Protective Effect of CXCR4 Antagonist DBPR807 against Ischemia-Reperfusion Injury in a Rat and Porcine Model of Myocardial Infarction: Potential Adjunctive Therapy for Percutaneous Coronary Intervention
Abstract
1. Introduction
2. Results
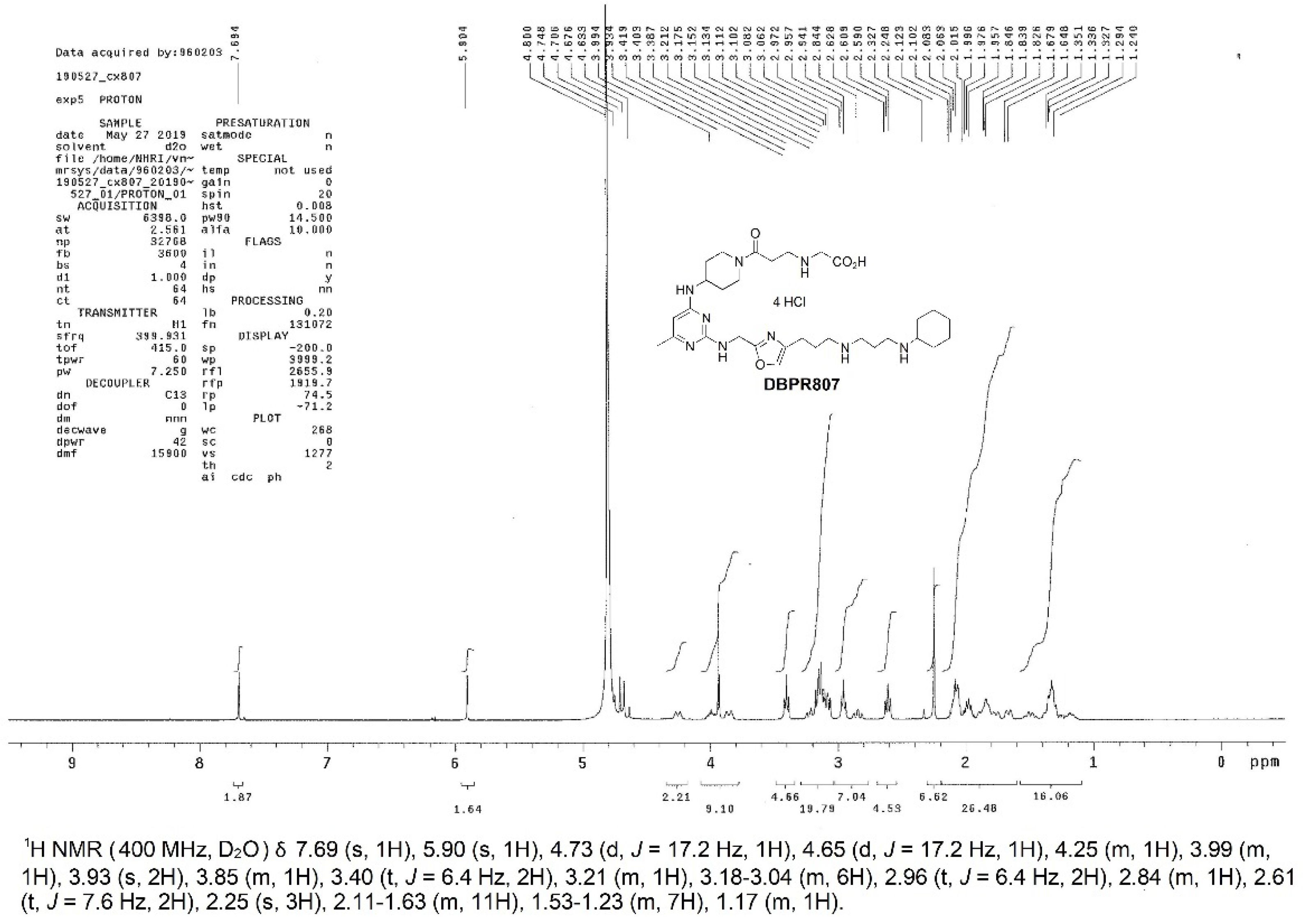
2.1. Synthesis, Characterization and Biological Activity of DBPR807
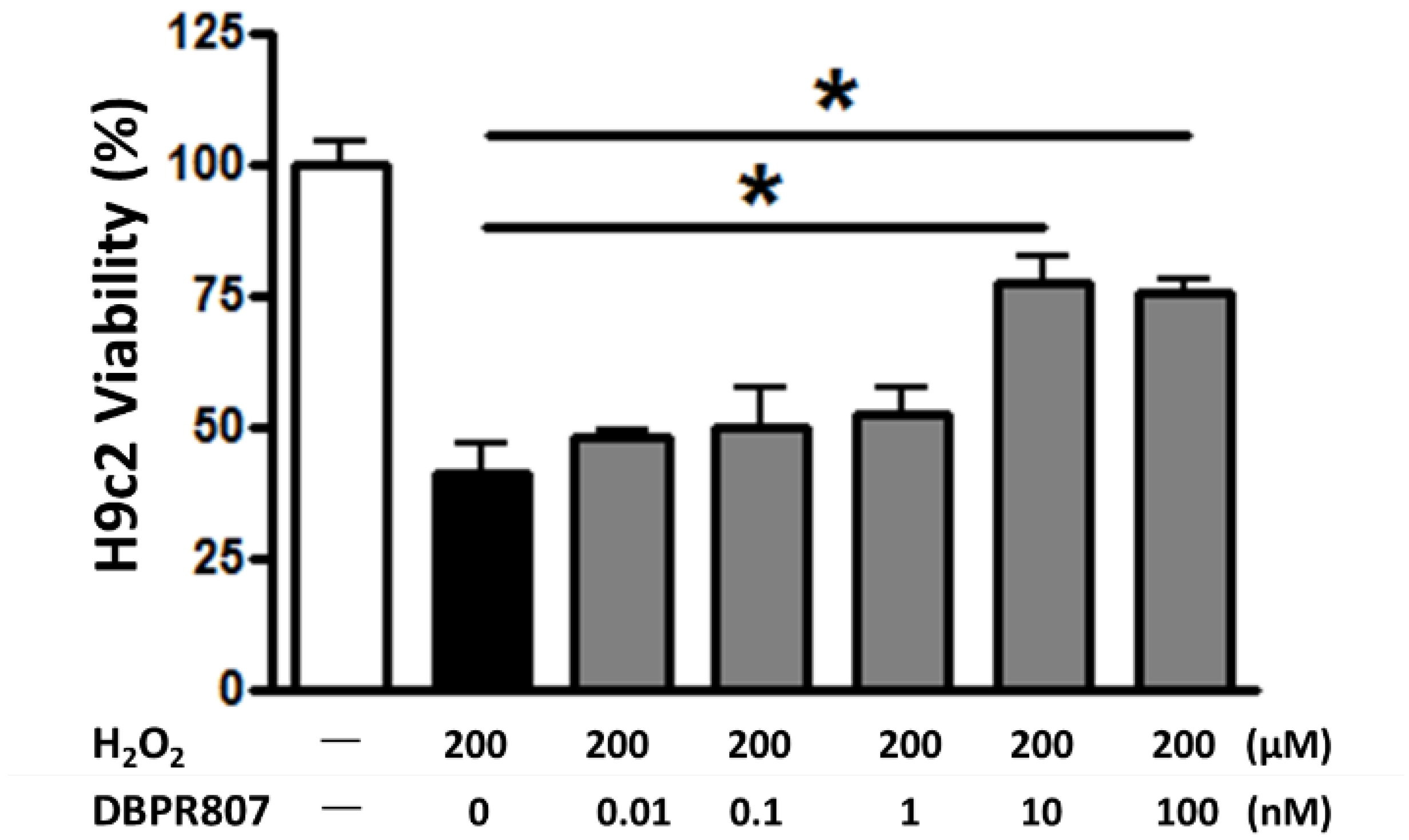
2.2. DBPR807 Reduced Damage from H2O2-Induced Oxidative Stress In Vitro
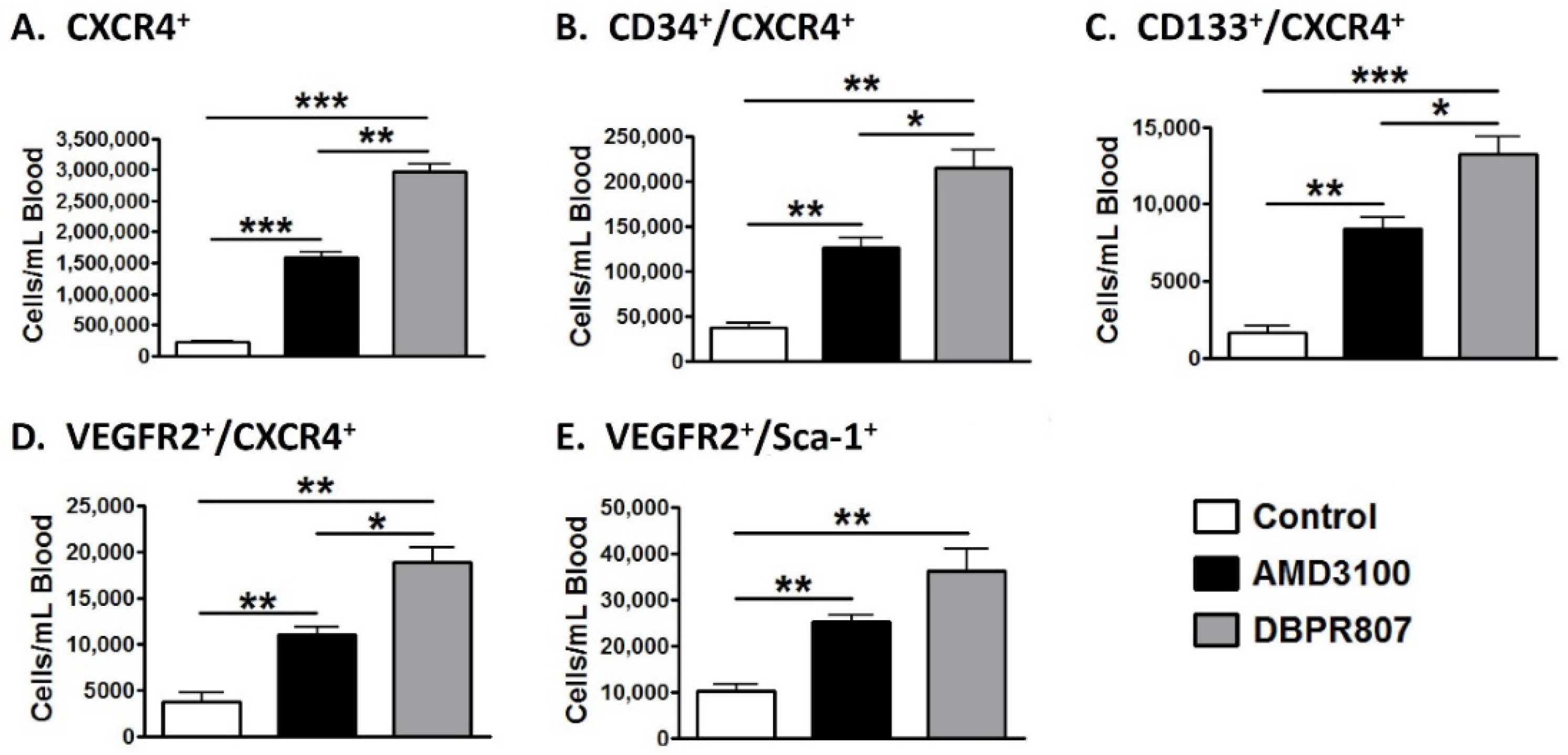
2.3. DBPR807 Mobilized CXCR4-Expressing Stem/Progenitors Cells in Mice
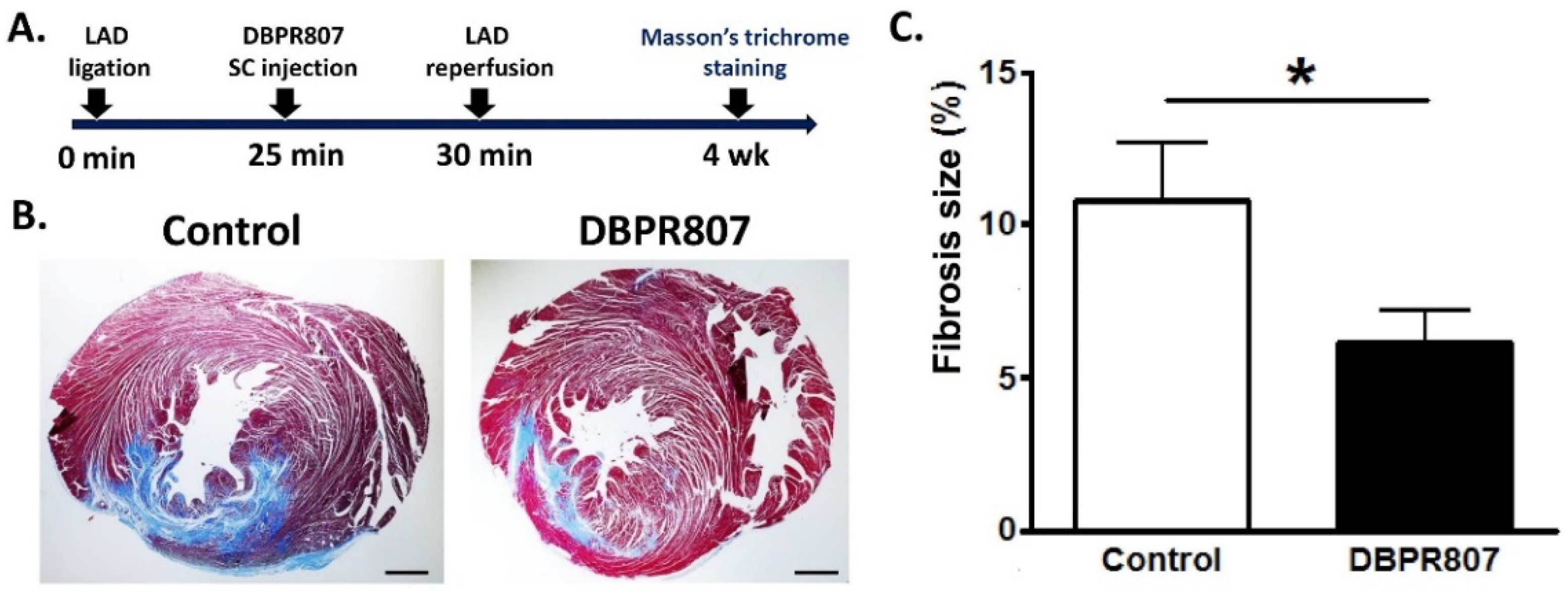
2.4. DBPR807 Alleviated Cardiac Damage after IRI in Rats
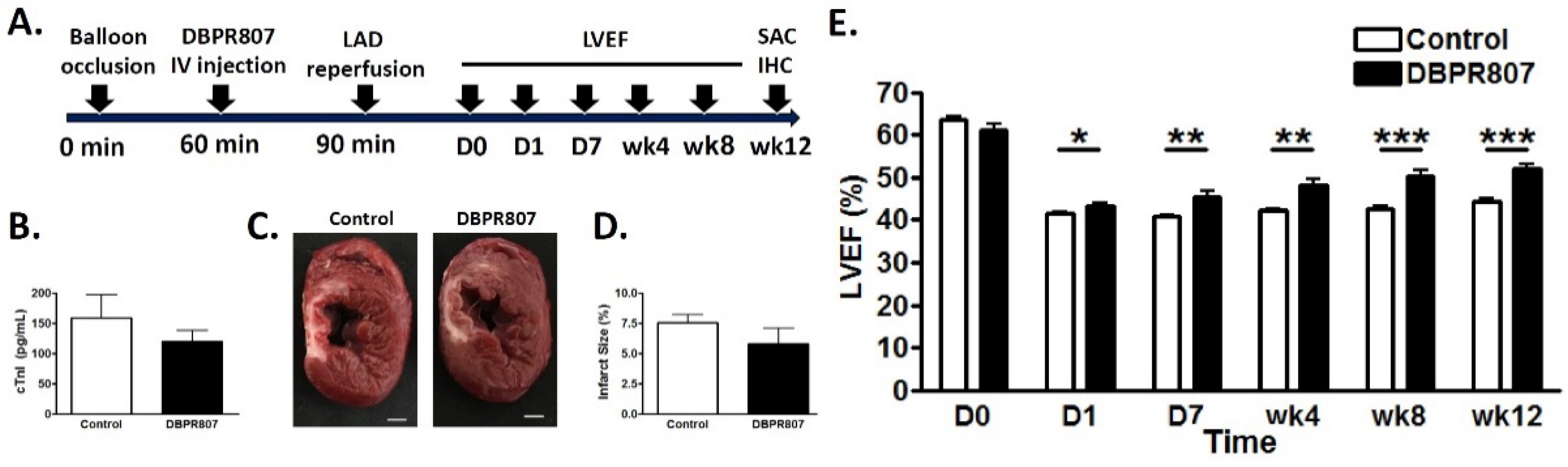
2.5. DBPR807 Ameliorated Recovery of Cardiac Function after IRI in Mini-Pigs
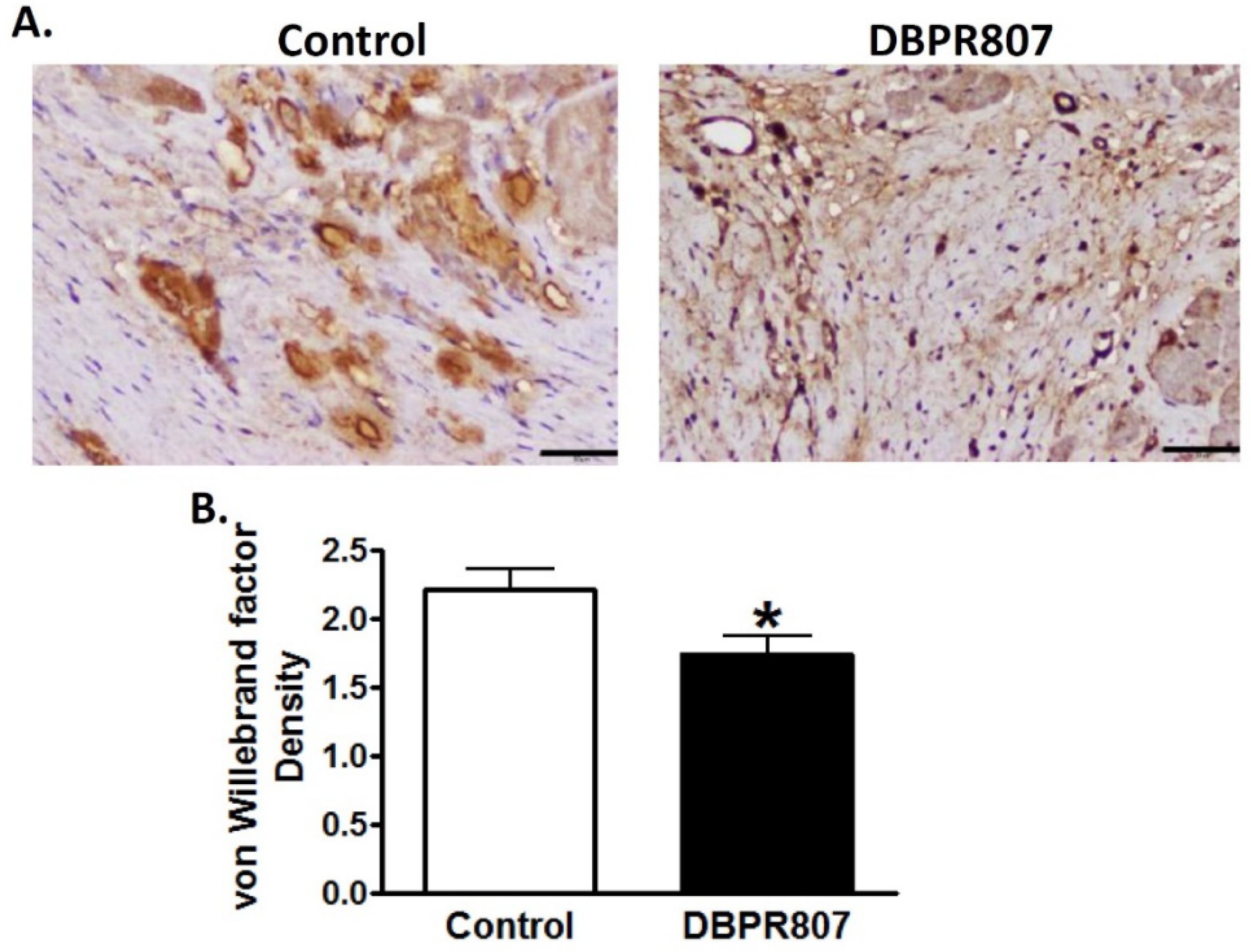
2.6. DBPR807 Preserved Density and Integrity of Capillary of Infarct Region after IRI in Mini-Pigs
2.7. Pharmacokinetic Studies on DBPR807 following IV and Administration SC
3. Discussion
4. Materials and Methods
4.1. Animals and Material
4.2. Cell Culture
4.3. H2O2-Induced Damage of Cardiac Myoblast Cells
4.4. Rat Model of Reperfused AMI
4.5. Histological Evaluation of the Infarct Size by TTC Staining in Rats
4.6. Histological Evaluation of the Fibrosis Size by Masson’s Trichrome Staining in Rats
4.7. Mini-Pig Model of Reperfused AMI
4.8. Determination of Blood Cardiac Troponin I in Mini-Pigs
4.9. Histological Evaluation of the Infarct Size by TTC Staining in Mini-Pigs
4.10. Determination of the LVEF in Mini-Pigs
4.11. Histological Evaluation of the Capillary Density and Integrity in Mini-Pigs
4.12. Pharmacokinetic and Toxicological Studies
4.13. Flow Cytometry Analysis
4.14. Statistics
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B

Appendix C

References
- Timmis, A.; Vardas, P.; Townsend, N.; Torbica, A.; Katus, H.; De Smedt, D.; Gale, C.P.; Maggioni, A.P.; Petersen, S.E.; Huculeci, R.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2021. Eur. Heart J. 2022, 43, 716–799. [Google Scholar] [CrossRef] [PubMed]
- Neri, M.; Riezzo, I.; Pascale, N.; Pomara, C.; Turillazzi, E. Ischemia/Reperfusion Injury following Acute Myocardial Infarction: A Critical Issue for Clinicians and Forensic Pathologists. Mediat. Inflamm. 2017, 2017, 7018393. [Google Scholar] [CrossRef]
- Wu, M.Y.; Yiang, G.T.; Liao, W.T.; Tsai, A.P.Y.; Cheng, Y.L.; Cheng, P.W.; Li, C.Y.; Li, C.J. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell. Physiol. Biochem. 2018, 46, 1650–1667. [Google Scholar] [CrossRef]
- Ibanez, B.; Heusch, G.; Ovize, M.; Van De Werf, F. Evolving Therapies for Myocardial Ischemia/Reperfusion Injury. J. Am. Coll. Cardiol. 2015, 65, 1454–1471. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Yellon, D.M. Myocardial Ischemia-Reperfusion Injury: A Neglected Therapeutic Target. J. Clin. Investig. 2013, 123, 92–100. [Google Scholar] [CrossRef]
- Abela, C.B.; Homer-Vanniasinkham, S. Clinical Implications of Ischaemia-Reperfusion Injury. Pathophysiology 2003, 9, 229–240. [Google Scholar] [CrossRef]
- Yellon, D.M.; Hausenloy, D.J. Myocardial Reperfusion Injury. N. Engl. J. Med. 2007, 357, 1121–1135. [Google Scholar] [CrossRef]
- Turer, A.T.; Hill, J.A. Pathogenesis of Myocardial Ischemia-Reperfusion Injury and Rationale for Therapy. Am. J. Cardiol. 2010, 37, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Chemokines in Ischemia and Reperfusion. Thromb. Haemost. 2007, 97, 738–747. [Google Scholar] [CrossRef]
- Wang, Y.; Dembowsky, K.; Chevalier, E.; Stüve, P.; Korf-Klingebiel, M.; Lochner, M.; Napp, L.C.; Frank, H.; Brinkmann, E.; Kanwischer, A.; et al. C-X-C Motif Chemokine Receptor 4 Blockade Promotes Tissue Repair after Myocardial Infarction by Enhancing Regulatory T Cell Mobilization and Immune-Regulatory Function. Circulation 2019, 139, 1798–1812. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.J.; Wu, K.J.; Wang, Y.S.; Song, J.S.; Wu, C.H.; Jan, J.J.; Bae, E.; Chen, H.; Shia, K.S.; Wang, Y. Protective Effect of CXCR4 Antagonist CX807 in a Rat Model of Hemorrhagic Stroke. Int. J. Mol. Sci. 2020, 21, 7085. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.J.; Yu, S.J.; Shia, K.S.; Wu, C.H.; Song, J.S.; Kuan, H.H.; Yeh, K.C.; Chen, C.T.; Bae, E.; Wang, Y. A Novel CXCR4 Antagonist CX549 Induces Neuroprotection in Stroke Brain. Cell Transplant. 2017, 26, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Shintani, S.; Murohara, T.; Ikeda, H.; Ueno, T.; Honma, T.; Katoh, A.; Sasaki, K.; Shimada, T.; Oike, Y.; Imaizumi, T. Mobilization of Endothelial Progenitor Cells in Patients with Acute Myocardial Infarction. Circulation 2001, 103, 2776–2779. [Google Scholar] [CrossRef]
- Porto, I.; Leone, A.M.; De Maria, G.L.; Hamilton Craig, C.; Tritarelli, A.; Camaioni, C.; Natale, L.; Niccoli, G.; Biasucci, L.M.; Crea, F. Are Endothelial Progenitor Cells Mobilized by Myocardial Ischemia or Myocardial Necrosis? A Cardiac Magnetic Resonance Study. Atherosclerosis 2011, 216, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Massa, M.; Rosti, V.; Ferrario, M.; Campanelli, R.; Ramajoli, I.; Rosso, R.; De Ferrari, G.M.; Ferlini, M.; Goffredo, L.; Bertoletti, A.; et al. Increased Circulating Hematopoietic and Endothelial Progenitor Cells in the Early Phase of Acute Myocardial Infarction. Blood 2005, 105, 199–206. [Google Scholar] [CrossRef]
- Regueiro, A.; Cuadrado-Godia, E.; Bueno-Betí, C.; Diaz-Ricart, M.; Oliveras, A.; Novella, S.; Gené, G.G.; Jung, C.; Subirana, I.; Ortiz-Pérez, J.T.; et al. Mobilization of Endothelial Progenitor Cells in Acute Cardiovascular Events in the PROCELL Study: Time-Course after Acute Myocardial Infarction and Stroke. J. Mol. Cell. Cardiol. 2015, 80, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Fish, J.E.; White, M.D.; Yu, S.; Smyth, J.W.; Shaw, R.M.; DiMaio, J.M.; Srivastava, D. Stromal Cell-Derived Factor-1alpha is Cardioprotective after Myocardial Infarction. Circulation 2008, 117, 2224–2231. [Google Scholar] [CrossRef]
- Kong, D.; Melo, L.G.; Gnecchi, M.; Zhang, L.; Mostoslavsky, G.; Liew, C.C.; Pratt, R.E.; Dzau, V.J. Cytokine-Induced Mobilization of Circulating Endothelial Progenitor Cells Enhances Repair of Injured Arteries. Circulation 2004, 110, 2039–2046. [Google Scholar] [CrossRef] [PubMed]
- Kalka, C.; Masuda, H.; Takahashi, T.; Kalka-Moll, W.M.; Silver, M.; Kearney, M.; Li, T.; Isner, J.M.; Asahara, T. Transplantation of ex vivo Expanded Endothelial Progenitor Cells for Therapeutic Neovascularization. Proc. Natl. Acad. Sci. USA 2000, 97, 3422–3427. [Google Scholar] [CrossRef] [PubMed]
- Murayama, T.; Tepper, O.M.; Silver, M.; Ma, H.; Losordo, D.W.; Isner, J.M.; Asahara, T.; Kalka, C. Determination of Bone Marrow-Derived Endothelial Progenitor Cell Significance in Angiogenic Growth Factor-Induced Neovascularization in vivo. Exp. Hematol. 2002, 30, 967–972. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Mao, M.; Zhao, M.; Xiao, X.; Sun, W.; Guo, J.; Liu, C.; Yang, D.; Qiao, J.; et al. Velvet Antler Mobilizes Endothelial Progenitor Cells to Promote Angiogenesis and Repair Vascular Endothelial Injury in Rats Following Myocardial Infarction. Front. Physiol. 2019, 9, 1940. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zhao, X.Y.; Jiang, R.C.; Chen, X.H.; Zhu, X.; Chen, K.F.; Chen, S.Y.; Zhang, X.L.; Qin, Y.; Liu, Y.H.; et al. Increased Expression of Sonic Hedgehog Restores Diabetic Endothelial Progenitor Cells and Improves Cardiac Repair after Acute Myocardial Infarction in Diabetic Mice. Int. J. Mol. Med. 2019, 44, 1091–1105. [Google Scholar] [CrossRef] [PubMed]
- Jujo, K.; Hamada, H.; Iwakura, A.; Thorne, T.; Sekiguchi, H.; Clarke, T.; Ito, A.; Misener, S.; Tanaka, T.; Klyachko, E.; et al. CXCR4 Blockade Augments Bone Marrow Progenitor Cell Recruitment to the Neovasculature and Reduces Mortality after Myocardial Infarction. Proc. Natl. Acad. Sci. USA 2010, 107, 11008–11013. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.T.; Jui, H.Y.; Huang, Y.H.; Su, M.Y.; Wu, Y.W.; Tseng, W.Y.; Hsu, M.C.; Chiang, B.L.; Wu, K.K.; Lee, C.M. CXCR4 Antagonist TG-0054 Mobilizes Mesenchymal Stem Cells, Attenuates Inflammation, and Preserves Cardiac Systolic Function in a Porcine Model of Myocardial Infarction. Cell Transplant. 2015, 24, 1313–1328. [Google Scholar] [CrossRef]
- Jujo, K.; Ii, M.; Sekiguchi, H.; Klyachko, E.; Misener, S.; Tanaka, T.; Tongers, J.; Roncalli, J.; Renault, M.A.; Thorne, T.; et al. CXC-Chemokine Receptor 4 Antagonist AMD3100 Promotes Cardiac Functional Recovery after Ischemia/Reperfusion Injury via Endothelial Nitric Oxide Synthase-Dependent Mechanism. Circulation 2013, 127, 63–73. [Google Scholar] [CrossRef]
- Song, J.S.; Chang, C.C.; Wu, C.H.; Dinh, T.K.; Jan, J.J.; Huang, K.W.; Chou, M.C.; Shiue, T.Y.; Yeh, K.C.; Ke, Y.Y.; et al. A Highly Selective and Potent CXCR4 Antagonist for Hepatocellular Carcinoma Treatment. Proc. Natl. Acad. Sci. USA 2021, 118, e2015433118. [Google Scholar] [CrossRef]
- Grdović, N.; Dinić, S.; Mihailović, M.; Uskoković, A.; Jovanović, J.A.; Poznanović, G.; Wagner, L.; Vidaković, M. CXC Chemokine Ligand 12 Protects Pancreatic β-cells from Necrosis through Akt Kinase-Mediated Modulation of Poly(ADP-Ribose) Polymerase-1 Activity. PLoS ONE 2014, 9, e101172. [Google Scholar] [CrossRef]
- Jones, K.J.; Chetram, M.A.; Bethea, D.A.; Bryant, L.K.; Odero-Marah, V.; Hinton, C.V. Cysteine (C)-X-C Receptor 4 Regulates NADPH Oxidase-2 During Oxidative Stress in Prostate Cancer Cells. Cancer Microenviron. 2013, 6, 277–288. [Google Scholar] [CrossRef]
- Yoder, M.C. Endothelial Progenitor Cell: A Blood Cell by Many Other Names May Serve Similar Functions. J. Mol. Med. 2013, 91, 285–295. [Google Scholar] [CrossRef]
- Lee, H.J.; Choi, C.W.; Kim, E.K.; Kim, H.S.; Kim, B.I.; Choi, J.H. Granulocyte Colony-Stimulating Factor Reduces Hyperoxia-Induced Alveolarization Inhibition by Increasing Angiogenic Factors. Neonatology 2012, 101, 278–284. [Google Scholar] [CrossRef]
- Westvik, T.S.; Fitzgerald, T.N.; Muto, A.; Maloney, S.P.; Pimiento, J.M.; Fancher, T.T.; Magri, D.; Westvik, H.H.; Nishibe, T.; Velazquez, O.C.; et al. Limb Ischemia After Iliac Ligation in Aged Mice Stimulates Angiogenesis without Arteriogenesis. J. Vasc. Surg. 2009, 49, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Krueger, M.A.; Huke, S.S.; Glenny, R.W. Visualizing Regional Myocardial Blood Flow in the Mouse. Circ. Res. 2013, 112, e88–e97. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Tang, M.; Liu, Y.; Hu, L.; Gu, Y. Three-Dimensional Visualization of Coronary Microvasculature in Rats with Myocardial Infarction. Microvasc. Res. 2020, 130, 103990. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.A.; Spinale, F.G. Large Animal Models of Heart Failure: A Critical Link in the Translation of Basic Science to Clinical Practice. Circ. Heart Fail. 2009, 2, 262–271. [Google Scholar] [CrossRef]
- Milani-Nejad, N.; Janssen, P.M.L. Small and Large Animal Models in Cardiac Contraction Research: Advantages and Disadvantages. Pharmacol. Ther. 2014, 141, 235–249. [Google Scholar] [CrossRef]
- Nguyen, P.K.; Wu, J.C. Large Animal Models of Ischemic Cardiomyopathy: Are They Enough to Bridge the Translational Gap? J. Nucl. Cardiol. 2015, 22, 666–672. [Google Scholar] [CrossRef]
- Singhal, A.K.; Symons, J.D.; Boudina, S.; Jaishy, B.; Shiu, Y.T. Role of Endothelial Cells in Myocardial Ischemia-Reperfusion Injury. Vasc. Dis. Prev. 2010, 7, 1–14. [Google Scholar] [CrossRef]
- Buja, L.M. Myocardial Ischemia and Reperfusion Injury. Cardiovasc. Pathol. 2005, 14, 170–175. [Google Scholar] [CrossRef]
- Tsou, L.K.; Huang, Y.H.; Song, J.S.; Ke, Y.Y.; Huang, J.K.; Shia, K.S. Harnessing CXCR4 Antagonists in Stem Cell Mobilization, HIV infection, Ischemic Diseases and Oncology. Med. Res. Rev. 2018, 38, 1188–1234. [Google Scholar] [CrossRef]
- Wu, C.H.; Song, J.S.; Kuan, H.H.; Wu, S.H.; Chou, M.C.; Jan, J.J.; Tsou, L.K.; Ke, Y.Y.; Chen, C.T.; Yeh, K.C.; et al. Development of Stem Cell Mobilizing Agents Targeting CXCR4 Receptors for Peripheral Blood Stem Cell Transplantation and Beyond. J. Med. Chem. 2018, 61, 818–833. [Google Scholar] [CrossRef]
- Wu, C.H.; Song, J.S.; Chang, K.H.; Jan, J.J.; Chen, C.T.; Chou, M.C.; Yeh, K.C.; Wong, Y.C.; Tseng, C.T.; Wu, S.H.; et al. Stem Cell Mobilizers Targeting Chemokine Receptor CXCR4: Renoprotective Application in Acute Kidney Injury. J. Med. Chem. 2015, 58, 2315–2325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kim, H.; Park, B.W.; Noh, M.; Kim, Y.; Park, J.; Park, J.H.; Kim, J.J.; Sim, W.S.; Ban, K.; et al. Cu06-1004 Enhances Vascular Integrity and Improves Cardiac Remodeling by Suppressing Edema and Inflammation in Myocardial Ischemia–Reperfusion Injury. Exp. Mol. Med. 2022, 54, 23–34. [Google Scholar] [CrossRef]


| DBPR807 | T1/2 (h) | AUC (0-Inf) (ng/mL × h) |
|---|---|---|
| 6 mg/kg, SC, Mice a | 1.0 | 16,499 ± 878 |
| 3 mg/kg, IV, Mini-Pig b | 4.0 | 37,845 ± 3745 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, K.-C.; Lee, C.-J.; Song, J.-S.; Wu, C.-H.; Yeh, T.-K.; Wu, S.-H.; Hsieh, T.-C.; Chen, Y.-T.; Tseng, H.-Y.; Huang, C.-L.; et al. Protective Effect of CXCR4 Antagonist DBPR807 against Ischemia-Reperfusion Injury in a Rat and Porcine Model of Myocardial Infarction: Potential Adjunctive Therapy for Percutaneous Coronary Intervention. Int. J. Mol. Sci. 2022, 23, 11730. https://doi.org/10.3390/ijms231911730
Yeh K-C, Lee C-J, Song J-S, Wu C-H, Yeh T-K, Wu S-H, Hsieh T-C, Chen Y-T, Tseng H-Y, Huang C-L, et al. Protective Effect of CXCR4 Antagonist DBPR807 against Ischemia-Reperfusion Injury in a Rat and Porcine Model of Myocardial Infarction: Potential Adjunctive Therapy for Percutaneous Coronary Intervention. International Journal of Molecular Sciences. 2022; 23(19):11730. https://doi.org/10.3390/ijms231911730
Chicago/Turabian StyleYeh, Kai-Chia, Chia-Jui Lee, Jen-Shin Song, Chien-Huang Wu, Teng-Kuang Yeh, Szu-Huei Wu, Tsung-Chin Hsieh, Yen-Ting Chen, Huan-Yi Tseng, Chen-Lung Huang, and et al. 2022. "Protective Effect of CXCR4 Antagonist DBPR807 against Ischemia-Reperfusion Injury in a Rat and Porcine Model of Myocardial Infarction: Potential Adjunctive Therapy for Percutaneous Coronary Intervention" International Journal of Molecular Sciences 23, no. 19: 11730. https://doi.org/10.3390/ijms231911730
APA StyleYeh, K.-C., Lee, C.-J., Song, J.-S., Wu, C.-H., Yeh, T.-K., Wu, S.-H., Hsieh, T.-C., Chen, Y.-T., Tseng, H.-Y., Huang, C.-L., Chen, C.-T., Jan, J.-J., Chou, M.-C., Shia, K.-S., & Chiang, K.-H. (2022). Protective Effect of CXCR4 Antagonist DBPR807 against Ischemia-Reperfusion Injury in a Rat and Porcine Model of Myocardial Infarction: Potential Adjunctive Therapy for Percutaneous Coronary Intervention. International Journal of Molecular Sciences, 23(19), 11730. https://doi.org/10.3390/ijms231911730






