Tick-Borne Encephalitis Virus RNA Found in Frozen Goat’s Milk in a Family Outbreak
Abstract
:1. Introduction
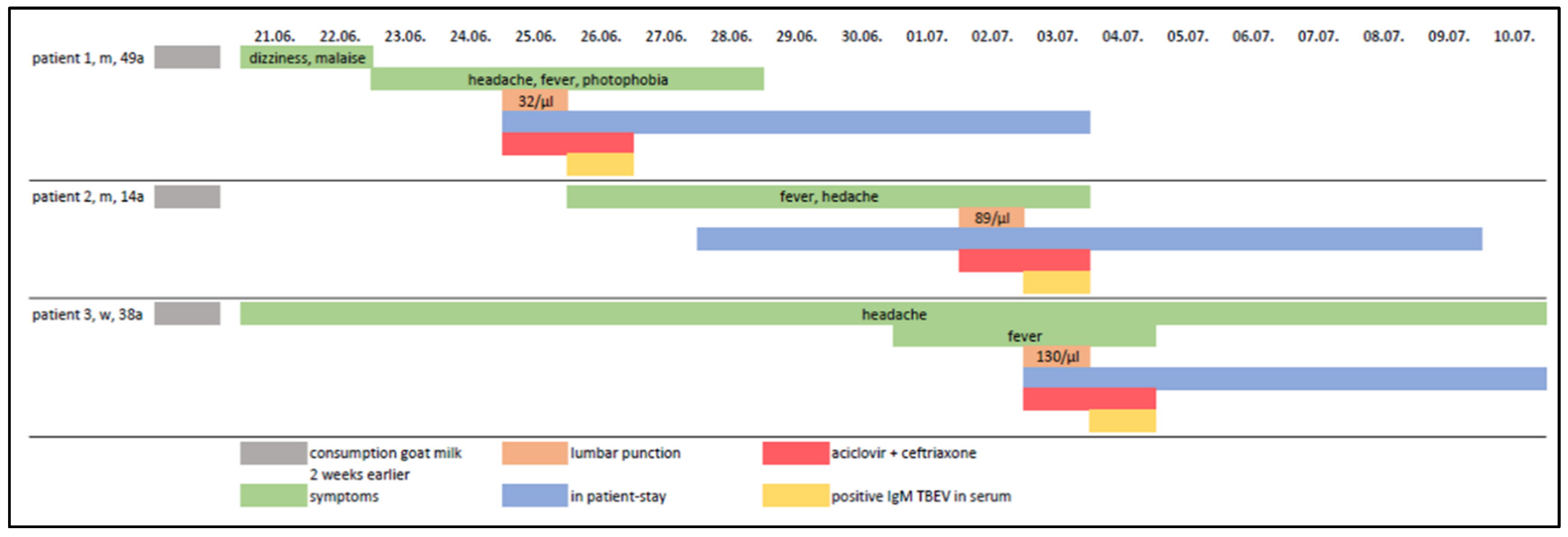
2. Case Series-Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bogovic, P.; Strle, F. Tick-borne encephalitis: A review of epidemiology, clinical characteristics, and management. World J. Clin. Cases 2015, 3, 430–441. [Google Scholar] [CrossRef]
- Kaiser, R. Tick-borne encephalitis. Infect. Dis. Clin. N. Am. 2008, 22, 561–575. [Google Scholar] [CrossRef]
- Kaiser, R. FrühsommerMeningoenzephalitis (FSME), S1-Leitlinie. In Leitlinien für Diagnostik und Therapie in der Neurologie; Deutsche Gesellschaft für Neurologie e.V.: Berlin, Germany, 2020; Available online: www.dgn.org/leitlinien (accessed on 9 August 2022).
- Süss, J. Tick-borne encephalitis 2010: Epidemiology, risk areas, and virus strains in Europe and Asia—An overview. Ticks Tick Borne Dis. 2011, 2, 2–15. [Google Scholar] [CrossRef]
- Wilhelmsson, P.; Jaenson, T.G.T.; Olsen, B.; Waldenström, J.; Lindgren, P.E. Migratory birds as disseminators of ticks and the tick-borne pathogens Borrelia bacteria and tick-borne encephalitis (TBE) virus: A seasonal study at Ottenby Bird Observatory in South-eastern Sweden. Parasit Vectors 2020, 13, 607. [Google Scholar] [CrossRef] [PubMed]
- Beauté, J.; Spiteri, G.; Warns-Petit, E.; Zeller, H. Tick-borne encephalitis in Europe, 2012 to 2016. Eurosurveillance 2018, 23, 1800201. [Google Scholar] [CrossRef] [Green Version]
- Günther, G.; Haglund, M.; Lindquist, L.; Forsgren, M.; Sköldenberg, B. Tick-bone encephalitis in Sweden in relation to aseptic meningo-encephalitis of other etiology: A prospective study of clinical course and outcome. J. Neurol. 1997, 244, 230–238. [Google Scholar] [CrossRef]
- Mickiene, A.; Laiskonis, A.; Günther, G.; Vene, S.; Lundkvist, A.; Lindquist, L. Tickborne encephalitis in an area of high endemicity in lithuania: Disease severity and long-term prognosis. Clin. Infect. Dis. 2002, 35, 650–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taba, P.; Schmutzhard, E.; Forsberg, P.; Lutsar, I.; Ljøstad, U.; Mygland, Å.; Levchenko, I.; Strle, F.; Steiner, I. EAN consensus review on prevention, diagnosis and management of tick-borne encephalitis. Eur. J. Neurol. 2017, 24, 1214-e61. [Google Scholar] [CrossRef]
- Factsheet about Tick-Borne Encephalitis (TBE), Stockholm: European Centre for Disease Prevention and Control. Available online: https://www.ecdc.europa.eu/en/tick-borne-encephalitis/facts/factsheet (accessed on 9 August 2022).
- Ventura Spagnolo, E.; Mondello, C.; Roccuzzo, S.; Cardia, L.; Raffino, C. A lethal Tick-Borne Encephalitis (TBE) due to TBE Virus in Sicily (Italy): A case of IgG+/IgM-response? Clin. Ter. 2018, 169, e145–e148. [Google Scholar] [CrossRef]
- Dekker, M.; Laverman, G.D.; de Vries, A.; Reimerink, J.; Geeraedts, F. Emergence of tick-borne encephalitis (TBE) in the Netherlands. Ticks Tick Borne Dis. 2019, 10, 176–179. [Google Scholar] [CrossRef]
- Paulsen, K.M.; Stuen, S.; Das Neves, C.; Suhel, F.; Gurung, D.; Soleng, A.; Stiasny, K.; Vikse, R.; Andreassen, K.; Granquist, E.G. Tick-borne encephalitis virus in cows and unpasteurized cow milk from Norway. Zoonoses Public Health 2019, 66, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Vikse, R.; Paulsen, K.M.; Edgar, K.S.; Pettersson, J.H.; Ottesen, P.S.; Okbaldet, Y.B.; Kiran, N.; Lamsal, A.; Lindstedt, H.E.H.; Pedersen, B.N.; et al. Geographical distribution and prevalence of tick-borne encephalitis virus in questing Ixodes ricinus ticks and phylogeographic structure of the Ixodes ricinus vector in Norway. Zoonoses Public Health 2020, 67, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Buczek, A.M.; Buczek, W.; Buczek, A.; Wysokińska-Miszczuk, J. Food-Borne Transmission of Tick-Borne Encephalitis Virus-Spread, Consequences, and Prophylaxis. Int. J. Environ. Res. Public Health 2022, 19, 1812. [Google Scholar] [CrossRef] [PubMed]
- Kríz, B.; Benes, C.; Daniel, M. Alimentary transmission of tick-borne encephalitis in the Czech Republic (1997–2008). Epidemiol. Mikrobiol. Imunol. 2009, 58, 98–103. [Google Scholar]
- Brockmann, S.; Oehme, R.; Buckenmaier, T.; Beer, M.; Jeffery-Smith, A.; Spannenkrebs, M.; Haag-Milz, S.; Wagner-Wiening, C.; Schlegel, C.; Fritz, J.; et al. A cluster of two human cases of tick-borne encephalitis (TBE) transmitted by unpasteurised goat milk and cheese in Germany, May 2016. Eurosurveillance 2018, 23, 17-00336. [Google Scholar] [CrossRef] [Green Version]
- Böhm, B.; Schade, B.; Bauer, B.; Hoffmann, B.; Hoffmann, D.; Ziegler, U.; Beer, M.; Klaus, C.; Weissenböck, H.; Böttcher, J. Tick-borne encephalitis in a naturally infected sheep. BMC Vet. Res. 2017, 13, 267. [Google Scholar] [CrossRef] [Green Version]
- Bagó, Z.; Bauder, B.; Kolodziejek, J.; Nowotny, N.; Weissenböck, H. Tickborne encephalitis in a mouflon (Ovis Ammon Musimon). Vet. Rec. 2002, 150, 218–220. [Google Scholar] [CrossRef]
- Rushton, J.O.; Lecollinet, S.; Hubálek, Z.; Svobodová, P.; Lussy, H.; Nowotny, N. Tick-borne encephalitis virus in horses, Austria, 2011. Emerg. Infect. Dis. 2013, 19, 635–637. [Google Scholar] [CrossRef]
- Fouché, N.; Oesch, S.; Ziegler, U.; Gerber, V. Clinical Presentation and Laboratory Diagnostic Work-Up of a Horse with Tick-Borne Encephalitis in Switzerland. Viruses 2021, 13, 1474. [Google Scholar] [CrossRef]
- Pfeffer, M.; Dobler, G. Tick-borne encephalitis virus in dogs-is this an issue? Parasit. Vectors 2011, 4, 59. [Google Scholar] [CrossRef] [Green Version]
- Süss, J.; Gelpi, E.; Klaus, C.; Bagon, A.; Liebler-Tenorio, E.M.; Budka, H.; Stark, B.; Müller, W.; Hotzel, H. Tickborne encephalitis in naturally exposed monkey (Macaca sylvanus). Emerg. Infect. Dis. 2007, 13, 905–907. [Google Scholar] [CrossRef] [PubMed]
- Da Rold, G.; Obber, F.; Monne, I.; Milani, A.; Ravagnan, S.; Toniolo, F.; Sgubin, S.; Zamperin, G.; Foiani, G.; Vascellari, M.; et al. Clinical Tick-Borne Encephalitis in a Roe Deer (Capreolus capreolus L.). Viruses 2022, 14, 300. [Google Scholar] [CrossRef] [PubMed]
- Offerdahl, D.K.; Clancy, N.G.; Bloom, M.E. Stability of a Tick-Borne Flavivirus in Milk. Front. Bioeng. Biotechnol. 2016, 4, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rónai, Z.; Egyed, L. Survival of Tick-Borne Encephalitis Virus in Goat Cheese and Milk. Food Environ. Virol. 2020, 12, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Ilic, M.; Barbic, L.; Bogdanic, M.; Tabain, I.; Savic, V.; Licina, M.L.K.; Kaic, B.; Jungic, A.; Vucelja, M.; Angelov, V.; et al. Tick-borne encephalitis outbreak following raw goat milk consumption in a new micro-location, Croatia, June 2019. Ticks Tick Borne Dis. 2020, 11, 101513. [Google Scholar] [CrossRef]
- Blaškovič, D. The Epidemic of Encephalitis in Roznava Natural Focus of Infection; Slovak Academy of Sciences: Bratislava, Czechoslovakia, 1954; p. 314. [Google Scholar]
- European Centre for Disease Prevention and Control. Tick-Borne Encephalitis. In Annual Epidemiological Report for 2017; ECDC: Stockholm, Sweden, 2019. [Google Scholar]
- Kubinski, M.; Beicht, J.; Gerlach, T.; Volz, A.; Sutter, G.; Rimmelzwaan, G.F. Tick-Borne Encephalitis Virus: A Quest for Better Vaccines against a Virus on the Rise. Vaccines 2020, 8, 451. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mylonaki, E.; Seiberl, M.; Jones, N.; Bernhard, H.; Otto, F.; Pilz, G.; Trinka, E.; Wipfler, P. Tick-Borne Encephalitis Virus RNA Found in Frozen Goat’s Milk in a Family Outbreak. Int. J. Mol. Sci. 2022, 23, 11632. https://doi.org/10.3390/ijms231911632
Mylonaki E, Seiberl M, Jones N, Bernhard H, Otto F, Pilz G, Trinka E, Wipfler P. Tick-Borne Encephalitis Virus RNA Found in Frozen Goat’s Milk in a Family Outbreak. International Journal of Molecular Sciences. 2022; 23(19):11632. https://doi.org/10.3390/ijms231911632
Chicago/Turabian StyleMylonaki, Eirini, Michael Seiberl, Neil Jones, Heike Bernhard, Ferdinand Otto, Georg Pilz, Eugen Trinka, and Peter Wipfler. 2022. "Tick-Borne Encephalitis Virus RNA Found in Frozen Goat’s Milk in a Family Outbreak" International Journal of Molecular Sciences 23, no. 19: 11632. https://doi.org/10.3390/ijms231911632
APA StyleMylonaki, E., Seiberl, M., Jones, N., Bernhard, H., Otto, F., Pilz, G., Trinka, E., & Wipfler, P. (2022). Tick-Borne Encephalitis Virus RNA Found in Frozen Goat’s Milk in a Family Outbreak. International Journal of Molecular Sciences, 23(19), 11632. https://doi.org/10.3390/ijms231911632





