Investigation into the Antibacterial Mechanism of Biogenic Tellurium Nanoparticles and Precursor Tellurite
Abstract
1. Introduction
2. Results
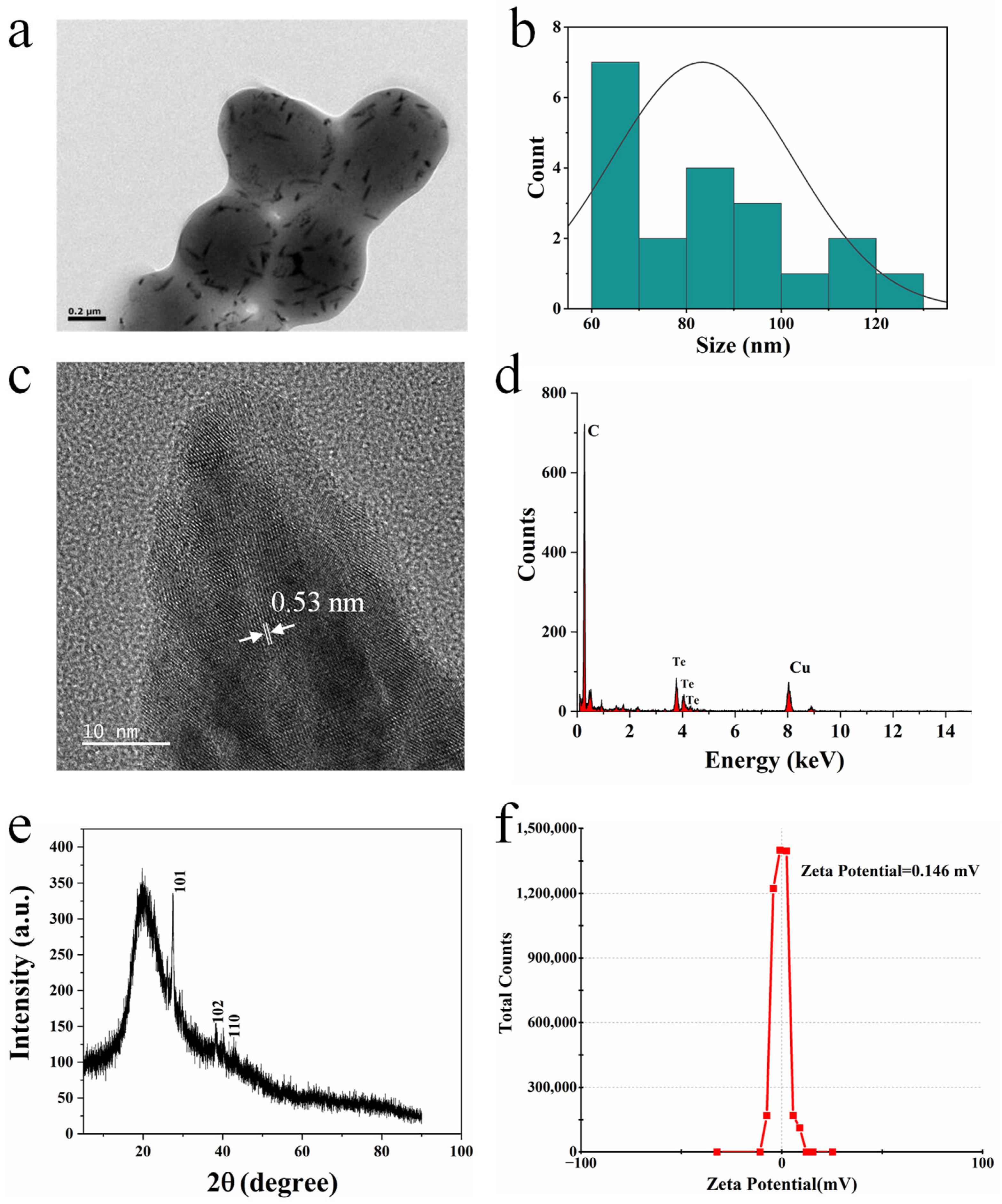
2.1. Biosynthesis of Rod-Shaped Te Nanoparticles (BioTe)
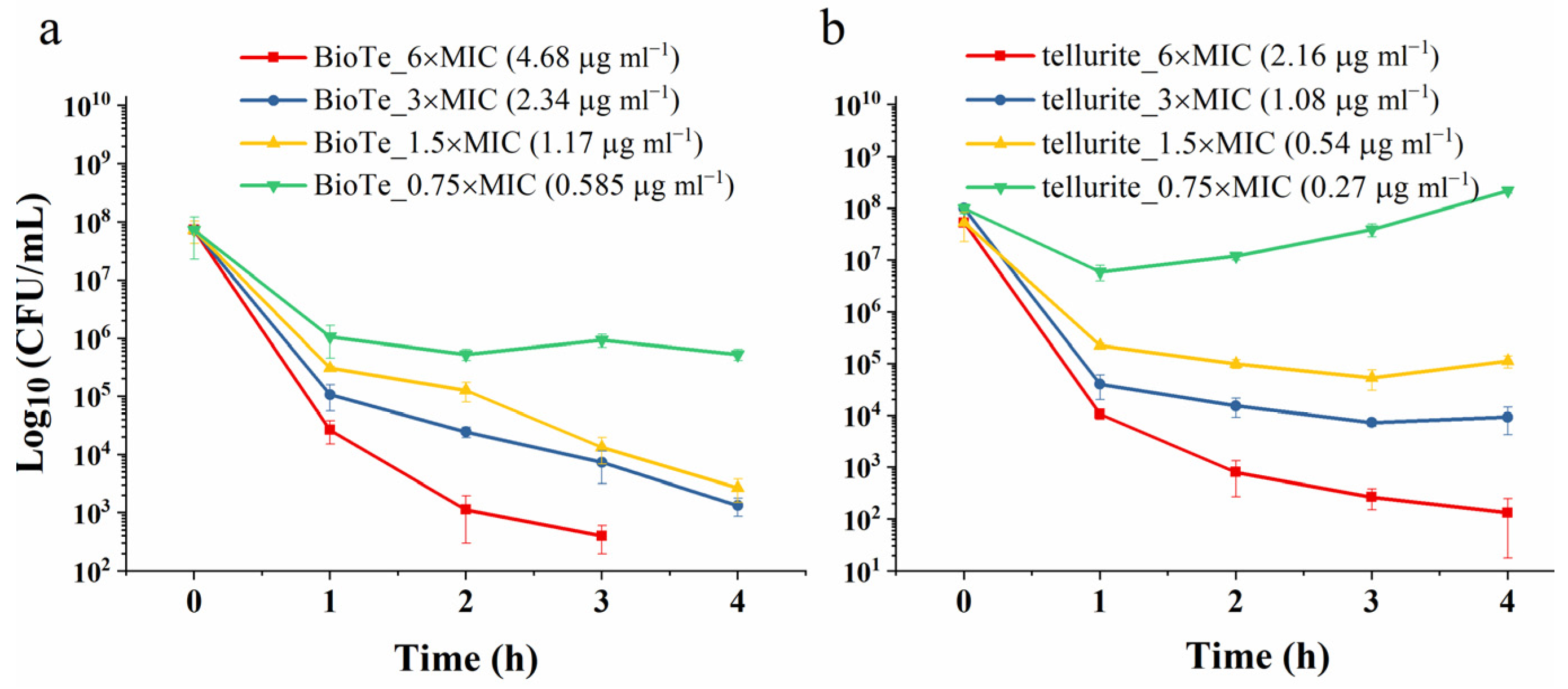
2.2. Antibacterial Activity of BioTe
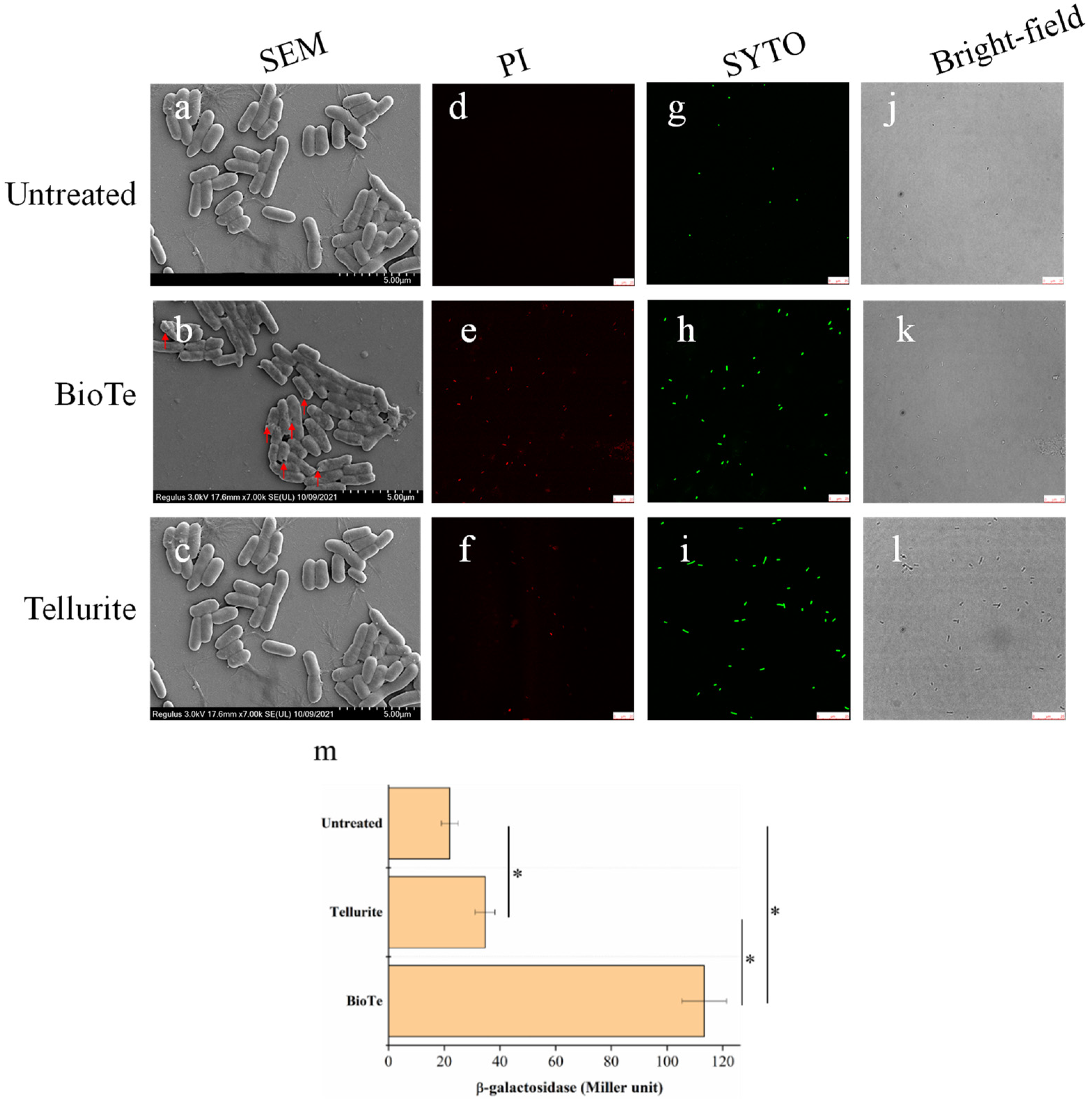
2.3. BioTe Causes Membrane Damage of E. coli Cells
2.4. Involvement of ROS and DNA Damage in Antibacterial Action of BioTe
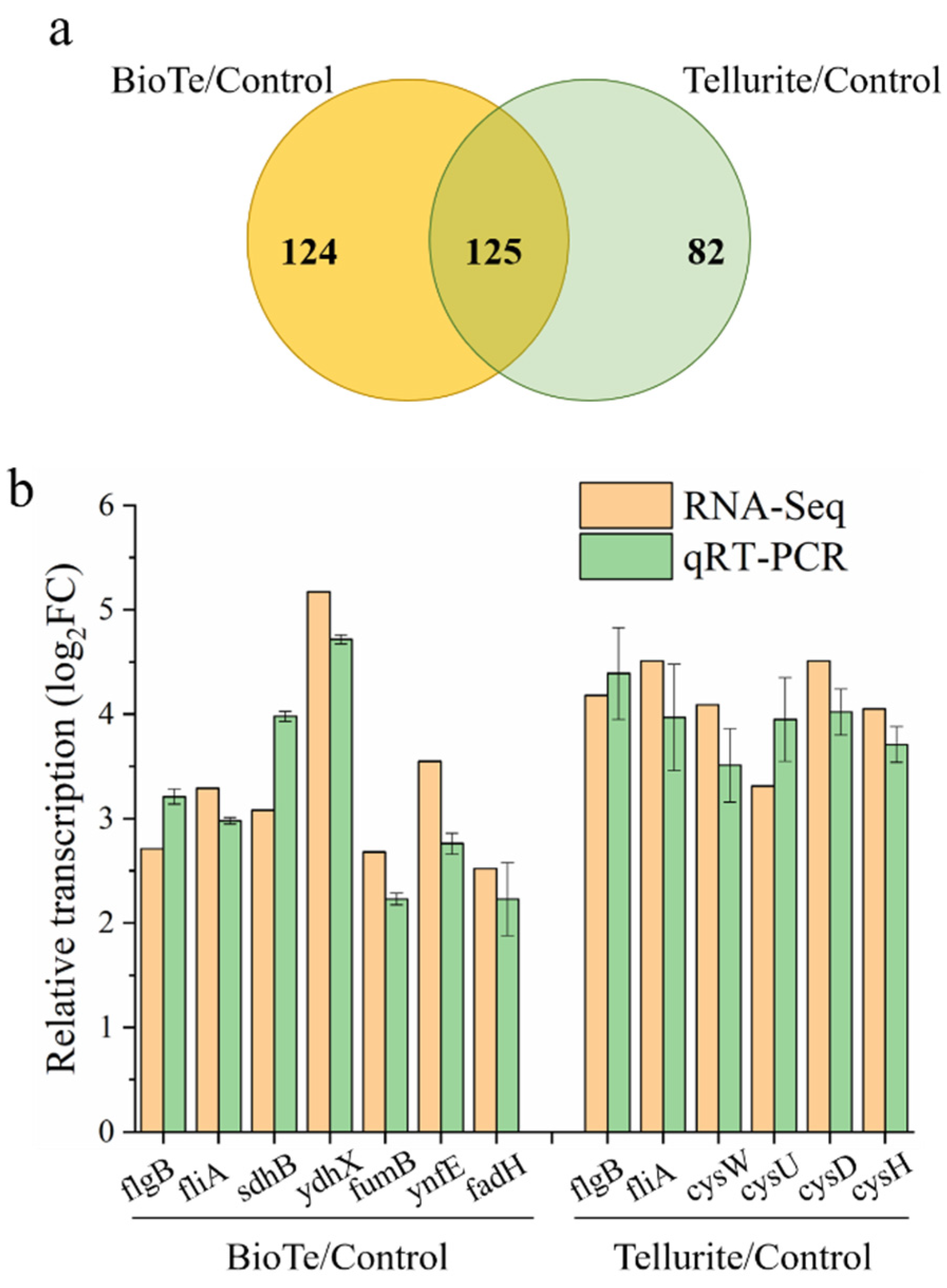
2.5. Global Response of Transcription in E. coli to Stresses of BioTe and Tellurite
3. Discussion
4. Materials and Methods
4.1. Strains and Cultivation
4.2. Biosynthesis of BioTe
4.3. Characterization of BioTe
4.4. Analysis of Antibacterial Activity
4.5. Analysis of Membrane Permeability
4.6. Measurement of ROS
4.7. Analysis of Interaction with DNA and DNA Damage
4.8. RNA Sequencing for Transcriptome Analysis
4.9. RNA Extraction and qRT-PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Makabenta, J.M.V.; Nabawy, A.; Li, C.H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2021, 19, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.W.; Zhang, L.F. Nanomaterials arising amid antibiotic resistance. Nat. Rev. Microbiol. 2021, 19, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Pervez, M.N.; Balakrishnan, M.; Hasan, S.W.; Choo, K.H.; Zhao, Y.P.; Cai, Y.J.; Zarra, T.; Belgiorno, V.; Naddeo, V. A critical review on nanomaterials membrane bioreactor (NMs-MBR) for wastewater treatment. npj Clean Water 2020, 3, 43. [Google Scholar] [CrossRef]
- Zambonino, M.C.; Quizhpe, E.M.; Jaramillo, F.E.; Rahman, A.; Santiago Vispo, N.; Jeffryes, C.; Dahoumane, S.A. Green Synthesis of Selenium and Tellurium Nanoparticles: Current Trends, Biological Properties and Biomedical Applications. Int. J. Mol. Sci. 2021, 22, 989. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, X.; Kislyakov, I.M.; Dong, N.; Zhang, S.; Wang, G.; Fan, J.; Zou, X.; Du, J.; Leng, Y.; et al. Bacterially synthesized tellurium nanostructures for broadband ultrafast nonlinear optical applications. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Liang, X.; Perez, M.A.M.-J.; Nwoko, K.C.; Egbers, P.; Feldmann, J.; Csetenyi, L.; Gadd, G.M. Fungal formation of selenium and tellurium nanoparticles. Appl. Microbiol. Biotechnol. 2019, 103, 7241–7259. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Nikhil, E.V.R.; Braganca, J.M.; Kowshik, M. Anti-bacterial TeNPs biosynthesized by haloarcheaon Halococcus salifodinae BK3. Extremophiles 2015, 19, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Shakibaie, M.; Adeli-Sardou, M.; Mohammadi-Khorsand, T.; Zeydabadi-Nejad, M.; Amirafzali, E.; Amirpour-Rostami, S.; Ameri, A.; Forootanfar, H. Antimicrobial and Antioxidant Activity of the Biologically Synthesized Tellurium Nanorods; A Preliminary In vitro Study. Iran J. Biotechnol. 2017, 15, 268–276. [Google Scholar] [CrossRef]
- Zonaro, E.; Lampis, S.; Turner, R.J.; Qazi, S.J.; Vallini, G. Biogenic selenium and tellurium nanoparticles synthesized by environmental microbial isolates efficaciously inhibit bacterial planktonic cultures and biofilms. Front. Microbiol. 2015, 6, 584. [Google Scholar] [CrossRef]
- Abo Elsoud, M.M.; Al-Hagar, O.E.A.; Abdelkhalek, E.S.; Sidkey, N.M. Synthesis and investigations on tellurium myconanoparticles. Biotechnol. Rep. (Amst. Neth.) 2018, 18, e00247. [Google Scholar] [CrossRef]
- Vavrova, S.; Struharnanska, E.; Turna, J.; Stuchlik, S. Tellurium: A Rare Element with Influence on Prokaryotic and Eukaryotic Biological Systems. Int. J. Mol. Sci. 2021, 22, 5924. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Sakthivel, N. Biological synthesis of metal nanoparticles by microbes. Adv. Colloid Interface 2010, 156, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Lv, M.; Xiu, P.; Huynh, T.; Zhang, M.; Castelli, M.; Liu, Z.; Huang, Q.; Fan, C.; Fang, H.; et al. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat. Nanotechnol. 2013, 8, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano 2012, 6, 5164–5173. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Salih, S.; Roh, D.; Lacharme-Lora, L.; Parry, M.; Hardiman, B.; Keehan, R.; Grummer, R.; Winterhager, E.; Gokhale, P.J.; et al. Signalling of DNA damage and cytokines across cell barriers exposed to nanoparticles depends on barrier thickness. Nat. Nanotechnol. 2011, 6, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Peiris, M.K.; Gunasekara, C.P.; Jayaweera, P.M.; Arachchi, N.D.; Fernando, N. Biosynthesized silver nanoparticles: Are they effective antimicrobials? Mem. Inst. Oswaldo Cruz 2017, 112, 537–543. [Google Scholar] [CrossRef]
- Lu, Z.; Rong, K.; Li, J.; Yang, H.; Chen, R. Size-dependent antibacterial activities of silver nanoparticles against oral anaerobic pathogenic bacteria. J. Mater. Sci. Mater. Med. 2013, 24, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Boulos, L.; Prevost, M.; Barbeau, B.; Coallier, J.; Desjardins, R. LIVE/DEAD BacLight: Application of a new rapid staining method for direct enumeration of viable and total bacteria in drinking water. J. Microbiol. Methods 1999, 37, 77–86. [Google Scholar] [CrossRef]
- Diaz-Vasquez, W.A.; Abarca-Lagunas, M.J.; Cornejo, F.A.; Pinto, C.A.; Arenas, F.A.; Vasquez, C.C. Tellurite-mediated damage to the Escherichia coli NDH-dehydrogenases and terminal oxidases in aerobic conditions. Arch Biochem. Biophys. 2015, 566, 67–75. [Google Scholar] [CrossRef]
- Perez, J.M.; Calderon, I.L.; Arenas, F.A.; Fuentes, D.E.; Pradenas, G.A.; Fuentes, E.L.; Sandoval, J.M.; Castro, M.E.; Elias, A.O.; Vasquez, C.C. Bacterial toxicity of potassium tellurite: Unveiling an ancient enigma. PLoS ONE 2007, 2, e211. [Google Scholar] [CrossRef]
- Brynildsen, M.P.; Winkler, J.A.; Spina, C.S.; MacDonald, I.C.; Collins, J.J. Potentiating antibacterial activity by predictably enhancing endogenous microbial ROS production. Nat. Biotechnol. 2013, 31, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Drlica, K. Reactive oxygen species and the bacterial response to lethal stress. Curr. Opin. Microbiol. 2014, 21, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Doukyu, N.; Taguchi, K. Involvement of catalase and superoxide dismutase in hydrophobic organic solvent tolerance of Escherichia coli. AMB Express 2021, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Chaithawiwat, K.; Vangnai, A.; McEvoy, J.M.; Pruess, B.; Krajangpan, S.; Khan, E. Role of oxidative stress in inactivation of Escherichia coli BW25113 by nanoscale zero-valent iron. Sci. Total Environ. 2016, 565, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Zeng, J.; Wang, X.; Drlica, K.; Zhao, X. Post-stress bacterial cell death mediated by reactive oxygen species. Proc. Natl. Acad. Sci. USA 2019, 116, 10064–10071. [Google Scholar] [CrossRef]
- Hong, Y.Z.; Li, L.P.; Luan, G.; Drlica, K.; Zhao, X.L. Contribution of reactive oxygen species to thymineless death in Escherichia coli. Nat. Microbiol. 2017, 2, 1667–1675. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, F.; Sun, X.; Choi, K.Y.; Niu, G.; Zhang, G.; Guo, J.; Lee, S.; Chen, X. The genotype-dependent influence of functionalized multiwalled carbon nanotubes on fetal development. Biomaterials 2014, 35, 856–865. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, C.; Zhou, Q.Q.; Zhang, X.F.; Wang, L.Y.; Chang, H.B.; Li, H.P.; Oda, Y.; Xing, X.H. Quantitative evaluation of DNA damage and mutation rate by atmospheric and room-temperature plasma (ARTP) and conventional mutagenesis. Appl. Microbiol. Biotechnol. 2015, 99, 5639–5646. [Google Scholar] [CrossRef]
- Fang, X.; Sastry, A.; Mih, N.; Kim, D.; Tan, J.; Yurkovich, J.T.; Lloyd, C.J.; Gao, Y.; Yang, L.; Palsson, B.O. Global transcriptional regulatory network for Escherichia coli robustly connects gene expression to transcription factor activities. Proc. Natl. Acad. Sci. USA 2017, 114, 10286–10291. [Google Scholar] [CrossRef]
- Panacek, A.; Kvitek, L.; Smekalova, M.; Vecerova, R.; Kolar, M.; Roderova, M.; Dycka, F.; Sebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2018, 13, 65–71. [Google Scholar] [CrossRef]
- Stabryla, L.M.; Johnston, K.A.; Diemler, N.A.; Cooper, V.S.; Millstone, J.E.; Haig, S.J.; Gilbertson, L.M. Role of bacterial motility in differential resistance mechanisms of silver nanoparticles and silver ions. Nat. Nanotechnol. 2021, 16, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, D.E.; Fuentes, E.L.; Castro, M.E.; Perez, J.M.; Araya, M.A.; Chasteen, T.G.; Pichuantes, S.E.; Vasquez, C.C. Cysteine metabolism-related genes and bacterial resistance to potassium tellurite. J. Bacteriol. 2007, 189, 8953–8960. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kharwar, S.; Bhattacharjee, S.; Chakraborty, S.; Mishra, A.K. Regulation of sulfur metabolism, homeostasis and adaptive responses to sulfur limitation in cyanobacteria. Biologia 2021, 76, 2811–2835. [Google Scholar] [CrossRef]
- Panpatte, D.G.; Jhala, Y.K. Nanotechnology for Agriculture: Crop Production & Protection; Springer: Singapore, 2019. [Google Scholar]
- Rice, A.; Rooney, M.T.; Greenwood, A.I.; Cotten, M.L.; Wereszczynski, J. Lipopolysaccharide Simulations Are Sensitive to Phosphate Charge and Ion Parameterization. J. Chem. Theory Comput. 2020, 16, 1806–1815. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Ye, R.; Huang, J.; Wang, B.; Xie, Z.; Ou, X.; Yu, N.; Huang, C.; Hua, Y.; Zhou, R.; et al. Distinct lipid membrane interaction and uptake of differentially charged nanoplastics in bacteria. J. Nanobiotechnol. 2022, 20, 191. [Google Scholar] [CrossRef]
- Slomberg, D.L.; Lu, Y.; Broadnax, A.D.; Hunter, R.A.; Carpenter, A.W.; Schoenfisch, M.H. Role of size and shape on biofilm eradication for nitric oxide-releasing silica nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 9322–9329. [Google Scholar] [CrossRef]
- Luebke, J.L.; Arnold, R.J.; Giedroc, D.P. Selenite and tellurite form mixed seleno- and tellurotrisulfides with CstR from Staphylococcus aureus. Metallomics 2013, 5, 335–342. [Google Scholar] [CrossRef]
- Cheng, Y.Y.; Wu, C.; Wu, J.Y.; Jia, H.L.; Wang, M.Y.; Wang, H.Y.; Zou, S.M.; Sun, R.R.; Jia, R.; Xiao, Y.Z. FlrA Represses Transcription of the Biofilm-Associated bpfA Operon in Shewanella putrefaciens. Appl. Environ. Microbiol. 2017, 83, e02410-16. [Google Scholar] [CrossRef]
- Eruslanov, E.; Kusmartsev, S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol. Biol. 2010, 594, 57–72. [Google Scholar] [CrossRef]
- Lundblad, J.R.; Laurance, M.; Goodman, R.H. Fluorescence polarization analysis of protein-DNA and protein-protein interactions. Mol. Endocrinol. 1996, 10, 607–612. [Google Scholar] [CrossRef]
- Bustin, S.A. A-Z of Quantitative PCR; International University Line: La Jolla, CA, USA, 2004; Volume xxix, 882p. [Google Scholar]
- Zare, B.; Faramarzi, M.A.; Sepehrizadeh, Z.; Shakibaie, M.; Rezaie, S.; Shahverdi, A.R. Biosynthesis and recovery of rod-shaped tellurium nanoparticles and their bactericidal activities. Mater. Res. Bull. 2012, 47, 3719–3725. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, A.; Ren, Q.; Wu, Y.; Wu, C.; Cheng, Y. Investigation into the Antibacterial Mechanism of Biogenic Tellurium Nanoparticles and Precursor Tellurite. Int. J. Mol. Sci. 2022, 23, 11697. https://doi.org/10.3390/ijms231911697
Tang A, Ren Q, Wu Y, Wu C, Cheng Y. Investigation into the Antibacterial Mechanism of Biogenic Tellurium Nanoparticles and Precursor Tellurite. International Journal of Molecular Sciences. 2022; 23(19):11697. https://doi.org/10.3390/ijms231911697
Chicago/Turabian StyleTang, Aiguo, Qianwen Ren, Yaling Wu, Chao Wu, and Yuanyuan Cheng. 2022. "Investigation into the Antibacterial Mechanism of Biogenic Tellurium Nanoparticles and Precursor Tellurite" International Journal of Molecular Sciences 23, no. 19: 11697. https://doi.org/10.3390/ijms231911697
APA StyleTang, A., Ren, Q., Wu, Y., Wu, C., & Cheng, Y. (2022). Investigation into the Antibacterial Mechanism of Biogenic Tellurium Nanoparticles and Precursor Tellurite. International Journal of Molecular Sciences, 23(19), 11697. https://doi.org/10.3390/ijms231911697





