Exploring Amodiaquine’s Repurposing Potential in Breast Cancer Treatment—Assessment of In-Vitro Efficacy & Mechanism of Action
Abstract
1. Introduction
2. Results
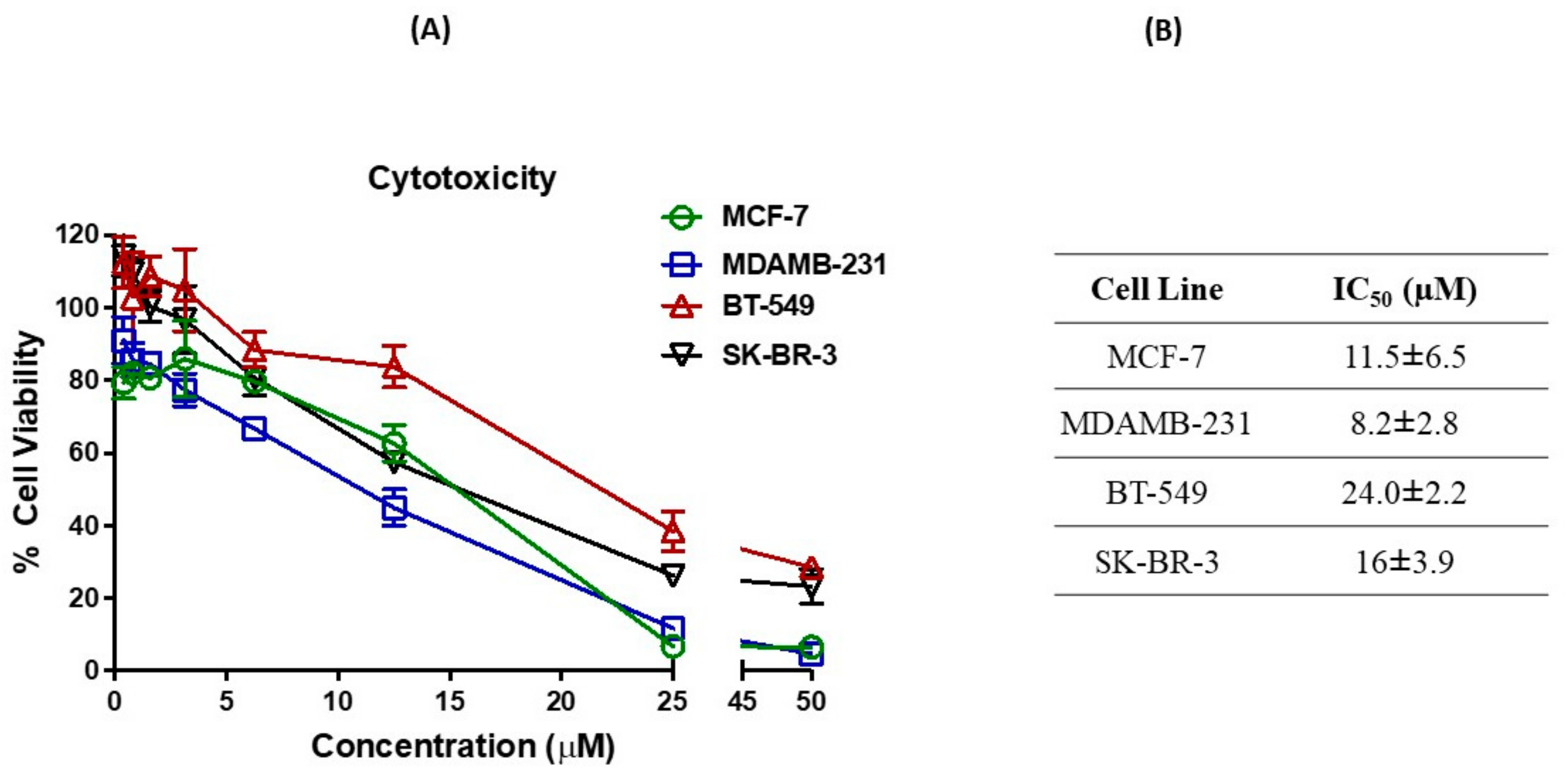
2.1. Cytotoxicity against Breast Cancer Cell Lines
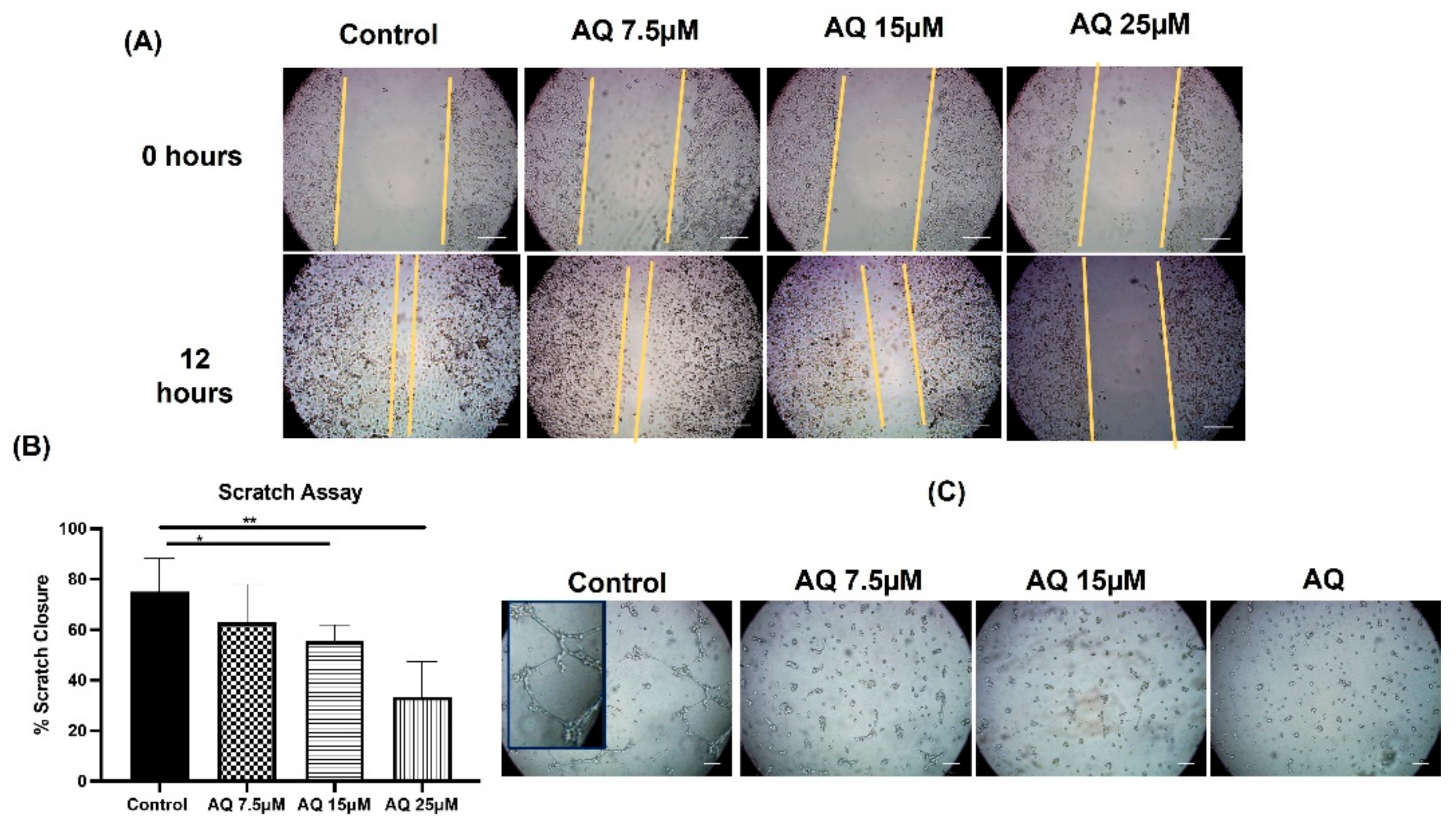
2.2. Scratch Assay
2.3. Vasculogenic Mimicry Assay
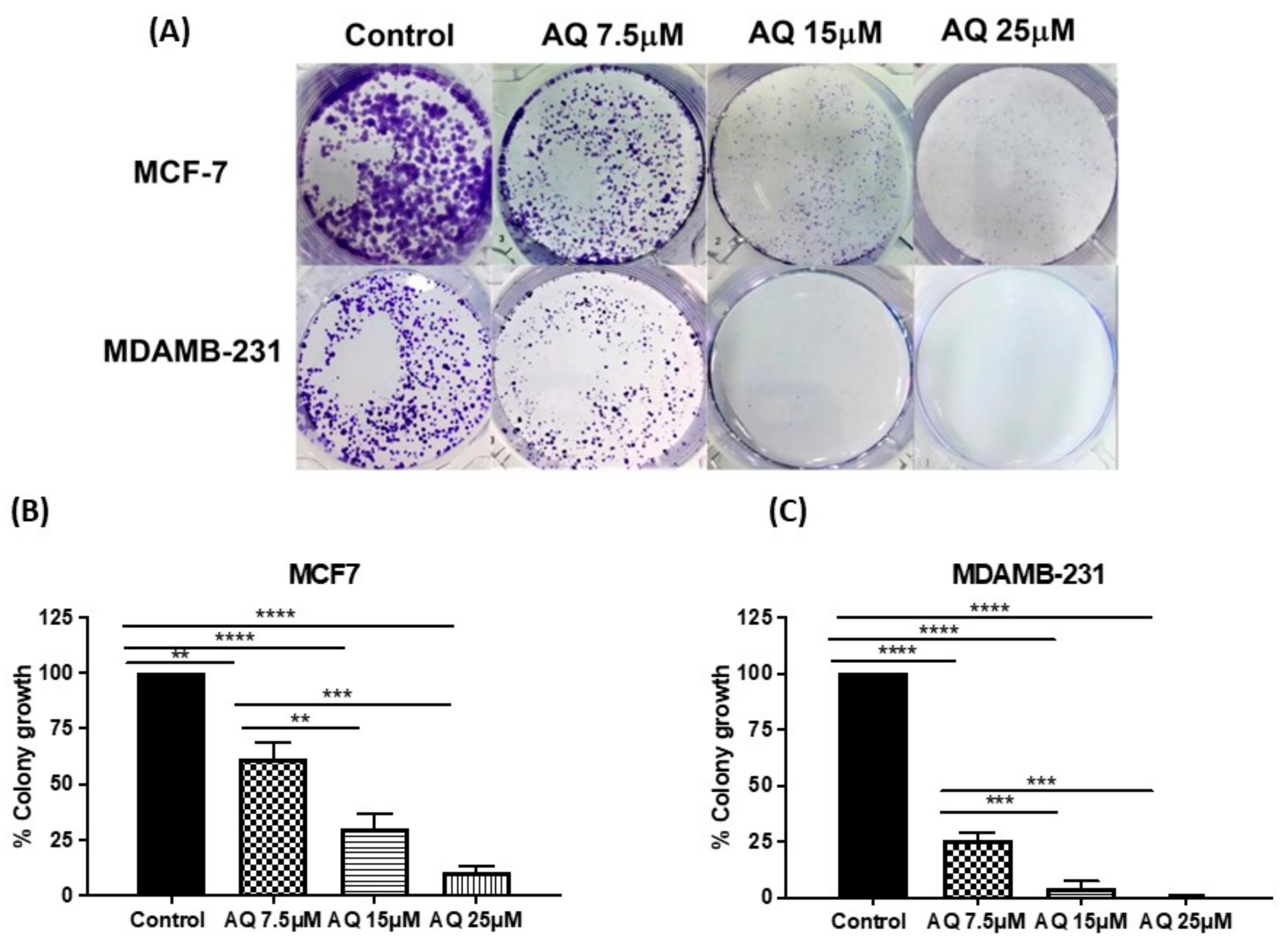
2.4. Clonogenic Assay
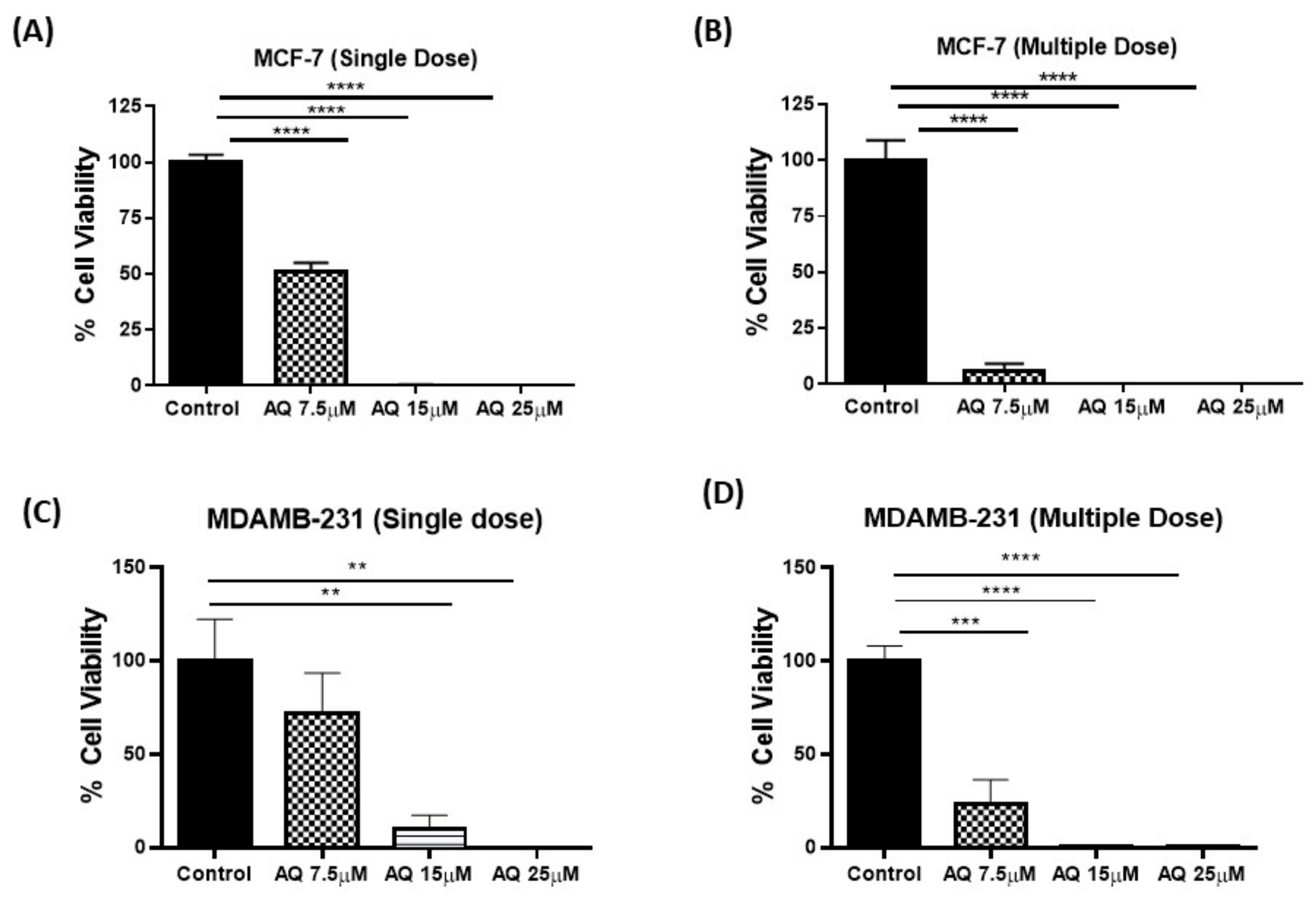
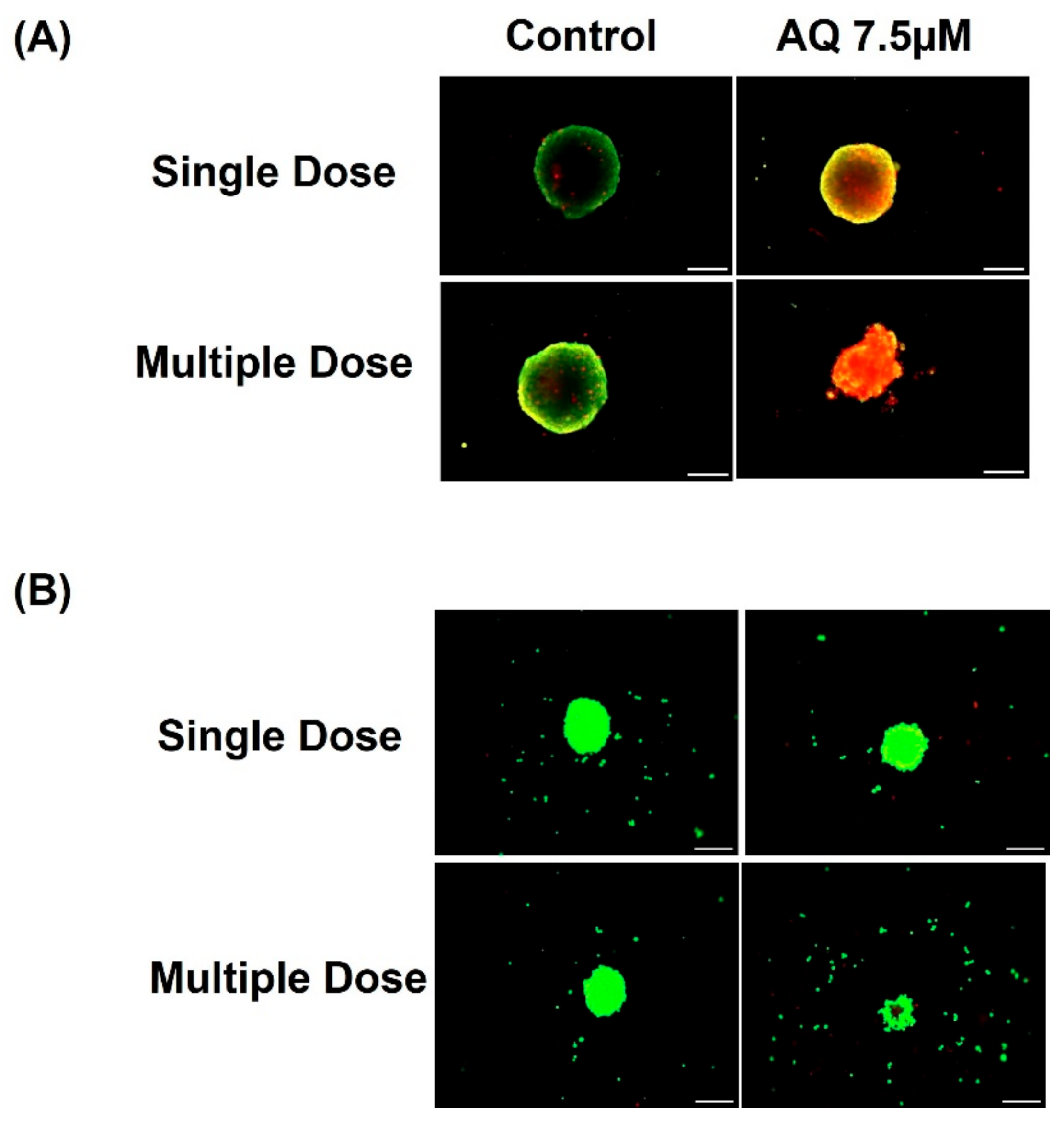
2.5. 3D Spheroid Studies
2.6. CellTiter-Glo Luminescent Cell Viability Assay
2.7. Live-Dead Cell Assay
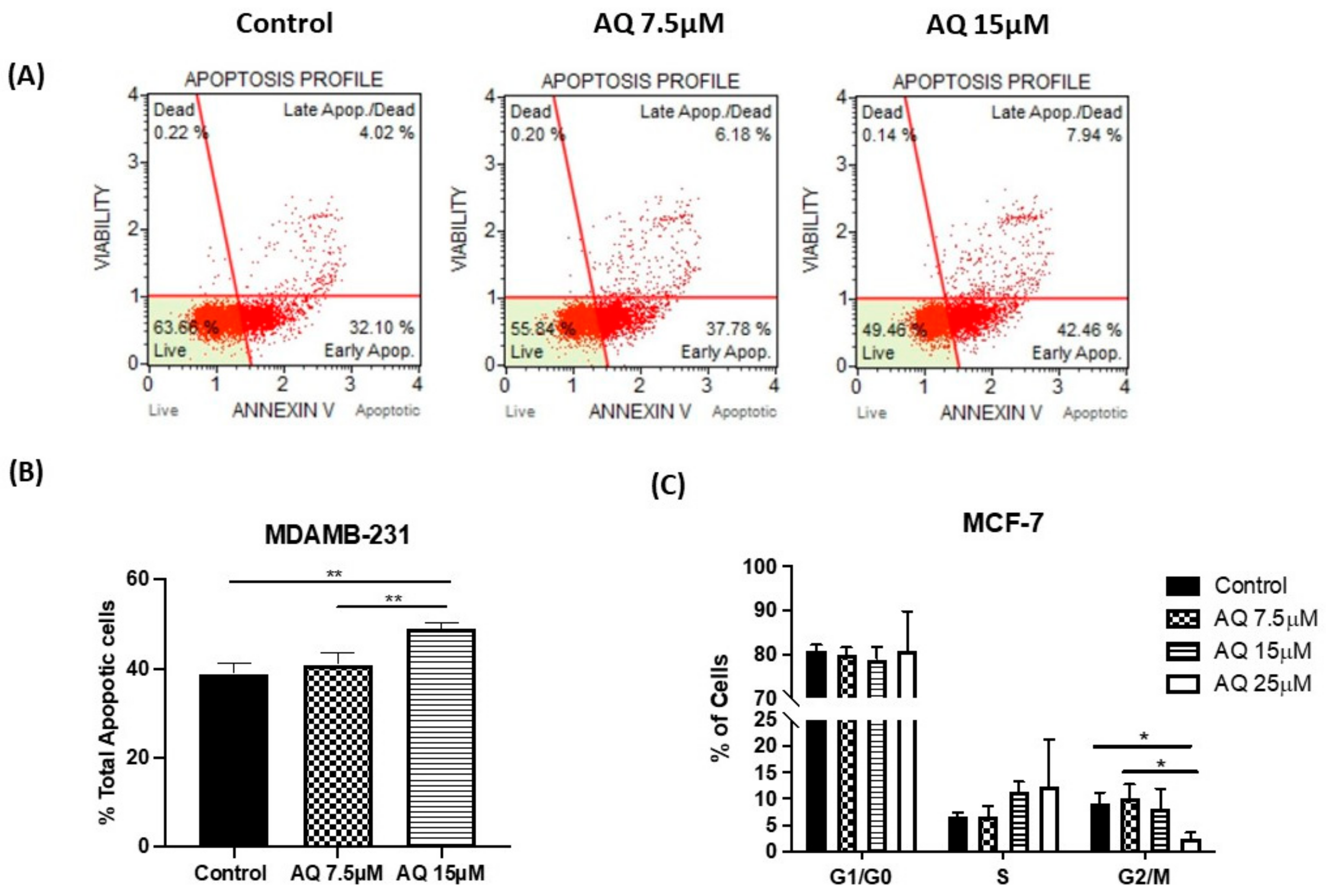
2.8. Annexin V and Dead Cell Assay
2.9. Cell Cycle Analysis
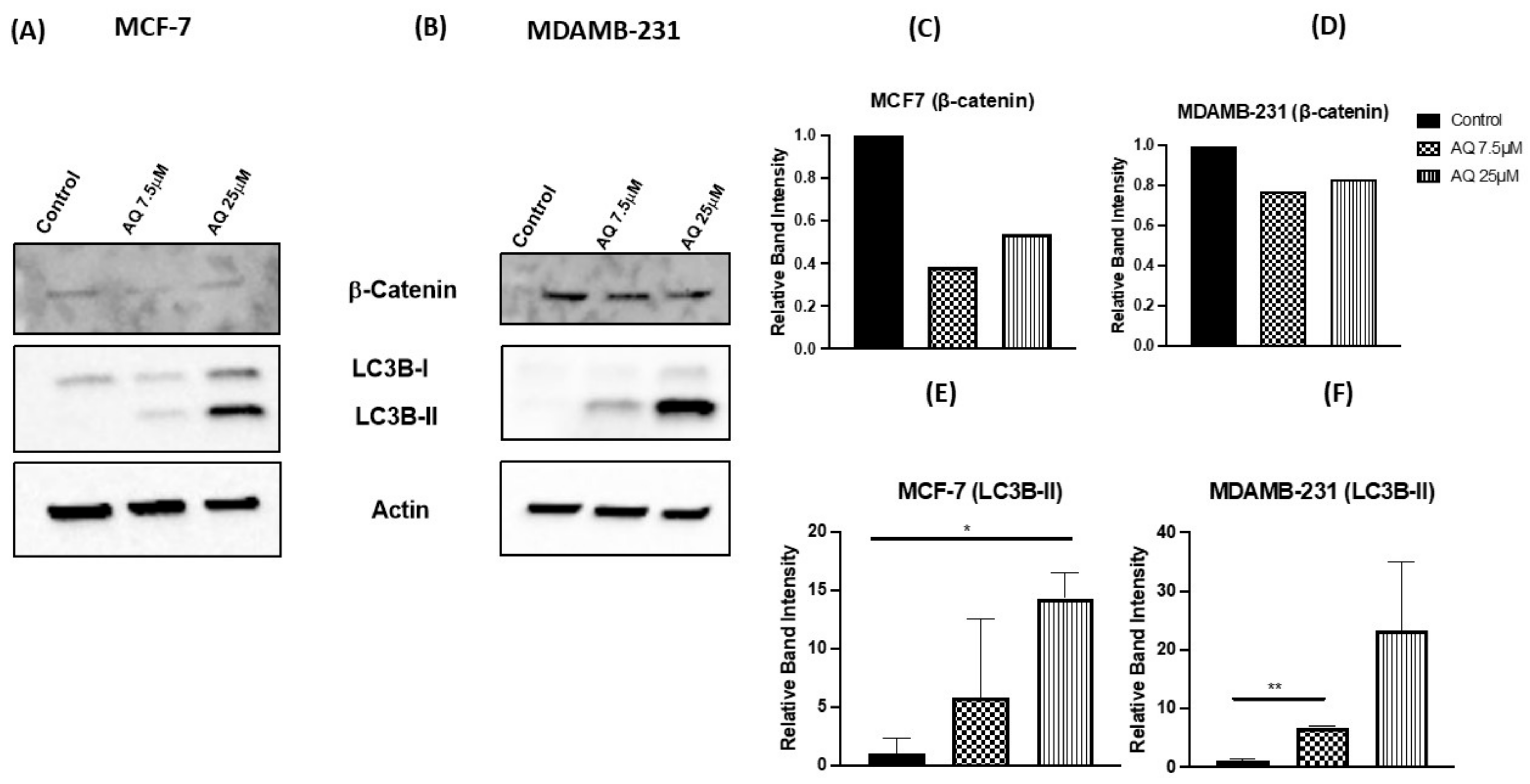
2.10. Western Blot and Protein Expression Studies
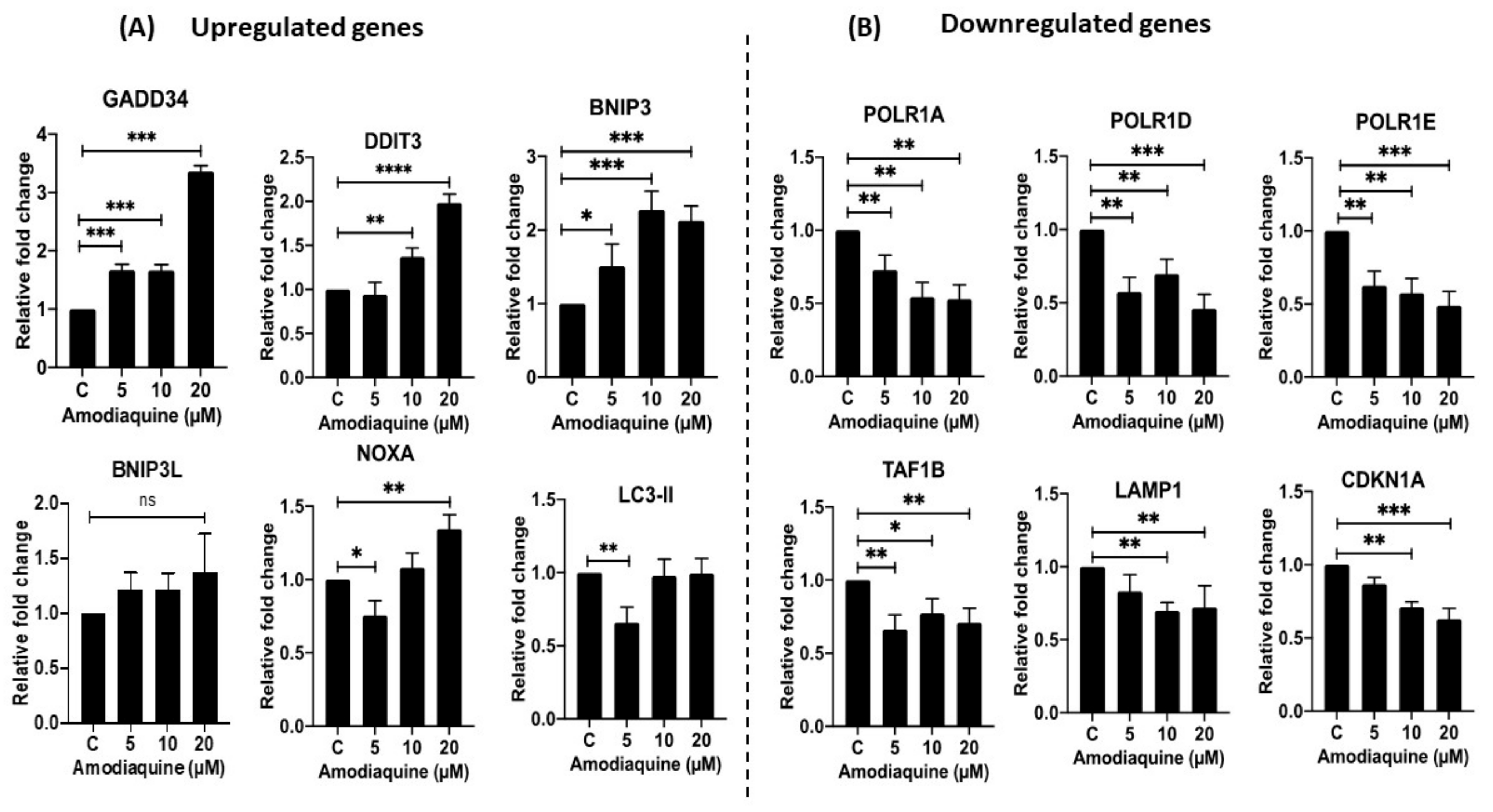
2.11. Gene Expression Analysis
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Materials
4.2. Methods
4.2.1. Cytotoxicity Studies
4.2.2. Scratch Assay
4.2.3. Vasculogenic Mimicry Assay
4.2.4. Clonogenic Assay
4.2.5. 3D Spheroid Study
4.2.6. CellTiter-Glo 3D Luminescent Cell Viability Assay
4.2.7. Live-Dead Cell Assay
4.2.8. Muse Annexin V and Dead Cell Assay
4.2.9. Cell Cycle Analysis
4.2.10. Western Blot Analysis
4.2.11. RNA Extraction
4.2.12. cDNA Synthesis
4.2.13. Gene Expression Analysis
4.2.14. Data Analysis and Statistical Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Fang, X.; Cao, J.; Shen, A. Advances in Anti-Breast Cancer Drugs and the Application of Nano-Drug Delivery Systems in Breast Cancer Therapy. J. Drug Deliv. Sci. Technol. 2020, 57, 101662. [Google Scholar] [CrossRef]
- Momenimovahed, Z.; Salehiniya, H. Epidemiological Characteristics of and Risk Factors for Breast Cancer in the World. Breast Cancer 2019, 11, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Breast Cancer Statistics. Susan G. Komen®. Available online: https://www.komen.org/breast-cancer/facts-statistics/breast-cancer-statistics/ (accessed on 21 July 2022).
- Onitilo, A.A.; Engel, J.M.; Greenlee, R.T.; Mukesh, B.N. Breast Cancer Subtypes Based on ER/PR and Her2 Expression: Comparison of Clinicopathologic Features and Survival. Clin. Med. Res. 2009, 7, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Types of Breast Cancer|Memorial Sloan Kettering Cancer Center. Available online: https://www.mskcc.org/cancer-care/types/breast/types-breast (accessed on 21 July 2022).
- Types of Breast Cancer: Triple Negative, ER-Positive, HER2-Positive. Available online: https://www.webmd.com/breast-cancer/breast-cancer-types-er-positive-her2-positive (accessed on 21 July 2022).
- Tong, C.W.S.; Wu, M.; Cho, W.C.S.; To, K.K.W. Recent Advances in the Treatment of Breast Cancer. Front. Oncol. 2018, 8, 227. [Google Scholar] [CrossRef]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. J. Cancer 2017, 8, 3131–3141. [Google Scholar] [CrossRef]
- Aggarwal, S.; Verma, S.S.; Aggarwal, S.; Gupta, S.C. Drug Repurposing for Breast Cancer Therapy: Old Weapon for New Battle. Semin. Cancer Biol. 2019, 68, 8–20. [Google Scholar] [CrossRef]
- Triple-Negative Breast Cancer|Details, Diagnosis, and Signs. Available online: https://www.cancer.org/cancer/breast-cancer/about/types-of-breast-cancer/triple-negative.html (accessed on 21 July 2022).
- Survival Rates for Breast Cancer. Available online: https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-survival-rates.html (accessed on 21 July 2022).
- Kuo, M.T. Roles of Multidrug Resistance Genes in Breast Cancer Chemoresistance. Adv. Exp. Med. Biol. 2007, 608, 23–30. [Google Scholar] [CrossRef]
- Nedeljković, M.; Damjanović, A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer—How We Can Rise to the Challenge. Cells 2019, 8, 957. [Google Scholar] [CrossRef]
- Jana, D.; Zhao, Y. Strategies for Enhancing Cancer Chemodynamic Therapy Performance. Exploration 2022, 2, 20210238. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug Repurposing: Progress, Challenges and Recommendations. Nat. Rev. Drug Discov. 2018, 18, 41–58. [Google Scholar] [CrossRef]
- Parvathaneni, V.; Kulkarni, N.S.; Muth, A.; Gupta, V. Drug Repurposing: A Promising Tool to Accelerate the Drug Discovery Process. Drug Discov. Today 2019, 24, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Pantziarka, P.; Bouche, G.; Meheus, L.; Sukhatme, V.; Sukhatme, V.P.; Vikas, P. The Repurposing Drugs in Oncology (ReDO) Project. Ecancermedicalscience 2014, 8, 442. [Google Scholar] [CrossRef] [PubMed]
- Talevi, A.; Bellera, C.L. Challenges and Opportunities with Drug Repurposing: Finding Strategies to Find Alternative Uses of Therapeutics. Expert Opin. Drug Discov. 2020, 15, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Parvathaneni, V.; Gupta, V. Utilizing Drug Repurposing against COVID-19—Efficacy, Limitations, and Challenges. Life Sci. 2020, 259, 118275. [Google Scholar] [CrossRef] [PubMed]
- Hooft van Huijsduijnen, R.; Guy, R.K.; Chibale, K.; Haynes, R.K.; Peitz, I.; Kelter, G.; Phillips, M.A.; Vennerstrom, J.L.; Yuthavong, Y.; Wells, T.N.C. Anticancer Properties of Distinct Antimalarial Drug Classes. PLoS ONE 2013, 8, e82962. [Google Scholar] [CrossRef] [PubMed]
- Janku, F.; McConkey, D.J.; Hong, D.S.; Kurzrock, R. Autophagy as a Target for Anticancer Therapy. Nat. Rev. Clin. Oncol. 2011, 8, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Kamat, S.; Kumari, M. Repurposing Chloroquine Against Multiple Diseases with Special Attention to SARS-CoV-2 and Associated Toxicity. Front. Pharmacol. 2021, 12, 576093. [Google Scholar] [CrossRef]
- Pellegrini, P.; Strambi, A.; Zipoli, C.; Hägg-Olofsson, M.; Buoncervello, M.; Linder, S.; De Milito, A. Acidic Extracellular PH Neutralizes the Autophagy-Inhibiting Activity of Chloroquine. Autophagy 2014, 10, 562–571. [Google Scholar] [CrossRef]
- Parvathaneni, V.; Shukla, S.K.; Kulkarni, N.S.; Gupta, V. Development and Characterization of Inhalable Transferrin Functionalized Amodiaquine Nanoparticles—Efficacy in Non-Small Cell Lung Cancer (NSCLC) Treatment. Int. J. Pharm. 2021, 608, 121038. [Google Scholar] [CrossRef]
- Qiao, S.; Tao, S.; Rojo de la Vega, M.; Park, S.L.; Vonderfecht, A.A.; Jacobs, S.L.; Zhang, D.D.; Wondrak, G.T. The Antimalarial Amodiaquine Causes Autophagic-Lysosomal and Proliferative Blockade Sensitizing Human Melanoma Cells to Starvation- and Chemotherapy-Induced Cell Death. Autophagy 2013, 9, 2087–2102. [Google Scholar] [CrossRef]
- Goodall, M.L.; Wang, T.; Martin, K.R.; Kortus, M.G.; Kauffman, A.L.; Trent, J.M.; Gately, S.; MacKeigan, J.P. Development of Potent Autophagy Inhibitors That Sensitize Oncogenic BRAF V600E Mutant Melanoma Tumor Cells to Vemurafenib. Autophagy 2014, 10, 1120–1136. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, N.S.; Parvathaneni, V.; Shukla, S.K.; Barasa, L.; Perron, J.C.; Yoganathan, S.; Muth, A.; Gupta, V. Tyrosine Kinase Inhibitor Conjugated Quantum Dots for Non-Small Cell Lung Cancer (NSCLC) Treatment. Eur. J. Pharm. Sci. 2019, 133, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, B.; Parvathaneni, V.; Kulkarni, N.S.; Shukla, S.K.; Damon, J.K.; Sarode, A.; Kanabar, D.; Garcia, J.V.; Mitragotri, S.; Muth, A.; et al. Cyclodextrin Modified Erlotinib Loaded PLGA Nanoparticles for Improved Therapeutic Efficacy against Non-Small Cell Lung Cancer. Int. J. Biol. Macromol. 2019, 122, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Parvathaneni, V.; Goyal, M.; Kulkarni, N.S.; Shukla, S.K.; Gupta, V. Nanotechnology Based Repositioning of an Anti-Viral Drug for Non-Small Cell Lung Cancer (NSCLC). Pharm. Res. 2020, 37, 123. [Google Scholar] [CrossRef]
- Kabała-Dzik, A.; Rzepecka-Stojko, A.; Kubina, R.; Jastrzębska-Stojko, Ż.; Stojko, R.; Wojtyczka, R.D.; Stojko, J. Migration Rate Inhibition of Breast Cancer Cells Treated by Caffeic Acid and Caffeic Acid Phenethyl Ester: An In Vitro Comparison Study. Nutrients 2017, 9, 1144. [Google Scholar] [CrossRef]
- Ge, H.; Luo, H. Overview of Advances in Vasculogenic Mimicry—A Potential Target for Tumor Therapy. Cancer Manag. Res. 2018, 10, 2429–2437. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor Angiogenesis: Causes, Consequences, Challenges and Opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Shen, Y.; Quan, J.; Wang, M.; Li, S.; Yang, J.; Lv, M.; Chen, Z.; Zhang, L.; Zhao, X.; Yang, J. Tumor Vasculogenic Mimicry Formation as an Unfavorable Prognostic Indicator in Patients with Breast Cancer. Oncotarget 2017, 8, 56408–56416. [Google Scholar] [CrossRef]
- Xu, M.-R.; Wei, P.-F.; Suo, M.-Z.; Hu, Y.; Ding, W.; Su, L.; Zhu, Y.-D.; Song, W.-J.; Tang, G.-H.; Zhang, M.; et al. Brucine Suppresses Vasculogenic Mimicry in Human Triple-Negative Breast Cancer Cell Line MDA-MB-231. Biomed. Res. Int. 2019, 2019, 6543230. [Google Scholar] [CrossRef]
- Parvathaneni, V.; Kulkarni, N.S.; Chauhan, G.; Shukla, S.K.; Elbatanony, R.; Patel, B.; Kunda, N.K.; Muth, A.; Gupta, V. Development of Pharmaceutically Scalable Inhaled Anti-Cancer Nanotherapy—Repurposing Amodiaquine for Non-Small Cell Lung Cancer (NSCLC). Mater. Sci. Eng. C 2020, 115, 111139. [Google Scholar] [CrossRef]
- Elangovan, S.; Hsieh, T.-C.; Wu, J.M. Growth Inhibition of Human MDA-MB-231 Breast Cancer Cells by δ-Tocotrienol Is Associated with Loss of Cyclin D1/CDK4 Expression and Accompanying Changes in the State of Phosphorylation of the Retinoblastoma Tumor Suppressor Gene Product. Anticancer. Res. 2008, 7, 2641–2647. [Google Scholar]
- Huang, Z.; Yu, P.; Tang, J. Characterization of Triple-Negative Breast Cancer MDA-MB-231 Cell Spheroid Model. Onco. Targets Ther. 2020, 13, 5395–5405. [Google Scholar] [CrossRef]
- Shukla, S.K.; Kulkarni, N.S.; Chan, A.; Parvathaneni, V.; Farrales, P.; Muth, A.; Gupta, V. Metformin-Encapsulated Liposome Delivery System: An Effective Treatment Approach against Breast Cancer. Pharmaceutics 2019, 11, 559. [Google Scholar] [CrossRef]
- Balsa, L.M.; Ruiz, M.C.; Santa Maria de la Parra, L.; Baran, E.J.; León, I.E. Anticancer and Antimetastatic Activity of Copper(II)-Tropolone Complex against Human Breast Cancer Cells, Breast Multicellular Spheroids and Mammospheres. J. Inorg. Biochem. 2020, 204, 110975. [Google Scholar] [CrossRef]
- Froehlich, K.; Haeger, J.-D.; Heger, J.; Pastuschek, J.; Photini, S.M.; Yan, Y.; Lupp, A.; Pfarrer, C.; Mrowka, R.; Schleußner, E.; et al. Generation of Multicellular Breast Cancer Tumor Spheroids: Comparison of Different Protocols. J. Mammary Gland Biol. Neoplasia 2016, 21, 89–98. [Google Scholar] [CrossRef]
- Nunes, A.S.; Costa, E.C.; Barros, A.S.; de Melo-Diogo, D.; Correia, I.J. Establishment of 2D Cell Cultures Derived From 3D MCF-7 Spheroids Displaying a Doxorubicin Resistant Profile. Biotechnol. J. 2019, 14, e1800268. [Google Scholar] [CrossRef]
- Rodríguez, C.E.; Berardi, D.E.; Abrigo, M.; Todaro, L.B.; Bal de Kier Joffé, E.D.; Fiszman, G.L. Breast Cancer Stem Cells Are Involved in Trastuzumab Resistance through the HER2 Modulation in 3D Culture. J. Cell Biochem. 2018, 119, 1381–1391. [Google Scholar] [CrossRef]
- Brancato, V.; Gioiella, F.; Imparato, G.; Guarnieri, D.; Urciuolo, F.; Netti, P.A. 3D Breast Cancer Microtissue Reveals the Role of Tumor Microenvironment on the Transport and Efficacy of Free-Doxorubicin In Vitro. Acta Biomater. 2018, 75, 200–212. [Google Scholar] [CrossRef]
- Liang, C.-C.; Park, A.Y.; Guan, J.-L. In Vitro Scratch Assay: A Convenient and Inexpensive Method for Analysis of Cell Migration in Vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]
- Veldwijk, M.R.; Neumaier, C.; Gerhardt, A.; Giordano, F.A.; Sütterlin, M.; Herskind, C.; Wenz, F. Comparison of the Proliferative and Clonogenic Growth Capacity of Wound Fluid from Breast Cancer Patients Treated with and without Intraoperative Radiotherapy. Transl. Cancer Res. 2015, 4, 173–177. [Google Scholar] [CrossRef]
- Parvathaneni, V.; Kulkarni, N.S.; Shukla, S.K.; Farrales, P.T.; Kunda, N.K.; Muth, A.; Gupta, V. Systematic Development and Optimization of Inhalable Pirfenidone Liposomes for Non-Small Cell Lung Cancer Treatment. Pharmaceutics 2020, 12, 206. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, N.S.; Vaidya, B.; Parvathaneni, V.; Bhanja, D.; Gupta, V. Repurposing Quinacrine for Treatment of Malignant Mesothelioma: In-Vitro Therapeutic and Mechanistic Evaluation. Int. J. Mol. Sci. 2020, 21, 6306. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Kulkarni, N.S.; Farrales, P.; Kanabar, D.D.; Parvathaneni, V.; Kunda, N.K.; Muth, A.; Gupta, V. Sorafenib Loaded Inhalable Polymeric Nanocarriers against Non-Small Cell Lung Cancer. Pharm. Res. 2020, 37, 67. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Chan, A.; Parvathaneni, V.; Gupta, V. Metformin-Loaded Chitosomes for Treatment of Malignant Pleural Mesothelioma—A Rare Thoracic Cancer. Int. J. Biol. Macromol. 2020, 160, 128–141. [Google Scholar] [CrossRef]
- Shukla, S.K.; Sarode, A.; Wang, X.; Mitragotri, S.; Gupta, V. Particle Shape Engineering for Improving Safety and Efficacy of Doxorubicin—A Case Study of Rod-Shaped Carriers in Resistant Small Cell Lung Cancer. Biomater. Adv. 2022, 137, 212850. [Google Scholar] [CrossRef]
- Gills, J.J.; Lopiccolo, J.; Tsurutani, J.; Shoemaker, R.H.; Best, C.J.M.; Abu-Asab, M.S.; Borojerdi, J.; Warfel, N.A.; Gardner, E.R.; Danish, M.; et al. Nelfinavir, A Lead HIV Protease Inhibitor, Is a Broad-Spectrum, Anticancer Agent That Induces Endoplasmic Reticulum Stress, Autophagy, and Apoptosis in Vitro and in Vivo. Clin. Cancer Res. 2007, 13, 5183–5194. [Google Scholar] [CrossRef]
- Wu, X.; Deng, G.; Hao, X.; Li, Y.; Zeng, J.; Ma, C.; He, Y.; Liu, X.; Wang, Y. A Caspase-Dependent Pathway Is Involved in Wnt/β-Catenin Signaling Promoted Apoptosis in Bacillus Calmette-Guerin Infected RAW264.7 Macrophages. Int. J. Mol. Sci. 2014, 15, 5045–5062. [Google Scholar] [CrossRef]
- Espinoza, J.A.; Zisi, A.; Kanellis, D.C.; Carreras-Puigvert, J.; Henriksson, M.; Hühn, D.; Watanabe, K.; Helleday, T.; Lindström, M.S.; Bartek, J. The Antimalarial Drug Amodiaquine Stabilizes P53 through Ribosome Biogenesis Stress, Independently of Its Autophagy-Inhibitory Activity. Cell Death Differ. 2019, 27, 773–789. [Google Scholar] [CrossRef]
- Vakilinezhad, M.A.; Amini, A.; Dara, T.; Alipour, S. Methotrexate and Curcumin Co-Encapsulated PLGA Nanoparticles as a Potential Breast Cancer Therapeutic System: In Vitro and in Vivo Evaluation. Colloids Surf. B Biointerfaces 2019, 184, 110515. [Google Scholar] [CrossRef]
- Luque-Bolivar, A.; Pérez-Mora, E.; Villegas, V.E.; Rondón-Lagos, M. Resistance and Overcoming Resistance in Breast Cancer. Breast Cancer 2020, 12, 211–229. [Google Scholar] [CrossRef]
- Gordon, A. The Increasing Efficacy of Breast Cancer Treatment. Clin. Oncol. 1997, 9, 338–342. [Google Scholar] [CrossRef]
- Salentin, S.; Adasme, M.F.; Heinrich, J.C.; Haupt, V.J.; Daminelli, S.; Zhang, Y.; Schroeder, M. From Malaria to Cancer: Computational Drug Repositioning of Amodiaquine Using PLIP Interaction Patterns. Sci. Rep. 2017, 7, 11401. [Google Scholar] [CrossRef]
- Hassan, F.; Mohammed, G.; El-Hiti, G.A.; Alshanon, A.; Yousif, E. Cytotoxic Effects of Tamoxifen in Breast Cancer Cells. J. Unexplored Med. Data 2018, 3, 3. [Google Scholar] [CrossRef][Green Version]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular Principles of Metastasis: A Hallmark of Cancer Revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Shin, C.S.; Kwak, B.; Han, B.; Park, K. Development of an in Vitro 3D Tumor Model to Study Therapeutic Efficiency of an Anticancer Drug. Mol. Pharm. 2013, 10, 2167–2175. [Google Scholar] [CrossRef]
- Vaidya, B.; Kulkarni, N.S.; Shukla, S.K.; Parvathaneni, V.; Chauhan, G.; Damon, J.K.; Sarode, A.; Garcia, J.V.; Kunda, N.; Mitragotri, S.; et al. Development of Inhalable Quinacrine Loaded Bovine Serum Albumin Modified Cationic Nanoparticles: Repurposing Quinacrine for Lung Cancer Therapeutics. Int. J. Pharm. 2020, 577, 118995. [Google Scholar] [CrossRef]
- Chen, K.; Shi, W. Autophagy Regulates Resistance of Non-Small Cell Lung Cancer Cells to Paclitaxel. Tumor. Biol. 2016, 37, 10539–10544. [Google Scholar] [CrossRef]
- Liu, G.; Pei, F.; Yang, F.; Li, L.; Amin, A.D.; Liu, S.; Buchan, J.R.; Cho, W.C. Role of Autophagy and Apoptosis in Non-Small-Cell Lung Cancer. Int. J. Mol. Sci. 2017, 18, 367. [Google Scholar] [CrossRef]
- Redmann, M.; Benavides, G.A.; Berryhill, T.F.; Wani, W.Y.; Ouyang, X.; Johnson, M.S.; Ravi, S.; Barnes, S.; Darley-Usmar, V.M.; Zhang, J. Inhibition of Autophagy with Bafilomycin and Chloroquine Decreases Mitochondrial Quality and Bioenergetic Function in Primary Neurons. Redox Biol. 2016, 11, 73–81. [Google Scholar] [CrossRef]
- Yu, S.; Wang, Z.; Su, Z.; Song, J.; Zhou, L.; Sun, Q.; Liu, S.; Li, S.; Li, Y.; Wang, M.; et al. Gigantol Inhibits Wnt/β-Catenin Signaling and Exhibits Anticancer Activity in Breast Cancer Cells. BMC Complement Altern. Med. 2018, 18, 59. [Google Scholar] [CrossRef]
- Abderrahman, B.; Maximov, P.Y.; Curpan, R.F.; Hanspal, J.S.; Fan, P.; Xiong, R.; Tonetti, D.A.; Thatcher, G.R.J.; Jordan, V.C. Pharmacology and Molecular Mechanisms of Clinically Relevant Estrogen Estetrol and Estrogen Mimic BMI-135 for the Treatment of Endocrine-Resistant Breast Cancer. Mol. Pharmacol. 2020, 98, 364–381. [Google Scholar] [CrossRef]
- Samuel, S.M.; Varghese, E.; Koklesová, L.; Líšková, A.; Kubatka, P.; Büsselberg, D. Counteracting Chemoresistance with Metformin in Breast Cancers: Targeting Cancer Stem Cells. Cancers 2020, 12, 2482. [Google Scholar] [CrossRef]
- Campbell, E.J.; Dachs, G.U.; Morrin, H.R.; Davey, V.C.; Robinson, B.A.; Vissers, M.C.M. Activation of the Hypoxia Pathway in Breast Cancer Tissue and Patient Survival Are Inversely Associated with Tumor Ascorbate Levels. BMC Cancer 2019, 19, 307. [Google Scholar] [CrossRef]
- Avena, P.; Anselmo, W.; Whitaker-Menezes, D.; Wang, C.; Pestell, R.G.; Lamb, R.S.; Hulit, J.; Casaburi, I.; Andò, S.; Martinez-Outschoorn, U.E.; et al. Compartment-Specific Activation of PPARγ Governs Breast Cancer Tumor Growth, via Metabolic Reprogramming and Symbiosis. Cell Cycle 2013, 12, 1360–1370. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Chen, S.; Feng, W.; Li, Z.; Luo, Y.; Zhu, X. A STING-Related Prognostic Score Predicts High-Risk Patients of Colorectal Cancer and Provides Insights into Immunotherapy. Ann. Transl. Med. 2021, 9, 14. [Google Scholar] [CrossRef]
- Sun, X.; Hu, Y.; Wu, J.; Shi, L.; Zhu, L.; Xi, P.-W.; Wei, J.-F.; Ding, Q. RBMS2 Inhibits the Proliferation by Stabilizing P21 MRNA in Breast Cancer. J. Exp. Clin. Cancer Res. 2018, 37, 298. [Google Scholar] [CrossRef]
- Yang, Y.; Roy, A.; Zhao, Y.; Undzys, E.; Li, S.-D. Comparison of Tumor Penetration of Podophyllotoxin–Carboxymethylcellulose Conjugates with Various Chemical Compositions in Tumor Spheroid Culture and In Vivo Solid Tumor. Bioconjugate Chem. 2017, 28, 1505–1518. [Google Scholar] [CrossRef]
- Lazzari, G.; Couvreur, P.; Mura, S. Multicellular Tumor Spheroids: A Relevant 3D Model for the in Vitro Preclinical Investigation of Polymer Nanomedicines. Polym. Chem. 2017, 8, 4947–4969. [Google Scholar] [CrossRef]
- Lagies, S.; Schlimpert, M.; Neumann, S.; Wäldin, A.; Kammerer, B.; Borner, C.; Peintner, L. Cells Grown in Three-Dimensional Spheroids Mirror in Vivo Metabolic Response of Epithelial Cells. Commun. Biol. 2020, 3, 246. [Google Scholar] [CrossRef]
- Geissmann, Q. OpenCFU, a New Free and Open-Source Software to Count Cell Colonies and Other Circular Objects. PLoS ONE 2013, 8, e54072. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parvathaneni, V.; Chilamakuri, R.; Kulkarni, N.S.; Baig, N.F.; Agarwal, S.; Gupta, V. Exploring Amodiaquine’s Repurposing Potential in Breast Cancer Treatment—Assessment of In-Vitro Efficacy & Mechanism of Action. Int. J. Mol. Sci. 2022, 23, 11455. https://doi.org/10.3390/ijms231911455
Parvathaneni V, Chilamakuri R, Kulkarni NS, Baig NF, Agarwal S, Gupta V. Exploring Amodiaquine’s Repurposing Potential in Breast Cancer Treatment—Assessment of In-Vitro Efficacy & Mechanism of Action. International Journal of Molecular Sciences. 2022; 23(19):11455. https://doi.org/10.3390/ijms231911455
Chicago/Turabian StyleParvathaneni, Vineela, Rameswari Chilamakuri, Nishant S. Kulkarni, Nabeela F. Baig, Saurabh Agarwal, and Vivek Gupta. 2022. "Exploring Amodiaquine’s Repurposing Potential in Breast Cancer Treatment—Assessment of In-Vitro Efficacy & Mechanism of Action" International Journal of Molecular Sciences 23, no. 19: 11455. https://doi.org/10.3390/ijms231911455
APA StyleParvathaneni, V., Chilamakuri, R., Kulkarni, N. S., Baig, N. F., Agarwal, S., & Gupta, V. (2022). Exploring Amodiaquine’s Repurposing Potential in Breast Cancer Treatment—Assessment of In-Vitro Efficacy & Mechanism of Action. International Journal of Molecular Sciences, 23(19), 11455. https://doi.org/10.3390/ijms231911455







