Applicability of the MASK-Air® App to Severe Asthma Treated with Biologic Molecules: A Pilot Study
Abstract
1. Introduction
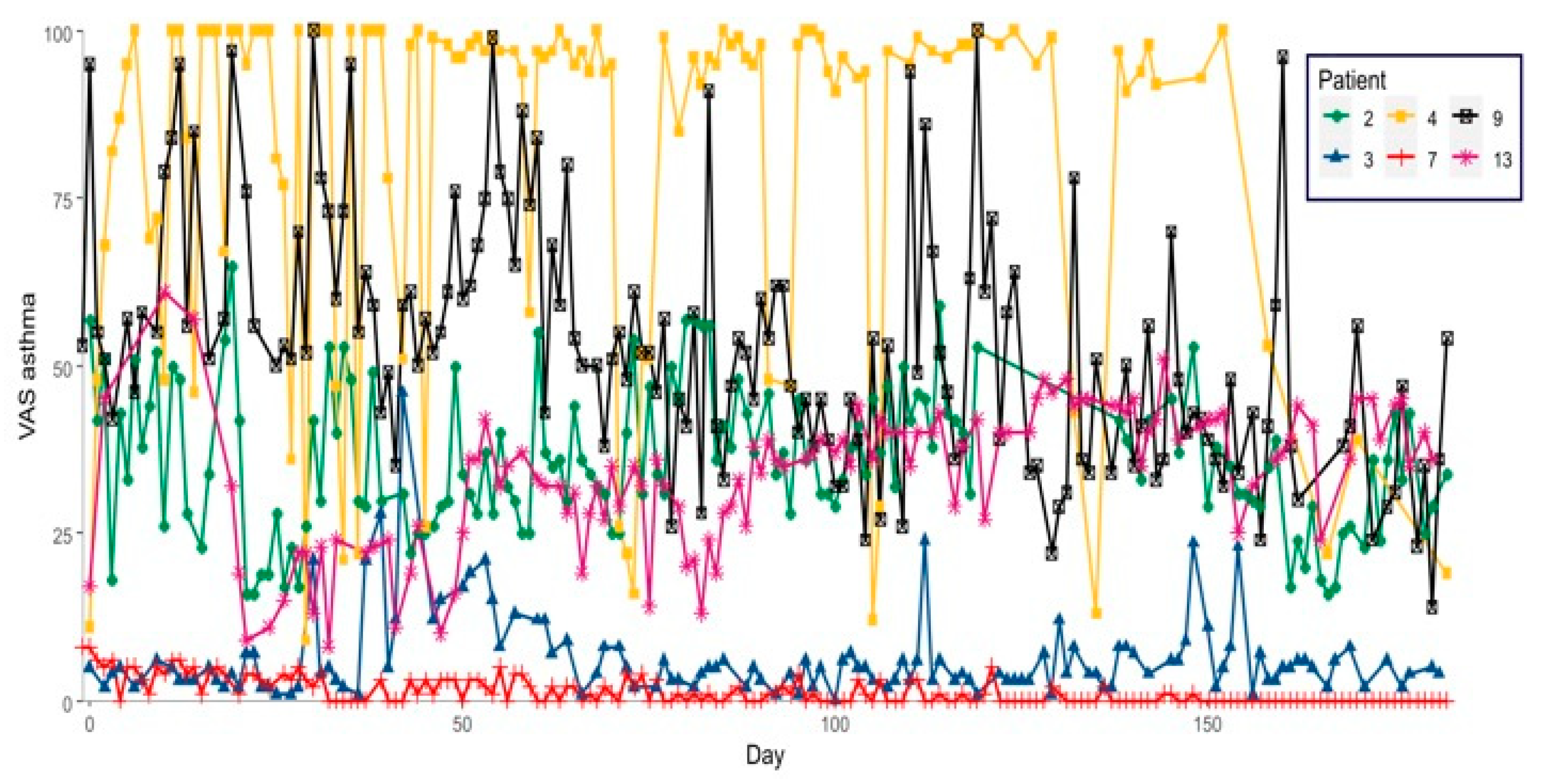
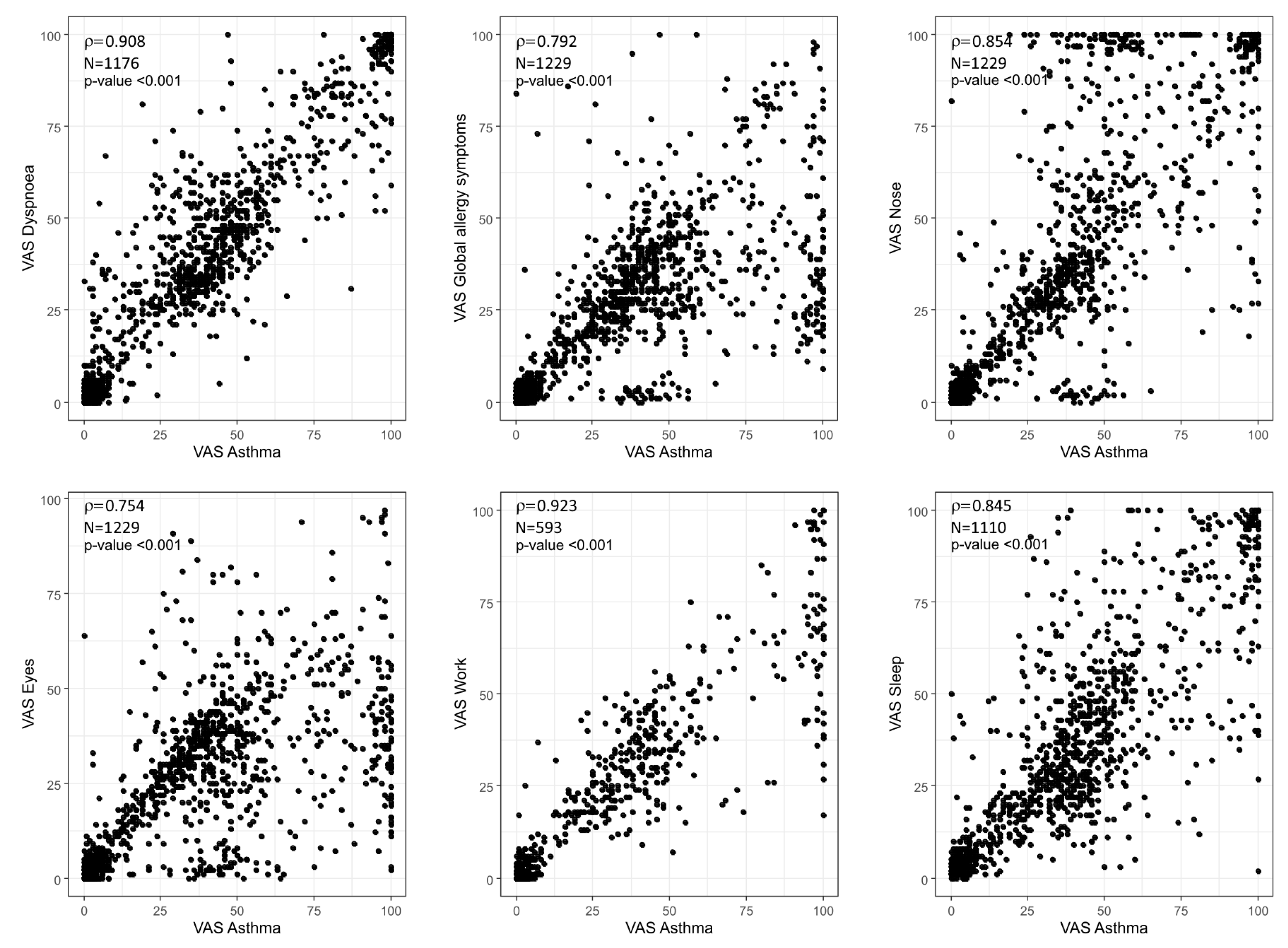
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Setting
4.3. Participants
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AHA | Active and Healthy Ageing |
| CRSwNP | Chronic rhinosinusitis with nasal polyps |
| EGPA | Eosinophilic granulomatosis with polyangiitis |
| EIP | European Innovation Partnership |
| FEV1 | Forced expiratory volume in the first second |
| FVC | Forced vital capacity |
| GINA | Global Initiative for Asthma |
| ICPs | Integrated Care Pathways |
| ICS | Inhaled corticosteroids |
| LABA | Long-acting beta-agonist |
| LAMA | Long-acting muscarinic agonist |
| MASK | Mobile Airways Sentinel NetworK |
| mMPR | modified medication possession ratio |
| OSAS | Obstructive Sleep Apnea Syndrome |
| PRO | patient-reported outcome |
| SIgE | Serum specific IgE |
| VAS | visual analogue scales |
References
- Reddel, H.K.; FitzGerald, J.M.; Bateman, E.D.; Bacharier, L.B.; Becker, A.; Brusselle, G.; Buhl, R.; Cruz, A.A.; Fleming, L.; Inoue, H.; et al. GINA 2019: A fundamental change in asthma management: Treatment of asthma with short-acting bronchodilators alone is no longer recommended for adults and adolescents. Eur. Respir. J. 2019, 53, 1901046. [Google Scholar] [CrossRef] [PubMed]
- Portnoy, J.M.; Waller, M.; De Lurgio, S.; Dinakar, C. Telemedicine is as effective as in-person visits for patients with asthma. Ann. Allergy Asthma Immunol. 2016, 117, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Chavannes, N.H.; Guldemond, N.; Haahtela, T.; Hellings, P.W.; Sheikh, A. Realising the potential of mHealth to improve asthma and allergy care: How to shape the future. Eur. Respir. J. 2017, 49, 1700447. [Google Scholar] [CrossRef]
- Denton, E.; Hore-Lacy, F.; Radhakrishna, N.; Gilbert, A.; Tay, T.; Lee, J.; Dabscheck, E.; Harvey, E.S.; Bulathsinhala, L.; Fingleton, J.; et al. Severe Asthma Global Evaluation (SAGE): An electronic platform for severe asthma. J. Allergy Clin. Immunol. Pract. 2019, 7, 1440–1449. [Google Scholar] [CrossRef]
- Tay, T.R.; Lee, J.; Radhakrishna, N.; Hore-Lacy, F.; Stirling, R.; Hoy, R.; Dabscheck, E.; O’Hehir, R.; Hew, M. A structured approach to specialist-referred difficult asthma patients improves control of comorbidities and enhances asthma outcomes. J. Allergy Clin. Immunol. Pract. 2017, 5, 956–964.e3. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.; Price, D.; Musgrave, S.D.; Malhotra, S.; Lee, A.J.; Ayansina, D.; Sheikh, A.; Tarassenko, L.; Pagliari, C.; Pinnock, H.; et al. Clinical and cost effectiveness of mobile phone supported self monitoring of asthma: Multicentre randomised controlled trial. BMJ 2012, 344, e1756. [Google Scholar] [CrossRef]
- Kagen, S.; Garland, A. Asthma and allergy mobile Apps in 2018. Curr. Allergy Asthma Rep. 2019, 19, 6. [Google Scholar] [CrossRef]
- Ljungberg, H.; Carleborg, A.; Gerber, H.; Öfverström, C.; Wolodarski, J.; Menshi, F.; Engdahl, M.; Eduards, M.; Nordlund, B. Clinical effect on uncontrolled asthma using a novel digital automated self-management solution: A physician-blinded randomised controlled crossover trial. Eur. Respir. J. 2019, 54, 1900983. [Google Scholar] [CrossRef]
- Moore, A.; Preece, A.; Sharma, R.; Heaney, L.G.; Costello, R.W.; Wise, R.A.; Ludwig-Sengpiel, A.; Mosnaim, G.; Rees, J.; Tomlinson, R.; et al. A randomised controlled trial of the effect of a connected inhaler system on medication adherence in uncontrolled asthmatic patients. Eur. Respir. J. 2021, 57, 2003103. [Google Scholar] [CrossRef]
- Bousquet, J.; Michel, J.; Standberg, T.; Crooks, G.; Iakovidis, I.; Iglesia, M. The european innovation partnership on active and healthy ageing: The european geriatric medicine introduces the EIP on AHA Column. Eur. Geriatr. Med. 2014, 5, 361–362. [Google Scholar] [CrossRef]
- Bousquet, J.; Addis, A.; Adcock, I.; Agache, I.; Agusti, A.; Alonso, A.; Annesi-Maesano, I.; Anto, J.M.; Bachert, C.; Baena-Cagnani, C.E.; et al. Integrated care pathways for airway diseases (AIRWAYS-ICPs). Eur. Respir. J. 2014, 44, 304–323. [Google Scholar] [CrossRef]
- Bousquet, J.; Anto, J.M.; Bachert, C.; Bosnic-Anticevich, S.; Erhola, M.; Haahtela, T.; Hellings, P.W.; Kuna, P.; Pfaar, O.; Samolinski, B.; et al. From ARIA guidelines to the digital transformation of health in rhinitis and asthma multimorbidity. Eur. Respir. J. 2019, 54, 1901023. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; MASK Study Group; Arnavielhe, S.; Bedbrook, A.; Bewick, M.; Laune, D.; Mathieu-Dupas, E.; Murray, R.; Onorato, G.L.; Pépin, J.L.; et al. MASK 2017: ARIA digitally-enabled, integrated, person-centred care for rhinitis and asthma multimorbidity using real-world-evidence. Clin. Transl. Allergy 2018, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Agache, I.; Aliberti, M.R.; Angles, R.; Annesi-Maesano, I.; Anto, J.M.; Arnavielhe, S.; Asayag, E.; Bacci, E.; Bedbrook, A.; et al. Transfer of innovation on allergic rhinitis and asthma multimorbidity in the elderly (MACVIA-ARIA)—EIP on AHA twinning reference site (GARD research demonstration project). Allergy 2018, 73, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Ventura, M.T.; Giuliano, A.F.M.; Buquicchio, R.; Bedbrook, A.; Czarlewski, W.; Laune, D.; Patella, V.; Canonica, G.W.; Bousquet, J. Implementation of the MASK-Air® App for Rhinitis and Asthma in Older Adults: MASK@Puglia Pilot Study. Int. Arch. Allergy Immunol. 2022, 183, 45–50. [Google Scholar] [CrossRef]
- Benfante, A.; Principe, S.; Battaglia, S.; Scichilone, N. Are biological drugs effective and safe in older severe asthmatics? Expert Opin. Drug Saf. 2019, 18, 369–380. [Google Scholar] [CrossRef]
- Agusta, F.; Battaglia, S.; Benfante, A.; Spatafora, M.; Scichilone, N. Challenges in the pharmacological treatment of geriatric asthma. Expert Rev. Clin. Pharmacol. 2016, 9, 917–926. [Google Scholar] [CrossRef]
- Sousa-Pinto, B.; Fonseca, J.A.; Gemicioglu, B.; Regateiro, F.; Scichilone, N.; Ventura, M.T.; Bousquet, J. Patient-reported outcome measures (PROMs) using the MASK-air ® app in severe asthma. Allergy 2022, 77, 1600–1602. [Google Scholar] [CrossRef]
- Agache, I.; Eguiluz-Gracia, I.; Cojanu, C.; Laculiceanu, A.; del Giacco, S.; Zemelka-Wiacek, M.; Kosowska, A.; Akdis, C.A.; Jutel, M. Advances and highlights in asthma in 2021. Allergy 2021, 76, 3390–3407. [Google Scholar] [CrossRef]
- Tan, R.; Cvetkovski, B.; Kritikos, V.; O’Hehir, R.E.; Lourenço, O.; Bousquet, J.; Bosnic-Anticevich, S. Identifying an effective mobile health application for the self-management of allergic rhinitis and asthma in Australia. J. Asthma 2019, 57, 1128–1139. [Google Scholar] [CrossRef]
- Caminati, M.; Vaia, R.; Furci, F.; Guarnieri, G.; Senna, G. Uncontrolled asthma: Unmet needs in the management of patients. J. Asthma Allergy 2021, 14, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Anto, J.M.; Bachert, C.; Haahtela, T.; Zuberbier, T.; Czarlewski, W.; Bedbrook, A.; Bosnic-Anticevich, S.; Canonica, G.W.; Cardona, V.; et al. ARIA digital anamorphosis: Digital transformation of health and care in airway diseases from research to practice. Allergy 2021, 76, 168–190. [Google Scholar] [CrossRef] [PubMed]
- Menditto, E.; Costa, E.; Midao, L.; Bosnic-Anticevich, S.; Novellino, E.; Bialek, S.; Briedis, V.; Mair, A.; Rajabian-Soderlund, R.; Arnavielhe, S.; et al. Adherence to treatment in allergic rhinitis using mobile technology. The MASK Study. Clin. Exp. Allergy 2019, 49, 442–460. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.F.; Wenzel, S.E.; Brozek, J.L.; Bush, A.; Castro, M.; Sterk, P.J.; Adcock, I.M.; Bateman, E.D.; Bel, E.H.; Bleecker, E.R.; et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 2014, 43, 343–373. [Google Scholar] [CrossRef]
- Park, J.Y.E.; Li, J.; Howren, A.; Tsao, N.W.; De Vera, M. Mobile phone Apps targeting medication adherence: Quality assessment and content analysis of user reviews. JMIR mHealth uHealth 2019, 7, e11919. [Google Scholar] [CrossRef]
- Vieira-Marques, P.; Almeida, R.; Teixeira, J.F.; Valente, J.; Jácome, C.; Cachim, A.; Guedes, R.; Pereira, A.; Jacinto, T.; Fonseca, J.A. InspirerMundi-Remote monitoring of inhaled medication adherence through objective verification based on combined image processing techniques. Methods Inf. Med. 2021, 60, e9–e19. [Google Scholar] [CrossRef]
- Jacome, C.; Almeida, R.; Pereira, A.M.; Amaral, R.; Mendes, S.; Alves-Correia, M.; Vidal, C.; Freire, S.L.; Brea, P.M.; Araújo, L.; et al. Feasibility and acceptability of an asthma App to monitor medication adherence: Mixed methods study. JMIR mHealth uHealth 2021, 9, e26442. [Google Scholar] [CrossRef]
- Doshi, H.; Hsia, B.; Shahani, J.; Mowrey, W.; Jariwala, S.P. Impact of technology-based interventions on patient-reported outcomes in asthma: A systematic review. J. Allergy Clin. Immunol. Pract. 2021, 9, 2336–2341. [Google Scholar] [CrossRef]
- Snoswell, C.L.; Rahja, M.; Lalor, A.F. A Systematic review and meta-analysis of change in health-related quality of life for interactive telehealth interventions for patients with asthma. Value Health 2021, 24, 291–302. [Google Scholar] [CrossRef]
- Bousquet, J.; Devillier, P.; Arnavielhe, S.; Bedbrook, A.; Alexis-Alexandre, G.; Van Eerd, M.; Murray, R.; Canonica, G.W.; Illario, M.; Menditto, E.; et al. Treatment of allergic rhinitis using mobile technology with real-world data: The MASK observational pilot study. Allergy 2018, 73, 1763–1774. [Google Scholar] [CrossRef]
- Laune, D.; Arnavielhe, S.; Viart, F.; Bedbrook, A.; Mercier, J.; Lun San Luk, G.; deVries, G.; Spreux, O.; Bousquet, J. Adaptation of the general data protection regulation (GDPR) to a smartphone app for rhinitis and asthma [MASK-air(R)]. Rev. Mal. Respir. 2019, 36, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Juniper, E.F.; Bousquet, J.; Abetz, L.; Bateman, E.D. Identifying “well-controlled” and “not well-controlled” asthma using the Asthma Control Questionnaire. Respir. Med. 2006, 100, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Di Fraia, M.; Tripodi, S.; Arasi, S.; Dramburg, S.; Castelli, S.; Villalta, D.; Buzzulini, F.; Sfika, I.; Villella, V.; Potapova, E.; et al. Adherence to prescribed E-Diary recording by patients with seasonal allergic rhinitis: Observational study. J. Med. Internet Res. 2020, 22, e16642. [Google Scholar] [CrossRef] [PubMed]
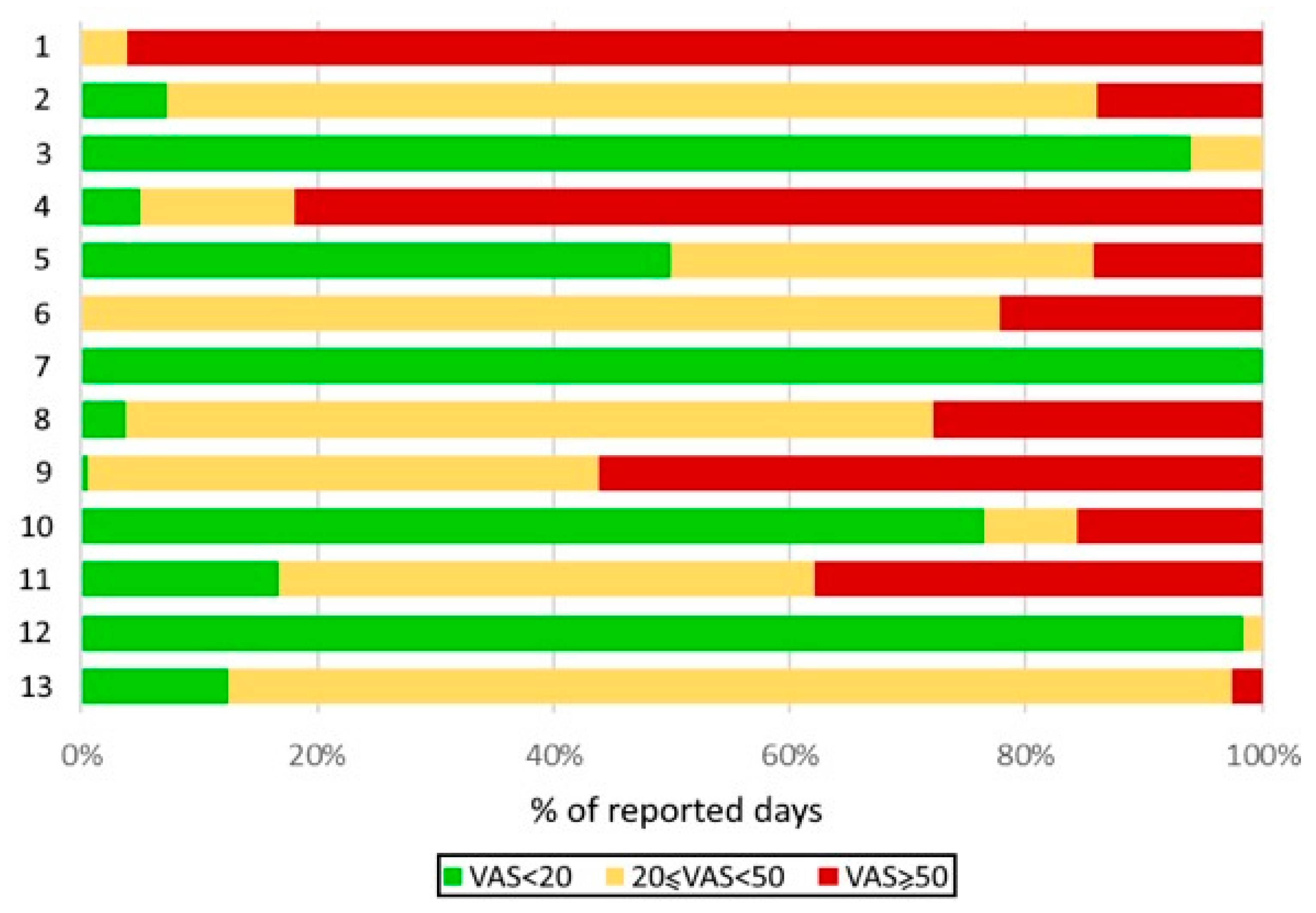
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 44 | 43 | 37 | 21 | 55 | 56 | 59 | 66 | 41 | 18 | 48 | 59 | 62 |
| Sex | M | M | F | F | M | F | F | M | F | F | F | F | F |
| GINA step | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Rhinitis | + | + | + | + | + | + | + | + | + | + | + | + | + |
| CRSwNP | + | +EGPA | + | + | + | + | |||||||
| Conjunctivitis | + | + | |||||||||||
| OSAS | + | + | |||||||||||
| FEV1 (% pred) | 80 | 101 | 83 | 91 | 71 | 89 | 40 | 122 | 125 | 82 | 73 | 70 | 104 |
| FEV1/FVC | 55 | 125 | 84 | 112 | 62 | 75 | 50 | 107 | 106 | 103 | 90 | 105 | 99 |
| Eos/mm3 | 100 | 700 | 300 | 60 | 0 | 170 | 30 | 950 | 160 | 0 | 760 | 250 | |
| Total IgE | 25 | 761 | 300 | 84 | 15 | 27 | 238 | 30 | 20 | 518 | 130 | 66 | 81 |
| ICS/LABA | + | + | + | + | + | + | + | + | + | + | + | + | + |
| LAMA | + | + | + | + | + | + | |||||||
| Other meds | + | + | + | + | + | + | |||||||
| Omalizumab | + | + | + | ||||||||||
| Mepolizumab | + | + | + | + | |||||||||
| Benralizumab | + | + | + | + | + | ||||||||
| Dupilumab | + |
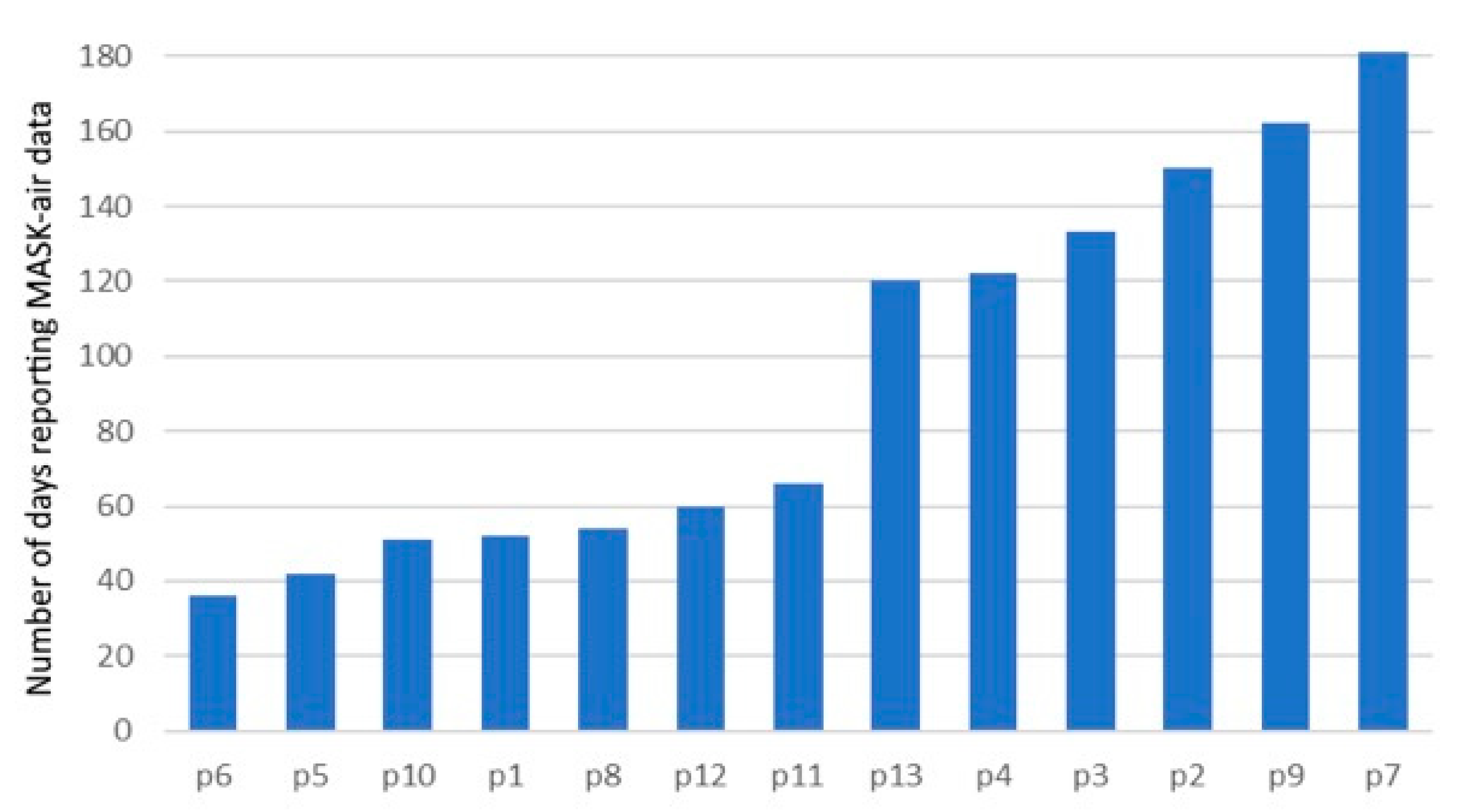
| Patient Number | Number of MASK-Air® Reporting Days | Number of Days Reporting Treatment with the Usual Asthma Long-Acting Medication (% a) | Number of Days Reporting Treatment with the Usual Asthma Long-Acting Medication, with No Additional Asthma Medication Used (%) |
|---|---|---|---|
| 6 | 36 | 32 (88.9) | 32 (88.9) |
| 5 | 42 | 40 (95.2) | 40 (95.2) |
| 10 | 51 | 51 (100) | 37 (72.6) |
| 1 | 52 | 51 (98.1) | 25 (48.1) |
| 8 | 54 | 54 (100) | 54 (100) |
| 12 | 60 | 58 (96.7) | 58 (96.7) |
| 11 | 66 | 40 (60.6) | 40 (60.6) |
| 13 | 120 | 96 (80.0) | 93 (77.5) |
| 4 | 122 | 117 (95.9) | 112 (91.8) |
| 3 | 133 | 127 (95.5) | 126 (94.7) |
| 2 | 150 | 149 (99.3) | 140 (93.3) |
| 9 | 162 | 159 (98.2) | 133 (82.1) |
| 7 | 181 | 177 (97.8) | 177 (97.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benfante, A.; Sousa-Pinto, B.; Pillitteri, G.; Battaglia, S.; Fonseca, J.; Bousquet, J.; Scichilone, N. Applicability of the MASK-Air® App to Severe Asthma Treated with Biologic Molecules: A Pilot Study. Int. J. Mol. Sci. 2022, 23, 11470. https://doi.org/10.3390/ijms231911470
Benfante A, Sousa-Pinto B, Pillitteri G, Battaglia S, Fonseca J, Bousquet J, Scichilone N. Applicability of the MASK-Air® App to Severe Asthma Treated with Biologic Molecules: A Pilot Study. International Journal of Molecular Sciences. 2022; 23(19):11470. https://doi.org/10.3390/ijms231911470
Chicago/Turabian StyleBenfante, Alida, Bernardo Sousa-Pinto, Gianluca Pillitteri, Salvatore Battaglia, Joao Fonseca, Jean Bousquet, and Nicola Scichilone. 2022. "Applicability of the MASK-Air® App to Severe Asthma Treated with Biologic Molecules: A Pilot Study" International Journal of Molecular Sciences 23, no. 19: 11470. https://doi.org/10.3390/ijms231911470
APA StyleBenfante, A., Sousa-Pinto, B., Pillitteri, G., Battaglia, S., Fonseca, J., Bousquet, J., & Scichilone, N. (2022). Applicability of the MASK-Air® App to Severe Asthma Treated with Biologic Molecules: A Pilot Study. International Journal of Molecular Sciences, 23(19), 11470. https://doi.org/10.3390/ijms231911470








