Dissecting Stemness in Aggressive Intracranial Meningiomas: Prognostic Role of SOX2 Expression
Abstract
1. Introduction
2. Results
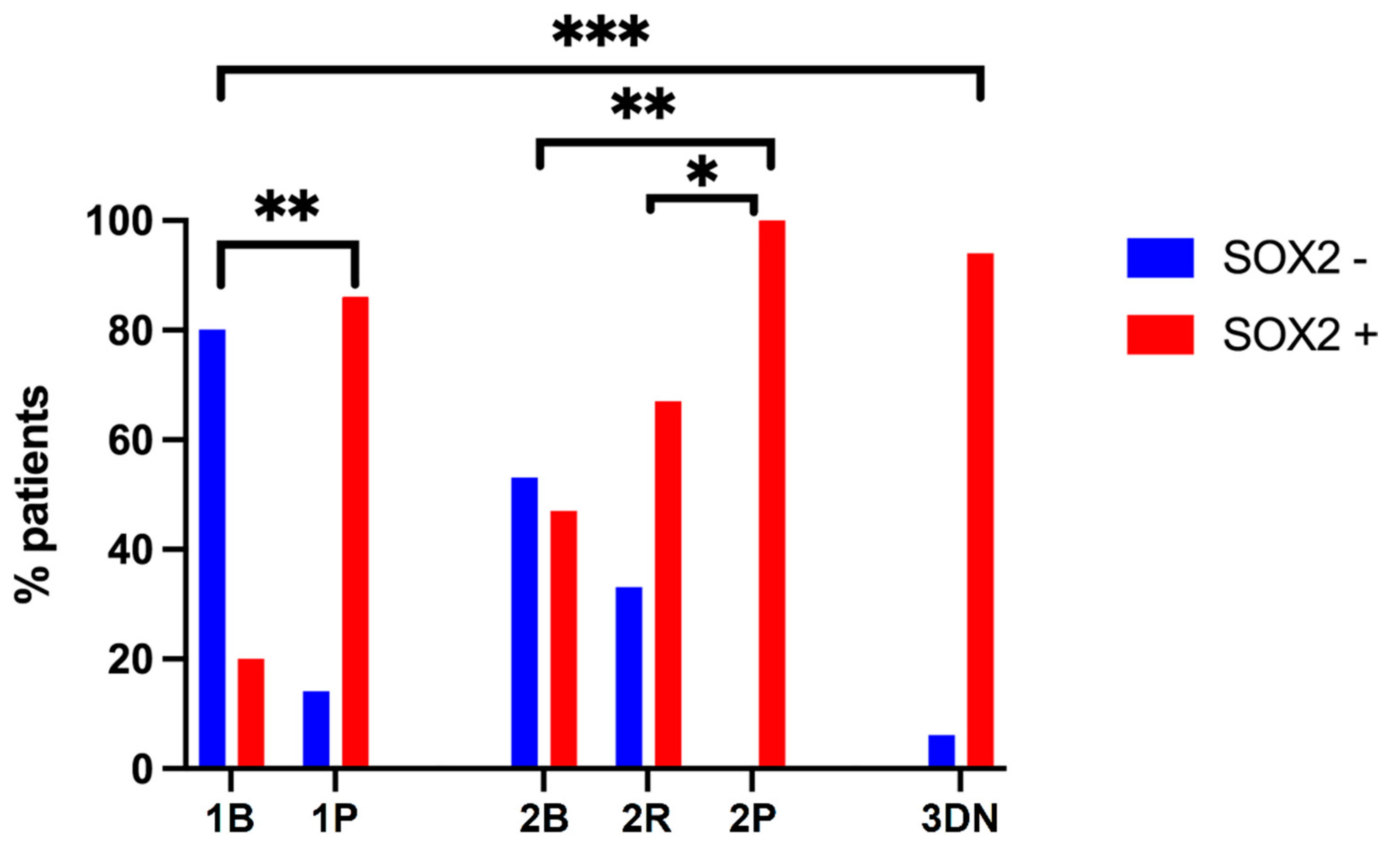
2.1. Clinical Features and Subgrouping of Patients
2.2. Validation of SOX2 IHC by RT-PCR
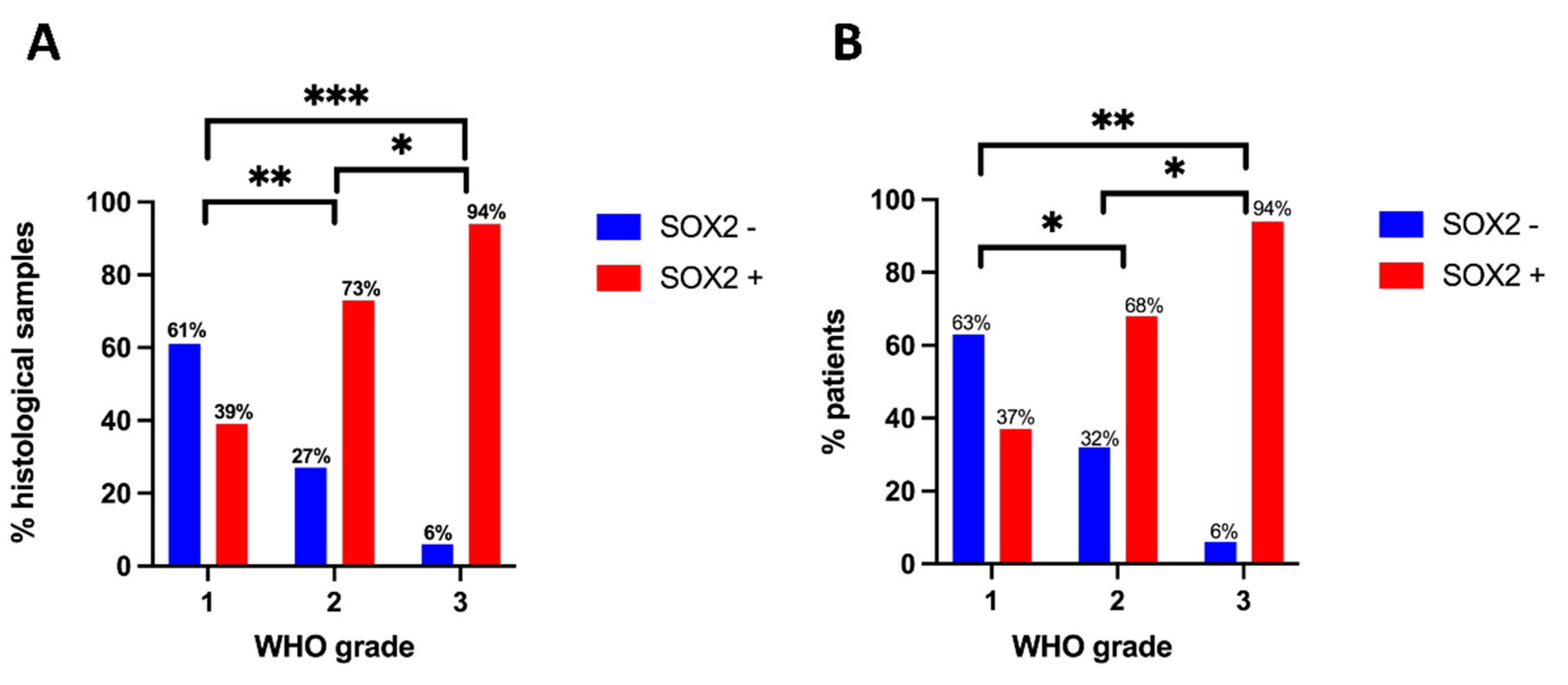
2.3. SOX2 Immunostaining and Meningioma Grade
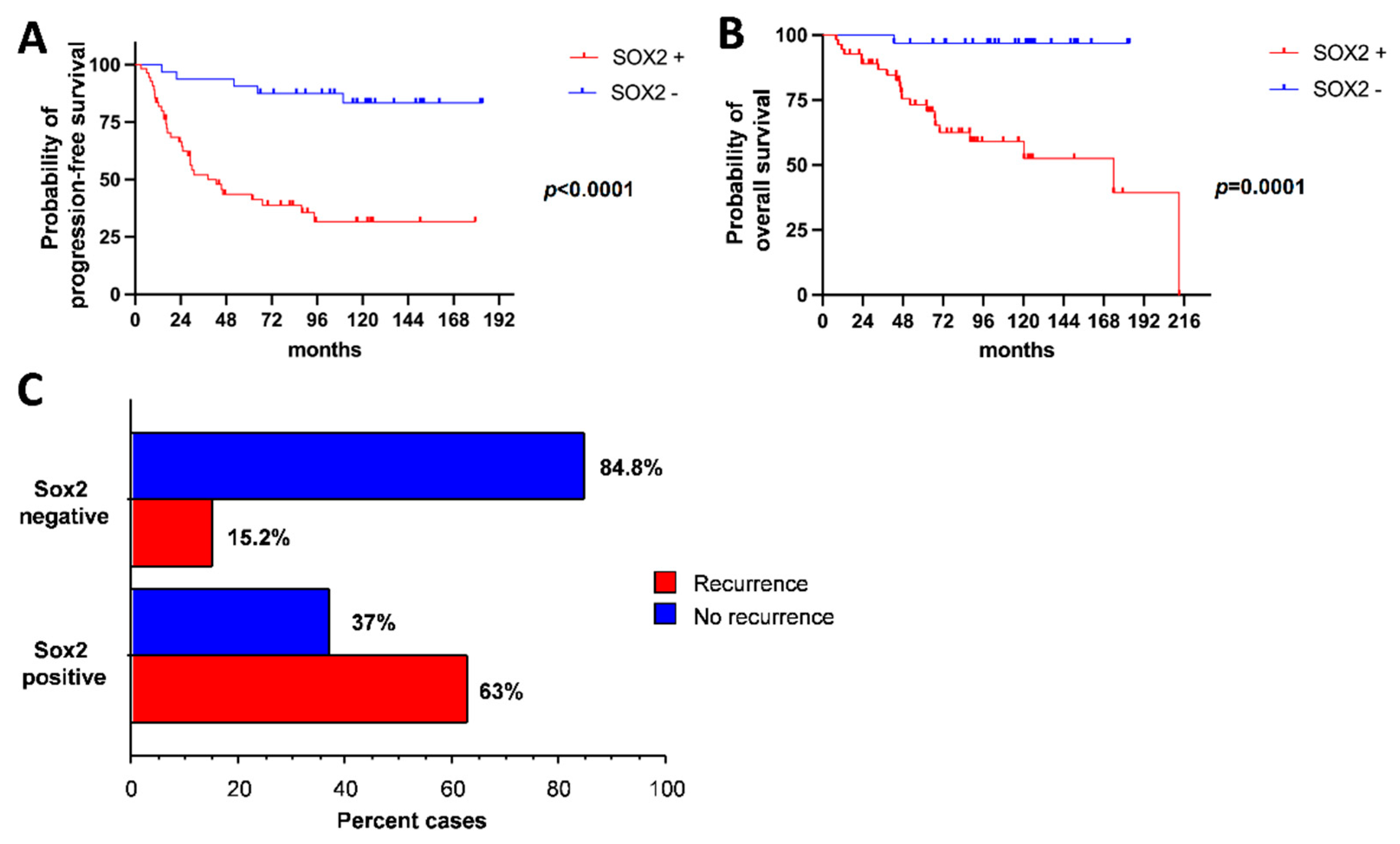
2.4. SOX2 Expression Correlates with PFS, OS and Recurrence Risk
3. Discussion
4. Materials and Methods
4.1. Patient Enrollment
4.2. Data Collection and Follow-Up
4.3. Immunohistochemistry for SOX2
4.4. mRNA Extraction and Semiquantitative Real Time (RT)-PCR for SOX2
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- San-Miguel, T.; Navarro, L.; Megías, J.; Muñoz-Hidalgo, L.; Gil-Benso, R.; Roldán, P.; López-Ginés, C.; Cerdá-Nicolás, M. Epigenetic changes underlie the aggressiveness of histologically benign meningiomas that recur. Hum. Pathol. 2019, 84, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Fioravanzo, A.; Caffo, M.; Di Bonaventura, R.; Gardiman, M.P.; Ghimenton, C.; Ius, T.; Maffeis, V.; Martini, M.; Nicolato, A.; Pallini, R.; et al. A Risk Score Based on 5 Clinico-Pathological Variables Predicts Recurrence of Atypical Meningiomas. J. Neuropathol. Exp. Neurol. 2020, 79, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Mansouri, S.; Mamatjan, Y.; Liu, J.; Nassiri, F.; Suppiah, S.; Singh, O.; Aldape, K.; Zadeh, G. Programmed death ligand-1 (PD-L1) expression in meningioma; prognostic significance and its association with hypoxia and NFKB2 expression. Sci. Rep. 2020, 10, 14115. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board (Ed.) Central Nervous System Tumours; International Agency for Research on Cancer: Lyon, France, 2021; ISBN 978-92-832-4508-7.
- Rath, P.; Miller, D.C.; Litofsky, N.S.; Anthony, D.C.; Feng, Q.; Franklin, C.; Pei, L.; Free, A.; Liu, J.; Ren, M.; et al. Isolation and characterization of a population of stem-like progenitor cells from an atypical meningioma. Exp. Mol. Pathol. 2011, 90, 179–188. [Google Scholar] [CrossRef]
- Shivapathasundram, G.; Wickremesekera, A.C.; Brasch, H.D.; Marsh, R.; Tan, S.T.; Itinteang, T. Expression of Embryonic Stem Cell Markers on the Microvessels of WHO Grade I Meningioma. Front. Surg. 2018, 5, 65. [Google Scholar] [CrossRef]
- Shivapathasundram, G.; Wickremesekera, A.C.; Tan, S.T.; Itinteang, T. Tumour stem cells in meningioma: A review. J. Clin. Neurosci. 2018, 47, 66–71. [Google Scholar] [CrossRef]
- Pallini, R.; Ricci-Vitiani, L.; Banna, G.L.; Signore, M.; Lombardi, D.; Todaro, M.; Stassi, G.; Martini, M.; Maira, G.; Larocca, L.M.; et al. Cancer stem cell analysis and clinical outcome in patients with glioblastoma multiforme. Clin. Cancer Res. 2008, 14, 8205–8212. [Google Scholar] [CrossRef]
- Novak, D.; Hüser, L.; Elton, J.J.; Umansky, V.; Altevogt, P.; Utikal, J. SOX2 in development and cancer biology. Semin. Cancer Biol. 2020, 67, 74–82. [Google Scholar] [CrossRef]
- Chaudhary, S.; Islam, Z.; Mishra, V.; Rawat, S.; Ashraf, G.M.; Kolatkar, P.R. Sox2: A Regulatory Factor in Tumorigenesis and Metastasis. Curr. Protein Pept. Sci. 2019, 20, 495–504. [Google Scholar] [CrossRef]
- Grimm, D.; Bauer, J.; Wise, P.; Krüger, M.; Simonsen, U.; Wehland, M.; Infanger, M.; Corydon, T.J. The role of SOX family members in solid tumours and metastasis. Semin. Cancer Biol. 2020, 67, 122–153. [Google Scholar] [CrossRef]
- Barone, C.; Buccarelli, M.; Alessandrini, F.; Pagin, M.; Rigoldi, L.; Sambruni, I.; Favaro, R.; Ottolenghi, S.; Pallini, R.; Ricci-Vitiani, L.; et al. Sox2-dependent maintenance of mouse oligodendroglioma involves the Sox2-mediated downregulation of Cdkn2b, Ebf1, Zfp423, and Hey2. Glia 2021, 69, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Jagga, B.; Edwards, M.; Pagin, M.; Wagstaff, K.M.; Aragão, D.; Roman, N.; Nanson, J.D.; Raidal, S.R.; Dominado, N.; Stewart, M.; et al. Structural basis for nuclear import selectivity of pioneer transcription factor SOX2. Nat. Commun. 2021, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liu, T.; Fei, W.; Yue, X.-G. Correlation between SOX2 and Survivin clinical features in patients with salivary adenoid cystic carcinoma. J. Infect. Public Health 2019, 12, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, M.; Temme, A.; Senner, V.; Ebner, R.; Schwind, S.; Stevanovic, S.; Wehner, R.; Schackert, G.; Schackert, H.K.; Fussel, M.; et al. Identification of SOX2 as a novel glioma-associated antigen and potential target for T cell-based immunotherapy. Br. J. Cancer 2007, 96, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Sofela, A.A.; McGavin, L.; Whitfield, P.C.; Hanemann, C.O. Biomarkers for differentiating grade II meningiomas from grade I: A systematic review. Br. J. Neurosurg. 2021, 35, 696–702. [Google Scholar] [CrossRef]
- Al-Mefty, O.; Kadri, P.A.S.; Pravdenkova, S.; Sawyer, J.R.; Stangeby, C.; Husain, M. Malignant progression in meningioma: Documentation of a series and analysis of cytogenetic findings. J. Neurosurg. 2004, 101, 210–218. [Google Scholar] [CrossRef]
- Sahm, F.; Schrimpf, D.; Olar, A.; Koelsche, C.; Reuss, D.; Bissel, J.; Kratz, A.; Capper, D.; Schefzyk, S.; Hielscher, T.; et al. TERT Promoter Mutations and Risk of Recurrence in Meningioma. J. Natl. Cancer Inst. 2016, 108, djv370. [Google Scholar] [CrossRef]
- Nassiri, F.; Mamatjan, Y.; Suppiah, S.; Badhiwala, J.H.; Mansouri, S.; Karimi, S.; Saarela, O.; Poisson, L.; Gepfner-Tuma, I.; Schittenhelm, J.; et al. DNA methylation profiling to predict recurrence risk in meningioma: Development and validation of a nomogram to optimize clinical management. Neuro-Oncology 2019, 21, 901–910. [Google Scholar] [CrossRef]
- Freitag, D.; McLean, A.L.; Simon, M.; Koch, A.; Grube, S.; Walter, J.; Kalff, R.; Ewald, C. NANOG overexpression and its correlation with stem cell and differentiation markers in meningiomas of different WHO grades. Mol. Carcinog. 2017, 56, 1953–1964. [Google Scholar] [CrossRef]
- Kamachi, Y.; Kondoh, H. Sox proteins: Regulators of cell fate specification and differentiation. Development 2013, 140, 4129–4144. [Google Scholar] [CrossRef]
- Mansouri, S.; Nejad, R.; Karabork, M.; Ekinci, C.; Solaroglu, I.; Aldape, K.D.; Zadeh, G. Sox2: Regulation of expression and contribution to brain tumors. CNS Oncol. 2016, 5, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Ikushima, H.; Todo, T.; Ino, Y.; Takahashi, M.; Miyazawa, K.; Miyazono, K. Autocrine TGF-beta signaling maintains tumorigenicity of glioma-initiating cells through Sry-related HMG-box factors. Cell Stem Cell 2009, 5, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Gangemi, R.M.R.; Griffero, F.; Marubbi, D.; Perera, M.; Capra, M.C.; Malatesta, P.; Ravetti, G.L.; Zona, G.L.; Daga, A.; Corte, G. SOX2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity. Stem Cells 2009, 27, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Emlet, D.R.; Gupta, P.; Holgado-Madruga, M.; Del Vecchio, C.A.; Mitra, S.S.; Han, S.-Y.; Li, G.; Jensen, K.C.; Vogel, H.; Xu, L.W.; et al. Targeting a glioblastoma cancer stem-cell population defined by EGF receptor variant III. Cancer Res. 2014, 74, 1238–1249. [Google Scholar] [CrossRef] [PubMed]
- Berezovsky, A.D.; Poisson, L.M.; Cherba, D.; Webb, C.P.; Transou, A.D.; Lemke, N.W.; Hong, X.; Hasselbach, L.A.; Irtenkauf, S.M.; Mikkelsen, T.; et al. Sox2 promotes malignancy in glioblastoma by regulating plasticity and astrocytic differentiation. Neoplasia 2014, 16, 193–206. [Google Scholar] [CrossRef][Green Version]
- Phi, J.H.; Park, S.-H.; Kim, S.-K.; Paek, S.H.; Kim, J.H.; Lee, Y.J.; Cho, B.-K.; Park, C.-K.; Lee, D.-H.; Wang, K.-C. Sox2 expression in brain tumors: A reflection of the neuroglial differentiation pathway. Am. J. Surg. Pathol. 2008, 32, 103–112. [Google Scholar] [CrossRef]
- Lengerke, C.; Fehm, T.; Kurth, R.; Neubauer, H.; Scheble, V.; Müller, F.; Schneider, F.; Petersen, K.; Wallwiener, D.; Kanz, L.; et al. Expression of the embryonic stem cell marker SOX2 in early-stage breast carcinoma. BMC Cancer 2011, 11, 42. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Xu, Z.; Ahmad, A.; Li, E.; Wang, Y.; Qin, S.; Wang, Q. Expression of Sox2 and Oct4 and their clinical significance in human non-small-cell lung cancer. Int. J. Mol. Sci. 2012, 13, 7663–7675. [Google Scholar] [CrossRef]
- Fang, X.; Yoon, J.-G.; Li, L.; Yu, W.; Shao, J.; Hua, D.; Zheng, S.; Hood, L.; Goodlett, D.R.; Foltz, G.; et al. The SOX2 response program in glioblastoma multiforme: An integrated ChIP-seq, expression microarray, and microRNA analysis. BMC Genom. 2011, 12, 11. [Google Scholar] [CrossRef]
- Marziali, G.; Signore, M.; Buccarelli, M.; Grande, S.; Palma, A.; Biffoni, M.; Rosi, A.; D’Alessandris, Q.G.; Martini, M.; Larocca, L.M.; et al. Metabolic/Proteomic Signature Defines Two Glioblastoma Subtypes With Different Clinical Outcome. Sci. Rep. 2016, 6, 21557. [Google Scholar] [CrossRef]
- Huang, R.Y.; Bi, W.L.; Weller, M.; Kaley, T.; Blakeley, J.; Dunn, I.; Galanis, E.; Preusser, M.; McDermott, M.; Rogers, L.; et al. Proposed response assessment and endpoints for meningioma clinical trials: Report from the Response Assessment in Neuro-Oncology Working Group. Neuro-Oncology 2019, 21, 26–36. [Google Scholar] [CrossRef] [PubMed]


| Parameter | All Patients | Group | |||||
|---|---|---|---|---|---|---|---|
| 1B | 1P | 2B | 2R | 2P | 3DN | ||
| N | 87 | 20 | 7 | 19 | 12 | 13 | 16 |
| F:M | 42:45 0.9 | 13:7 1.9 | 3:4 0.8 | 11:8 1.4 | 4:8 0.5 | 3:10 0.3 | 8:8 1 |
| Age at diagnosis (years) | 61.0 ± 11.5 | 57.8 ± 8.9 | 57.3 ± 16.9 | 59.0 ± 11.9 | 61.4 ± 10.7 | 60.2 ± 9.1 | 69.4 ± 11.1 |
| N surgeries, median (range) | 1 (1–6) | 1 | 2 (2–6) | 1 | 3 (2–5) | 2 (2–6) | 1 (1–3) |
| Follow-up (months) | 87.5 ± 47.9 | 136.0 ± 21.3 | 88.1 ± 67.9 | 102.0 ± 22.8 | 73.8 ± 28.1 | 57.5 ± 43.5 | 43.8 ± 40.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Bonaventura, R.; Martini, M.; Cenci, T.; Caccavella, V.M.; Barresi, V.; Gessi, M.; Albanese, A.; Lauretti, L.; Pallini, R.; D'Alessandris, Q.G.; et al. Dissecting Stemness in Aggressive Intracranial Meningiomas: Prognostic Role of SOX2 Expression. Int. J. Mol. Sci. 2022, 23, 11690. https://doi.org/10.3390/ijms231911690
Di Bonaventura R, Martini M, Cenci T, Caccavella VM, Barresi V, Gessi M, Albanese A, Lauretti L, Pallini R, D'Alessandris QG, et al. Dissecting Stemness in Aggressive Intracranial Meningiomas: Prognostic Role of SOX2 Expression. International Journal of Molecular Sciences. 2022; 23(19):11690. https://doi.org/10.3390/ijms231911690
Chicago/Turabian StyleDi Bonaventura, Rina, Maurizio Martini, Tonia Cenci, Valerio Maria Caccavella, Valeria Barresi, Marco Gessi, Alessio Albanese, Liverana Lauretti, Roberto Pallini, Quintino Giorgio D'Alessandris, and et al. 2022. "Dissecting Stemness in Aggressive Intracranial Meningiomas: Prognostic Role of SOX2 Expression" International Journal of Molecular Sciences 23, no. 19: 11690. https://doi.org/10.3390/ijms231911690
APA StyleDi Bonaventura, R., Martini, M., Cenci, T., Caccavella, V. M., Barresi, V., Gessi, M., Albanese, A., Lauretti, L., Pallini, R., D'Alessandris, Q. G., & Olivi, A. (2022). Dissecting Stemness in Aggressive Intracranial Meningiomas: Prognostic Role of SOX2 Expression. International Journal of Molecular Sciences, 23(19), 11690. https://doi.org/10.3390/ijms231911690









