Improved Adipose Tissue Function after Single Anastomosis Duodeno-Ileal Bypass with Sleeve-Gastrectomy (SADI-S) in Diet-Induced Obesity
Abstract
1. Introduction
2. Results
2.1. Effect of SG and SADI-S on Adiposity, Energy Expenditure and Metabolic Profile
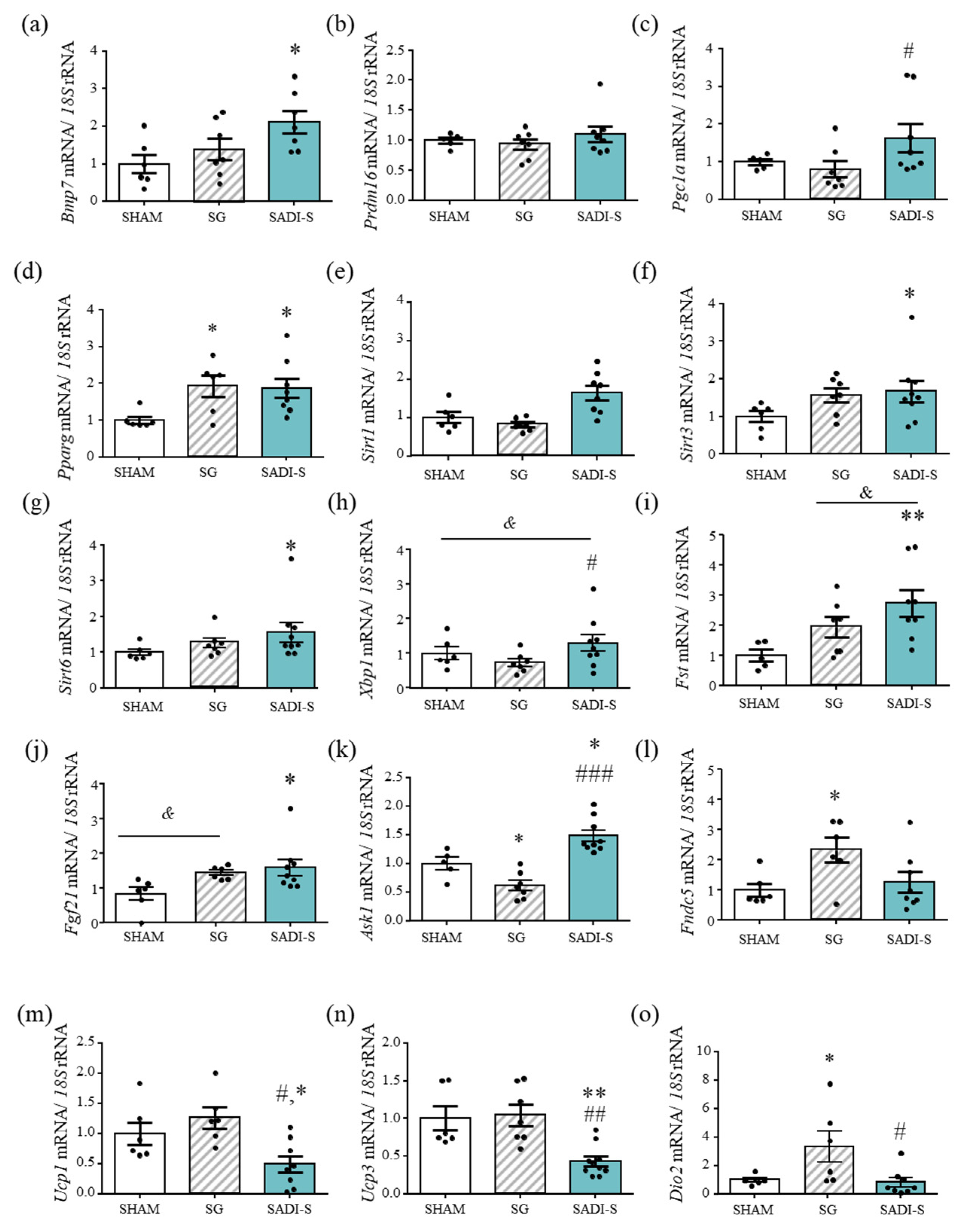
2.2. Impact of the Bariatric Surgery on Markers of Brown Adipocyte Function
2.3. Effect of SG and SADI-S on Factors Involved in Fat Browning in Subcutaneous Adipose Tissue
2.4. Improved Beige Adipose Tissue Charateristics after SG and SADI-S
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Experimental Animals
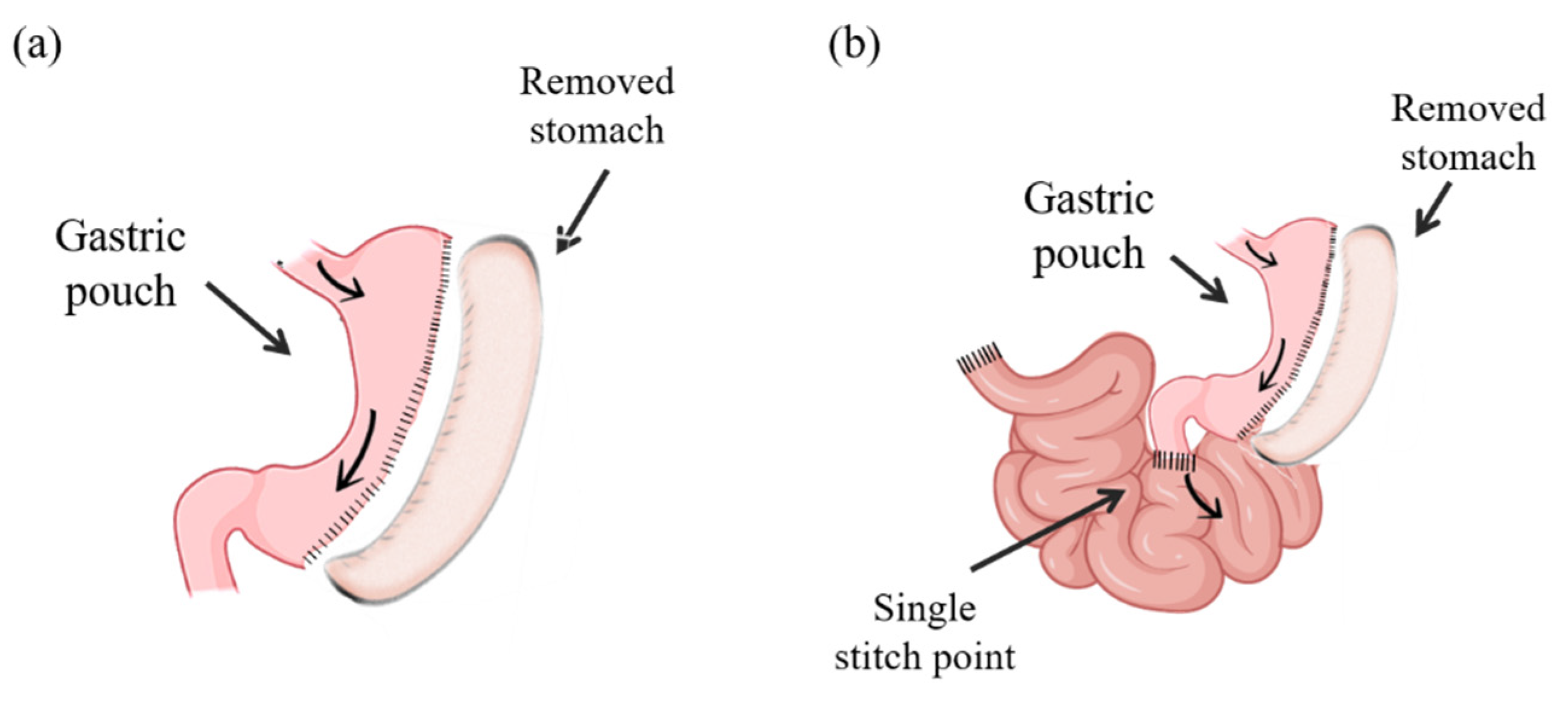
5.2. Surgical Procedures
5.3. Temperature Measurements
5.4. Oxygen Consumption and Daily Energy Expenditure
5.5. Blood Measurements
5.6. RNA Extraction and Real-Time PCR
5.7. Determination of Adipocyte Surface Area
5.8. Immunohistochemistry of UCP-1
5.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Catalán, V.; Avilés-Olmos, I.; Rodríguez, A.; Becerril, S.; Fernández-Formoso, J.A.; Kiortsis, D.; Portincasa, P.; Gómez-Ambrosi, J.; Frühbeck, G. Time to Consider the “Exposome Hypothesis” in the Development of the Obesity Pandemic. Nutrients 2022, 14, 1597. [Google Scholar] [CrossRef] [PubMed]
- Mingrone, G.; Panunzi, S.; De Gaetano, A.; Guidone, C.; Iaconelli, A.; Nanni, G.; Castagneto, M.; Bornstein, S.; Rubino, F. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2015, 386, 964–973. [Google Scholar] [CrossRef]
- Bray, G.A.; Frühbeck, G.; Ryan, D.H.; Wilding, J.P. Management of obesity. Lancet 2016, 387, 1947–1956. [Google Scholar] [CrossRef]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Aminian, A.; Brethauer, S.A.; Navaneethan, S.D.; Singh, R.P.; Pothier, C.E.; Nissen, S.E.; et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes—5-Year Outcomes. N. Engl. J. Med. 2017, 376, 641–651. [Google Scholar] [CrossRef]
- Kashyap, S.R.; Gatmaitan, P.; Brethauer, S.; Schauer, P. Bariatric surgery for type 2 diabetes: Weighing the impact for obese patients. Cleve Clin. J. Med. 2010, 77, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Rubino, F.; Nathan, D.M.; Eckel, R.H.; Schauer, P.R.; Alberti, K.G.M.; Zimmet, P.Z.; Del Prato, S.; Ji, L.; Sadikot, S.M.; Herman, W.H.; et al. Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: A Joint Statement by International Diabetes Organizations. Surg. Obes. Relat. Dis. 2016, 12, 1144–1162. [Google Scholar] [CrossRef] [PubMed]
- Batterham, R.L.; Cummings, D.E. Mechanisms of Diabetes Improvement Following Bariatric/Metabolic Surgery. Diabetes Care 2016, 39, 893–901. [Google Scholar] [CrossRef]
- Rosen, E.D.; Spiegelman, B.M. What We Talk About When We Talk About Fat. Cell 2014, 156, 20–44. [Google Scholar] [CrossRef]
- Rodríguez, A.; Becerril, S.; Valentí, V.; Moncada, R.; Méndez-Giménez, L.; Ramírez, B.; Lancha, A.; Martin, M.; Burrell, M.A.; Catalán, V.; et al. Short-Term Effects of Sleeve Gastrectomy and Caloric Restriction on Blood Pressure in Diet-Induced Obese Rats. Obes. Surg. 2012, 22, 1481–1490. [Google Scholar] [CrossRef]
- Rodríguez, A.; Becerril, S.; Valentí, V.; Ramírez, B.; Martin, M.; Méndez-Giménez, L.; Lancha, A.; Calderón, P.D.S.; Catalán, V.; Burrell, M.A.; et al. Sleeve Gastrectomy Reduces Blood Pressure in Obese (fa/fa) Zucker Rats. Obes. Surg. 2011, 22, 309–315. [Google Scholar] [CrossRef]
- Moncada, R.; Becerril, S.; Rodríguez, A.; Méndez-Giménez, L.; Ramírez, B.; Catalán, V.; Gómez-Ambrosi, J.; Gil, M.J.; Fernández, S.; Cienfuegos, J.A.; et al. Sleeve Gastrectomy Reduces Body Weight and Improves Metabolic Profile also in Obesity-Prone Rats. Obes. Surg. 2015, 26, 1537–1548. [Google Scholar] [CrossRef] [PubMed]
- Assaad, H.; Yao, K.; Tekwe, C.D.; Feng, S.; Bazer, F.W.; Zhou, L.; Carroll, R.J.; Meininger, C.J.; Wu, G. Analysis of energy expenditure in diet-induced obese rats. Front. Biosci. 2014, 19, 967–985. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Ramírez, B.; Becerril, S.; Salvador, J.; Colina, I.; Gómez-Ambrosi, J. Adiponectin-leptin Ratio is a Functional Biomarker of Adipose Tissue Inflammation. Nutrients 2019, 11, 454. [Google Scholar] [CrossRef]
- Asada, R.; Kanemoto, S.; Matsuhisa, K.; Hino, K.; Cui, M.; Cui, X.; Kaneko, M.; Imaizumi, K. IRE1α-XBP1 is a novel branch in the transcriptional regulation of Ucp1 in brown adipocytes. Sci. Rep. 2015, 5, 16580. [Google Scholar] [CrossRef] [PubMed]
- Hattori, K.; Naguro, I.; Okabe, K.; Funatsu, T.; Furutani, S.; Takeda, K.; Ichijo, H. ASK1 signalling regulates brown and beige adipocyte function. Nat. Commun. 2016, 7, 11158. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Kim, S.-J.; Park, S.-S.; Chang, C.; Kim, E. TR4 activates FATP1 gene expression to promote lipid accumulation in 3T3-L1 adipocytes. FEBS Lett. 2011, 585, 2763–2767. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Kazantzis, M.; Doege, H.; Ortegon, A.M.; Tsang, B.; Falcon, A.; Stahl, A. Fatty Acid Transport Protein 1 Is Required for Nonshivering Thermogenesis in Brown Adipose Tissue. Diabetes 2006, 55, 3229–3237. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, Y.; Yuan, W.; Wang, M.; Zhou, Y.; Chen, K.; Huang, Q. Downregulation of osteopontin inhibits browning of white adipose tissues through PI3K-AKT pathway in C57BL/6 mice. Eur. J. Pharmacol. 2020, 866, 172822. [Google Scholar] [CrossRef]
- Liu, Y.; Maekawa, T.; Yoshida, K.; Kaneda, H.; Chatton, B.; Wakana, S.; Ishii, S. The transcription factor ATF7 mediates in vitro fertilization-induced gene expression changes in mouse liver. FEBS Open Bio. 2017, 7, 1598–1610. [Google Scholar] [CrossRef]
- Cinti S Transdifferentiation properties of adipocytes in the adipose organ. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E977–E986. [CrossRef]
- Wu, J.; Boström, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.-H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige Adipocytes Are a Distinct Type of Thermogenic Fat Cell in Mouse and Human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Seale, P. Control of brown and beige fat development. Nat. Rev. Mol. Cell Biol. 2016, 17, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Reges, O.; Greenland, P.; Dicker, D.; Leibowitz, M.; Hoshen, M.; Gofer, I.; Rasmussen-Torvik, L.; Balicer, R. Association of Bariatric Surgery Using Laparoscopic Banding, Roux-en-Y Gastric Bypass, or Laparoscopic Sleeve Gastrectomy vs Usual Care Obesity Management With All-Cause Mortality. JAMA 2018, 319, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Welbourn, R.; Hollyman, M.; Kinsman, R.; Dixon, J.; Liem, R.; Ottosson, J.; Ramos, A.; Våge, V.; Al-Sabah, S.; Brown, W.; et al. Bariatric Surgery Worldwide: Baseline Demographic Description and One-Year Outcomes from the Fourth IFSO Global Registry Report 2018. Obes. Surg. 2018, 29, 782–795. [Google Scholar] [CrossRef] [PubMed]
- Rubino, F.; Gagner, M. Potential of Surgery for Curing Type 2 Diabetes Mellitus. Ann. Surg. 2002, 236, 554–559. [Google Scholar] [CrossRef]
- Stylopoulos, N.; Hoppin, A.G.; Kaplan, L.M. Roux-en-Y Gastric Bypass Enhances Energy Expenditure and Extends Lifespan in Diet-induced Obese Rats. Obesity 2009, 17, 1839–1847. [Google Scholar] [CrossRef]
- Bueter, M.; Löwenstein, C.; Olbers, T.; Wang, M.; Cluny, N.L.; Bloom, S.R.; Sharkey, K.A.; Lutz, T.A.; Le Roux, C.W. Gastric bypass increases energy expenditure in rats. Gastroenterology 2010, 138, 1845–1853. [Google Scholar] [CrossRef]
- Hao, Z.; Mumphrey, M.B.; Townsend, R.L.; Morrison, C.D.; Münzberg, H.; Ye, J.; Berthoud, H.-R. Body Composition, Food Intake, and Energy Expenditure in a Murine Model of Roux-en-Y Gastric Bypass Surgery. Obes. Surg. 2016, 26, 2173–2182. [Google Scholar] [CrossRef]
- Das, S.K.; Roberts, S.B.; McCrory, M.A.; Hsu, L.G.; Shikora, S.A.; Kehayias, J.J.; Dallal, G.E.; Saltzman, E. Long-term changes in energy expenditure and body composition after massive weight loss induced by gastric bypass surgery. Am. J. Clin. Nutr. 2003, 78, 22–30. [Google Scholar] [CrossRef]
- Harms, M.; Seale, P. Brown and beige fat: Development, function and therapeutic potential. Nat. Med. 2013, 19, 1252–1263. [Google Scholar] [CrossRef]
- Seale, P.; Conroe, H.M.; Estall, J.; Kajimura, S.; Frontini, A.; Ishibashi, J.; Cohen, P.; Cinti, S.; Spiegelman, B.M. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J. Clin. Investig. 2011, 121, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.-H.; Kokkotou, E.; Schulz, T.J.; Huang, T.L.; Winnay, J.N.; Taniguchi, C.M.; Tran, T.T.; Suzuki, R.; Espinoza, D.O.; Yamamoto, Y.; et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 2008, 454, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, J.; Petrovic, N.; Lindgren, E.M.; Jacobsson, A.; Cannon, B. PPARg in the control of brown adipocyte differentiation. Biochim. Biophys Acta 2005, 1740, 293–304. [Google Scholar] [CrossRef]
- Uldry, M.; Yang, W.; St-Pierre, J.; Lin, J.; Seale, P.; Spiegelman, B.M. Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab. 2006, 3, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Feige, J.N.; Lagouge, M.; Canto, C.; Strehle, A.; Houten, S.M.; Milne, J.C.; Lambert, P.D.; Mataki, C.; Elliott, P.J.; Auwerx, J. Specific SIRT1 Activation Mimics Low Energy Levels and Protects against Diet-Induced Metabolic Disorders by Enhancing Fat Oxidation. Cell Metab. 2008, 8, 347–358. [Google Scholar] [CrossRef]
- Giralt, A.; Hondares, E.; Villena, J.A.; Ribas, F.; Diaz-Delfin, J.; Giralt, M.; Iglesias, R.; Villarroya, F. Peroxisome proliferator-activated receptor-gamma coactivator-1alpha controls transcription of the Sirt3 gene, an essential component of the thermogenic brown adipocyte phenotype. J. Biol. Chem. 2011, 286, 16958–16966. [Google Scholar] [CrossRef]
- Wu, D.; Bang, I.H.; Park, B.-H.; Bae, E.J. Loss of Sirt6 in adipocytes impairs the ability of adipose tissue to adapt to intermittent fasting. Exp. Mol. Med. 2021, 53, 1298–1306. [Google Scholar] [CrossRef]
- Gómez-Ambrosi, J.; Gallego-Escuredo, J.M.; Catalán, V.; Rodríguez, A.; Domingo, P.; Moncada, R.; Valentí, V.; Salvador, J.; Giralt, M.; Villarroya, F.; et al. FGF19 and FGF21 serum concentrations in human obesity and type 2 diabetes behave differently after diet- or surgically-induced weight loss. Clin. Nutr. 2016, 36, 861–868. [Google Scholar] [CrossRef]
- Alvarez-Sola, G.; Uriarte, I.; Latasa, M.U.; Fernández-Barrena, M.G.; Urtasun, R.; Elizalde, M.; Barcena-Varela, M.; Jiménez, M.; Chang, H.C.; Barbero, R.; et al. Fibroblast growth factor 15/19 (FGF15/19) protects from diet-induced hepatic steatosis: Development of an FGF19-based chimeric molecule to promote fatty liver regeneration. Gut 2017, 66, 1818–1828. [Google Scholar] [CrossRef]
- Fisher, F.M.; Kleiner, S.; Douris, N.; Fox, E.C.; Mepani, R.J.; Verdeguer, F.; Wu, J.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E.; et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012, 26, 271–281. [Google Scholar] [CrossRef]
- Tsuchida, A.; Yamauchi, T.; Ito, Y.; Hada, Y.; Maki, T.; Takekawa, S.; Kamon, J.; Kobayashi, M.; Suzuki, R.; Hara, K.; et al. Insulin/Foxo1 Pathway Regulates Expression Levels of Adiponectin Receptors and Adiponectin Sensitivity. J. Biol. Chem. 2004, 279, 30817–30822. [Google Scholar] [CrossRef] [PubMed]
- Abegg, K.; Corteville, C.; Bueter, M.; A Lutz, T. Alterations in energy expenditure in Roux-en-Y gastric bypass rats persist at thermoneutrality. Int. J. Obes. 2016, 40, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhuang, J.; Cui, Q.; Jiang, S.; Tao, W.; Chen, W.; Yu, S.; Wu, L.; Yang, W.; Liu, F.; et al. Two Bariatric Surgical Procedures Differentially Alter the Intestinal Microbiota in Obesity Patients. Obes. Surg. 2020, 30, 2345–2361. [Google Scholar] [CrossRef]
- Sánchez-Alcoholado, L.; Gutiérrez-Repiso, C.; Gómez-Pérez, A.M.; García-Fuentes, E.; Tinahones, F.J.; Moreno-Indias, I. Gut Microbiota Adaptation after Weight Loss by Roux-en-Y Gastric Bypass or Sleeve Gastrectomy Bariatric Surgeries. Surg. Obes. Relat. Dis. 2019, 15, 1888–1895. Available online: http://www.ncbi.nlm.nih.gov/pubmed/31648978 (accessed on 23 April 2020). [CrossRef] [PubMed]
- Worthmann, A.; John, C.; Rühlemann, M.C.; Baguhl, M.; Heinsen, F.-A.; Schaltenberg, N.; Heine, M.; Schlein, C.; Evangelakos, I.; Mineo, C.; et al. Cold-induced conversion of cholesterol to bile acids in mice shapes the gut microbiome and promotes adaptive thermogenesis. Nat. Med. 2017, 23, 839–849. [Google Scholar] [CrossRef]
- Ziętak, M.; Kovatcheva-Datchary, P.; Markiewicz, L.H.; Ståhlman, M.; Kozak, L.P.; Bäckhed, F. Altered Microbiota Contributes to Reduced Diet-Induced Obesity upon Cold Exposure. Cell Metab. 2016, 23, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Gomez-Ambrosi, J. Rationale for the existence of additional adipostatic hormones. FASEB J. 2001, 15, 1996–2006. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Gómez-Ambrosi, J. Control of body weight: A physiologic and transgenic perspective. Diabetologia 2003, 46, 143–172. [Google Scholar] [CrossRef]
- Sabater-Masdeu, M.; Moreno-Navarrete, J.M.; Ortega, F.J.; Pardo, G.; Salvador, J.; Ricart, W.; Frühbeck, G.; Fernández-Real, J.M. Circulating Pigment Epithelium-Derived Factor Levels Are Associated with Insulin Resistance and Decrease after Weight Loss. J. Clin. Endocrinol. Metab. 2010, 95, 4720–4728. [Google Scholar] [CrossRef]
- Shook, R.P.; A Hand, G.; E Paluch, A.; Wang, X.; Moran, R.G.; Hebert, J.R.; Jakicic, J.M.; Blair, S.N. High respiratory quotient is associated with increases in body weight and fat mass in young adults. Eur. J. Clin. Nutr. 2015, 70, 1197–1202. [Google Scholar] [CrossRef]
- Carrasco, F.; Papapietro, K.; Csendes, A.; Salazar, G.; Echenique, C.; Lisboa, C.; Díaz, E.; Rojas, J. Changes in Resting Energy Expenditure and Body Composition after Weight Loss following Roux-en-Y Gastric Bypass. Obes. Surg. 2007, 17, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Tamboli, R.A.; Hossain, H.A.; Marks, P.A.; Eckhauser, A.W.; Rathmacher, J.A.; Phillips, S.E.; Buchowski, M.S.; Chen, K.Y.; Abumrad, N.N. Body Composition and Energy Metabolism Following Roux-en-Y Gastric Bypass Surgery. Obesity 2010, 18, 1718–1724. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zheng, L.; Guo, J.; Shi, W.; Zhao, F.; Yang, C.; Dai, Q.; Wang, B.; Li, Y. Increased Resting Energy Expenditure/Body Weight and Decreased Respiratory Quotient Correlate with Satisfactory Weight Loss After Sleeve Gastrectomy: A 6-Month Follow-Up. Obes. Surg. 2020, 30, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G. Bariatric and metabolic surgery: A shift in eligibility and success criteria. Nat. Rev. Endocrinol. 2015, 11, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Kit, B.K.; Orpana, H.; Graubard, B.I. Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA 2013, 309, 71–82. [Google Scholar] [CrossRef]
- Chang, S.H.; Stoll, C.R.; Song, J.; Varela, J.E.; Eagon, C.J.; Colditz, G.A. The effectiveness and risks of bariatric surgery: An updated systematic review and meta-analysis, 2003–2012. JAMA Surg. 2014, 149, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Valentí, V.; Martin, M.; Ramírez, B.; Gómez-Ambrosi, J.; Rodríguez, A.; Catalán, V.; Becerril, S.; Lancha, A.; Fernández, S.; Cienfuegos, J.A.; et al. Sleeve Gastrectomy Induces Weight Loss in Diet-Induced Obese Rats Even if High-Fat Feeding Is Continued. Obes. Surg. 2010, 21, 1438–1443. [Google Scholar] [CrossRef]
- Stępień, M.; Gaudichon, C.; Azzout-Marniche, D.; Fromentin, G.; Tomé, D.; Even, P. Postprandial Nutrient Partitioning but Not Energy Expenditure Is Modified in Growing Rats during Adaptation to a High-Protein Diet. J. Nutr. 2010, 140, 939–945. [Google Scholar] [CrossRef]
- Muruzabal, F.J.; Frühbeck, G.; Gomez-Ambrosi, J.; Archanco, M.; A Burrell, M. Immunocytochemical detection of leptin in non-mammalian vertebrate stomach. Gen. Comp. Endocrinol. 2002, 128, 149–152. [Google Scholar] [CrossRef]
- Fortuño, A.; Rodríguez, A.; Gómez-Ambrosi, J.; Muñiz, P.; Salvador, J.; Díez, J.; Frühbeck, G. Leptin Inhibits Angiotensin II-Induced Intracellular Calcium Increase and Vasoconstriction in the Rat Aorta. Endocrinology 2002, 143, 3555–3560. [Google Scholar] [CrossRef]
- Pulido, M.R.; Diaz-Ruiz, A.; Jiménez-Gómez, Y.; Garcia-Navarro, S.; Gracia-Navarro, F.; Tinahones, F.; López-Miranda, J.; Frühbeck, G.; Vázquez-Martínez, R.; Malagón, M.M. Rab18 Dynamics in Adipocytes in Relation to Lipogenesis, Lipolysis and Obesity. PLoS ONE 2011, 6, e22931. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Gena, P.; Méndez-Giménez, L.; Rosito, A.; Valentí, V.; Rotellar, F.; Sola, I.; Moncada, R.; Silva, C.; Svelto, M.; et al. Reduced hepatic aquaporin-9 and glycerol permeability are related to insulin resistance in non-alcoholic fatty liver disease. Int. J. Obes. 2013, 38, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Catalán, V.; Gómez-Ambrosi, J.; Rotellar, F.; Silva, C.; Rodríguez, A.; Salvador, J.; Gil, M.; Cienfuegos, J.; Frühbeck, G. Validation of Endogenous Control Genes in Human Adipose Tissue: Relevance to Obesity and Obesity-associated Type 2 Diabetes Mellitus. Horm. Metab. Res. 2007, 39, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Becerril, S.; Rodríguez, A.; Catalán, V.; Méndez-Giménez, L.; Ramírez, B.; Sáinz, N.; Llorente, M.; Unamuno, X.; Gómez-Ambrosi, J.; Frühbeck, G. Targeted disruption of the iNOS gene improves adipose tissue inflammation and fibrosis in leptin-deficient ob/ob mice: Role of tenascin C. Int. J. Obes. 2018, 42, 1458–1470. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ambrosi, J.; Becerril, S.; Oroz, P.; Zabalza, S.; Rodríguez, A.; Muruzabal, F.J.; Archanco, M.; Gil, M.J.; Burrell, M.A.; Frühbeck, G. Reduced adipose tissue mass and hypoleptinemia in iNOS deficient mice: Effect of LPS on plasma leptin and adiponectin concentrations. FEBS Lett. 2004, 577, 351–356. [Google Scholar] [CrossRef] [PubMed]



| Determination | Sham Surgery (n = 13) | SG (n = 10) | SADI-S (n = 9) | p |
|---|---|---|---|---|
| Final body weight (g) | 529 ± 8 | 510 ± 7 | 345 ± 18 c,f | <0.001 |
| Rectal temperature (°C) | 36.5 ± 0.1 | 37.0 ± 0.1 b | 36.7 ± 0.1 | 0.010 |
| BAT temperature (°C) | 37.5 ± 0.2 | 38.1 ± 0.1 a | 37.6 ± 0.2 | 0.044 |
| VO2 (mL/min/kg0.75) | 8.72 ± 0.10 | 8.69 ± 0.06 | 8.68 ± 0.04 | 0.979 |
| VCO2 (mL/min/kg0.75) | 9.59 ± 0.12 | 9.09 ± 0.07 b | 8.34 ± 0.04 c,f | <0.001 |
| Epididymal WAT (g/100 g BW) | 1.19 ± 0.07 | 1.31 ± 0.10 | 0.39 ± 0.16 c,f | <0.001 |
| Subcutaneous WAT (g/100 g BW) | 0.92 ± 0.07 | 0.94 ± 0.08 | 0.29 ± 0.08 c,f | <0.001 |
| Perirenal WAT (g/100 g BW) | 1.30 ± 0.10 | 1.31 ± 0.08 | 0.40 ± 0.18 c,f | <0.001 |
| BAT (g/100 g BW) | 0.157 ± 0.017 | 0.145 ± 0.007 | 0.056 ± 0.008 c,f | <0.001 |
| Total WAT (g/100 g BW) | 3.41 ± 0.21 | 3.44 ± 0.16 | 0.91 ± 0.37 c,f | <0.001 |
| Glucose (mg/dL) | 73 ± 2 | 79 ± 2 | 59 ± 3 c,f | <0.001 |
| Insulin (ng/mL) | 3.6 ± 0.5 | 2.9 ± 0.4 | 1.4 ± 0.2 b,e | <0.01 |
| HOMA | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.2 ± 0.1 b,e | <0.001 |
| QUICKI | 0.43 ± 0.02 | 0.42 ± 0.01 | 0.53 ± 0.01 c,f | <0.001 |
| FFA (mg/dL) | 19 ± 1 | 23 ± 1 | 18 ± 2 d | 0.084 |
| TG (mg/dL) | 118 ± 14 | 93 ± 8 | 51 ± 8 b,d | <0.001 |
| Cholesterol (mg/dL) | 56 ± 3 | 51 ± 4 | 36 ± 4 b,d | 0.003 |
| Glycerol (mg/dL) | 0.019 ± 0.002 | 0.018 ± 0.001 | 0.015 ± 0.001 | 0.115 |
| Adipo-IR index | 26.2 ± 0.2 | 28.2 ± 0.2 | 8.3 ± 0.1 a,e | 0.002 |
| Leptin (ng/mL) | 4.3 ± 0.4 | 3.3 ± 0.4 | 0.4 ± 0.1 c,f | <0.001 |
| Adiponectin (ng/mL) | 9.8 ± 0.4 | 11.4 ± 0.6 | 6.5 ± 0.8 c,f | <0.001 |
| Adpn/leptin ratio | 2.50 ± 0.21 | 3.24 ± 0.34 | 15.98 ± 3.01 c,f | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becerril, S.; Tuero, C.; Cienfuegos, J.A.; Rodríguez, A.; Catalán, V.; Ramírez, B.; Valentí, V.; Moncada, R.; Unamuno, X.; Gómez-Ambrosi, J.; et al. Improved Adipose Tissue Function after Single Anastomosis Duodeno-Ileal Bypass with Sleeve-Gastrectomy (SADI-S) in Diet-Induced Obesity. Int. J. Mol. Sci. 2022, 23, 11641. https://doi.org/10.3390/ijms231911641
Becerril S, Tuero C, Cienfuegos JA, Rodríguez A, Catalán V, Ramírez B, Valentí V, Moncada R, Unamuno X, Gómez-Ambrosi J, et al. Improved Adipose Tissue Function after Single Anastomosis Duodeno-Ileal Bypass with Sleeve-Gastrectomy (SADI-S) in Diet-Induced Obesity. International Journal of Molecular Sciences. 2022; 23(19):11641. https://doi.org/10.3390/ijms231911641
Chicago/Turabian StyleBecerril, Sara, Carlota Tuero, Javier A. Cienfuegos, Amaia Rodríguez, Victoria Catalán, Beatriz Ramírez, Víctor Valentí, Rafael Moncada, Xabier Unamuno, Javier Gómez-Ambrosi, and et al. 2022. "Improved Adipose Tissue Function after Single Anastomosis Duodeno-Ileal Bypass with Sleeve-Gastrectomy (SADI-S) in Diet-Induced Obesity" International Journal of Molecular Sciences 23, no. 19: 11641. https://doi.org/10.3390/ijms231911641
APA StyleBecerril, S., Tuero, C., Cienfuegos, J. A., Rodríguez, A., Catalán, V., Ramírez, B., Valentí, V., Moncada, R., Unamuno, X., Gómez-Ambrosi, J., & Frühbeck, G. (2022). Improved Adipose Tissue Function after Single Anastomosis Duodeno-Ileal Bypass with Sleeve-Gastrectomy (SADI-S) in Diet-Induced Obesity. International Journal of Molecular Sciences, 23(19), 11641. https://doi.org/10.3390/ijms231911641








