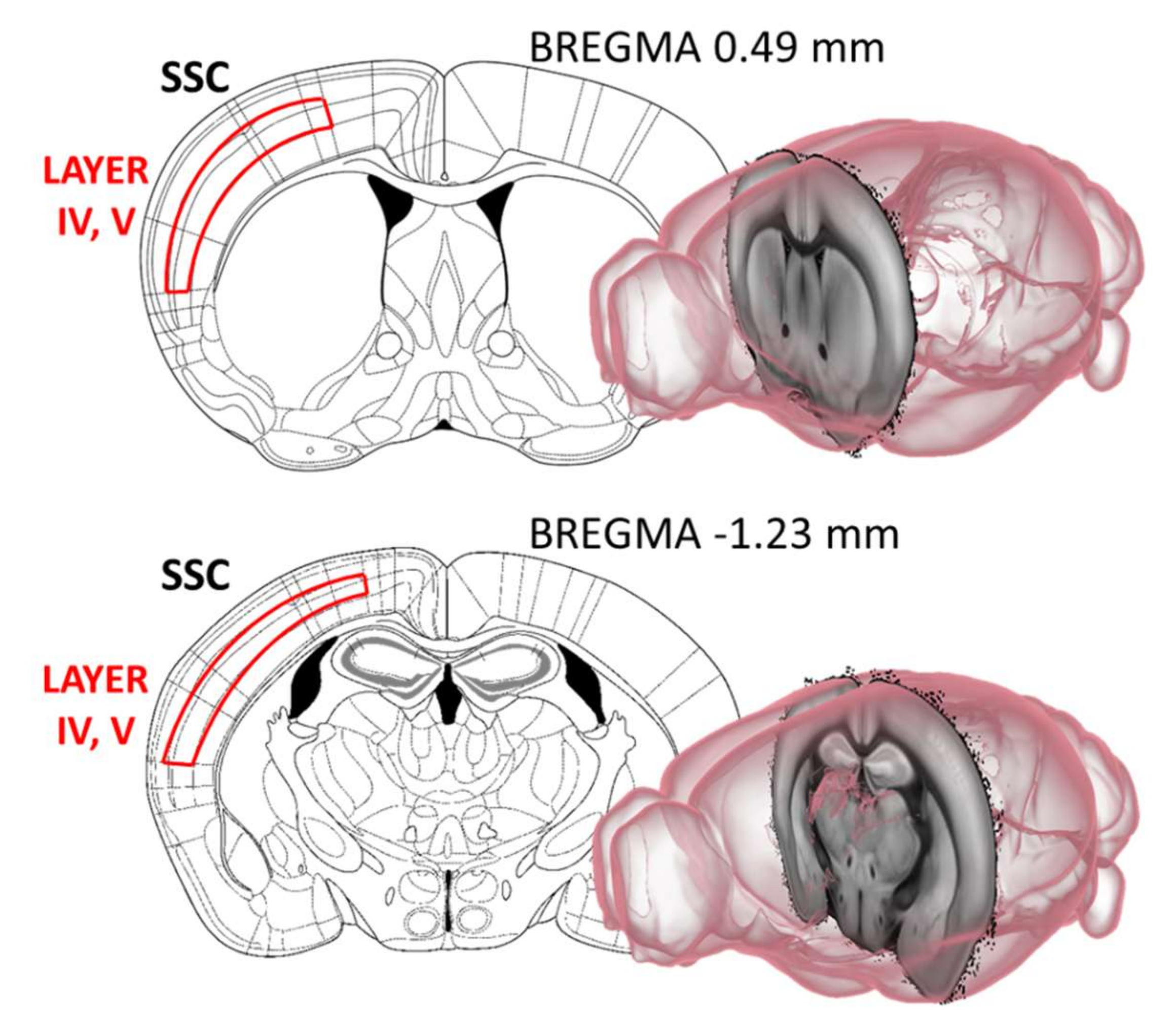
2.4. Acetylcholinesterase (AChE) Immunohistochemistry
2.5. Dexamethasone Suppression Test
3. Discussion
3.1. Cognitive Tests
3.2. Anxiety-like Behavior
3.3. Depression-like Behavior
3.4. Acetylcholinesterase (AChE) Immunohistochemistry
3.5. Dexamethasone Suppression
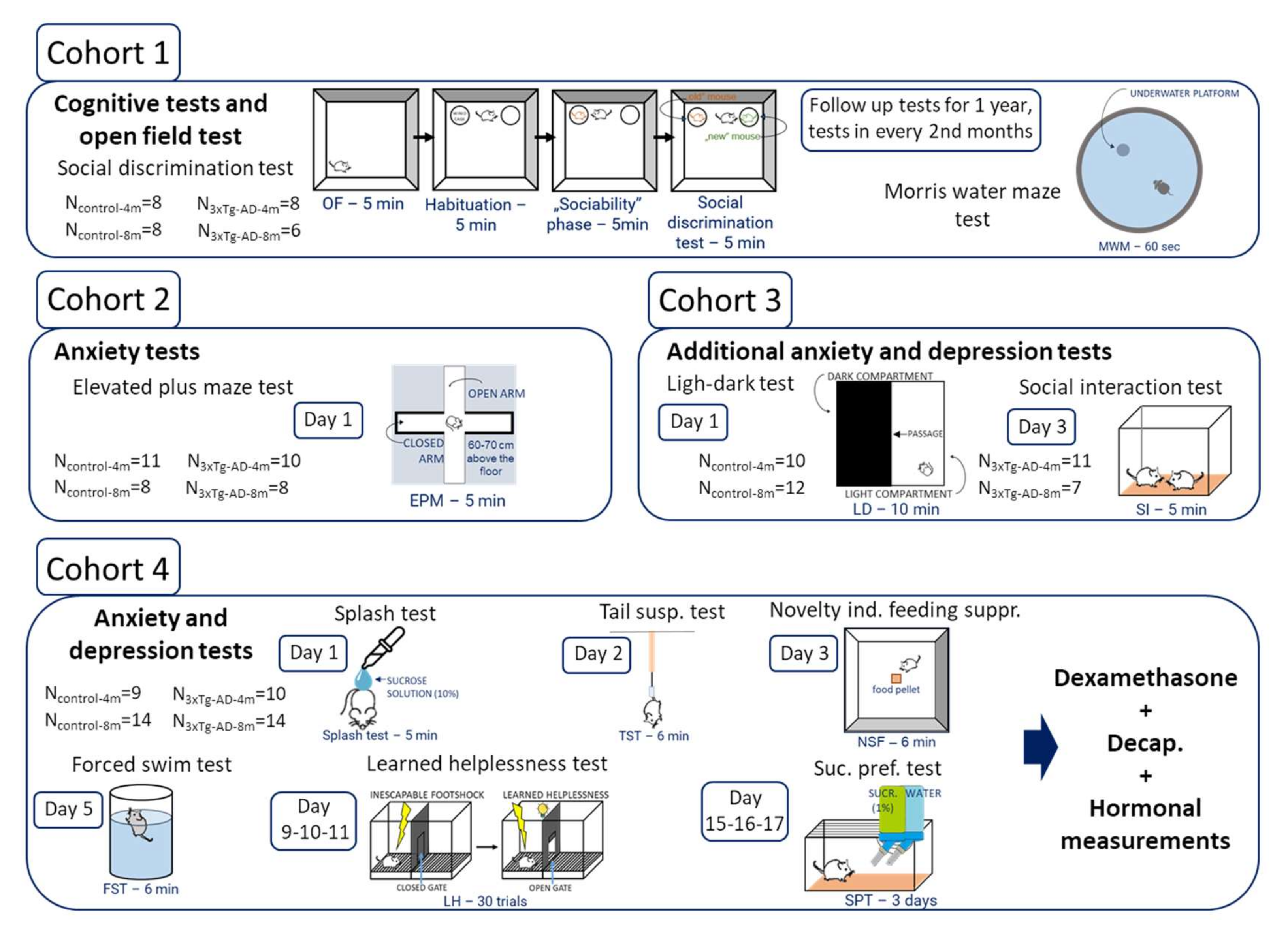
4. Materials and Methods
4.1. Animals
4.2. Behavioral Tests
4.2.1. Cognitive Tests
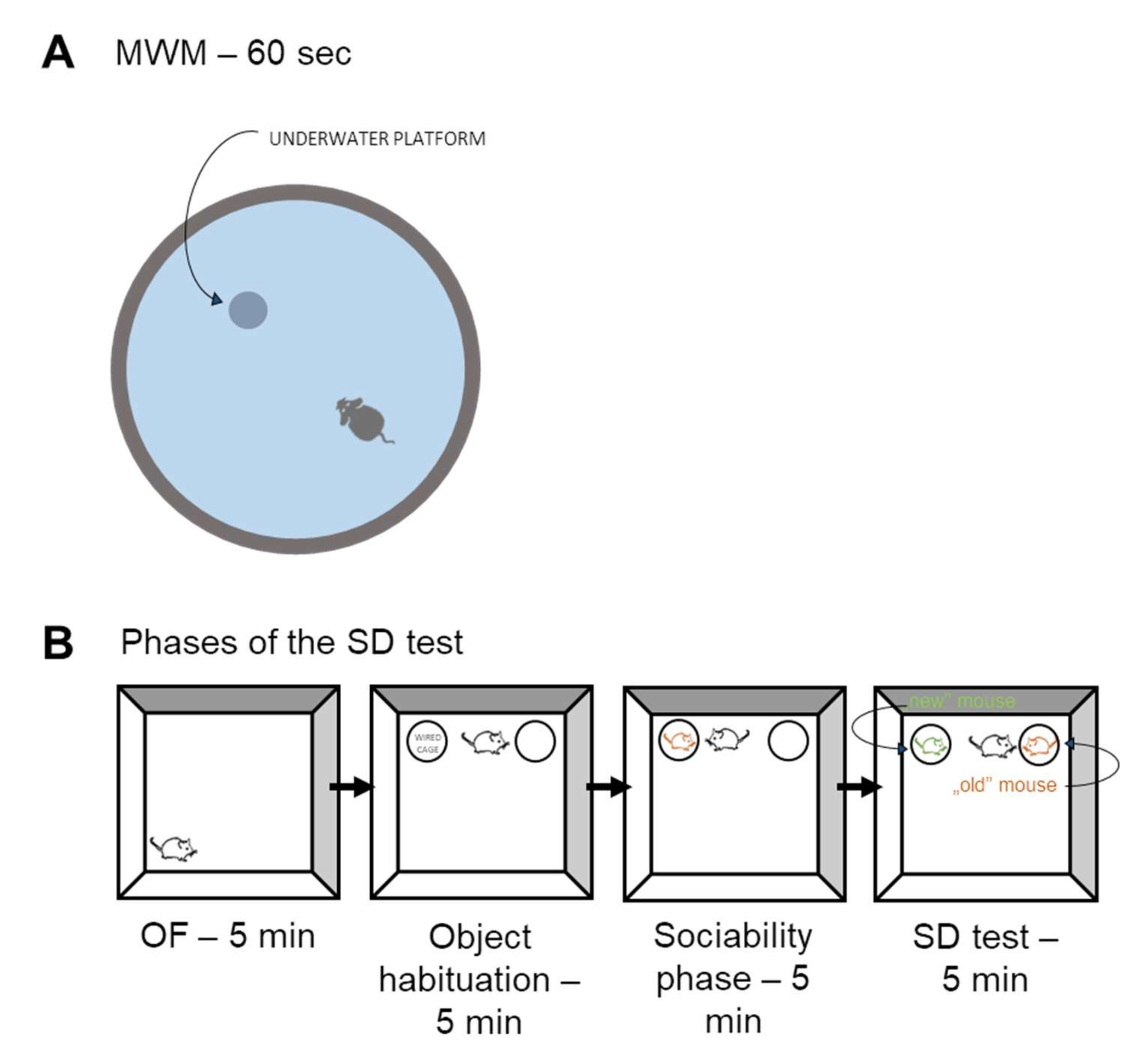
Morris Water Maze (MWM) Test
Social Discrimination (SD) Test
4.2.2. Tests of Anxiety-Like Behavior
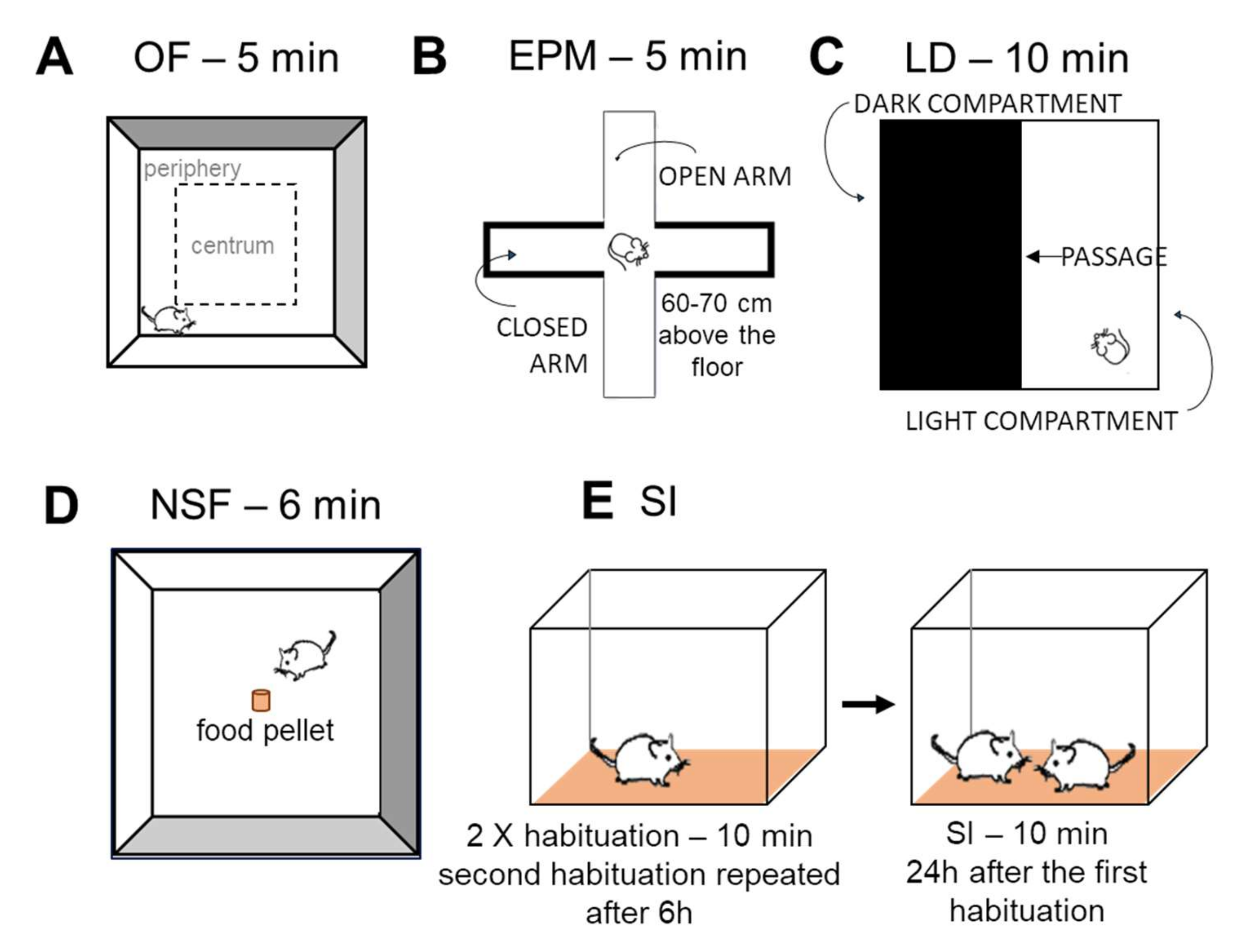
Open Field (OF) Test
Elevated Plus Maze (EPM)
Light-Dark (LD) Test
Novelty Suppressed Feeding (NSF) Test
Social Interaction (SI) Test
4.2.3. Tests of Depression-like Behavior
Forced Swim Test (FST)
Tail Suspension Test (TST)
Splash Test
Sucrose Preference Test (SPT)
4.3. Immunohistochemistry
4.3.1. Cholinergic Fiber Labelling in the Somatosensory and Motor Cortex
4.3.2. Analysis of Histological Data
4.4. Dexamethasone Suppression Test
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Test | Main Finding in 3xTg-AD Mice Compared to Controls | Age of the Mice (Months) | Author(s) |
| Morris Water Maze (MWM) | normal | 2 | [15] |
| no learning and memory impairment, reduced cognitive flexibility, higher speed | 6 | [30] | |
| no circadian variation in performance | [31] | ||
| no proper control | [55] | ||
| impaired performance | [56,57] | ||
| no difference | 7 | [58] | |
| learning impairment | 4 and 7 | [59] | |
| learning impairment | 8 | [60] | |
| longer swimming paths during learning | 10 and 17 | [61] | |
| impaired performance | 10 10 | [62] | |
| same learning curve | [63] | ||
| Social discrimination test (SD) | no significant difference no proper control group | 6 | [17] [64] |
| Open field (OF) | altered thigmotaxis with reduction of horizontal and vertical activities for 2 months | 2, 4 and 6 | [29] |
| impaired locomotion | 3, 6, 9, 12 | [21] | |
| no difference (4 m) less horizontal activity (7 m) | 4 and 7 | [59] | |
| less movement | [30] | ||
| more time to entering periphery | 6 | [37] | |
| less time in the center and same distance travelled | [20] | ||
| more freezing and bizarre behaviors | [65] | ||
| less activity | 7 | [62] | |
| less activity in both ages | 10 and 17 | [66] | |
| less distance travel for all ages | 12 and 18 | [27] | |
| less total distance walked | 12 | [29] | |
| less rearing behaviors more time in the periphery | 18 | [67] | |
| Elevated Plus Maze (EPM) | more time in the open arms | 3, 6, 9 and 12 | [21] |
| decreased number of entries and less time spend in the open arms; no difference in the total distance travel | 6 | [20] | |
| more latency to move | [37] | ||
| less time spent in the open arms | 9 | [22] | |
| no proper control | 18 | [68] | |
| Light-dark box (LD) | less time spent entering and less distance traveled in the light compartment | 6 | [20] |
| more freezing time in the light compartment | [57] | ||
| more time in the light compartment lower latency in entering the dark | [65] | ||
| more latency to enter the light compartment (7 m) | 4 and 7 | [59] | |
| lower latency of stretch attendance (7 m) | 7 and 11 | [65] | |
| same time spent in the light compartment frequency of changing compartments was lower | 20 | [23] | |
| Novelty suppressed feeding (NSF) | no data available | - | - |
| decreased social interaction | 9 | [24] | |
| Social interaction (SI) | increased social interaction (18m) | 12 and 18 | [27] |
| decreased social interaction | 12 and 14 | [25] | |
| Forced swim test (FST) | more time immobile | 11 | [69] |
| less time immobile and more time in swimming | 12 | [28] | |
| more time immobile | 18 | [67] | |
| Tail suspension test (TST) | more time spent in immobility | 18 | [67] |
| Sucrose preference test (SPT) | decreased sucrose preference | 9 | [22] |
| decreased sucrose preference | 18 | [67] | |
| Splash test | no data available | - | - |
| Learned helplessness (LH) | no data available | - | - |
References
- Novais, F.; Starkstein, S. Phenomenology of Depression in Alzheimer’s Disease. J. Alzheimer’s Dis. 2015, 47, 845–855. [Google Scholar] [CrossRef]
- Tsuno, N.; Homma, A. What is the association between depression and Alzheimer’s disease? Expert Rev. Neurother. 2009, 9, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.F.; Tan, L.; Wang, H.F.; Jiang, T.; Tan, M.S.; Tan, L.; Xu, W.; Li, J.Q.; Wang, J.; Lai, T.J.; et al. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: Systematic review and meta-analysis. J. Affect. Disord. 2016, 190, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Mitic, M.; Lazarevic-Pasti, T. Does the application of acetylcholinesterase inhibitors in the treatment of Alzheimer’s disease lead to depression? Expert Opin. Drug Metab. Toxicol. 2021, 17, 841–856. [Google Scholar] [CrossRef] [PubMed]
- Oddo, S.; Caccamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; LaFerla, F.M. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular Abeta and synaptic dysfunction. Neuron 2003, 39, 409–421. [Google Scholar] [CrossRef]
- Belfiore, R.; Rodin, A.; Ferreira, E.; Velazquez, R.; Branca, C.; Caccamo, A.; Oddo, S. Temporal and regional progression of Alzheimer’s disease-like pathology in 3xTg-AD mice. Aging Cell 2019, 18, e12873. [Google Scholar] [CrossRef]
- Stover, K.R.; Campbell, M.A.; Van Winssen, C.M.; Brown, R.E. Early detection of cognitive deficits in the 3xTg-AD mouse model of Alzheimer’s disease. Behav. Brain Res. 2015, 289, 29–38. [Google Scholar] [CrossRef]
- Sterniczuk, R.; Antle, M.C.; Laferla, F.M.; Dyck, R.H. Characterization of the 3xTg-AD mouse model of Alzheimer’s disease: Part 2. Behavioral and cognitive changes. Brain Res. 2010, 1348, 149–155. [Google Scholar] [CrossRef]
- Bromley-Brits, K.; Deng, Y.; Song, W. Morris water maze test for learning and memory deficits in Alzheimer’s disease model mice. J. Vis. Exp. 2011, 20, 2920. [Google Scholar] [CrossRef]
- Scearce-Levie, K. Monitoring spatial learning and memory in Alzheimer’s disease mouse models using the Morris Water Maze. Methods Mol. Biol. 2011, 670, 191–205. [Google Scholar]
- Plotsky, P.M.; Owens, M.J.; Nemeroff, C.B. Psychoneuroendocrinology of depression. Hypothalamic-pituitary-adrenal axis. Psychiatr. Clin. N. Am. 1998, 21, 293–307. [Google Scholar] [CrossRef]
- Barden, N. Implication of the hypothalamic-pituitary-adrenal axis in the physiopathology of depression. J. Psychiatry Neurosci. 2004, 29, 185–193. [Google Scholar] [PubMed]
- Evanson, N.K.; Tasker, J.G.; Hill, M.N.; Hillard, C.J.; Herman, J.P. Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology 2010, 151, 4811–4819. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.C.; Davis, J.M. DST studies in psychotic depression: A meta-analysis. Am. J. Psychiatry 1997, 154, 1497–1503. [Google Scholar] [CrossRef]
- Billings, L.M.; Oddo, S.; Green, K.N.; McGaugh, J.L.; LaFerla, F.M. Intraneuronal Abeta causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron 2005, 45, 675–688. [Google Scholar] [CrossRef]
- Mitrano, D.A.; Houle, S.E.; Pearce, P.; Quintanilla, R.M.; Lockhart, B.K.; Genovese, B.C.; Schendzielos, R.A.; Croushore, E.E.; Dymond, E.M.; Bogenpohl, J.W.; et al. Olfactory dysfunction in the 3xTg-AD model of Alzheimer’s disease. IBRO Neurosci. Rep. 2021, 10, 51–61. [Google Scholar] [CrossRef]
- Nguyen, E.T.; Selmanovic, D.; Maltry, M.; Morano, R.; Franco-Villanueva, A.; Estrada, C.M.; Solomon, M.B. Endocrine stress responsivity and social memory in 3xTg-AD female and male mice: A tale of two experiments. Horm. Behav. 2020, 126, 104852. [Google Scholar] [CrossRef]
- Pavisic, I.M.; Nicholas, J.M.; Pertzov, Y.; O’Connor, A.; Liang, Y.; Collins, J.D.; Lu, K.; Weston, P.S.J.; Ryan, N.S.; Husain, M.; et al. Visual short-term memory impairments in presymptomatic familial Alzheimer’s disease: A longitudinal observational study. Neuropsychologia 2021, 162, 108028. [Google Scholar] [CrossRef]
- Heinz, D.E.; Schottle, V.A.; Nemcova, P.; Binder, F.P.; Ebert, T.; Domschke, K.; Wotjak, C.T. Exploratory drive, fear, and anxiety are dissociable and independent components in foraging mice. Transl. Psychiatry 2021, 11, 318. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Xing, R.Z.; Luo, X.B.; Xu, H.; Chang, R.C.; Zou, L.Y.; Liu, J.J.; Yang, X.F. Anxiety-like behavior and dysregulation of miR-34a in triple transgenic mice of Alzheimer’s disease. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2853–2862. [Google Scholar]
- Pairojana, T.; Phasuk, S.; Suresh, P.; Huang, S.P.; Pakaprot, N.; Chompoopong, S.; Hsieh, T.C.; Liu, I.Y. Age and gender differences for the behavioral phenotypes of 3xTg alzheimer’s disease mice. Brain Res. 2021, 1762, 147437. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cao, X.; Yin, J.; Gao, W.R.; Li, W.R.; Qi, J.S.; Wu, M.N. Chronic sleep deprivation exacerbates cognitive and pathological impairments in APP/PS1/tau triple transgenic Alzheimer’s disease model mice. Sheng Li Xue Bao 2021, 73, 471–481. [Google Scholar] [PubMed]
- Arsenault, D.; Dal-Pan, A.; Tremblay, C.; Bennett, D.A.; Guitton, M.J.; De Koninck, Y.; Tonegawa, S.; Calon, F. PAK inactivation impairs social recognition in 3xTg-AD Mice without increasing brain deposition of tau and Abeta. J. Neurosci. 2013, 33, 10729–10740. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Lin, Y.F.; Chen, K.C.; Yang, Y.K.; Hsiao, Y.H. Collapsin response mediator protein 5 (CRMP5) causes social deficits and accelerates memory loss in an animal model of Alzheimer’s disease. Neuropharmacology 2019, 157, 107673. [Google Scholar] [CrossRef]
- Torres-Lista, V.; Lopez-Pousa, S.; Gimenez-Llort, L. Impact of Chronic Risperidone Use on Behavior and Survival of 3xTg-AD Mice Model of Alzheimer’s Disease and Mice With Normal Aging. Front. Pharmacol. 2019, 10, 1061. [Google Scholar] [CrossRef]
- Torres-Lista, V.; Gimenez-Llort, L. Vibrating Tail, Digging, Body/Face Interaction, and Lack of Barbering: Sex-Dependent Behavioral Signatures of Social Dysfunction in 3xTg-AD Mice as Compared to Mice with Normal Aging. J. Alzheimer’s Dis. 2019, 69, 969–977. [Google Scholar] [CrossRef]
- Bories, C.; Guitton, M.J.; Julien, C.; Tremblay, C.; Vandal, M.; Msaid, M.; De Koninck, Y.; Calon, F. Sex-dependent alterations in social behaviour and cortical synaptic activity coincide at different ages in a model of Alzheimer’s disease. PLoS ONE 2012, 7, e46111. [Google Scholar] [CrossRef]
- Torres-Lista, V.; Gimenez-Llort, L. Persistence of behaviours in the Forced Swim Test in 3xTg-AD mice at advanced stages of disease. Behav. Process. 2014, 106, 118–121. [Google Scholar] [CrossRef]
- Torres-Lista, V.; Parrado-Fernandez, C.; Alvarez-Monton, I.; Frontinan-Rubio, J.; Duran-Prado, M.; Peinado, J.R.; Johansson, B.; Alcain, F.J.; Gimenez-Llort, L. Neophobia, NQO1 and SIRT1 as premorbid and prodromal indicators of AD in 3xTg-AD mice. Behav. Brain Res. 2014, 271, 140–146. [Google Scholar] [CrossRef]
- Suresh, P.; Jasmin, S.; Yen, Y.; Hsu, H.J.; Varinthra, P.; Pairojana, T.; Chen, C.C.; Liu, I.Y. Attenuation of HECT-E3 ligase expression rescued memory deficits in 3xTg-AD mice. Front. Aging Neurosci. 2022, 14, 916904. [Google Scholar] [CrossRef]
- Bachli, H.; Steiner, M.A.; Habersetzer, U.; Wotjak, C.T. Increased water temperature renders single-housed C57BL/6J mice susceptible to antidepressant treatment in the forced swim test. Behav. Brain Res. 2008, 187, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Tournissac, M.; Bourassa, P.; Martinez-Cano, R.D.; Vu, T.M.; Hebert, S.S.; Planel, E.; Calon, F. Repeated cold exposures protect a mouse model of Alzheimer’s disease against cold-induced tau phosphorylation. Mol. Metab. 2019, 22, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Y.; Yin, C.Y.; Zhu, L.J.; Zhu, X.H.; Xu, C.; Luo, C.X.; Chen, H.; Zhu, D.Y.; Zhou, Q.G. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat. Protoc. 2018, 13, 1686–1698. [Google Scholar] [CrossRef] [PubMed]
- Falangola, M.F.; Nie, X.; Ward, R.; Dhiman, S.; Voltin, J.; Nietert, P.J.; Jensen, J.H. Diffusion MRI detects basal forebrain cholinergic abnormalities in the 3xTg-AD mouse model of Alzheimer’s disease. Magn. Reson. Imaging 2021, 83, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Clinton, L.K.; Billings, L.M.; Green, K.N.; Caccamo, A.; Ngo, J.; Oddo, S.; McGaugh, J.L.; LaFerla, F.M. Age-dependent sexual dimorphism in cognition and stress response in the 3xTg-AD mice. Neurobiol. Dis. 2007, 28, 76–82. [Google Scholar] [CrossRef]
- Arranz, L.; De Castro, N.M.; Baeza, I.; Gimenez-Llort, L.; De la Fuente, M. Effect of environmental enrichment on the immunoendocrine aging of male and female triple-transgenic 3xTg-AD mice for Alzheimer’s disease. J. Alzheimer’s Dis. 2011, 25, 727–737. [Google Scholar] [CrossRef]
- Hebda-Bauer, E.K.; Simmons, T.A.; Sugg, A.; Ural, E.; Stewart, J.A.; Beals, J.L.; Wei, Q.; Watson, S.J.; Akil, H. 3xTg-AD mice exhibit an activated central stress axis during early-stage pathology. J. Alzheimer’s Dis. 2013, 33, 407–422. [Google Scholar] [CrossRef]
- Fazekas, C.L.; Balazsfi, D.; Horvath, H.R.; Balogh, Z.; Aliczki, M.; Puhova, A.; Balagova, L.; Chmelova, M.; Jezova, D.; Haller, J.; et al. Consequences of VGluT3 deficiency on learning and memory in mice. Physiol. Behav. 2019, 212, 112688. [Google Scholar] [CrossRef]
- Fazekas, C.L.; Bellardie, M.; Torok, B.; Sipos, E.; Toth, B.; Baranyi, M.; Sperlagh, B.; Dobos-Kovacs, M.; Chaillou, E.; Zelena, D. Pharmacogenetic excitation of the median raphe region affects social and depressive-like behavior and core body temperature in male mice. Life Sci. 2021, 286, 120037. [Google Scholar] [CrossRef]
- Chaves, T.; Torok, B.; Fazekas, C.L.; Correia, P.; Sipos, E.; Varkonyi, D.; Hellinger, A.; Erk, D.; Zelena, D. Median raphe region GABAergic neurons contribute to social interest in mouse. Life Sci. 2022, 289, 120223. [Google Scholar] [CrossRef]
- Crawley, J.; Goodwin, F.K. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol. Biochem. Behav. 1980, 13, 167–170. [Google Scholar] [CrossRef]
- Francois, M.; Canal Delgado, I.; Shargorodsky, N.; Leu, C.S.; Zeltser, L. Assessing the effects of stress on feeding behaviors in laboratory mice. eLife 2022, 11, e70271. [Google Scholar] [CrossRef] [PubMed]
- Powell, T.R.; Fernandes, C.; Schalkwyk, L.C. Depression-Related Behavioral Tests. Curr. Protoc. Mouse Biol. 2012, 2, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Can, A.; Dao, D.T.; Terrillion, C.E.; Piantadosi, S.C.; Bhat, S.; Gould, T.D. The tail suspension test. J. Vis. Exp. 2012, 59, e3769. [Google Scholar] [CrossRef]
- Isingrini, E.; Camus, V.; Le Guisquet, A.M.; Pingaud, M.; Devers, S.; Belzung, C. Association between repeated unpredictable chronic mild stress (UCMS) procedures with a high fat diet: A model of fluoxetine resistance in mice. PLoS ONE 2010, 5, e10404. [Google Scholar] [CrossRef] [PubMed]
- Palucha-Poniewiera, A.; Podkowa, K.; Rafalo-Ulinska, A.; Branski, P.; Burnat, G. The influence of the duration of chronic unpredictable mild stress on the behavioural responses of C57BL/6J mice. Behav. Pharmacol. 2020, 31, 574–582. [Google Scholar] [CrossRef]
- Otrokocsi, L.; Kittel, A.; Sperlagh, B. P2X7 Receptors Drive Spine Synapse Plasticity in the Learned Helplessness Model of Depression. Int. J. Neuropsychopharmacol. 2017, 20, 813–822. [Google Scholar] [CrossRef]
- Abraham, I.; Harkany, T.; Horvath, K.M.; Veenema, A.H.; Penke, B.; Nyakas, C.; Luiten, P.G. Chronic corticosterone administration dose-dependently modulates Abeta(1-42)- and NMDA-induced neurodegeneration in rat magnocellular nucleus basalis. J. Neuroendocrinol. 2000, 12, 486–494. [Google Scholar] [CrossRef]
- Hedreen, J.C.; Bacon, S.J.; Price, D.L. A modified histochemical technique to visualize acetylcholinesterase-containing axons. J. Histochem. Cytochem. 1985, 33, 134–140. [Google Scholar] [CrossRef]
- Harkany, T.; Grosche, J.; Mulder, J.; Horvath, K.M.; Keijser, J.; Hortobagyi, T.; Luiten, P.G.; Hartig, W. Short-term consequences of N-methyl-D-aspartate excitotoxicity in rat magnocellular nucleus basalis: Effects on in vivo labelling of cholinergic neurons. Neuroscience 2001, 108, 611–627. [Google Scholar] [CrossRef]
- Bakker, R.; Tiesinga, P.; Kotter, R. The Scalable Brain Atlas: Instant Web-Based Access to Public Brain Atlases and Related Content. Neuroinformatics 2015, 13, 353–366. [Google Scholar] [CrossRef]
- Avitsur, R.; Grinshpahet, R.; Goren, N.; Weinstein, I.; Kirshenboim, O.; Chlebowski, N. Prenatal SSRI alters the hormonal and behavioral responses to stress in female mice: Possible role for glucocorticoid resistance. Horm. Behav. 2016, 84, 41–49. [Google Scholar] [CrossRef]
- Torok, B.; Fodor, A.; Zsebok, S.; Sipos, E.; Zelena, D. The Effect of Vasopressin Antagonists on Maternal-Separation-Induced Ultrasonic Vocalization and Stress-Hormone Level Increase during the Early Postnatal Period. Brain Sci. 2021, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Carvalho da Silva, A.M.; Lemos, C.; Silva, H.B.; Ferreira, I.L.; Tome, A.R.; Rego, A.C.; Cunha, R.A. Simultaneous Alteration of the Circadian Variation of Memory, Hippocampal Synaptic Plasticity, and Metabolism in a Triple Transgenic Mouse Model of Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 835885. [Google Scholar] [CrossRef]
- Chen, T.F.; Lee, S.H.; Zheng, W.R.; Hsu, C.C.; Cho, K.H.; Kuo, L.W.; Chou, C.C.; Chiu, M.J.; Tee, B.L.; Cheng, T.J. White matter pathology in alzheimer’s transgenic mice with chronic exposure to low-level ambient fine particulate matter. Part. Fibre Toxicol. 2022, 19, 44. [Google Scholar] [CrossRef] [PubMed]
- Bareiss, S.K.; Johnston, T.; Lu, Q.; Tran, T.D. The effect of exercise on early sensorimotor performance alterations in the 3xTg-AD model of Alzheimer’s disease. Neurosci. Res. 2022, 178, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Espana, J.; Gimenez-Llort, L.; Valero, J.; Minano, A.; Rabano, A.; Rodriguez-Alvarez, J.; LaFerla, F.M.; Saura, C.A. Intraneuronal beta-amyloid accumulation in the amygdala enhances fear and anxiety in Alzheimer’s disease transgenic mice. Biol. Psychiatry 2010, 67, 513–521. [Google Scholar] [CrossRef]
- Gannon, O.J.; Robison, L.S.; Salinero, A.E.; Abi-Ghanem, C.; Mansour, F.M.; Kelly, R.D.; Tyagi, A.; Brawley, R.R.; Ogg, J.D.; Zuloaga, K.L. High-fat diet exacerbates cognitive decline in mouse models of Alzheimer’s disease and mixed dementia in a sex-dependent manner. J. Neuroinflamm. 2022, 19, 110. [Google Scholar] [CrossRef]
- Garcia-Mesa, Y.; Lopez-Ramos, J.C.; Gimenez-Llort, L.; Revilla, S.; Guerra, R.; Gruart, A.; Laferla, F.M.; Cristofol, R.; Delgado-Garcia, J.M.; Sanfeliu, C. Physical exercise protects against Alzheimer’s disease in 3xTg-AD mice. J. Alzheimer’s Dis. 2011, 24, 421–454. [Google Scholar] [CrossRef]
- Cao, H.; Zuo, C.; Gu, Z.; Huang, Y.; Yang, Y.; Zhu, L.; Jiang, Y.; Wang, F. High frequency repetitive transcranial magnetic stimulation alleviates cognitive deficits in 3xTg-AD mice by modulating the PI3K/Akt/GLT-1 axis. Redox Biol. 2022, 54, 102354. [Google Scholar] [CrossRef]
- Halagappa, V.K.; Guo, Z.; Pearson, M.; Matsuoka, Y.; Cutler, R.G.; Laferla, F.M.; Mattson, M.P. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol. Dis. 2007, 26, 212–220. [Google Scholar] [CrossRef]
- Nelson, R.L.; Guo, Z.; Halagappa, V.M.; Pearson, M.; Gray, A.J.; Matsuoka, Y.; Brown, M.; Martin, B.; Iyun, T.; Maudsley, S.; et al. Prophylactic treatment with paroxetine ameliorates behavioral deficits and retards the development of amyloid and tau pathologies in 3xTgAD mice. Exp. Neurol. 2007, 205, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Morin, J.P.; Ceron-Solano, G.; Velazquez-Campos, G.; Pacheco-Lopez, G.; Bermudez-Rattoni, F.; Diaz-Cintra, S. Spatial Memory Impairment is Associated with Intraneural Amyloid-beta Immunoreactivity and Dysfunctional Arc Expression in the Hippocampal-CA3 Region of a Transgenic Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2016, 51, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Barnett, A.; David, E.; Rohlman, A.; Nikolova, V.D.; Moy, S.S.; Vetreno, R.P.; Coleman, L.G., Jr. Adolescent Binge Alcohol Enhances Early Alzheimer’s Disease Pathology in Adulthood Through Proinflammatory Neuroimmune Activation. Front. Pharmacol. 2022, 13, 884170. [Google Scholar] [CrossRef]
- Baeta-Corral, R.; Gimenez-Llort, L. Bizarre behaviors and risk assessment in 3xTg-AD mice at early stages of the disease. Behav. Brain Res. 2014, 258, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Ramos, K.; Moreno-Castilla, P.; Castro-Cruz, M.; McGaugh, J.L.; Martinez-Coria, H.; LaFerla, F.M.; Bermudez-Rattoni, F. Restoration of dopamine release deficits during object recognition memory acquisition attenuates cognitive impairment in a triple transgenic mice model of Alzheimer’s disease. Learn. Mem. 2012, 19, 453–460. [Google Scholar] [CrossRef]
- Romano, A.; Pace, L.; Tempesta, B.; Lavecchia, A.M.; Macheda, T.; Bedse, G.; Petrella, A.; Cifani, C.; Serviddio, G.; Vendemiale, G.; et al. Depressive-like behavior is paired to monoaminergic alteration in a murine model of Alzheimer’s disease. Int. J. Neuropsychopharmacol. 2014, 18, pyu20. [Google Scholar] [CrossRef]
- Cross, D.J.; Huber, B.R.; Silverman, M.A.; Cline, M.M.; Gill, T.B.; Cross, C.G.; Cook, D.G.; Minoshima, S. Intranasal Paclitaxel Alters Alzheimer’s Disease Phenotypic Features in 3xTg-AD Mice. J. Alzheimer’s Dis. 2021, 83, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Wei, G.; Peng, S.; Qu, Z.; Yang, Y.; Yang, Q.; Huang, X.; Liu, J.; Zhuang, Z.; Yang, X. Melatonin ameliorates anxiety and depression-like behaviors and modulates proteomic changes in triple transgenic mice of Alzheimer’s disease. Biofactors 2017, 43, 593–611. [Google Scholar] [CrossRef]
| Test | Main Finding in 3xTg-AD Mice Compared to Controls | Interpretation |
|---|---|---|
| Morris water maze (MWM) | ↑ Latency to find the platform (8-m) | Progressive long-term memory loss |
| Social discrimination (SD) | 3xTg-AD DI did not differ from 0 | Short-term memory impairment |
| Open field (OF) | ↓ Time with active movement | Anxiety-like behavior |
| Elevated plus maze (EPM) | - | - |
| Light-dark box (LD) | ↓ Motivation to explore | Anxiety-like behavior |
| Novelty suppressed feeding (NSF) | ↑ Immobility | Anxiety-like behavior |
| Social interaction (SI) | ↓ Social behavior (8-m) | Progressive anxiety-like behavior |
| Forced swim test (FST) | ↑ Swimming | Stress coping impairment? |
| Tail suspension test (TST) | - | - |
| Sucrose preference test (SPT) | - | - |
| Splash | ↓ Grooming | Depression-like behavior |
| Learned helplessness (LH) | ↓ Escape latency | Stress-coping impairment? |
| Dexamethasone suppression | Non-suppression (4-m) | Depression-like behavior |
| AChE immunohistochemistry in cortex | ↓ Fiber density (8-m) | Progressive fiber density decrease |
| Test | 4-Month | 8-Month | ||
|---|---|---|---|---|
| 3xTg-AD | Control | 3xTg-AD | Control | |
| Morris Water Maze (MWM) | 8 | 8 | 6 | 8 |
| Social discrimination (SD) | 8 | 8 | 6 | 8 |
| Open field (OF) | 8 | 8 | 6 | 8 |
| Elevated plus maze (EPM) | 10 | 11 | 8 | 8 |
| Light-dark box (LD) | 11 | 10 | 7 | 12 |
| Novelty suppressed feeding (NSF) | 8 | 10 | 10 | 14 |
| Social interaction (SI) | 11 | 10 | 7 | 12 |
| Forced swim test (FST) | 8 | 10 | 13 | 14 |
| Tail suspension test (TST) | 9 | 10 | 14 | 14 |
| Splash | 9 | 10 | 12 | 13 |
| Sucrose preference test (SPT) | 9 | 10 | 14 | 14 |
| Learned helplessness (LH) | 9 | 10 | 14 | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Várkonyi, D.; Török, B.; Sipos, E.; Fazekas, C.L.; Bánrévi, K.; Correia, P.; Chaves, T.; Farkas, S.; Szabó, A.; Martínez-Bellver, S.; et al. Investigation of Anxiety- and Depressive-like Symptoms in 4- and 8-Month-Old Male Triple Transgenic Mouse Models of Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 10816. https://doi.org/10.3390/ijms231810816
Várkonyi D, Török B, Sipos E, Fazekas CL, Bánrévi K, Correia P, Chaves T, Farkas S, Szabó A, Martínez-Bellver S, et al. Investigation of Anxiety- and Depressive-like Symptoms in 4- and 8-Month-Old Male Triple Transgenic Mouse Models of Alzheimer’s Disease. International Journal of Molecular Sciences. 2022; 23(18):10816. https://doi.org/10.3390/ijms231810816
Chicago/Turabian StyleVárkonyi, Dorottya, Bibiána Török, Eszter Sipos, Csilla Lea Fazekas, Krisztina Bánrévi, Pedro Correia, Tiago Chaves, Szidónia Farkas, Adrienn Szabó, Sergio Martínez-Bellver, and et al. 2022. "Investigation of Anxiety- and Depressive-like Symptoms in 4- and 8-Month-Old Male Triple Transgenic Mouse Models of Alzheimer’s Disease" International Journal of Molecular Sciences 23, no. 18: 10816. https://doi.org/10.3390/ijms231810816
APA StyleVárkonyi, D., Török, B., Sipos, E., Fazekas, C. L., Bánrévi, K., Correia, P., Chaves, T., Farkas, S., Szabó, A., Martínez-Bellver, S., Hangya, B., & Zelena, D. (2022). Investigation of Anxiety- and Depressive-like Symptoms in 4- and 8-Month-Old Male Triple Transgenic Mouse Models of Alzheimer’s Disease. International Journal of Molecular Sciences, 23(18), 10816. https://doi.org/10.3390/ijms231810816







