A Call for Drug Therapies for the Treatment of Social Behavior Disorders in Dementia: Systematic Review of Evidence and State of the Art
Abstract
1. Introduction
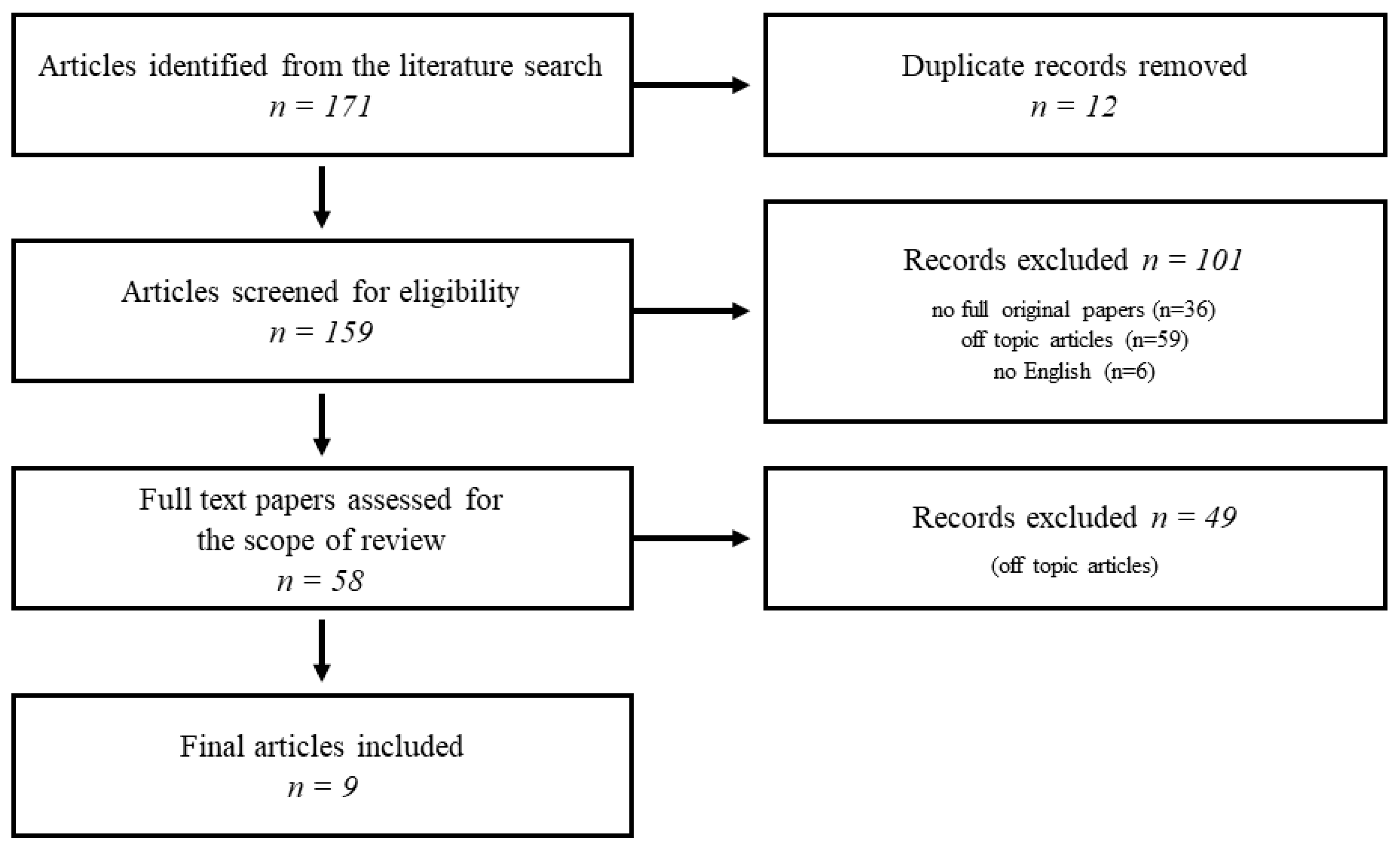
2. Methods
3. Literature Search Results
3.1. Drugs Tested in Animal Models
3.2. Drugs Tested in Clinical Trials
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frith, C.D. Social cognition. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 2033–2039. [Google Scholar] [CrossRef] [PubMed]
- Cerami, C.; Cappa, S.F. The behavioral variant of frontotemporal dementia: Linking neuropathology to social cognition. Neurol. Sci. 2013, 34, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Piguet, O.; Kumfor, F. Frontotemporal dementias: Main syndromes and underlying brain changes. Curr. Opin. Neurol. 2020, 33, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Bora, E.; Walterfang, M.; Velakoulis, D. Theory of mind in behavioural-variant frontotemporal dementia and Alzheimer’s disease: A meta-analysis. J. Neurol. Neurosurg. Psychiatry 2015, 86, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Bora, E.; Velakoulis, D.; Walterfang, M. Meta-Analysis of Facial Emotion Recognition in Behavioral variant frontotemporal dementia: Comparison with Alzheimer disease and healthy controls. J. Geriatr. Psychiatry Neurol. 2016, 29, 205–211. [Google Scholar] [CrossRef]
- Bora, E.; Yener, G.G. Meta-analysis of social cognition in Mild Cognitive Impairment. J. Geriatr. Psychiatry Neurol. 2017, 30, 206–213. [Google Scholar] [CrossRef]
- Fornaro, M.; Solmi, M.; Stubbs, B.; Veronese, N.; Monaco, F.; Novello, S.; Fusco, A.; Anastasia, A.; De Berardis, D.; Carvalho, A.F.; et al. Prevalence and correlates of major depressive disorder, bipolar disorder and schizophrenia among nursing home residents without dementia: Systematic review and meta-analysis. Br. J. Psychiatry J. Ment. Sci. 2020, 216, 6–15. [Google Scholar] [CrossRef]
- Kim, H.K.; Nunes, P.V.; Oliveira, K.C.; Young, L.T.; Lafer, B. Neuropathological relationship between major depression and dementia: A hypothetical model and review. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 67, 51–57. [Google Scholar] [CrossRef]
- Porcelli, S.; Van Der Wee, N.; van der Werff, S.; Aghajani, M.; Glennon, J.C.; van Heukelum, S.; Mogavero, F.; Lobo, A.; Olivera, F.J.; Lobo, E.; et al. Social brain, social dysfunction and social withdrawal. Neurosci. Biobehav. Rev. 2019, 97, 10–33. [Google Scholar] [CrossRef]
- Diehl-Schmid, J.; Pohl, C.; Perneczky, R.; Förstl, H.; Kurz, A. Behavioral disturbances in the course of frontotemporal dementia. Dement. Geriatr. Cogn. Disord. 2006, 22, 352–357. [Google Scholar] [CrossRef]
- Riedijk, S.; Duivenvoorden, H.; Rosso, S.; Van Swieten, J.; Niermeijer, M.; Tibben, A. Frontotemporal dementia: Change of familial caregiver burden and partner relation in a Dutch cohort of 63 patients. Dement. Geriatr. Cogn. Disord. 2008, 26, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Johnen, A.; Bertoux, M. Psychological and cognitive markers of behavioral variant frontotemporal dementia-A clinical neuropsychologist’s view on diagnostic criteria and beyond. Front. Neurol. 2019, 10, 594. [Google Scholar] [CrossRef] [PubMed]
- Boutoleau-Bretonnière, C.; Vercelletto, M.; Volteau, C.; Renou, P.; Lamy, E. Zarit burden inventory and activities of daily living in the behavioral variant of frontotemporal dementia. Dement. Geriatr. Cogn. Disord. 2008, 25, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Mioshi, E.; Bristow, M.; Cook, R.; Hodges, J.R. Factors underlying caregiver stress in frontotemporal dementia and Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2009, 27, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Riedijk, S.R.; de Vugt, M.E.; Duivenvoorden, H.J.; Niermeijer, M.F.; van Swieten, J.C.; Verhey, F.R.J.; Tibben, A. Caregiver burden, health-related quality of life and coping in dementia caregivers: A comparison of frontotemporal dementia and Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2006, 22, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Mioshi, E.; Foxe, D.; Leslie, F.; Savage, S.; Hsieh, S.; Miller, L.; Hodges, J.R.; Piguet, O. The impact of dementia severity on caregiver burden in frontotemporal dementia and Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2013, 27, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Mourik, J.C.; Rosso, S.M.; Niermeijer, M.F.; Duivenvoorden, H.J.; Van Swieten, J.C.; Tibben, A. Frontotemporal dementia: Behavioral symptoms and caregiver distress. Dement. Geriatr. Cogn. Disord. 2004, 18, 299–306. [Google Scholar] [CrossRef]
- Gitlin, L.N.; Kales, H.C.; Lyketsos, C.G. Nonpharmacologic management of behavioral symptoms in dementia. JAMA 2012, 308, 2020–2029. [Google Scholar] [CrossRef]
- Shnall, A.; Agate, A.; Grinberg, A.; Huijbregts, M.; Nguyen, M.Q.; Chow, T.W. Development of supportive services for frontotemporal dementias through community engagement. Int. Rev. Psychiatry 2013, 25, 246–252. [Google Scholar] [CrossRef]
- Pressman, P.S.; Miller, B.L. Diagnosis and management of behavioral variant frontotemporal dementia. Biol. Psychiatry 2014, 75, 574–581. [Google Scholar] [CrossRef]
- Bickart, K.C.; Dickerson, B.C.; Barrett, L.F. The amygdala as a hub in brain networks that support social life. Neuropsychologia 2014, 63, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Arioli, M.; Cattaneo, Z.; Ricciardi, E.; Canessa, N. Overlapping and specific neural correlates for empathizing, affective mentalizing, and cognitive mentalizing: A coordinate-based meta-analytic study. Hum. Brain Mapp. 2021, 42, 4777–4804. [Google Scholar] [CrossRef]
- Blakemore, C.B. Cyclandelate in the treatment of multi-infarct dementia. Interim findings from a multicentre study in general practice. Drugs 1987, 33, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Ditzler, K. Efficacy and tolerability of memantine in patients with dementia syndrome. A double-blind, placebo-controlled trial. Arzneimittelforschung 1991, 41, 773–780. [Google Scholar] [PubMed]
- Jesso, S.; Morlog, D.; Ross, S.; Pell, M.D.; Pasternak, S.H.; Mitchell, D.G.V.; Kertesz, A.; Finger, E.C. The effects of oxytocin on social cognition and behaviour in frontotemporal dementia. Brain 2011, 134, 2493–2501. [Google Scholar] [CrossRef] [PubMed]
- Finger, E.C.; MacKinley, J.; Blair, M.; Oliver, L.D.; Jesso, S.; Tartaglia, M.C.; Borrie, M.; Wells, J.; Dziobek, I.; Pasternak, S.; et al. Oxytocin for frontotemporal dementia: A randomized dose-finding study of safety and tolerability. Neurology 2015, 84, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Finger, E.; Berry, S.; Cummings, J.; Coleman, K.; Hsiung, R.; Feldman, H.H.; Boxer, A. Adaptive crossover designs for assessment of symptomatic treatments targeting behaviour in neurodegenerative disease: A phase 2 clinical trial of intranasal oxytocin for frontotemporal dementia (FOXY). Alzheimer’s. Res. Ther. 2018, 10, 102. [Google Scholar] [CrossRef]
- Oliver, L.D.; Stewart, C.; Coleman, K.; Kryklywy, J.H.; Bartha, R.; Mitchell, D.G.V.; Finger, E.C. Neural effects of oxytocin and mimicry in frontotemporal dementia: A randomized crossover study. Neurology 2020, 95, e2635–e2647. [Google Scholar] [CrossRef]
- Meyer-Lindenberg, A.; Domes, G.; Kirsch, P.; Heinrichs, M. Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nat. Rev. Neurosci. 2011, 12, 524–538. [Google Scholar] [CrossRef]
- Kosfeld, M.; Heinrichs, M.; Zak, P.J.; Fischbacher, U.; Fehr, E. Oxytocin increases trust in humans. Nature 2005, 435, 673–676. [Google Scholar] [CrossRef]
- Baumgartner, T.; Heinrichs, M.; Vonlanthen, A.; Fischbacher, U.; Fehr, E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron 2008, 58, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Domes, G.; Heinrichs, M.; Gläscher, J.; Büchel, C.; Braus, D.F.; Herpertz, S.C. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol. Psychiatry 2007, 62, 1187–1190. [Google Scholar] [CrossRef] [PubMed]
- Guastella, A.J.; Mitchell, P.B.; Dadds, M.R. Oxytocin increases gaze to the eye region of human faces. Biol. Psychiatry 2008, 63, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Hurlemann, R.; Patin, A.; Onur, O.A.; Cohen, M.X.; Baumgartner, T.; Metzler, S.; Dziobek, I.; Gallinat, J.; Wagner, M.; Maier, W.; et al. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J. Neurosci. 2010, 30, 4999–5007. [Google Scholar] [CrossRef]
- De Berardis, D.; Marini, S.; Iasevoli, F.; Tomasetti, C.; De Bartolomeis, A.; Mazza, M.; Valchera, A.; Fornaro, M.; Cavuto, M.; Srinivasan, V.; et al. The role of intranasal oxytocin in the treatment of patients with schizophrenia: A systematic review. CNS Neurol. Disord. Drug Targets 2013, 12, 252–264. [Google Scholar] [CrossRef]
- Winterton, A.; Westlye, L.T.; Steen, N.E.; Andreassen, O.A.; Quintana, D.S. Improving the precision of intranasal oxytocin research. Nat. Hum. Behav. 2021, 5, 9–18. [Google Scholar] [CrossRef]
- Leng, G.; Leng, R.I.; Ludwig, M. Oxytocin-a social peptide? Deconstructing the evidence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2022, 377, 20210055. [Google Scholar] [CrossRef]
- Piguet, O.; Ahmed, R.M.; Kumfor, F. The Role of Oxytocin in Social Circuits and Social Behavior in Dementia. In Oxytocin, Methods and Protocols Methods in Molecular Biology; Werry, E.L., Reekie, T.A., Kassiou, M., Eds.; Springer Science+Business Media, LLC: New York Plaza, NY, USA, 2022; Volume 2384, pp. 67–80. ISBN 978-1-0716-1758-8. [Google Scholar]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- National Institutes of Health. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. 2014. Available online: https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort (accessed on 9 September 2022).
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 26, 43. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Schluesener, H.J. Oral administration of histone deacetylase inhibitor MS-275 ameliorates neuroinflammation and cerebral amyloidosis and improves behavior in a mouse model. J. Neuropathol. Exp. Neurol. 2013, 72, 178–185. [Google Scholar] [CrossRef]
- Castro, A.A.; Wiemes, B.P.; Matheus, F.C.; Lapa, F.R.; Viola, G.G.; Santos, A.R.; Tasca, C.I.; Prediger, R.D. Atorvastatin improves cognitive, emotional and motor impairments induced by intranasal 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) administration in rats, an experimental model of Parkinson’s disease. Brain Res. 2013, 1513, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.R.; Magen, I.; Bove, N.; Zhu, C.; Lemesre, V.; Dutta, G.; Elias, C.J.; Lester, H.A.; Chesselet, M.F. Chronic nicotine improves cognitive and social impairment in mice overexpressing wild type α-synuclein. Neurobiol. Dis. 2018, 117, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Abel, T.; Zukin, R.S. Epigenetic targets of HDAC inhibition in neurodegenerative and psychiatric disorders. Curr. Opin. Pharmacol. 2008, 8, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Gräff, J.; Rei, D.; Guan, J.S.; Wang, W.Y.; Seo, J.; Hennig, K.M.; Nieland, T.J.F.; Fass, D.M.; Kao, P.F.; Kahn, M.; et al. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature 2012, 483, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Wesson, D.W.; Wilson, D.A. Age and gene overexpression interact to abolish nesting behavior in Tg2576 amyloid precursor protein (APP) mice. Behav. Brain Res. 2011, 216, 408–413. [Google Scholar] [CrossRef]
- Bhat, A.; Dalvi, H.; Jain, H.; Rangaraj, N.; Singh, S.B.; Srivastava, S. Perspective insights of repurposing the pleiotropic efficacy of statins in neurodegenerative disorders: An expository appraisal. Curr. Res. Pharmacol. Drug Discov. 2020, 2, 100012. [Google Scholar] [CrossRef]
- Jick, H.; Zornberg, G.L.; Jick, S.S.; Seshadri, S.; Drachman, D.A. Statins and the risk of dementia. Lancet 2000, 356, 1627–1631. [Google Scholar] [CrossRef]
- Wolozin, B.; Wang, S.W.; Li, N.C.; Lee, A.; Lee, T.A.; Kazis, L.E. Simvastatin is associated with a reduced incidence of dementia and Parkinson’s disease. BMC Med. 2007, 5, 20. [Google Scholar] [CrossRef]
- Ludka, F.K.; Zomkowski, A.D.E.; Cunha, M.P.; Dal-Cim, T.; Zeni, A.L.B.; Rodrigues, A.L.S.; Tasca, C.I. Acute atorvastatin treatment exerts antidepressant-like effect in mice via the L-arginine-nitric oxide-cyclic guanosine monophosphate pathway and increases BDNF levels. Eur. Neuropsychopharmacol. 2013, 23, 400–412. [Google Scholar] [CrossRef]
- Dantzer, R.; Bluthe, R.M.; Koob, G.F.; Le Moal, M. Modulation of social memory in male rats by neurohypophyseal peptides. Psychopharmacology 1987, 91, 363–368. [Google Scholar] [CrossRef]
- Wang, H.F.; Yu, J.T.; Tang, S.W.; Jiang, T.; Tan, C.C.; Meng, X.F.; Wang, C.; Tan, M.S.; Tan, L. Efficacy and safety of cholinesterase inhibitors and memantine in cognitive impairment in Parkinson’s disease, Parkinson’s disease dementia, and dementia with Lewy bodies: Systematic review with meta-analysis and trial sequential analysis. J. Neurol. Neurosurg. Psychiatry 2015, 86, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Magen, I.; Torres, E.R.; Dinh, D.; Chung, A.; Masliah, E.; Chesselet, M.F. Social Cognition Impairments in Mice Overexpressing Alpha-Synuclein Under the Thy1 Promoter, a Model of Pre-manifest Parkinson’s Disease. J. Parkinsons Dis. 2015, 5, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Weyer, G.; Eul, A.; Milde, K.; Wierich, W.; Herrmann, W.M. Cyclandelate in the treatment of patients with mild to moderate primary degenerative dementia of the Alzheimer type or vascular dementia: Experience from a placebo controlled multi-center study. Pharmacopsychiatry 2000, 33, 89–97. [Google Scholar] [CrossRef]
- Blakemore, C.B. Improvement in certain aspects of behaviour of elderly patients treated by cyclandelate. In Assessment in Cerebrovascular Insufficiency; Stocker, G., Ed.; Georg Thieme Verlag: Stuttgart, Germany, 1971. [Google Scholar]
- Collegium Internationale Psychiatrie (CIPS). Sandoz Clinical Assessment Geriatric Scale; CIPS: Beltz, Weinheim, 1986. [Google Scholar]
- Rascovsky, K.; Hodges, J.R.; Knopman, D.; Mendez, M.F.; Kramer, J.H.; Neuhaus, J.; Van Swieten, J.C.; Seelaar, H.; Dopper, E.G.P.; Onyike, C.U.; et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011, 134, 2456–2477. [Google Scholar] [CrossRef] [PubMed]
- Tottenham, N.; Tanaka, J.W.; Leon, A.C.; McCarry, T.; Nurse, M.; Hare, T.A.; Marcus, D.J.; Westerlund, A.; Casey, B.J.; Nelson, C. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Res. 2009, 168, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Pell, M.D.; Paulmann, S.; Dara, C.; Alasseri, A.; Kotz, S.A. Factors in the recognition of vocally expressed emotions: A comparison of four languages. J. Phon. 2009, 37, 417–435. [Google Scholar] [CrossRef]
- Cummings, J.L. The Neuropsychiatric Inventory: Assessing psychopathology in dementia patients. Neurology 1997, 48, S10–S16. [Google Scholar] [CrossRef]
- Kertesz, A.; Davidson, W.; Fox, H. Frontal behavioral inventory: Diagnostic criteria for frontal lobe dementia. Can. J. Neurol. Sci. 1997, 24, 29–36. [Google Scholar] [CrossRef]
- Davis, M.H. A multidimensional approach to individual differences in empathy. In JSAS Catalog of Selected Documents in Psychology; American Psychological Association: Washington, DC, USA, 1980; Volume 85. [Google Scholar]
- Knopman, D.S.; Kramer, J.H.; Boeve, B.F.; Caselli, R.J.; Graff-Radford, N.R.; Mendez, M.F.; Miller, B.L.; Mercaldo, N. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain 2008, 131, 2957–2968. [Google Scholar] [CrossRef]
- Mioshi, E.; Hsieh, S.; Savage, S.; Hornberger, M.; Hodges, J.R. Clinical staging and disease progression in frontotemporal dementia. Neurology 2010, 74, 1591–1597. [Google Scholar] [CrossRef]
- Lennox, R.D.; Wolfe, R.N. Revision of the self-monitoring scale. J. Pers. Soc. Psychol. 1984, 46, 1349–1364. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.F.; Fong, S.S.; Shapira, J.S.; Jimenez, E.E.; Kaiser, N.C.; Kremen, S.A.; Tsai, P.H. Observation of social behavior in frontotemporal dementia. Am. J. Alzheimer’s Dis. Other Demen. 2014, 29, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Oliver, L.D.; Mitchell, D.G.; Dziobek, I.; MacKinley, J.; Coleman, K.; Rankin, K.P.; Finger, E.C. Parsing cognitive and emotional empathy deficits for negative and positive stimuli in frontotemporal dementia. Neuropsychologia 2015, 67, 14–26. [Google Scholar] [CrossRef]
- Dziobek, I.; Rogers, K.; Fleck, S.; Bahnemann, M.; Heekeren, H.R.; Wolf, O.T.; Convit, A. Dissociation of cognitive and emotional empathy in adults with Asperger syndrome using the Multifaceted Empathy Test (MET). J. Autism Dev. Disord. 2008, 38, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Mozaz, M.; Rothi, L.J.; Anderson, J.M.; Crucian, G.P.; Heilman, K.M. Postural knowledge of transitive pantomimes and intransitive gestures. J. Int. Neuropsychol. Soc. 2002, 8, 958–962. [Google Scholar] [CrossRef]
- Soleimani, M.A.H.; Negarandeh, R.; Bastani, F.; Grey, R. Disrupted social connectedness in people with Parkinson’s disease. Br. J. Community Nurs. 2014, 19, 136–141. [Google Scholar] [CrossRef]
- Setién-Suero, E.; Murillo-García, N.; Sevilla-Ramos, M.; Abreu-Fernández, G.; Pozueta, A.; Ayesa-Arriola, R. Exploring the relationship between deficits in social cognition and neurodegenerative dementia: A systematic review. Front. Aging Neurosci. 2022, 14, 778093. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 2893, Cyclandelate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cyclandelate#section=Literature (accessed on 24 July 2022).
- Rammes, G.; Danysz, W.; Parsons, C.G. Pharmacodynamics of memantine: An update. Curr. Neuropharmacol. 2008, 6, 55–78. [Google Scholar] [CrossRef]
- Insel, T.R. The challenge of translation in social neuroscience: A review of oxytocin, vasopressin, and affiliative behavior. Neuron 2010, 65, 768–779. [Google Scholar] [CrossRef]
- Hollander, E.; Bartz, J.; Chaplin, W.; Phillips, A.; Sumner, J.; Soorya, L.; Anagnostou, E.; Wasserman, S. Oxytocin increases retention of social cognition in autism. Biol. Psychiatry 2007, 61, 498–503. [Google Scholar] [CrossRef]
- Marsh, A.A.; Yu, H.H.; Pine, D.S.; Blair, R.J. Oxytocin improves specific recognition of positive facial expressions. Psychopharmacology 2010, 209, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Diodati, D.; Cyn-Ang, L.; Kertesz, A.; Finger, E. Pathologic evaluation of the supraoptic and paraventricular nuclei in dementia. Can. J. Neurol. Sci. 2012, 39, 213–219. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Loup, F.; Tribollet, E.; Dubois-Dauphin, M.; Dreifuss, J.J. Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Res. 1991, 555, 220–232. [Google Scholar] [CrossRef]
- Boccia, M.L.; Petrusz, P.; Suzuki, K.; Marson, L.; Pedersen, C.A. Immunohistochemical localization of oxytocin receptors in human brain. Neuroscience 2013, 253, 155–164. [Google Scholar] [CrossRef]
- Whitwell, J.L.; Josephs, K.A. Recent advances in the imaging of frontotemporal dementia. Curr. Neurol. Neurosci. Rep. 2012, 12, 715–723. [Google Scholar] [CrossRef]
- Miller, J.B.; Banks, S.J.; Leger, G.C.; Cummings, J.L. Randomized controlled trials in frontotemporal dementia: Cognitive and behavioral outcomes. Transl. Neurodegener. 2014, 3, 12. [Google Scholar] [CrossRef]
- Richardson, E.; Burnell, J.; Adams, H.R.; Bohannon, R.W.; Bush, E.N.; Campbell, M.; Chen, W.H.; Coons, S.J.; Papadopoulos, E.; Reeve, B.R.; et al. Developing and implementing performance outcome assessments: Evidentiary, methodologic, and operational considerations. Ther. Innov. Regul. Sci. 2019, 53, 146–153. [Google Scholar] [CrossRef]
- Kiresuk, T.J.; Sherman, R.E. Goal attainment scaling: A general method for evaluating comprehensive community mental health programs. Community Ment. Health J. 1968, 4, 443–453. [Google Scholar] [CrossRef]
- Cotta Ramusino, M.; Perini, G.; Vaghi, G.; Dal Fabbro, B.; Capelli, M.; Picascia, M.; Franciotta, D.; Farina, L.; Ballante, E.; Costa, A. Correlation of frontal atrophy and CSF tau levels with neuropsychiatric symptoms in patients with cognitive impairment: A memory clinic experience. Front. Aging Neurosci. 2021, 13, 595758. [Google Scholar] [CrossRef]
- Boxer, A.L.; Gold, M.; Feldman, H.; Boeve, B.F.; Dickinson, S.L.; Fillit, H.; Ho, C.; Paul, R.; Pearlman, R.; Sutherland, M.; et al. New directions in clinical trials for frontotemporal lobar degeneration: Methods and outcome measures. Alzheimer’s Dement. 2020, 16, 131–143. [Google Scholar] [CrossRef]
- Insel, T.R. Translating Oxytocin Neuroscience to the Clinic: A National Institute of Mental Health perspective. Biol. Psychiatry 2016, 79, 153–154. [Google Scholar] [CrossRef] [PubMed]
- Einfeld, S.L.; Smith, E.; McGregor, I.S.; Steinbeck, K.; Taffe, J.; Rice, L.J.; Horstead, S.K.; Rogers, N.; Hodge, M.A.; Guastella, A.J. A double-blind randomized controlled trial of oxytocin nasal spray in Prader Willi syndrome. Am. J. Med. Genet. A 2014, 164, 2232–2239. [Google Scholar] [CrossRef] [PubMed]
- Yatawara, C.J.; Einfeld, S.L.; Hickie, I.B.; Davenport, T.A.; Guastella, A.J. The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: A randomized clinical crossover trial. Mol. Psychiatry 2016, 21, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Cacciotti-Saija, C.; Langdon, R.; Ward, P.B.; Hickie, I.B.; Scott, E.M.; Naismith, S.L.; Moore, L.; Alvares, G.A.; Redoblado Hodge, M.A.; Guastella, A.J. A double-blind randomized controlled trial of oxytocin nasal spray and social cognition training for young people with early psychosis. Schizophr. Bull. 2015, 41, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.M.; Penn, D.L.; Smedley, K.L.; Leserman, J.; Elliott, T.; Pedersen, C.A. A pilot six-week randomized controlled trial of oxytocin on social cognition and social skills in schizophrenia. Schizophr. Res. 2014, 156, 261–265. [Google Scholar] [CrossRef]
- Anagnostou, E.; Soorya, L.; Chaplin, W.; Bartz, J.; Halpern, D.; Wasserman, S.; Wang, A.T.; Pepa, L.; Tanel, N.; Kushki, A.; et al. Intranasal oxytocin versus placebo in the treatment of adults with autism spectrum disorders: A randomized controlled trial. Mol. Autism 2012, 3, 16. [Google Scholar] [CrossRef]
- Bales, K.L.; Perkeybile, A.M.; Conley, O.G.; Lee, M.H.; Guoynes, C.D.; Downing, G.M.; Yun, C.R.; Solomon, M.; Jacob, S.; Mendoza, S.P. Chronic intranasal oxytocin causes long-term impairments in partner preference formation in male prairie voles. Biol. Psychiatry 2013, 74, 180–188. [Google Scholar] [CrossRef]
- Huang, H.; Michetti, C.; Busnelli, M.; Managò, F.; Sannino, S.; Scheggia, D.; Giancardo, L.; Sona, D.; Murino, V.; Chini, B.; et al. Chronic and acute intranasal oxytocin produce divergent social effects in mice. Neuropsychopharmacology 2014, 39, 1102–1114. [Google Scholar] [CrossRef]
- Conti, F.; Sertic, S.; Reversi, A.; Chini, B. Intracellular trafficking of the human oxytocin receptor: Evidence of receptor recycling via a Rab4/Rab5 “short cycle”. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E532–E542. [Google Scholar] [CrossRef]
- Peters, S.; Slattery, D.A.; Uschold-Schmidt, N.; Reber, S.O.; Neumann, I.D. Dose-dependent effects of chronic central infusion of oxytocin on anxiety, oxytocin receptor binding and stress-related parameters in mice. Psychoneuroendocrinology 2014, 42, 225–236. [Google Scholar] [CrossRef]
- Van den Stock, J. Social cognition assessment for mild neurocognitive disorders. Alzheimer’s Dement. 2022, 18, 1439–1440. [Google Scholar] [CrossRef] [PubMed]
- Dodich, A.; Boccardi, M.; Sacco, L.; Monsch, A.U.; Démonet, J.F.; Filardi, M.; Logroscino, G.; Salmon, D.P.; Weinbtraub, S.; Dubois, B.; et al. Consortium for the Harmonization of Neuropsychological Assessment for Neurocognitive Disorders. Answer to “Social cognition assessment for mild neurocognitive disorders”. Alzheimer’s Dement. 2022, 18, 1441–1442. [Google Scholar] [CrossRef] [PubMed]
| Authors | Year | Study Design | Sample Features | Treatment and Scheduling | Social Behavior and Cognition Measures | Study Findings | Quality Assessment |
|---|---|---|---|---|---|---|---|
| Zhang et al. | 2013 | Case-control study to test drug efficacy | 6 treated APP/PS1-21 double transgenic mice 6 untreated APP/PS1-21 double transgenic mice 6 age- and sex-matched wild-type mice | 10-day oral administration of a histone deacetylase inhibitor (MS-275) | A nest construction assay to evaluate affiliative/social behavior | Improved nesting behavior in treated mice compared to non-treated ones | Middle Quality (SYRCLE Score = 5/10) |
| Castro et al. | 2013 | Case-control study to test drug efficacy | Two independent cohorts of Wistar rats (63 adult and juvenile males; 34 treated with intranasal administration of MPTP to induce Parkinson’s symptomatology) | 7-day pretreatment with oral atorvastatin (10 mg/kg/day) | Short-term social recognition task | Treatment with atorvastatin prevented the short-term social recognition memory impairments induced in the MPTP model | Middle Quality (SYRCLE Score = 5/10) |
| Subramaniam et al. | 2018 | Case-control study to test drug efficacy | 35 Thy1-aSynuclein transgenic mice 41 wild-type mice | 1 month of 0.4 mg/kg/h subcutaneous nicotine infusion | A social approach task | Improved social behavior in treated mice | Middle Quality (SYRCLE Score = 6/10) |
| Blakemore | 1987 | Open, multicenter clinical trial to test drug efficacy | 303 patients with mild-to-moderate multi-infarct dementia (>65 years old) | 12 weeks of oral administration of cyclandelate 1600 mg/day | Parkside Behavioural Rating Scale social cognition subscale | Improved social scores in treated patients | Low Quality (NIH Study QA Tool Score = 35%) |
| Ditzler | 1991 | Case-control study to test drug efficacy | 66 mild to moderate dementia patients (43 female and 23 male; mean age 72 years old) randomized in treatment and control groups | Memantine 10 mg (from day 1 to 3), memantine 20 mg (from day 4 to 7), and memantine 30 mg (from week 2 to 6) | Sandoz Clinical Assessment Geriatric scale (SCAG) socio-emotional subscales | Improved socio-affective behavior changes in treated compared to placebo group (already after 14 days and more pronounced after 6 weeks) | Middle Quality (NIH Study QA Tool Score = 50%) |
| Jesso et al. | 2011 | Placebo-controlled study to test drug efficacy | 20 bvFTD patients (64.4 ± 7.4 years old; 12.85 ± 3.3 years of education) randomized in treatment and placebo groups | Single dose of intranasal oxytocin (24 IU) | Facial Expression Recognition and Intensity task, vocal affect recognition task, Mind in the Eyes task | Significant reduced identification of anger and a trend of reduced fear recognition in treated vs. placebo group, and poorer Mind in the Eyes task accuracy in treated vs. placebo group after 20 min from drug administration. No significant effects of treatment after 2 weeks | Low Quality (NIH SQA Score = 40%) |
| Finger et al. | 2015 | Randomized, parallel-group, double-blind, placebo-controlled study to test drug safety and tolerability | 23 FTD patients randomized in 3 dosage escalation treatment groups (61.1 mean years of age; 12.9 mean years of education) and a placebo group (66.0 mean years of age; 13.6 mean years of education) | 1-week of intranasal oxytocin administration of 24, 48, or 72 IU twice a day | Interpersonal Reactivity Index (IRI) | Repeated doses of intranasal oxytocin are safe and well tolerated; after 72 IU, a trend towards an improvement of NPI and FBI apathy scores and IRI emphatic concern subscale | Middle Quality (NIH Study QA Tool Score = 60%) |
| Finger et al. | 2018 | Phase 2, adaptive, randomized, placebo-controlled, crossover trial to test dose-escalation design model | 60 FTD patients (stage 1) 40 additional FTD patients (stage 2) | In stage 1, patients would receive three different dose schedules of 72 IU intranasal oxytocin (daily, alternate days, or every third day dosing) or placebo in order to identify the most promising dose scheduling; then, after 6-week washout, patients would receive for 6 weeks the alternate drug (placebo or oxytocin) In stage 2, 40 additional patients would be enrolled in the most promising dose arm | Interpersonal Reactivity Index (IRI) empathic concern subscale, Revised Self-Monitoring Scale (rSMS) and objective ratings of emotional facial expression and naturalistic videotaped behaviors in patients using the Social Observation Checklist (tested at baseline, at the end of stage 1, after wash-out, and at the end of stage 2) | Adaptive crossover design may facilitate oxytocin dose selection and efficacy assessment for symptomatic treatment of social disorders in FTD | NA |
| Olivero et al. | 2020 | Randomized, placebo-controlled, crossover study to test fMRI activation | 28 FTD patients (including bvFTD and semantic variant PPA), mean age 64.29 (±7.88); 23 HC, mean age 61.39 (±7.04) | Single dose of intranasal oxytocin (72 IU) | View and Imitate Task, Multifaceted Empathy Test | Increased fMRI activity after oxytocin administration in treated compared to placebo group in fronto-limbic regions | Middle Quality (NIH Study QA Tool Score = 50%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerami, C.; Perini, G.; Panzavolta, A.; Cotta Ramusino, M.; Costa, A. A Call for Drug Therapies for the Treatment of Social Behavior Disorders in Dementia: Systematic Review of Evidence and State of the Art. Int. J. Mol. Sci. 2022, 23, 11550. https://doi.org/10.3390/ijms231911550
Cerami C, Perini G, Panzavolta A, Cotta Ramusino M, Costa A. A Call for Drug Therapies for the Treatment of Social Behavior Disorders in Dementia: Systematic Review of Evidence and State of the Art. International Journal of Molecular Sciences. 2022; 23(19):11550. https://doi.org/10.3390/ijms231911550
Chicago/Turabian StyleCerami, Chiara, Giulia Perini, Andrea Panzavolta, Matteo Cotta Ramusino, and Alfredo Costa. 2022. "A Call for Drug Therapies for the Treatment of Social Behavior Disorders in Dementia: Systematic Review of Evidence and State of the Art" International Journal of Molecular Sciences 23, no. 19: 11550. https://doi.org/10.3390/ijms231911550
APA StyleCerami, C., Perini, G., Panzavolta, A., Cotta Ramusino, M., & Costa, A. (2022). A Call for Drug Therapies for the Treatment of Social Behavior Disorders in Dementia: Systematic Review of Evidence and State of the Art. International Journal of Molecular Sciences, 23(19), 11550. https://doi.org/10.3390/ijms231911550







