The Antidepressant-like Activity, Effects on Recognition Memory Deficits, Bioavailability, and Safety after Chronic Administration of New Dual-Acting Small Compounds Targeting Neuropsychiatric Symptoms in Dementia
Abstract
1. Introduction
2. Results
2.1. Bioavailability of PQA-AZ4 and PQA-AZ6 in Rats
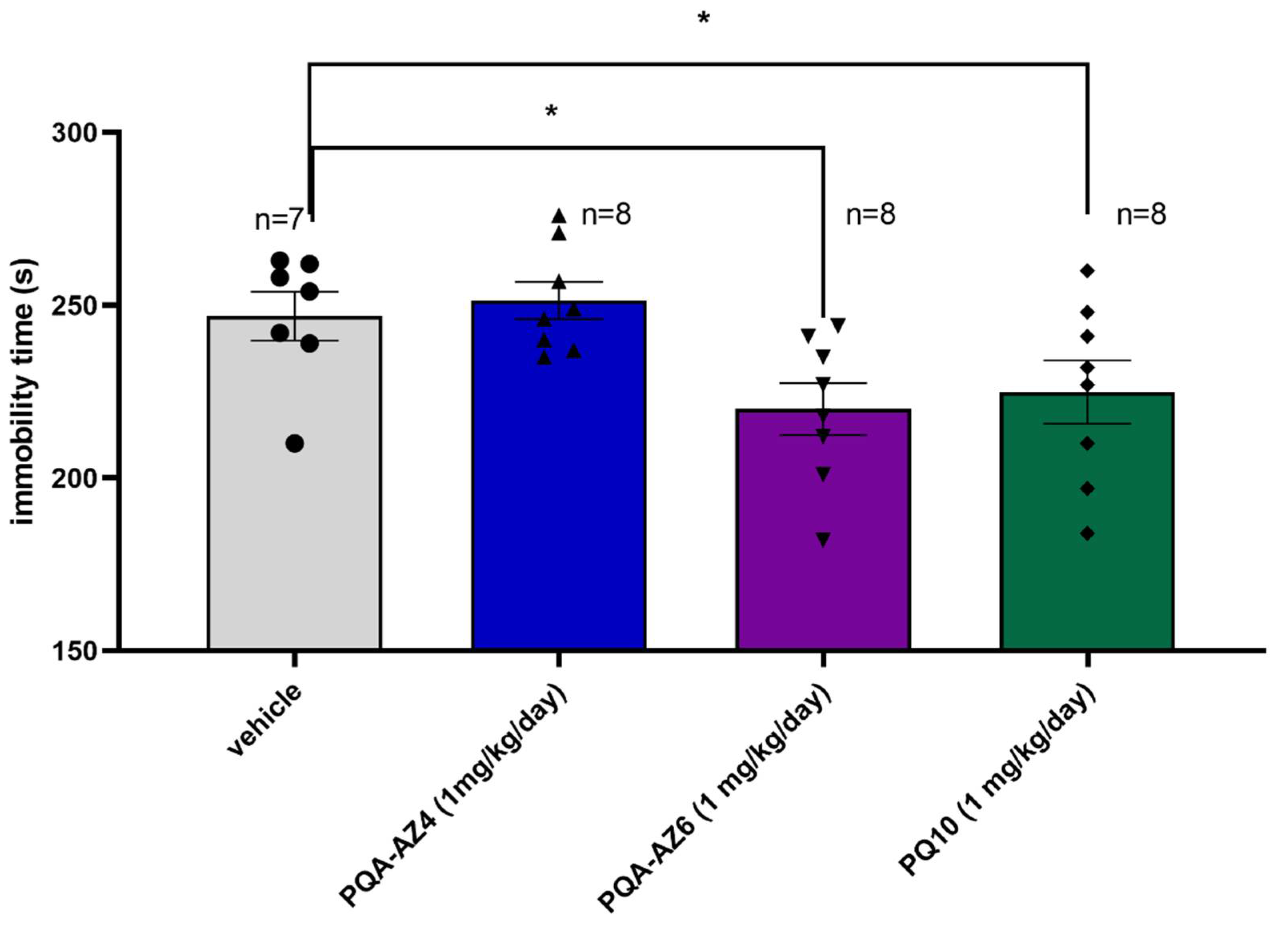
2.2. Antidepressant-like Activity of Repeated Administration of PQA-AZ4, PQA-AZ6 in the Forced Swim Test (FST) in Rats
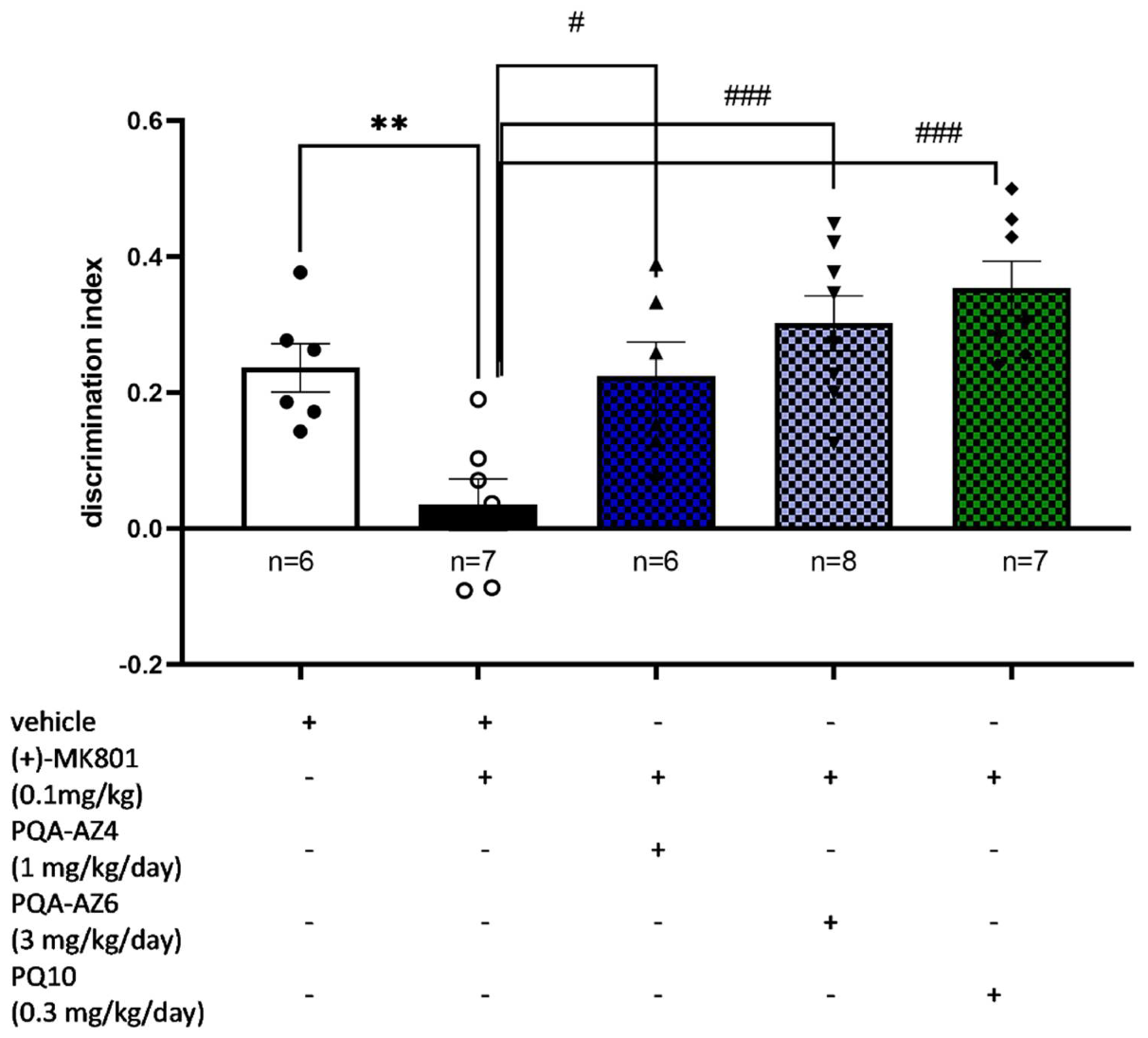
2.3. Memory-Enhancing Properties of Repeated Administration of PQA-AZ4, PQA-AZ6, and PQ-10 in the Novel Object Recognition (NOR) Test in (+)-MK-801 Treated Rats
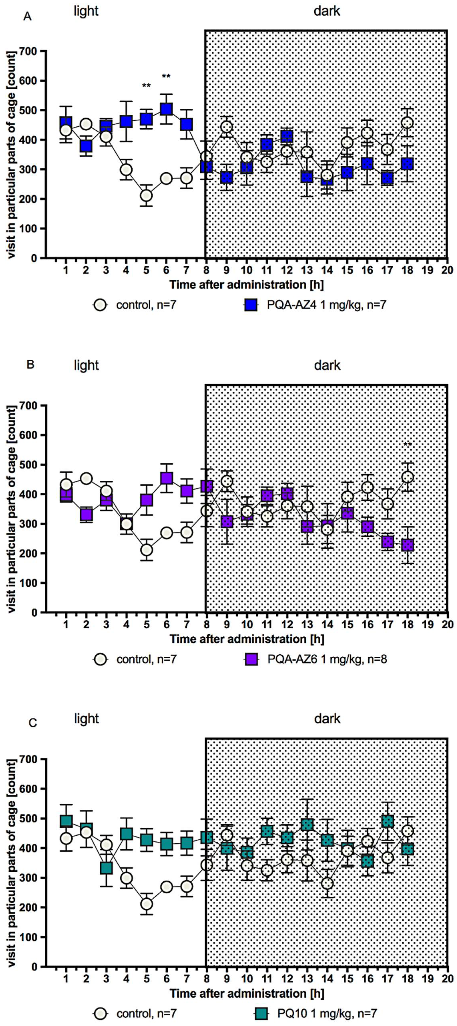
2.4. Effects of Repeated Administration of PQA-AZ4, PQA-AZ6, and PQ-10 on Spontaneous Locomotor Activity
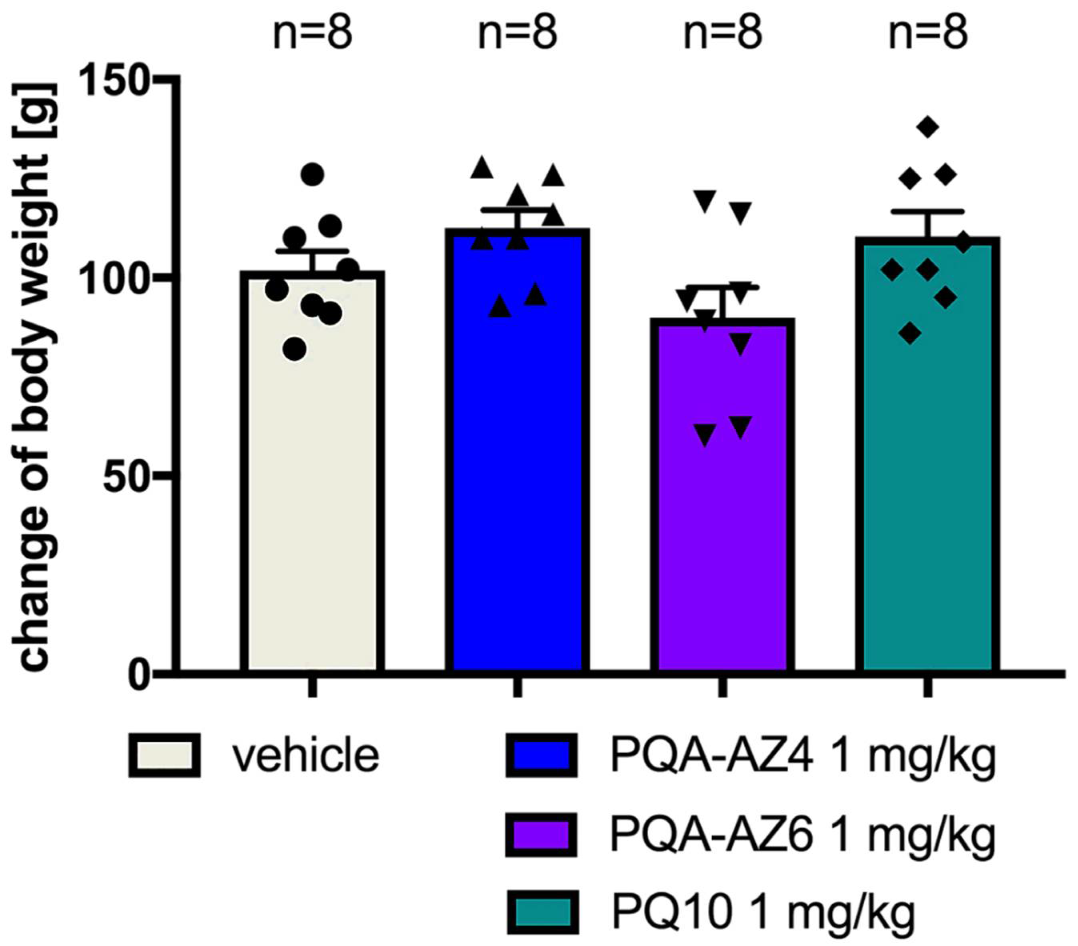
2.5. Effects of Repeated Administration of PQA-AZ4 and PQA-AZ6 on Body Weight of Rats
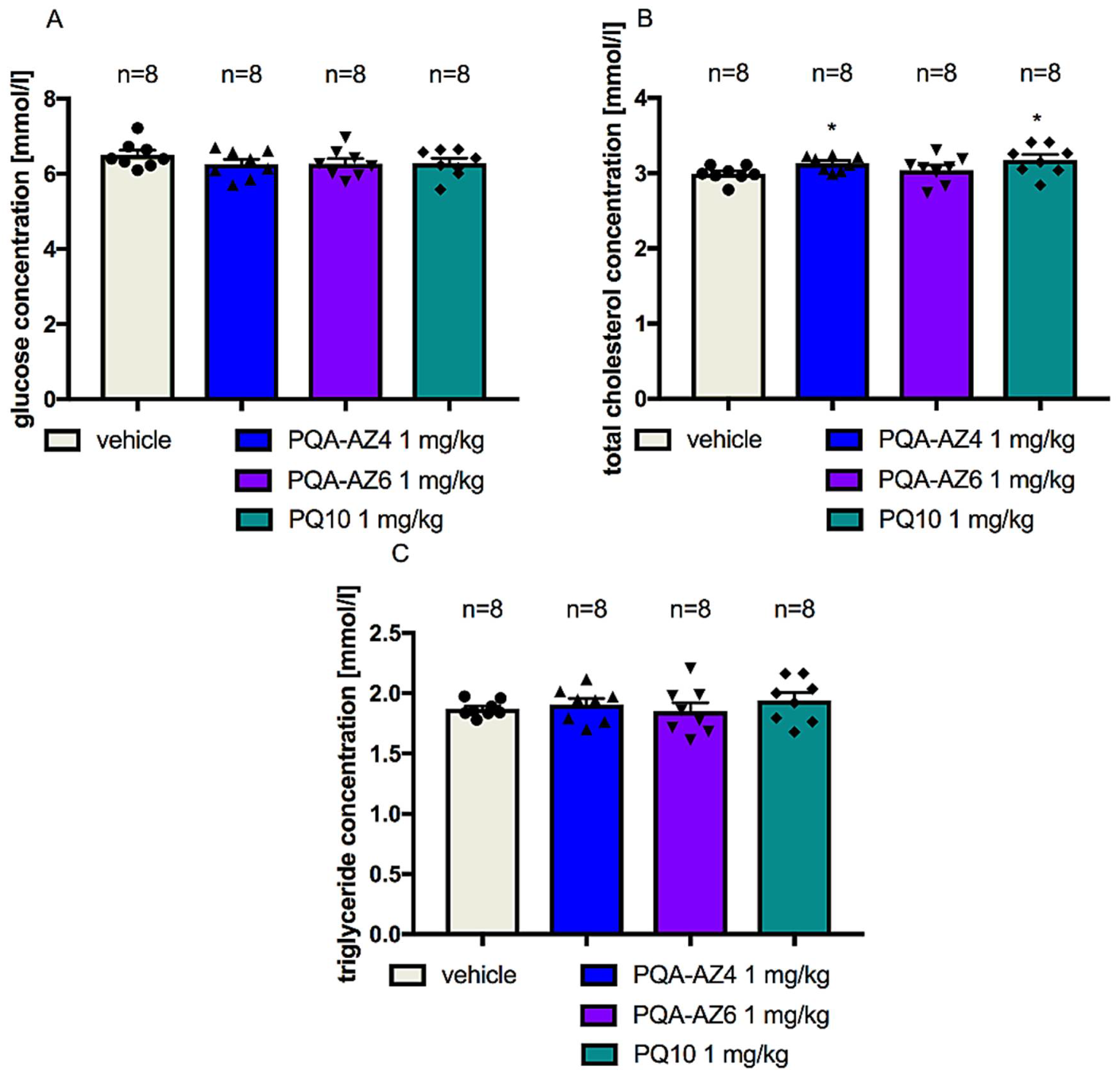
2.6. Effects of Repeated Administration of PQA-AZ4, PQA-AZ6, and PQ-10 on Serum Glucose, Triglyceride, and Cholesterol Concentrations in Rats
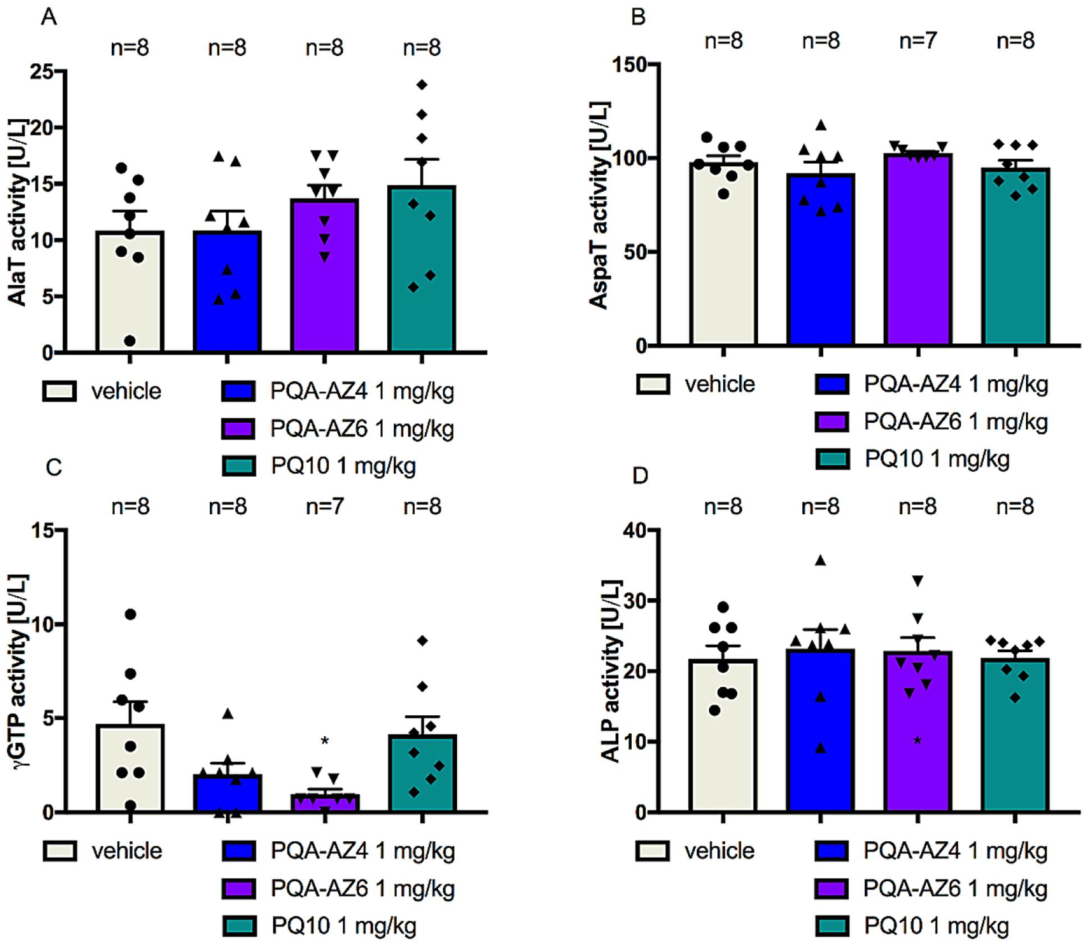
2.7. Effects of Repeated Administration of PQA-AZ4, PQA-AZ6, and PQ-10 Activities of Alanine Aminotransferase (AlaT), Aspartate Aminotransferase (AspaT), Gamma-Glutamyltransferase (γGTP), and Alkaline Phosphatase (ALP) in Rat Serum
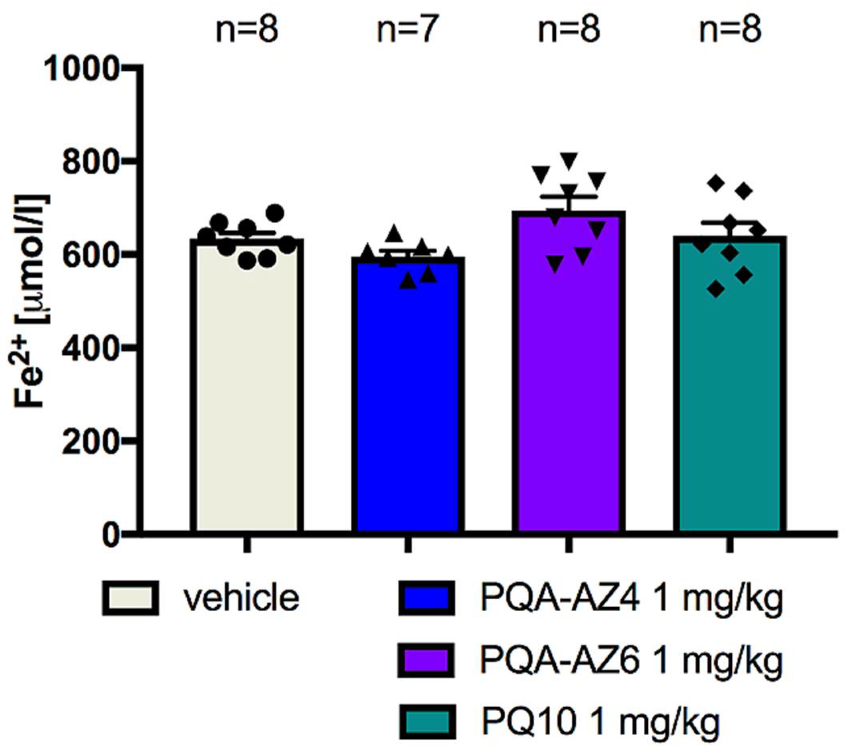
2.8. Ferric-Reducing Antioxidant Power after Chronic Administration of PQA-AZ4, PQA-AZ6, and PQ-10 to Rats
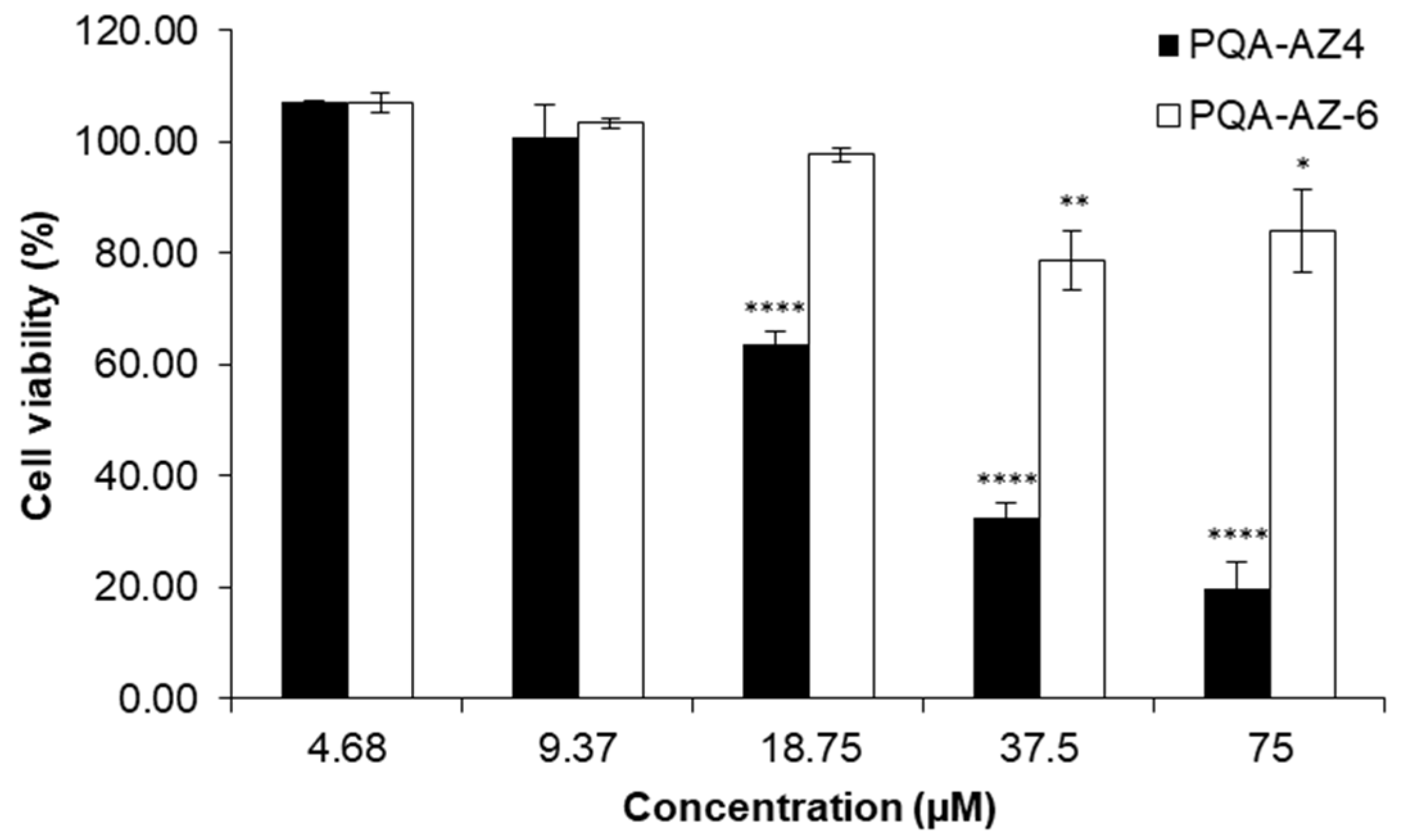
2.9. Cytotoxic Effects of PQA-AZ4 and PQA-AZ6 on Normal Human Dermal Fibroblast Cells (NHDF)
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Synthesized Compounds
4.3. LC/MS/MS Analysis and Pharmacokinetic Studies
4.4. Behavioral Studies
4.4.1. Forced Swim Test (FST)
4.4.2. Novel Object Recognition (NOR) Test
4.4.3. 18-Hour Spontaneous Activity Monitoring
4.4.4. Body Weight Measurement
4.5. Ex Vivo Biochemical Studies
4.5.1. Tissue Collection
4.5.2. Quantitative Real-Time PCR
4.5.3. Metabolic Parameters
4.5.4. Liver Enzymatic Activity
4.5.5. Ferric-Reducing Antioxidant Power (FRAP) Assay
4.6. Cytotoxic Effects of PQA-AZ4 and PQA-AZ-6 on Normal Human Dermal Fibroblast Cells
4.7. Data Analysis and Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- A decade of drug-likeness. Nat. Rev. Drug Discov. 2007, 6, 853. [CrossRef]
- Owens, J. Determining druggability. Nat. Rev. Drug Discov. 2007, 6, 187. [Google Scholar] [CrossRef]
- Ramsay, R.R.; Popovic-Nikolic, M.R.; Nikolic, K.; Uliassi, E.; Bolognesi, M.L. A perspective on multi-target drug discovery and design for complex diseases. Clin. Transl. Med. 2018, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Tejeda, J.F.; Sánchez-Ruiz, J.F.; Salazar, J.R.; Loza-Mejía, M.A. A Definition of “Multitargeticity”: Identifying Potential Multitarget and Selective Ligands Through a Vector Analysis. Front. Chem. 2020, 8, 176. [Google Scholar] [CrossRef] [PubMed]
- Sampietro, A.; Pérez-Areales, F.J.; Martínez, P.; Arce, E.M.; Galdeano, C.; Muñoz-Torrero, D. Unveiling the Multitarget Anti-Alzheimer Drug Discovery Landscape: A Bibliometric Analysis. Pharmaceuticals 2022, 15, 545. [Google Scholar] [CrossRef]
- Rossi, M.; Freschi, M.; De Camargo Nascente, L.; Salerno, A.; De Melo Viana Teixeira, S.; Nachon, F.; Chantegreil, F.; Soukup, O.; Prchal, L.; Malaguti, M.; et al. Sustainable Drug Discovery of Multi-Target-Directed Ligands for Alzheimer’s Disease. J. Med. Chem. 2021, 64, 4972–4990. [Google Scholar] [CrossRef] [PubMed]
- Rankovic, Z.; Morphy, R. CHAPTER 19: Multi-target Drug Discovery for Psychiatric Disorders. In Drug Discovery for Psychiatric; Rankovic, Z., Hargreaves, R., Bingham, M., Eds.; RSC Publishing: Cambridge, UK, 2012; pp. 510–533. [Google Scholar] [CrossRef]
- Kondej, M.; Stępnicki, P.; Kaczor, A.A. Multi-Target Approach for Drug Discovery against Schizophrenia. Int. J. Mol. Sci. 2018, 19, 3105. [Google Scholar] [CrossRef]
- Makhoba, X.H.; Viegas, C.; Mosa, R.A.; Viegas, F.P.D.; Pooe, O.J. Potential Impact of the Multi-Target Drug Approach in the Treatment of Some Complex Diseases. Drug Des. Devel. Ther. 2020, 14, 3235. [Google Scholar] [CrossRef] [PubMed]
- Zagórska, A.; Bucki, A.; Partyka, A.; Jastrzębska-Więsek, M.; Siwek, A.; Głuch-Lutwin, M.; Mordyl, B.; Jaromin, A.; Walczak, M.; Wesołowska, A.; et al. Design, synthesis, and behavioral evaluation of dual-acting compounds as phosphodiesterase type 10A (PDE10A) inhibitors and serotonin ligands targeting neuropsychiatric symptoms in dementia. Eur. J. Med. Chem. 2022, 233, 114218. [Google Scholar] [CrossRef] [PubMed]
- ICH M3 (R2) Non-Clinical Safety Studies for the Conduct of Human Clinical Trials for Pharmaceuticals|European Medicines Agency. Available online: https://www.ema.europa.eu/en/ich-m3-r2-non-clinical-safety-studies-conduct-human-clinical-trials-pharmaceuticals (accessed on 18 April 2022).
- Newman-Tancredi, A.; Depoortère, R.Y.; Kleven, M.S.; Kołaczkowski, M.; Zimmer, L. Translating biased agonists from molecules to medications: Serotonin 5-HT 1A receptor functional selectivity for CNS disorders. Pharmacol. Ther. 2022, 229, 107937. [Google Scholar] [CrossRef]
- Jankowska, A.; Świerczek, A.; Wyska, E.; Gawalska, A.; Bucki, A.; Pawłowski, M.; Chłoń-Rzepa, G. Advances in Discovery of PDE10A Inhibitors for CNS-Related Disorders. Part 1: Overview of the Chemical and Biological Research. Curr. Drug Targets 2019, 20, 122–143. [Google Scholar] [CrossRef]
- Zagorska, A.; Partyka, A.; Bucki, A.; Gawalskax, A.; Czopek, A.; Pawlowski, M. Phosphodiesterase 10 Inhibitors—Novel Perspectives for Psychiatric and Neurodegenerative Drug Discovery. Curr. Med. Chem. 2018, 25, 3455–3481. [Google Scholar] [CrossRef] [PubMed]
- Chłoń-Rzepa, G.; Zagórska, A.; Żmudzki, P.; Bucki, A.; Kołaczkowski, M.; Partyka, A.; Wesołowska, A.; Kazek, G.; Głuch-Lutwin, M.; Siwek, A.; et al. Aminoalkyl Derivatives of 8-Alkoxypurine-2,6-diones: Multifunctional 5-HT1A/5-HT7 Receptor Ligands and PDE Inhibitors with Antidepressant Activity. Arch. Pharm. 2016, 349, 889–903. [Google Scholar] [CrossRef] [PubMed]
- Marcinkowska, M.; Kotańska, M.; Zagórska, A.; Śniecikowska, J.; Kubacka, M.; Siwek, A.; Bucki, A.; Pawłowski, M.; Bednarski, M.; Sapa, J.; et al. Synthesis and biological evaluation of N-arylpiperazine derivatives of 4,4-dimethylisoquinoline-1,3(2H,4H)-dione as potential antiplatelet agents. J. Enzyme Inhib. Med. Chem. 2018, 33, 536–545. [Google Scholar] [CrossRef]
- Partyka, A.; Zagórska, A.; Kotańska, M.; Walczak, M.; Jastrzębska-Więsek, M.; Knutelska, J.; Bednarski, M.; Głuch-Lutwin, M.; Mordyl, B.; Janiszewska, P.; et al. Antidepressant-like activity and safety profile evaluation of 1H-imidazo[2,1-f]purine-2,4(3H,8H)-dione derivatives as 5-HT1A receptor partial agonists. PLoS ONE 2020, 15, e0237196. [Google Scholar] [CrossRef] [PubMed]
- Ramaker, M.J.; Dulawa, S.C. Identifying fast-onset antidepressants using rodent models. Mol. Psychiatry 2017, 22, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, C.; Duman, R.S. Stress, Depression, and Neuroplasticity: A Convergence of Mechanisms. Neuropsychopharmacology 2007, 33, 88–109. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Monteggia, L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry 2006, 59, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Nibuya, M.; Nestler, E.J.; Duman, R.S. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J. Neurosci. 1996, 16, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Nibuya, M.; Morinobu, S.; Duman, R.S. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J. Neurosci. 1995, 15, 7539–7547. [Google Scholar] [CrossRef]
- Ennaceur, A.; Delacour, J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 1988, 31, 47–59. [Google Scholar] [CrossRef]
- van der Staay, F.J.; Rutten, K.; Erb, C.; Blokland, A. Effects of the cognition impairer MK-801 on learning and memory in mice and rats. Behav. Brain Res. 2011, 220, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Adell, A.; Jiménez-Sánchez, L.; López-Gil, X.; Romón, T. Is the Acute NMDA Receptor Hypofunction a Valid Model of Schizophrenia? Schizophr. Bull. 2012, 38, 9. [Google Scholar] [CrossRef] [PubMed]
- Csernansky, J.G.; Martin, M.; Shah, R.; Bertchume, A.; Colvin, J.; Dong, H. Cholinesterase Inhibitors Ameliorate Behavioral Deficits Induced by MK-801 in Mice. Neuropsychopharmacology 2005, 30, 2135. [Google Scholar] [CrossRef] [PubMed]
- Boess, F.G.; De Vry, J.; Erb, C.; Flessner, T.; Hendrix, M.; Luithle, J.; Methfessel, C.; Riedl, B.; Schnizler, K.; Van Der Staay, F.J.; et al. The Novel 7 Nicotinic Acetylcholine Receptor Agonist N-[(3R)-1-Azabicyclo[2.2.2]oct-3-yl]-7-[2-(methoxy)phenyl]-1-benzofuran-2-carboxamide Improves Working and Recognition Memory in Rodents. J. Pharmacol. Exp. Ther. 2007, 321, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Boess, F.G.; Hendrix, M.; Van Der Staay, F.J.; Erb, C.; Schreiber, R.; Van Staveren, W.; De Vente, J.; Prickaerts, J.; Blokland, A.; Koenig, G. Inhibition of phosphodiesterase 2 increases neuronal cGMP, synaptic plasticity and memory performance. Neuropharmacology 2004, 47, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Reneerkens, O.A.H.; Rutten, K.; Bollen, E.; Hage, T.; Blokland, A.; Steinbusch, H.W.M.; Prickaerts, J. Inhibition of phoshodiesterase type 2 or type 10 reverses object memory deficits induced by scopolamine or MK-801. Behav. Brain Res. 2013, 236, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.H.F.; Kemp, J.A.; Priestley, T.; Knight, A.R.; Woodruff, G.N.; Iversen, L.L. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc. Natl. Acad. Sci. USA 1986, 83, 7104–7108. [Google Scholar] [CrossRef] [PubMed]
- Ellison, G. The N-methyl-d-aspartate antagonists phencyclidine, ketamine and dizocilpine as both behavioral and anatomical models of the dementias. Brain Res. Rev. 1995, 20, 250–267. [Google Scholar] [CrossRef]
- Bubeníková-Valešová, V.; Horáček, J.; Vrajová, M.; Höschl, C. Models of schizophrenia in humans and animals based on inhibition of NMDA receptors. Neurosci. Biobehav. Rev. 2008, 32, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Üçok, A.; Gaebel, W. Side effects of atypical antipsychotics: A brief overview. World Psychiatry 2008, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Zagórska, A. Phosphodiesterase 10 (PDE10) inhibitors: An updated patent review (2014-present). Expert Opin. Ther. Pat. 2020, 30, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Olson, H.; Betton, G.; Robinson, D.; Thomas, K.; Monro, A.; Kolaja, G.; Lilly, P.; Sanders, J.; Sipes, G.; Bracken, W.; et al. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul. Toxicol. Pharmacol. 2000, 32, 56–67. [Google Scholar] [CrossRef]
- Boone, L.; Meyer, D.; Cusick, P.; Ennulat, D.; Provencher Bolliger, A.; Everds, N.; Meador, V.; Elliott, G.; Honor, D.; Bounous, D.; et al. Selection and interpretation of clinical pathology indicators of hepatic injury in preclinical studies. Vet. Clin. Pathol. 2005, 34, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Non-Clinical Evaluation of Drug-Induced Liver Injury (DILI)|European Medicines Agency. Available online: https://www.ema.europa.eu/en/non-clinical-evaluation-drug-induced-liver-injury-dili (accessed on 18 April 2022).
- Aulbach, A.D.; Amuzie, C.J. Biomarkers in Nonclinical Drug Development, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128036204. [Google Scholar]
- ISO. ISO 10993-5:2009—Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. Available online: https://www.iso.org/standard/36406.html (accessed on 18 April 2022).
- Beria, I.; Ballinari, D.; Bertrand, J.A.; Borghi, D.; Bossi, R.T.; Brasca, M.G.; Cappella, P.; Caruso, M.; Ceccarelli, W.; Ciavolella, A.; et al. Identification of 4,5-dihydro-1 H -pyrazolo[4,3- h ]quinazoline Derivatives as a new class of orally and selective polo-like kinase 1 inhibitors. J. Med. Chem. 2010, 53, 3532–3551. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. “Behavioural despair” in rats and mice: Strain differences and the effects of imipramine. Eur. J. Pharmacol. 1978, 51, 291–294. [Google Scholar] [CrossRef]
- Zajdel, P.; Kos, T.; Marciniec, K.; Satała, G.; Canale, V.; Kamiński, K.; Hołuj, M.; Lenda, T.; Koralewski, R.; Bednarski, M.; et al. Novel multi-target azinesulfonamides of cyclic amine derivatives as potential antipsychotics with pro-social and pro-cognitive effects. Eur. J. Med. Chem. 2018, 145, 790–804. [Google Scholar] [CrossRef] [PubMed]
- Sudoł, S.; Cios, A.; Jastrzębska-więsek, M.; Honkisz-orzechowska, E.; Mordyl, B.; Wilczyńska-zawal, N.; Satała, G.; Kucwaj-brysz, K.; Partyka, A.; Latacz, G.; et al. The Phenoxyalkyltriazine Antagonists for 5-HT6 Receptor with Promising Procognitive and Pharmacokinetic Properties In Vivo in Search for a Novel Therapeutic Approach to Dementia Diseases. Int. J. Mol. Sci. 2021, 22, 10773. [Google Scholar] [CrossRef] [PubMed]
- Dudek, M.; Marcinkowska, M.; Bucki, A.; Olczyk, A.; Kołaczkowski, M. Idalopirdine—A small molecule antagonist of 5-HT6 with therapeutic potential against obesity. Metab. Brain Dis. 2015, 30, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Dudek, M.; Kuder, K.; Kołaczkowski, M.; Olczyk, A.; Żmudzka, E.; Rak, A.; Bednarski, M.; Pytka, K.; Sapa, J.; Kieć-Kononowicz, K. H3 histamine receptor antagonist pitolisant reverses some subchronic disturbances induced by olanzapine in mice. Metab. Brain Dis. 2016, 31, 1023–1029. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Filipczak, N.; Jaromin, A.; Piwoni, A.; Mahmud, M.; Sarisozen, C.; Torchilin, V.; Gubernator, J. A Triple Co-Delivery Liposomal Carrier That Enhances Apoptosis via an Intrinsic Pathway in Melanoma Cells. Cancers 2019, 11, 1982. [Google Scholar] [CrossRef] [PubMed]
- Fandzloch, M.; Jaromin, A.; Zaremba-Czogalla, M.; Wojtczak, A.; Lewińska, A.; Sitkowski, J.; Wiśniewska, J.; Łakomska, I.; Gubernator, J. Nanoencapsulation of a ruthenium(II) complex with triazolopyrimidine in liposomes as a tool for improving its anticancer activity against melanoma cell lines. Dalt. Trans. 2020, 49, 1207–1219. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]


| PQA-AZ4 | PQA-AZ6 | ||
|---|---|---|---|
| Structure | 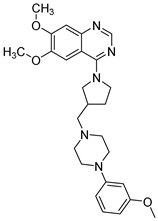 | 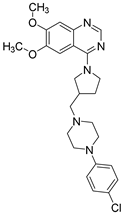 | |
| Chemical name | 6,7-Dimethoxy-4-(3-((4-(3-methoxyphenyl)piperazin-1-yl)methyl)pyrrolidin-1-yl)quinazoline | 4-(3-((4-(4-Chlorophenyl)piperazin-1-yl)methyl)pyrrolidin-1-yl)-6,7-dimethoxyquinazoline | |
| Affinity | 5-HT1AR 5-HT7R | Ki = 18.0 ± 1.8 nM | Ki = 346.0 ± 20.2 nM |
| Ki = 234.0 ± 15.0 nM | Ki = 136.0 ± 15.0 nM | ||
| Inhibition | PDE10A | IC50 = 7.07 µM | IC50 = 5.68 µM |
| i.v. | i.g. | |||
|---|---|---|---|---|
| Plasma | Brain | Plasma | Brain | |
| C0 [ng/mL] | 431.1 | - | - | - |
| AUC0→t [ng·min/mL] * [ng·min/g] | 42,859 | 40,806 | 7103 | 40,314 |
| t0.5 [min] | 67.3 | 220 | 92 | 214 |
| MRT [min] | 57.5 | 186 | 82.6 | 215 |
| Vss [L/kg] | 0.8 | - | - | - |
| Cl [mL/min/kg] | 10.8 | - | - | - |
| tmax [min] | - | 15 | 60 | 60 |
| Cmax [ng/mL] | - | 149.1 | 46.7 | 71.4 |
| F [%] | 8.29 | |||
| Brain/Plasma ratio | 0.9 | 5.68 | ||
| i.v. | i.g. | |||
|---|---|---|---|---|
| Plasma | Brain | Plasma | Brain | |
| C0 [ng/mL] | 101.6 | - | - | - |
| AUC0→t [ng·min/mL] * [ng·min/g] | 5616 | 48,321 | 8619 | 45,623 |
| t0.5 [min] | 54.2 | 597 | 216.8 | 1928 |
| MRT [min] | 44.5 | 444 | 66.5 | 719 |
| Vss [L/kg] | 4.8 | - | - | - |
| Cl [mL/min/kg] | 85.2 | - | - | - |
| tmax [min] | - | 15 | 15 | 15 |
| Cmax [ng/mL] | - | 113.4 | 99.5 | 341.5 |
| F [%] | 38.4 | |||
| Brain/Plasma ratio | 8.60 | 5.29 | ||
| Treatment | Dose (mg/kg) | Total Exploratory Time in T2 Session (s) |
|---|---|---|
| Vehicle + vehicle | 0 + 0 | 49.67 ± 4.63 |
| (+)-MK-801 + vehicle | 0.1 + 0 | 44.00 ± 4.05 |
| PQA-AZ4 + (+)-MK-801 | 1 + 0.1 | 28.83 ± 2.50 p < 0.01 vs. veh; p < 0.05 vs. (+)-MK-801 F(2,16) = 7.3773; p < 0.01 |
| Vehicle + vehicle | 0 + 0 | 49.67 ± 4.63 |
| (+)-MK-801 + vehicle | 0.1 + 0 | 44.00 ± 4.05 |
| PQA-AZ6 + (+)-MK-801 | 3 + 0.1 | 39.57 ± 4.39 F(2,17) = 1.3060; NS |
| Vehicle + vehicle | 0 + 0 | 49.67 ± 4.63 |
| (+)-MK-801 + vehicle | 0.1 + 0 | 44.00 ± 4.05 |
| PQ10 + (+)-MK-801 | 0.3 + 0.1 | 47.13 ± 5.69 F(2,18) = 0.3006; NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jastrzębska-Więsek, M.; Kotańska, M.; Grzeszczak, A.; Jaromin, A.; Walczak, M.; Partyka, A.; Gdula-Argasińska, J.; Smolik, M.; Zagórska, A. The Antidepressant-like Activity, Effects on Recognition Memory Deficits, Bioavailability, and Safety after Chronic Administration of New Dual-Acting Small Compounds Targeting Neuropsychiatric Symptoms in Dementia. Int. J. Mol. Sci. 2022, 23, 11452. https://doi.org/10.3390/ijms231911452
Jastrzębska-Więsek M, Kotańska M, Grzeszczak A, Jaromin A, Walczak M, Partyka A, Gdula-Argasińska J, Smolik M, Zagórska A. The Antidepressant-like Activity, Effects on Recognition Memory Deficits, Bioavailability, and Safety after Chronic Administration of New Dual-Acting Small Compounds Targeting Neuropsychiatric Symptoms in Dementia. International Journal of Molecular Sciences. 2022; 23(19):11452. https://doi.org/10.3390/ijms231911452
Chicago/Turabian StyleJastrzębska-Więsek, Magdalena, Magdalena Kotańska, Aleksandra Grzeszczak, Anna Jaromin, Maria Walczak, Anna Partyka, Joanna Gdula-Argasińska, Magdalena Smolik, and Agnieszka Zagórska. 2022. "The Antidepressant-like Activity, Effects on Recognition Memory Deficits, Bioavailability, and Safety after Chronic Administration of New Dual-Acting Small Compounds Targeting Neuropsychiatric Symptoms in Dementia" International Journal of Molecular Sciences 23, no. 19: 11452. https://doi.org/10.3390/ijms231911452
APA StyleJastrzębska-Więsek, M., Kotańska, M., Grzeszczak, A., Jaromin, A., Walczak, M., Partyka, A., Gdula-Argasińska, J., Smolik, M., & Zagórska, A. (2022). The Antidepressant-like Activity, Effects on Recognition Memory Deficits, Bioavailability, and Safety after Chronic Administration of New Dual-Acting Small Compounds Targeting Neuropsychiatric Symptoms in Dementia. International Journal of Molecular Sciences, 23(19), 11452. https://doi.org/10.3390/ijms231911452








