Biological Activity of fac-[Re(CO)3(phen)(aspirin)], fac-[Re(CO)3(phen)(indomethacin)] and Their Original Counterparts against Ishikawa and HEC-1A Endometrial Cancer Cells
Abstract
1. Introduction
2. Results
2.1. Effects of Aspirin, Indomethacin and Their Rhenium Derivatives on Endometrial Cancer Cell Viability
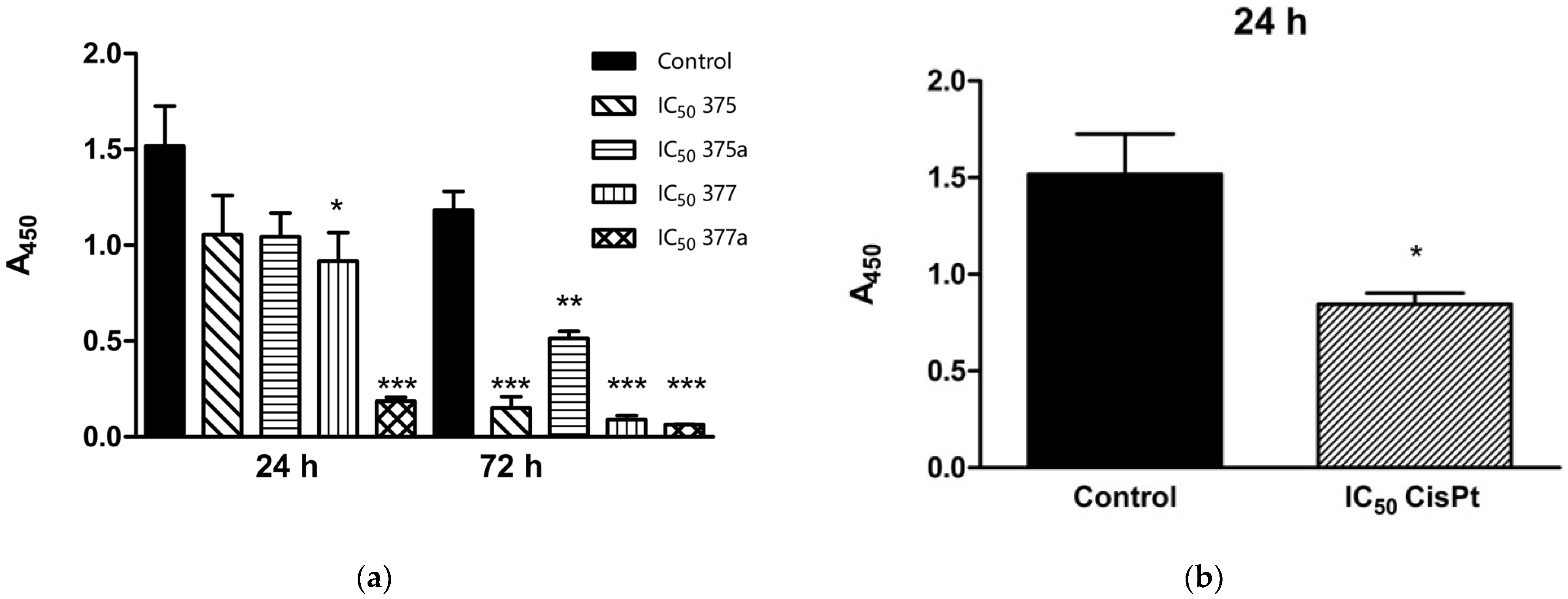
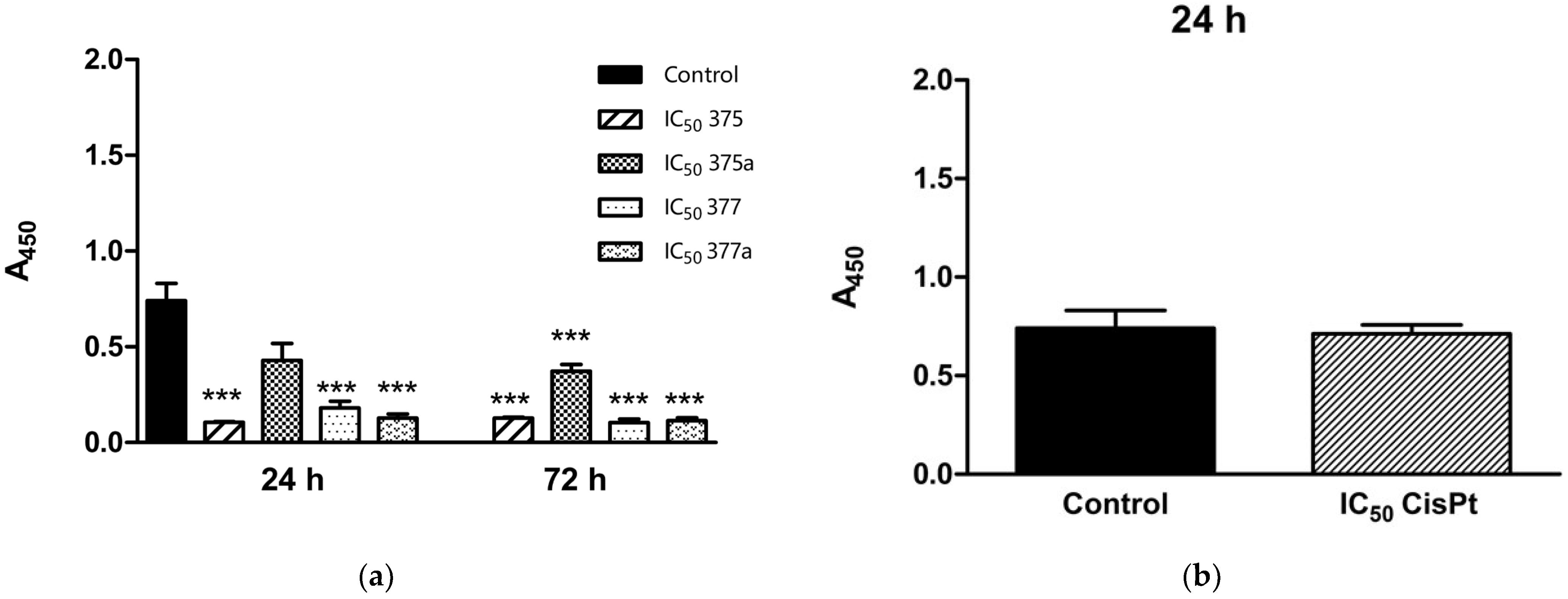
2.2. Inhibition of Endometrial Cancer Cell Proliferation by Aspirin, Indomethacin, and Their Rhenium Derivatives
2.3. The Effect of Aspirin, Indomethacin and Their Rhenium Derivatives on ROS Level in the Studied Endometrial Cancer Cells
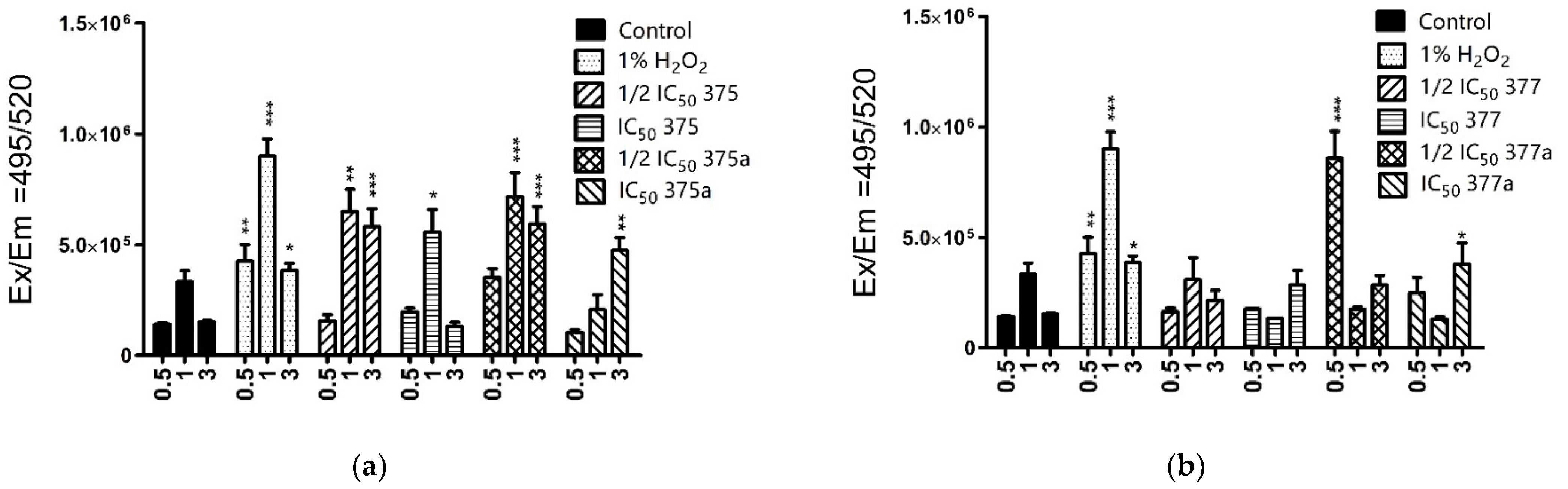
2.3.1. Cytosolic ROS Level Measurement
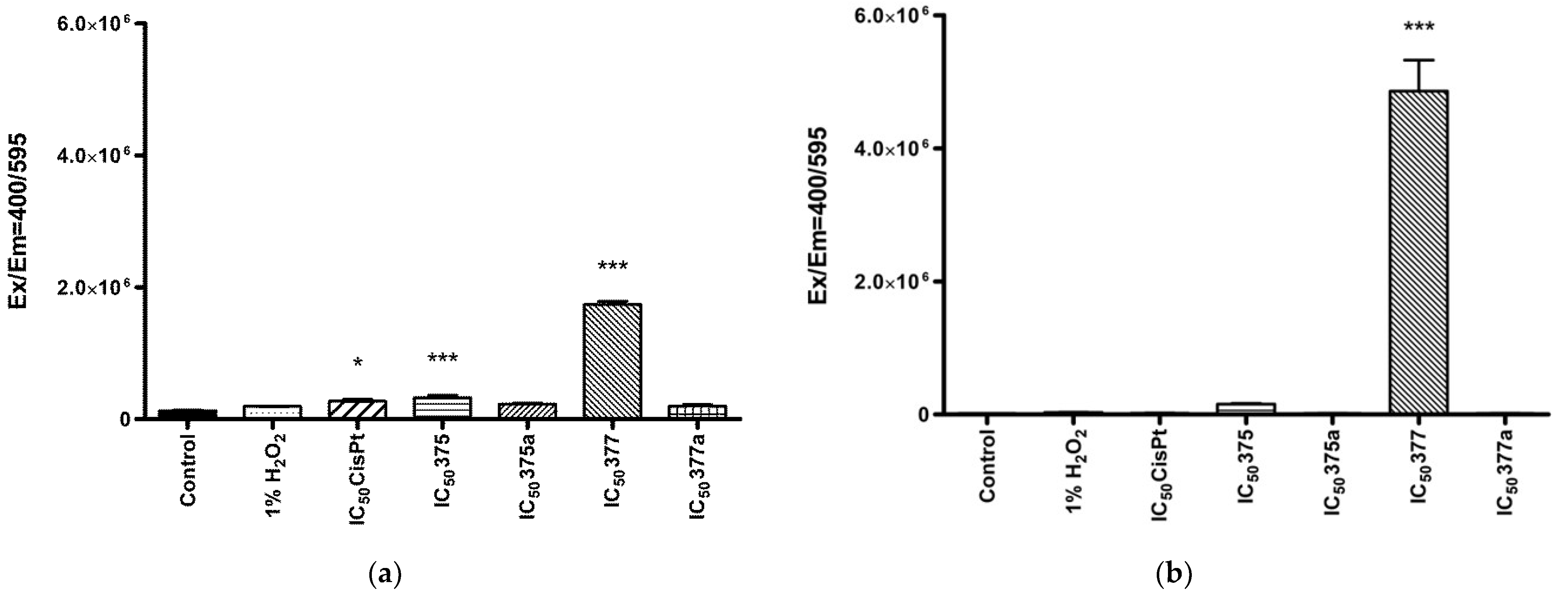
2.3.2. Mitochondrial ROS Level Measurement
3. Discussion
4. Materials and Methods
4.1. NSAIDs and Rhenium-NSAIDs Complexes
4.2. Human Cell Culture
4.3. Cell Viability Assay
4.4. BrdU Assay
4.5. Cellular ROS Level Measurement
4.6. Mitochondrial ROS Level Measurement
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, W.L.; Dewitt, D.L.; Garavito, R.M. Cyclooxygenases: Structural, Cellular, and Molecular Biology. Annu. Rev. Biochem. 2000, 69, 145–182. [Google Scholar] [CrossRef]
- De Leval, X.; Delarge, J.; Somers, F.; De Tullio, P.; Henrotin, Y.; Pirotte, B.; Dogne, J.-M. Recent advances in inducible cyclooxygenase (COX-2) inhibition. Curr. Med. Chem. 2000, 7, 1041–1062. [Google Scholar] [CrossRef]
- Macciò, A.; Madeddu, C. Inflammation and ovarian cancer. Cytokine 2012, 58, 133–147. [Google Scholar] [CrossRef]
- Deivendran, S.; Marzook, K.H.; Pillai, M.R. The Role of Inflammation in Cervical Cancer. Adv. Exp. Med. Biol. 2014, 816, 377–399. [Google Scholar] [CrossRef]
- Szewczyk, G.; Maciejewski, T.M.; Szukiewicz, D. Current progress in the inflammatory background of angiogenesis in gynecological cancers. Inflamm. Res. 2019, 68, 247–260. [Google Scholar] [CrossRef]
- Chubak, J.; Whitlock, E.P.; Williams, S.B.; Kamineni, A.; Burda, B.U.; Buist, D.S.; Anderson, M.L. Aspirin for the Prevention of Cancer Incidence and Mortality: Systematic Evidence Reviews for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2016, 164, 814–825. [Google Scholar] [CrossRef]
- Friis, S.; Riis, A.H.; Erichsen, R.; Baron, J.A.; Sørensen, H.T. Low-Dose Aspirin or Nonsteroidal Anti-inflammatory Drug Use and Colorectal Cancer Risk. Ann. Intern. Med. 2015, 163, 347–355. [Google Scholar] [CrossRef]
- Kehm, R.D.; Hopper, J.L.; John, E.M.; Phillips, K.-A.; MacInnis, R.J.; Dite, G.S.; Milne, R.L.; Liao, Y.; Zeinomar, N.; Knight, J.A.; et al. Regular use of aspirin and other non-steroidal anti-inflammatory drugs and breast cancer risk for women at familial or genetic risk: A cohort study. Breast Cancer Res. 2019, 21, 52. [Google Scholar] [CrossRef]
- Doat, S.; Cénée, S.; Trétarre, B.; Rebillard, X.; Lamy, P.-J.; Bringer, J.-P.; Iborra, F.; Murez, T.; Sanchez, M.; Menegaux, F. Nonsteroidal anti-inflammatory drugs (NSAIDs) and prostate cancer risk: Results from the EPICAP study. Cancer Med. 2016, 6, 2461–2470. [Google Scholar] [CrossRef]
- Chiu, L.; Tong, K.; Ooi, V. Cytostatic and cytotoxic effects of cyclooxygenase inhibitors and their synergy with docosahexaenoic acid on the growth of human skin melanoma A-375 cells. Biomed. Pharmacother. 2005, 59, S293–S297. [Google Scholar] [CrossRef]
- Marinov, L.; Georgieva, A.; Voynikov, Y.; Toshkova, R.; Nikolova, I.; Malchev, M. Cytotoxic and antiproliferative effects of the nonsteroidal anti-inflammatory drug diclofenac in human tumour cell lines. Biotechnol. Biotechnol. Equip. 2021, 35, 1118–1126. [Google Scholar] [CrossRef]
- Buzharevski, A.; Paskaš, S.; Sárosi, M.-B.; Laube, M.; Lönnecke, P.; Neumann, W.; Murganić, B.; Mijatović, S.; Maksimović-Ivanić, D.; Pietzsch, J.; et al. Carboranyl Derivatives of Rofecoxib with Cytostatic Activity against Human Melanoma and Colon Cancer Cells. Sci. Rep. 2020, 10, 4827. [Google Scholar] [CrossRef]
- Sostres, C.; Gargallo, C.J.; Lanas, A. Nonsteroidal anti-inflammatory drugs and upper and lower gastrointestinal mucosal damage. Arthritis Res. Ther. 2013, 15, 3. [Google Scholar] [CrossRef]
- Lim, Y.J.; Lee, J.S.; Ku, Y.S.; Hahm, K.-B. Rescue strategies against non-steroidal anti-inflammatory drug-induced gastroduodenal damage. J. Gastroenterol. Hepatol. 2009, 24, 1169–1178. [Google Scholar] [CrossRef]
- Dillon, C.T.; Hambley, T.W.; Kennedy, B.J.; Lay, P.A.; Zhou, Q.; Davies, N.M.; Biffin, J.R.; Regtop, H.L. Gastrointestinal Toxicity, Antiinflammatory Activity, and Superoxide Dismutase Activity of Copper and Zinc Complexes of the Antiinflammatory Drug Indomethacin. Chem. Res. Toxicol. 2003, 16, 28–37. [Google Scholar] [CrossRef]
- Skiba, J.; Kowalczyk, A.; Stączek, P.; Bernaś, T.; Trzybiński, D.; Woźniak, K.; Schatzschneider, U.; Czerwieniec, R.; Kowalski, K. Luminescent fac-[Re(CO)3(phen)] carboxylato complexes with non-steroidal anti-inflammatory drugs: Synthesis and mechanistic insights into the in vitro anticancer activity of fac-[Re(CO)3(phen)(aspirin)]. New J. Chem. 2019, 43, 573–583. [Google Scholar] [CrossRef]
- Reczek, C.R.; Chandel, N.S. The Two Faces of Reactive Oxygen Species in Cancer. Annu. Rev. Cancer Biol. 2017, 1, 79–98. [Google Scholar] [CrossRef]
- Tirichen, H.; Yaigoub, H.; Xu, W.; Wu, C.; Li, R.; Li, Y. Mitochondrial Reactive Oxygen Species and Their Contribution in Chronic Kidney Disease Progression Through Oxidative Stress. Front. Physiol. 2021, 12, 627837. [Google Scholar] [CrossRef]
- Mailloux, R.J. Mitochondrial Antioxidants and the Maintenance of Cellular Hydrogen Peroxide Levels. Oxidative Med. Cell. Longev. 2018, 2018, 7857251. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1. [Google Scholar] [CrossRef]
- Brasky, T.M.; Felix, A.S.; Cohn, D.E.; McMeekin, D.S.; Mutch, D.G.; Creasman, W.T.; Thaker, P.H.; Walker, J.L.; Moore, R.G.; Lele, S.B.; et al. Nonsteroidal Anti-inflammatory Drugs and Endometrial Carcinoma Mortality and Recurrence. J. Natl. Cancer Inst. 2017, 109, djw251. [Google Scholar] [CrossRef]
- Matsuo, K.; Cahoon, S.S.; Yoshihara, K.; Shida, M.; Kakuda, M.; Adachi, S.; Moeini, A.; Machida, H.; Garcia-Sayre, J.; Ueda, Y.; et al. Association of Low-Dose Aspirin and Survival of Women With Endometrial Cancer. Obstet. Gynecol. 2016, 128, 127–137. [Google Scholar] [CrossRef]
- Verdoodt, F.; Friis, S.; Dehlendorff, C.; Albieri, V.; Kjaer, S.K. Non-steroidal anti-inflammatory drug use and risk of endometrial cancer: A systematic review and meta-analysis of observational studies. Gynecol. Oncol. 2016, 140, 352–358. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Chen, X.; Zhang, F.; Li, X. Aspirin use and endometrial cancer risk: A meta-analysis and systematic review. Ann. Transl. Med. 2020, 8, 461. [Google Scholar] [CrossRef]
- Gao, J.; Niwa, K.; Sun, W.; Takemura, M.; Lian, Z.; Onogi, K.; Seishima, M.; Mori, H.; Tamaya, T. Non-steroidal anti-inflammatory drugs inhibit cellular proliferation and upregulate cyclooxygenase-2 protein expression in endometrial cancer cells. Cancer Sci. 2004, 95, 901–907. [Google Scholar] [CrossRef]
- Arango, H. Aspirin effects on endometrial cancer cell growth. Obstet. Gynecol. 2001, 97, 423–427. [Google Scholar] [CrossRef]
- Webb, P.M.; Na, R.; Weiderpass, E.; Adami, H.O.; Anderson, K.E.; Bertrand, K.A.; Botteri, E.; Brasky, T.M.; Brinton, L.A.; Chen, C.; et al. Use of aspirin, other nonsteroidal anti-inflammatory drugs and acetaminophen and risk of endometrial cancer: The Epidemiology of Endometrial Cancer Consortium. Ann. Oncol. 2019, 30, 310–316. [Google Scholar] [CrossRef]
- Abdulkareem, I.; Blair, M. Effects of indomethacin on expression of PTEN tumour suppressor in human cancers. Niger. Med. J. 2013, 54, 100–106. [Google Scholar] [CrossRef]
- Xiao, Y.; Teng, Y.; Zhang, R.; Luo, L. Antitumor effect of the selective COX-2 inhibitor celecoxib on endometrial adenocarcinoma in vitro and in vivo. Oncol. Lett. 2012, 4, 1219–1224. [Google Scholar] [CrossRef]
- Wongrakpanich, S.; Wongrakpanich, A.; Melhado, K.; Rangaswami, J. A Comprehensive Review of Non-Steroidal Anti-Inflammatory Drug Use in The Elderly. Aging Dis. 2018, 9, 143–150. [Google Scholar] [CrossRef]
- Henry, D.A. Side-effects of non-steroidal anti-inflammatory drugs. Baillière’s Clin. Rheumatol. 1988, 2, 425–454. [Google Scholar] [CrossRef]
- Dunlap, T.; Abdul-Hay, S.O.; Chandrasena, R.E.P.; Hagos, G.K.; Sinha, V.; Wang, Z.; Wang, H.; Thatcher, G.R.J. Nitrates and NO-NSAIDs in cancer chemoprevention and therapy: In vitro evidence querying the NO donor functionality. Nitric Oxide 2008, 19, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, H.N.; Piazza, G.A. Novel Therapeutics: NSAIDs, Derivatives, and Phosphodiesterases. Curr. Color. Cancer Rep. 2012, 8, 325–330. [Google Scholar] [CrossRef]
- Qandil, A.M. Prodrugs of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs), More Than Meets the Eye: A Critical Review. Int. J. Mol. Sci. 2012, 13, 17244–17274. [Google Scholar] [CrossRef]
- Hasegawa, K.; Ohashi, Y.; Ishikawa, K.; Yasue, A.; Kato, R.; Achiwa, Y.; Nishio, E.; Udagawa, Y. Expression of cyclooxygenase-2 in uterine endometrial cancer and anti-tumor effects of a selective COX-2 inhibitor. Int. J. Oncol. 2005, 26, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.-T.; Kang, S.; Kang, D.-H.; Yoo, K.-Y.; Park, I.-A.; Bang, Y.-J.; Kim, J.W.; Park, N.-H.; Kang, S.-B.; Lee, H.-P.; et al. Cyclooxygenase-2 and p53 Expressions in Endometrial Cancer. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1538–1542. [Google Scholar] [CrossRef]
- Abu Ali, H.; Fares, H.; Darawsheh, M.; Rappocciolo, E.; Akkawi, M.; Jaber, S. Synthesis, characterization and biological activity of new mixed ligand complexes of Zn(II) naproxen with nitrogen based ligands. Eur. J. Med. Chem. 2015, 89, 67–76. [Google Scholar] [CrossRef]
- O’Connor, M.; Kellett, A.; McCann, M.; Rosair, G.; McNamara, M.; Howe, O.; Creaven, B.S.; McClean, S.; Kia, A.F.-A.; O’Shea, D.; et al. Copper(II) Complexes of Salicylic Acid Combining Superoxide Dismutase Mimetic Properties with DNA Binding and Cleaving Capabilities Display Promising Chemotherapeutic Potential with Fast Acting in Vitro Cytotoxicity against Cisplatin Sensitive and Resistant Cancer Cell Lines. J. Med. Chem. 2012, 55, 1957–1968. [Google Scholar] [CrossRef]
- Wilczyński, M.; Danielska, J.; Wilczyński, J. An update of the classical Bokhman’s dualistic model of endometrial cancer. Menopausal Rev. 2016, 15, 63–68. [Google Scholar] [CrossRef]
- Kozak, J.; Wdowiak, P.; Maciejewski, R.; Torres, A. A guide for endometrial cancer cell lines functional assays using the measurements of electronic impedance. Cytotechnology 2018, 70, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Kasahara, K.; Kaneko, M.; Iwasaki, H.; Hayashi, K. Establishment of a new human endometrial adenocarcinoma cell line, Ishikawa cells, containing estrogen and progesterone receptors. Nihon Sanka Fujinka Gakkai Zasshi 1985, 37, 1103–1111. [Google Scholar] [PubMed]
- Nishida, M. Ishikawa Cells: Opening of In Vitro Hormone Research on Endometrial Carcinoma. In Cell and Molecular Biology of Endometrial Carcinoma; Springer: Tokyo, Japan, 2003; pp. 35–38. [Google Scholar] [CrossRef]
- Castro-Rivera, E.; Safe, S. Estrogen- and antiestrogen-responsiveness of HEC1A endometrial adenocarcinoma cells in culture. J. Steroid Biochem. Mol. Biol. 1998, 64, 287–295. [Google Scholar] [CrossRef]
- Glaab, W.E.; Risinger, J.I.; Umar, A.; Kunkel, T.A.; Barrett, J.C.; Tindall, K.R. Characterization of Distinct Human Endometrial Carcinoma Cell Lines Deficient in Mismatch Repair That Originated from a Single Tumor. J. Biol. Chem. 1998, 273, 26662–26669. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M. The Ishikawa cells from birth to the present. Hum. Cell 2002, 15, 104–117. [Google Scholar] [CrossRef]
- Kuźmycz, O.; Stączek, P. Prospects of NSAIDs administration as double-edged agents against endometrial cancer and pathological species of the uterine microbiome. Cancer Biol. Ther. 2020, 21, 486–494. [Google Scholar] [CrossRef]
- Nevadunsky, N.S.; Van Arsdale, A.; Strickler, H.D.; Spoozak, L.A.; Moadel, A.; Kaur, G.; Girda, E.; Goldberg, G.L.; Einstein, M.H. Association Between Statin Use and Endometrial Cancer Survival. Obstet. Gynecol. 2015, 126, 144–150. [Google Scholar] [CrossRef]
- Moxley, K.M.; McMeekin, D.S. Endometrial Carcinoma: A Review of Chemotherapy, Drug Resistance, and the Search for New Agents. Oncologist 2010, 15, 1026–1033. [Google Scholar] [CrossRef]
- Rouette, A.; Parent, S.; Girouard, J.; Leblanc, V.; Asselin, E. Cisplatin increases B-cell-lymphoma-2 expression via activation of protein kinase C and Akt2 in endometrial cancer cells. Int. J. Cancer 2012, 130, 1755–1767. [Google Scholar] [CrossRef]
- Ghosh, R.; Alajbegovic, A.; Gomes, A.V. NSAIDs and Cardiovascular Diseases: Role of Reactive Oxygen Species. Oxidative Med. Cell. Longev. 2015, 2015, 536962. [Google Scholar] [CrossRef]
- Kusuhara, H.; Komatsu, H.; Sumichika, H.; Sugahara, K. Reactive oxygen species are involved in the apoptosis induced by nonsteroidal anti-inflammatory drugs in cultured gastric cells. Eur. J. Pharmacol. 1999, 383, 331–337. [Google Scholar] [CrossRef]
- Adachi, M.; Sakamoto, H.; Kawamura, R.; Wang, W.; Imai, K.; Shinomura, Y. Nonsteroidal anti-inflammatory drugs and oxidative stress in cancer cells. Histol. Histopathol. 2007, 22, 437–442. [Google Scholar] [PubMed]

| 24 h | |||||||||
| IC50 | 373 | 373a | 375 | 375a | 376 | 376a | 377 | 377a | CisPt |
| µg/mL | na * | na | 11.83 | 180.6 | na | na | 18.45 | 117.9 | 4.072 |
| µM | na | na | 188.06 | 10,024.42 | na | na | 228.6 | 3295.3 | 13.57 |
| 72 h | |||||||||
| IC50 | 373 | 373a | 375 | 375a | 376 | 376a | 377 | 377a | CisPt |
| µg/mL | na | na | 9.208 | 313.4 | na | na | 8.695 | 169.5 | 12.31 |
| µM | na | na | 146.3 | 17,395.6 | na | na | 107.7 | 4737.4 | 41.03 |
| 24 h | |||||||||
| IC50 | 373 | 373a | 375 | 375a | 376 | 376a | 377 | 377a | CisPt |
| µg/mL | na * | na | 24.81 | 490.5 | na | na | 117.8 | 196.4 | 6.016 |
| µM | na | na | 394.06 | 27,255.8 | na | na | 1459.3 | 5489.3 | 20.05 |
| 72 h | |||||||||
| IC50 | 373 | 373a | 375 | 375a | 376 | 376a | 377 | 377a | CisPt |
| µg/mL | na | na | 7.702 | 309.3 | na | na | 11.18 | 228.1 | 3.083 |
| µM | na | na | 112.4 | 17,168 | na | na | 138.5 | 6375.2 | 10.27 |
| Compound | p < 0.05 | SD | Compound | p < 0.05 | SD | |
|---|---|---|---|---|---|---|
| 24 h | 375 at ½ IC50 | no * | ±0.005102 | 375a at ½ IC50 | * | ±0.00035 |
| 375 at ¼ IC50 | no | ±0.0002 | 375a at ¼ IC50 | no | ±0.00015 | |
| 377 at ½ IC50 | *** | ±0.0003 | 377a at ½ IC50 | *** | ±0.02975 | |
| 377 at ¼ IC50 | *** | ±0.1768 | 377a at ¼ IC50 | *** | ±0.1933 | |
| 72 h | 375 at ½ IC50 | *** | ±0.0308 | 375a at ½ IC50 | *** | ±0.1401 |
| 375 at ¼ IC50 | *** | ±0.01290 | 375a at ¼ IC50 | *** | ±0.01455 | |
| 377 at ½ IC50 | *** | ±0.0432 | 377a at ½ IC50 | *** | ±0.07570 | |
| 377 at ¼ IC50 | *** | ±0.0914 | 377a at ¼ IC50 | *** | ±0.1038 |
| Compound | p < 0.05 | SD | Compound | p < 0.05 | SD | |
|---|---|---|---|---|---|---|
| 24 h | 375 at ½ IC50 | * | ±0.0600 | 375a at ½ IC50 | no * | ±0.04030 |
| 375 at ¼ IC50 | no | ±0.3835 | 375a at ¼ IC50 | * | ±0.4275 | |
| 377 at ½ IC50 | no | ±0.1122 | 377a at ½ IC50 | no | ±0.1232 | |
| 377 at ¼ IC50 | no | ±0.1461 | 377a at ¼ IC50 | no | ±0.1284 | |
| 72 h | 375 at ½ IC50 | *** | ±0.0115 | 375a at ½ IC50 | *** | ±0.0886 |
| 375 at ¼ IC50 | *** | ±0.1675 | 375a at ¼ IC50 | *** | ±0.1061 | |
| 377 at ½ IC50 | *** | ±0.04905 | 377a at ½ IC50 | *** | ±0.05215 | |
| 377 at ¼ IC50 | *** | ±0.0171 | 377a at ¼ IC50 | ** | ±0.3529 |
| NSAIDs and Rhenium-NSAIDs Agents | ||
|---|---|---|
| Structure | Name/Formula | Molecular Weight [g/mol] |
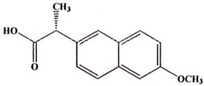 | Naproxen (373a) C14H14O3 | 230.26 |
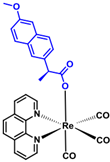 | Rhenium-naproxen (373) C29H21N2O6Re1 | 679.69 |
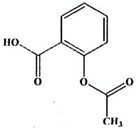 | Aspirin (375a) C9H8O4 | 180.16 |
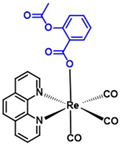 | Rhenium-aspirin (375) C24H15N2O7Re1 | 629.59 |
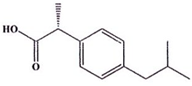 | Ibuprofen (376a) C14H22O2 | 206.28 |
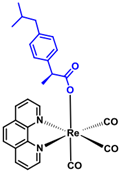 | Rhenium-ibuprofen (376) C28H25N2O5Re1 | 655.72 |
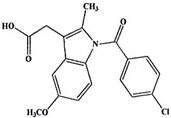 | Indomethacin (377a) C19H16Cl1N1O4 | 357.79 |
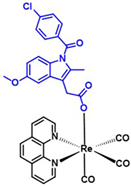 | Rhenium-indomethacin (377); C34H24Cl1N3O7Re1 | 807.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuźmycz, O.; Kowalczyk, A.; Stączek, P. Biological Activity of fac-[Re(CO)3(phen)(aspirin)], fac-[Re(CO)3(phen)(indomethacin)] and Their Original Counterparts against Ishikawa and HEC-1A Endometrial Cancer Cells. Int. J. Mol. Sci. 2022, 23, 11568. https://doi.org/10.3390/ijms231911568
Kuźmycz O, Kowalczyk A, Stączek P. Biological Activity of fac-[Re(CO)3(phen)(aspirin)], fac-[Re(CO)3(phen)(indomethacin)] and Their Original Counterparts against Ishikawa and HEC-1A Endometrial Cancer Cells. International Journal of Molecular Sciences. 2022; 23(19):11568. https://doi.org/10.3390/ijms231911568
Chicago/Turabian StyleKuźmycz, Olga, Aleksandra Kowalczyk, and Paweł Stączek. 2022. "Biological Activity of fac-[Re(CO)3(phen)(aspirin)], fac-[Re(CO)3(phen)(indomethacin)] and Their Original Counterparts against Ishikawa and HEC-1A Endometrial Cancer Cells" International Journal of Molecular Sciences 23, no. 19: 11568. https://doi.org/10.3390/ijms231911568
APA StyleKuźmycz, O., Kowalczyk, A., & Stączek, P. (2022). Biological Activity of fac-[Re(CO)3(phen)(aspirin)], fac-[Re(CO)3(phen)(indomethacin)] and Their Original Counterparts against Ishikawa and HEC-1A Endometrial Cancer Cells. International Journal of Molecular Sciences, 23(19), 11568. https://doi.org/10.3390/ijms231911568






