The Role of Forkhead Box O in Pathogenesis and Therapy of Diabetes Mellitus
Abstract
1. Introduction
2. Structure of FOXO
3. Post-Translational Changes—A Brief Overview
Phosphorylation
4. Acetylation and Deacetylation of FOXO
5. Ubiquitination
6. Methylation
7. Glycosylation
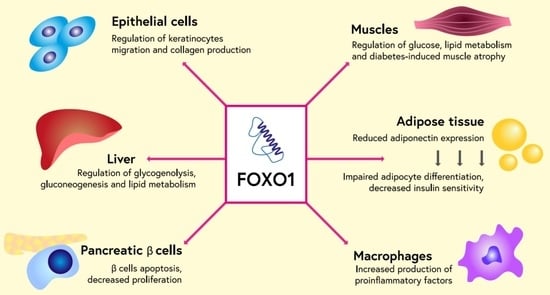
8. Role of FOXO Proteins in Diabetes and Its Complications
9. FOXO as a Therapeutic Target in Type 2 Diabetes and its Complications
1,25-Dihydroxyvitamin D
10. N1-Methylnicotinamide
11. Fucoxanthin
12. miR-233
13. Entacapone
14. Diazoxide
15. AS1842856
16. Atorvastatin
17. Resveratrol
18. Liraglutide
19. Metformin
20. Clinical Significance and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greer, E.L.; Brunet, A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene 2005, 24, 7410–7425. [Google Scholar] [CrossRef] [PubMed]
- Van Der Vos, K.E.; Coffer, P.J. FOXO-binding partners: It takes two to tango. Oncogene 2008, 27, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Tzivion, G.; Dobson, M.; Ramakrishnan, G. FoxO transcription factors; Regulation by AKT and 14-3-3 proteins. Biochim. Biophys. Acta 2011, 1813, 1938–1945. [Google Scholar] [CrossRef] [PubMed]
- Calissi, G.; Lam, E.W.-F.; Link, W. Therapeutic strategies targeting FOXO transcription factors. Nat. Rev. Drug Discov. 2021, 20, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, T.; Huang, P. Post-translational modifications of FOXO family proteins (Review). Mol. Med. Rep. 2016, 14, 4931–4941. [Google Scholar] [CrossRef]
- Yao, S.; Mahmud, Z.; Sachini, N.; Aimjongjun, S.; Saavedra-García, P.; Lam, E.W.-F. Characterization of FOXO Acetylation. Methods Mol. Biol. 2019, 1890, 77–90. [Google Scholar] [CrossRef]
- Yang, H.; Yan, B.; Liao, D.; Huang, S.; Qiu, Y. Acetylation of HDAC1 and degradation of SIRT1 form a positive feedback loop to regulate p53 acetylation during heat-shock stress. Cell Death Dis. 2015, 6, e1747. [Google Scholar] [CrossRef]
- Grumati, P.; Dikic, I. Ubiquitin signaling and autophagy. J. Biol. Chem. 2018, 293, 5404–5413. [Google Scholar] [CrossRef]
- Brenkman, A.B.; de Keizer, P.; Broek, N.J.F.V.D.; Jochemsen, A.G.; Burgering, T.B.M. Mdm2 Induces Mono-Ubiquitination of FOXO4. PLoS ONE 2008, 3, e2819. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Daitoku, H.; Takahashi, Y.; Namiki, K.; Hisatake, K.; Kako, K.; Mukai, H.; Kasuya, Y.; Fukamizu, A. Arginine Methylation of FOXO Transcription Factors Inhibits Their Phosphorylation by Akt. Mol. Cell 2008, 32, 221–231. [Google Scholar] [CrossRef]
- Butt, A.M.; Feng, D.; Idrees, M.; Tong, Y.; Lu, J. Computational identification and modeling of crosstalk between phosphorylation, O-β-glycosylation and methylation of FOXO3 and implications for cancer therapeutics. Int. J. Mol. Sci. 2012, 13, 2918–2938. [Google Scholar] [CrossRef]
- Ren, H.; Shao, Y.; Wu, C.; Ma, X.; Lv, C.; Wang, Q. Metformin alleviates oxidative stress and enhances autophagy in diabetic kidney disease via AMPK/SIRT1-FoxO1 pathway. Mol. Cell. Endocrinol. 2020, 500, 110628. [Google Scholar] [CrossRef]
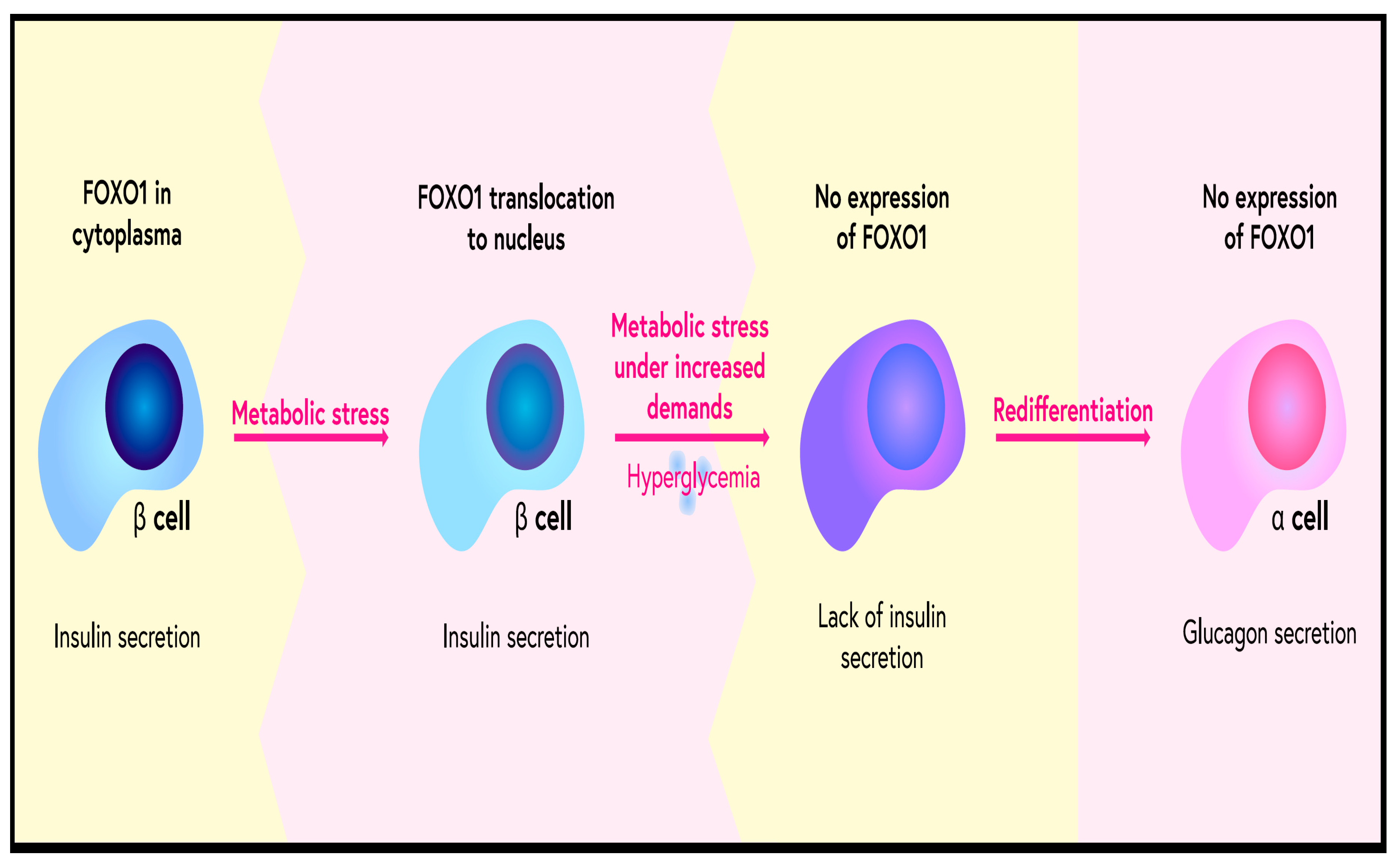
- Buteau, J.; Accili, D. Regulation of pancreatic beta-cell function by the forkhead protein FOXO1. Diabetes Obes. Metab. 2007, 9 (Suppl. 2), 140–146. [Google Scholar] [CrossRef] [PubMed]
- Talchai, C.; Xuan, S.; Lin, H.V.; Sussel, L.; Accili, D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β Cell failure. Cell 2012, 150, 1223–1234. [Google Scholar] [CrossRef]
- Honzawa, N.; Fujimoto, K. The Plasticity of Pancreatic β-Cells. Metabolites 2021, 11, 218. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guan, Y.; Xue, L.; Liu, J.; Yang, Z.; Nie, C.; Yan, Y.; Liu, S.; Sun, J.; Fan, M.; et al. l-Arabinose suppresses gluconeogenesis through modulating AMP-activated protein kinase in metabolic disorder mice. Food Funct. 2021, 12, 1745–1756. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Cai, Y.; Luo, J.; Liu, J.; Li, X.; Ying, F.; Xie, X.; Xu, A.; Ma, X.; Xia, Z. FOXO1 contributes to diabetic cardiomyopathy via inducing imbalanced oxidative metabolism in type 1 diabetes. J. Cell. Mol. Med. 2020, 24, 7850–7861. [Google Scholar] [CrossRef] [PubMed]
- Peserico, A.; Chiacchiera, F.; Grossi, V.; Matrone, A.; Latorre, D.; Simonatto, M.; Fusella, A.; Ryall, J.G.; Finley, L.W.S.; Haigis, M.C.; et al. A novel AMPK-dependent FoxO3A-SIRT3 intramitochondrial complex sensing glucose levels. Cell Mol. Life Sci. 2013, 70, 2015–2029. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Dong, H.H. FOXO integration of insulin signaling with glucose and lipid metabolism. J. Endocrinol. 2017, 233, R67–R79. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Ubaid, S. Role of Silent Information Regulator 1 (SIRT1) in Regulating Oxidative Stress and Inflammation. Inflammation 2020, 43, 1589–1598. [Google Scholar] [CrossRef]
- Kibbe, C.; Chen, J.; Xu, G.; Jing, G.; Shalev, A. FOXO1 Competes with carbohydrate response element-binding protein (ChREBP) and inhibits thioredoxin-interacting protein (TXNIP) transcription in pancreatic beta cells. J. Biol. Chem. 2013, 288, 23194–23202. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, Y.I.; Kitamura, T.; Kruse, J.P.; Raum, J.C.; Stein, R.; Gu, W.; Accili, D. FOXO1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab. 2005, 2, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Furuyama, T.; Kitayama, K.; Yamashita, H.; Mori, N. Forkhead transcription factor FOXO1 (FKHR)-dependent induction of PDK4 gene expression in skeletal muscle during energy deprivation. Biochem. J. 2003, 375, 365–371. [Google Scholar] [CrossRef]
- Gupta, A.; Stocker, H. FoxO suppresses endoplasmic reticulum stress to inhibit growth of Tsc1-deficient tissues under nutrient restriction. eLife 2020, 9. [Google Scholar] [CrossRef]
- Bowker-Kinley, M.M.; Davis, W.I.; Wu, P.; Harris, R.A.; Popov, K.M. Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochem. J. 1998, 329, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Gopal, K.; Saleme, B.; Al Batran, R.; Aburasayn, H.; Eshreif, A.; Ho, K.L.; Ma, W.K.; Almutairi, M.; Eaton, F.; Gandhi, M.; et al. FoxO1 regulates myocardial glucose oxidation rates via transcriptional control of pyruvate dehydrogenase kinase 4 expression. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H479–H490. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.X.; Heine, M.; Schlein, C.; Ramakrishnan, R.; Liu, J.; Belnavis, G.; Haimi, I.; Fischer, A.W.; Ginsberg, H.N.; Heeren, J.; et al. FoxO transcription factors are required for hepatic HDL cholesterol clearance. J. Clin. Investig. 2018, 128, 1615–1626. [Google Scholar] [CrossRef]
- Tsuchida, A.; Yamauchi, T.; Ito, Y.; Hada, Y.; Maki, T.; Takekawa, S.; Kamon, J.; Kobayashi, M.; Suzuki, R.; Hara, K.; et al. Insulin/Foxo1 pathway regulates expression levels of adiponectin receptors and adiponectin sensitivity. J. Biol. Chem. 2004, 279, 30817–30822. [Google Scholar] [CrossRef]
- Mezza, T.; Shirakawa, J.; Martinez, R.; Hu, J.; Giaccari, A.; Kulkarni, R.N. Nuclear Export of FoxO1 Is Associated with ERK Signaling in β-Cells Lacking Insulin Receptors. J. Biol. Chem. 2016, 291, 21485–21495. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Chen, C.-C.; Li, T.-K.; Wang, P.-H.; Liu, L.-R.; Chang, F.-Y.; Wang, Y.-C.; Yu, Y.-H.; Lin, S.-P.; Mersmann, H.J.; et al. Docosahexaenoic acid suppresses the expression of FoxO and its target genes. J. Nutr. Biochem. 2012, 23, 1609–1616. [Google Scholar] [CrossRef]
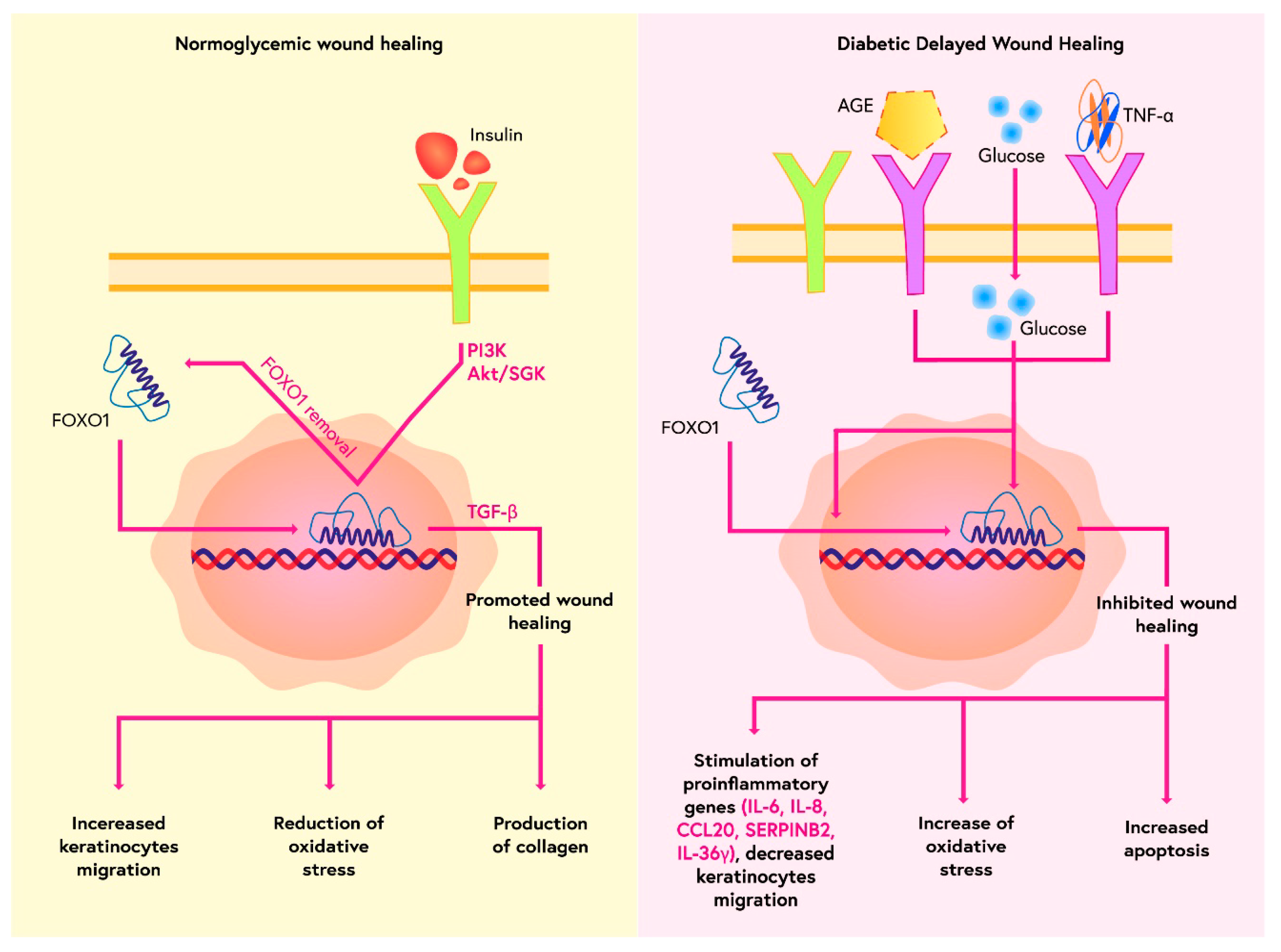
- Miao, C.; Li, Y.; Zhang, X. The functions of FoxO transcription factors in epithelial wound healing. Australas. J. Dermatol. 2019, 60, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Saoncella, S.; Tassone, B.; Deklic, E.; Avolio, F.; Jon, C.; Tornillo, G.; Luca, E.; Iorio, E.; Piva, R.; Cabodi, S.; et al. Nuclear Akt2 opposes limbal keratinocyte stem cell self-renewal by repressing a FOXO-mTORC1 signaling pathway. Stem Cells. 2014, 32, 754–769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lim, J.; Jeon, H.H.; Xu, F.; Tian, C.; Miao, F.; Hameedaldeen, A.; Graves, D.T. FOXO1 deletion in keratinocytes improves diabetic wound healing through MMP9 regulation. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ponugoti, B.; Xu, F.; Zhang, C.; Tian, C.; Pacios, S.; Graves, D.T. FOXO1 promotes wound healing through the up-regulation of TGF-β1 and prevention of oxidative stress. J. Cell Biol. 2013, 203, 327–343. [Google Scholar] [CrossRef] [PubMed]
- González-Molero, I.; Rojo-Martínez, G.; Morcillo, S.; Gutiérrez-Repiso, C.; Rubio-Martín, E.; Almaraz, M.C.; Olveira, G.; Soriguer, F. Vitamin D and incidence of diabetes: A prospective cohort study. Clin. Nutr. 2012, 31, 571–573. [Google Scholar] [CrossRef]
- Pittas, A.G.; Nathan, D.M.; Nelson, J.; Hu, F.; Mitri, J.; Dawson-Hughes, B.; Diabetes Prevention Program Research Group. Plasma 25-hydroxyvitamin D and progression to diabetes in patients at risk for diabetes: An ancillary analysis in the diabetes prevention program. Diabetes Care. 2012, 35, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, N.G.; Ye, Z.; Rickard, A.P.; Khaw, K.T.; Luben, R.; Langenberg, C.; Wareham, N.J. Circulating 25-hydroxyvitamin D concentration and the risk of type 2 diabetes: Results from the European Prospective Investigation into Cancer (EPIC)-Norfolk cohort and updated meta-analysis of prospective studies. Diabetologia 2012, 55, 2173–2182. [Google Scholar] [CrossRef]
- Borissova, A.M.; Tankova, T.; Kirilov, G.; Dakovska, L.; Kovacheva, R. The effect of vitamin D3 on insulin secretion and peripheral insulin sensitivity in type 2 diabetic patients. Int. J. Clin. Pract. 2003, 57, 258–261. [Google Scholar]
- Naharci, I.; Bozoglu, E.; Kocak, N.; Doganci, S.; Doruk, H.; Serdar, M. Effect of vitamin D on insulin sensitivity in elderly patients with impaired fasting glucose. Geriatr. Gerontol. Int. 2012, 12, 454–460. [Google Scholar] [CrossRef]
- Nazarian, S.; Peter, J.V.S.; Boston, R.C.; Jones, S.A.; Mariash, C.N. Vitamin D3 supplementation improves insulin sensitivity in subjects with impaired fasting glucose. Transl. Res. 2011, 158, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Jehle, S.; Lardi, A.; Felix, B.; Hulter, H.N.; Stettler, C.; Krapf, R. Effect of large doses of parenteral vitamin D on glycaemic control and calcium/phosphate metabolism in patients with stable type 2 diabetes mellitus: A randomised, placebo-controlled, prospective pilot study. Swiss. Med. Wkly. 2014, 144, w13942. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, S.; Villalta, S.A.; Agrawal, D.K. FOXO1 Mediates Vitamin D deficiency-induced insulin resistance in skeletal muscle. J. Bone Miner. Res. 2015, 31, 585–595. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, Y.; Xin, N.; Yuan, Y.; Zhang, Q.; Gong, P.; Wu, Y. 1α,25-Dihydroxyvitamin D3 promotes bone formation by promoting nuclear exclusion of the FoxO1 transcription factor in diabetic mice. J. Biol. Chem. 2016, 292, 20270–20280. [Google Scholar] [CrossRef]
- Guo, X.; Lin, H.; Liu, J.; Wang, D.; Li, D.; Jiang, C.; Tang, Y.; Wang, J.; Zhang, T.; Li, Y.; et al. 1,25-Dihydroxyvitamin D attenuates diabetic cardiac autophagy and damage by vitamin D receptor-mediated suppression of FoxO1 translocation. J. Nutr. Biochem. 2020, 80, 108380. [Google Scholar] [CrossRef] [PubMed]
- Watała, C.; Kaźmierczak, P.; Dobaczewski, M.; Przygodzki, T.; Bartuś, M.; Łomnicka, M.; Slominska, E.M.; Duračkova, Z.; Chlopicki, S. Anti-diabetic effects of 1-methylnicotinamide (MNA) in streptozocin-induced diabetes in rats. Pharmacol. Rep. 2009, 61, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Moreno-Navarrete, J.M.; Wei, X.; Kikukawa, Y.; Tzameli, I.; Prasad, D.; Lee, Y.; Asara, J.M.; Fernández-Real, J.M.; Maratos-Flier, E.; et al. Nicotinamide N-methyltransferase regulates hepatic nutrient metabolism through Sirt1 protein stabilization. Nat. Med. 2015, 21, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Y.; Liu, C.; Li, L.; Li, P. N1-Methylnicotinamide Improves Hepatic Insulin Sensitivity via Activation of SIRT1 and Inhibition of FOXO1 Acetylation. J. Diabetes Res. 2020, 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Murakami-Funayama, K.; Miyashita, K. Anti-obesity and anti-diabetic effects of fucoxanthin on diet-induced obesity conditions in a murine model. Mol. Med. Rep. 2009, 2, 897–902. [Google Scholar] [CrossRef]
- Yang, G.; Jin, L.; Zheng, D.; Tang, X.; Yang, J.; Fan, L.; Xie, X. Fucoxanthin alleviates oxidative stress through Akt/SIRT1/FOXO3α signaling to inhibit Hg-induced renal fibrosis in GMCs. Mar. Drugs 2019, 17, 702. [Google Scholar] [CrossRef]
- Li, A.; Peng, R.; Sun, Y.; Liu, H.; Peng, H.; Zhang, Z. LincRNA 1700020I14Rik alleviates cell proliferation and fibrosis in diabetic nephropathy via MIR-34a-5p/Sirt1/HIF-1α signaling. Cell Death Dis. 2018, 9, 461. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Cao, Q.; Wang, F.; Huang, L.-Y.; Sang, T.-T.; Liu, F.; Chen, S.-Y. SIRT1 Protects Against Oxidative Stress-Induced Endothelial Progenitor Cells Apoptosis by Inhibiting FOXO3a via FOXO3a Ubiquitination and Degradation. J. Cell. Physiol. 2015, 230, 2098–2107. [Google Scholar] [CrossRef]
- Kim-Muller, J.Y.; Zhao, S.; Srivastava, S.; Mugabo, Y.; Noh, H.L.; Kim, Y.R.; Madiraju, M.; Ferrante, A.W.; Skolnik, E.Y.; Prentki, M.; et al. Metabolic inflexibility impairs insulin secretion and results in MODY-like diabetes in triple FOXO-deficient mice. Cell Metab. 2014, 20, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Filios, S.R.; Shalev, A. β-cell microRNAs: Small but powerful. Diabetes. 2015, 64, 3631–3644. [Google Scholar] [CrossRef] [PubMed]
- Osmai, M.; Osmai, Y.; Bang-Berthelsen, C.H.; Pallesen, E.M.H.; Vestergaard, A.L.; Novotny, G.W.; Pociot, F.; Mandrup-Poulsen, T. MicroRNAs as regulators of beta-cell function and dysfunction. Diabetes/Metab. Res. Rev. 2016, 32, 334–349. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Deng, S.; Peng, J.; Wang, X.; Essandoh, K.; Mu, X.; Peng, T.; Meng, Z.-X.; Fan, G.-C. MicroRNA-223 is essential for maintaining functional β-cell mass during diabetes through inhibiting both FOXO1 and SOX6 pathways. J. Biol. Chem. 2019, 294, 10438–10448. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Xiao, W.; Ju, D.; Sun, B.; Hou, N.; Liu, Q.; Wang, Y.; Zhao, H.; Gao, C.; Zhang, S.; et al. Identification of entacapone as a chemical inhibitor of FTO mediating metabolic regulation through FOXO1. Sci. Transl. Med. 2019, 11, eaau7116. [Google Scholar] [CrossRef]
- Akao, M.; Ohler, A.; O’Rourke, B.; Marbán, E. Mitochondrial ATP-Sensitive potassium channels inhibit apoptosis induced by oxidative stress in cardiac cells. Circ. Res. 2001, 88, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Xu, Z.; Zhu, Q.; Thomas, C.; Kumar, R.; Feng, H.; Dostal, D.E.; White, M.F.; Baker, K.M.; Guo, S. Myocardial Loss of IRS1 and IRS2 Causes Heart Failure and Is Controlled by p38α MAPK During Insulin Resistance. Diabetes 2013, 62, 3887–3900. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Wang, J.; Li, Y.; Wei, S.; Su, F.; Zhang, S.; Duan, Y.; Wang, L.; Zhu, Q. Opening of mitoKATP improves cardiac function and inhibits apoptosis via the AKT-Foxo1 signaling pathway in diabetic cardiomyopathy. Int. J. Mol. Med. 2018, 42, 2709–2719. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, T.; Shigematsu, N.; Maruki, R.; Urano, Y.; Tanaka, H.; Shimaya, A.; Shimokawa, T.; Shibasaki, M. Discovery of Novel Forkhead Box O1 inhibitors for treating Type 2 diabetes: Improvement of fasting glycemia in diabetic db/db mice. Mol. Pharmacol. 2010, 78, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Fan, S.; Wang, D.; Huyan, T.; Chen, J.; Chen, J.; Su, J.; Li, X.; Wang, Z.; Xie, S.; et al. FOXO1 inhibition potentiates endothelial angiogenic functions in diabetes via suppression of ROCK1/Drp1-mediated mitochondrial fission. Biochim. Biophys. Acta. Mol. Basis. Dis. 2018, 1864, 2481–2494. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Wei, R.; Yang, J.; Liu, J.; Yang, K.; Wang, H.; Mu, Y.; Hong, T. FoxO1 inhibition promotes differentiation of human embryonic stem cells into insulin producing cells. Exp. Cell Res. 2018, 362, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Hwang, I.; Kim, S.-J.; Youn, S.-W.; Hur, J.; Kim, H.-S. Atorvastatin prevents endothelial dysfunction in high glucose condition through Skp2-mediated degradation of FOXO1 and ICAM-1. Biochem. Biophys. Res. Commun. 2018, 495, 2050–2057. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-Y.; Youn, S.-W.; Cho, H.-J.; Kwon, Y.-W.; Lee, S.-W.; Kim, S.-J.; Park, Y.-B.; Oh, B.-H.; Kim, H.-S. FOXO1 impairs whereas statin protects endothelial function in diabetes through reciprocal regulation of Krüppel-like factor 2. Cardiovasc. Res. 2013, 97, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Atkins, G.B.; Jain, M.K. Role of Krüppel-like transcription factors in endothelial biology. Circ. Res. 2007, 100, 1686–1695. [Google Scholar] [CrossRef]
- Jang, I.-A.; Kim, E.N.; Lim, J.H.; Kim, M.Y.; Ban, T.H.; Yoon, H.E.; Park, C.W.; Chang, Y.S.; Choi, B.S. Effects of resveratrol on the renin-angiotensin system in the aging kidney. Nutrients 2018, 10, 1741. [Google Scholar] [CrossRef]
- Galiniak, S.; Aebisher, D.; Bartusik-Aebisher, D. Health benefits of resveratrol administration. Acta Biochim. Pol. 2019, 66, 13–21. [Google Scholar] [CrossRef]
- Wang, X.; Meng, L.; Zhao, L.; Wang, Z.; Liu, H.; Liu, G.; Guan, G. Resveratrol ameliorates hyperglycemia-induced renal tubular oxidative stress damage via modulating the SIRT1/FOXO3a pathway. Diabetes Res. Clin. Pract. 2017, 126, 172–181. [Google Scholar] [CrossRef]
- Li, P.; Song, X.; Zhang, D.; Guo, N.; Wu, C.; Chen, K.; Liu, Y.; Yuan, L.; Chen, X.; Huang, X. Resveratrol improves left ventricular remodeling in chronic kidney disease via Sirt1-mediated regulation of FoxO1 activity and MnSOD expression. BioFactors 2020, 46, 168–179. [Google Scholar] [CrossRef]
- Chen, P.; Shi, X.; Xu, X.; Lin, Y.; Shao, Z.; Wu, R.; Huang, L. Liraglutide ameliorates early renal injury by the activation of renal FoxO1 in a type 2 diabetic kidney disease rat model. Diabetes Res. Clin. Pract 2018, 137, 173–182. [Google Scholar] [CrossRef]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef]
- Al-Masri, M.; Krishnamurthy, M.; Li, J.; Fellows, G.F.; Dong, H.H.; Goodyer, C.G.; Wang, R. Effect of forkhead box O1 (FOXO1) on beta cell development in the human fetal pancreas. Diabetologia 2010, 53, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, H.; Jia, C.Y.; Cheng, W.; Yu, M.; Peng, M.; Zhu, Y.; Zhao, Q.; Dong, Y.W.; Shao, K.; et al. MicroRNA-223 regulates FOXO1 expression and cell proliferation. FEBS Lett. 2012, 586, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Kousteni, S.; Kousteni, S. The PDK1-FOXO1 signaling in adipocytes controls systemic insulin sensitivity through the 5-lipoxygenase-leukotriene B4 axis. FOXO1, the transcriptional chief of staff of energy metabolism. Bone 2012, 50, 437–443. [Google Scholar] [CrossRef] [PubMed]
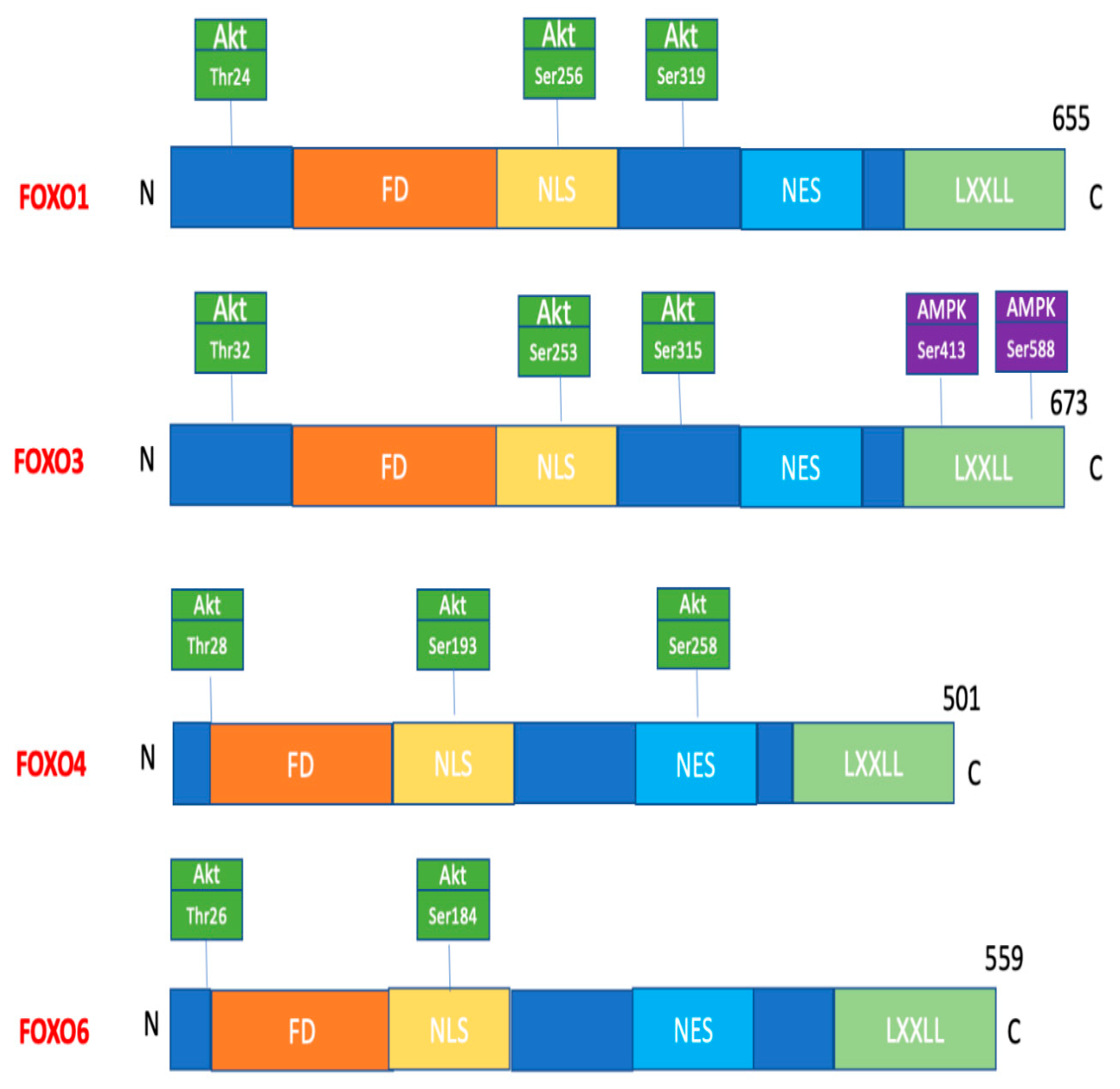
| Kinase/FOXO/Site of Phosphorylation | Physiological Effect of Phosphorylation |
|---|---|
| AKT FOXO1: Thr24, Ser256, Ser319 FOXO3: Thr32, Ser253, Ser315 FOXO4: Thr28, Ser193, Ser256 FOXO6: Thr26, Ser184 | FOXO retention in the cytoplasm |
| SGKs/Ser315 | FOXO retention in the cytoplasm |
| PERK/ Ser298 Ser301 Ser303 | FOXO retention in the cell nucleus, increase in transcriptional activity |
| MST1/Ser212 | Phosphorylation of FOXO3 translocation into the cell nucleus |
| AMPK Ser399, Ser413, Ser555, Ser588, Ser626 | Promoting interaction between cofactors and FOXO3 |
| JNK Thr447, Thr451 | Phosphorylation of FOXO4, translocation of FOXO into the cell nucleus |
| P38 Ser294, Ser425, Ser7 | Phosphorylation of FOXO3 in response to oxidative stress, translocation to the cell nucleus |
| ERK Ser294, ser344, ser425 | Phosphorylation of FOXO3, retention in the cytoplasm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchelek-Mysliwiec, M.; Nalewajska, M.; Turoń-Skrzypińska, A.; Kotrych, K.; Dziedziejko, V.; Sulikowski, T.; Pawlik, A. The Role of Forkhead Box O in Pathogenesis and Therapy of Diabetes Mellitus. Int. J. Mol. Sci. 2022, 23, 11611. https://doi.org/10.3390/ijms231911611
Marchelek-Mysliwiec M, Nalewajska M, Turoń-Skrzypińska A, Kotrych K, Dziedziejko V, Sulikowski T, Pawlik A. The Role of Forkhead Box O in Pathogenesis and Therapy of Diabetes Mellitus. International Journal of Molecular Sciences. 2022; 23(19):11611. https://doi.org/10.3390/ijms231911611
Chicago/Turabian StyleMarchelek-Mysliwiec, Malgorzata, Magdalena Nalewajska, Agnieszka Turoń-Skrzypińska, Katarzyna Kotrych, Violetta Dziedziejko, Tadeusz Sulikowski, and Andrzej Pawlik. 2022. "The Role of Forkhead Box O in Pathogenesis and Therapy of Diabetes Mellitus" International Journal of Molecular Sciences 23, no. 19: 11611. https://doi.org/10.3390/ijms231911611
APA StyleMarchelek-Mysliwiec, M., Nalewajska, M., Turoń-Skrzypińska, A., Kotrych, K., Dziedziejko, V., Sulikowski, T., & Pawlik, A. (2022). The Role of Forkhead Box O in Pathogenesis and Therapy of Diabetes Mellitus. International Journal of Molecular Sciences, 23(19), 11611. https://doi.org/10.3390/ijms231911611