Comparison of Homologous and Heterologous Booster SARS-CoV-2 Vaccination in Autoimmune Rheumatic and Musculoskeletal Patients
Abstract
:1. Introduction
2. Results
2.1. Clinical Characteristics
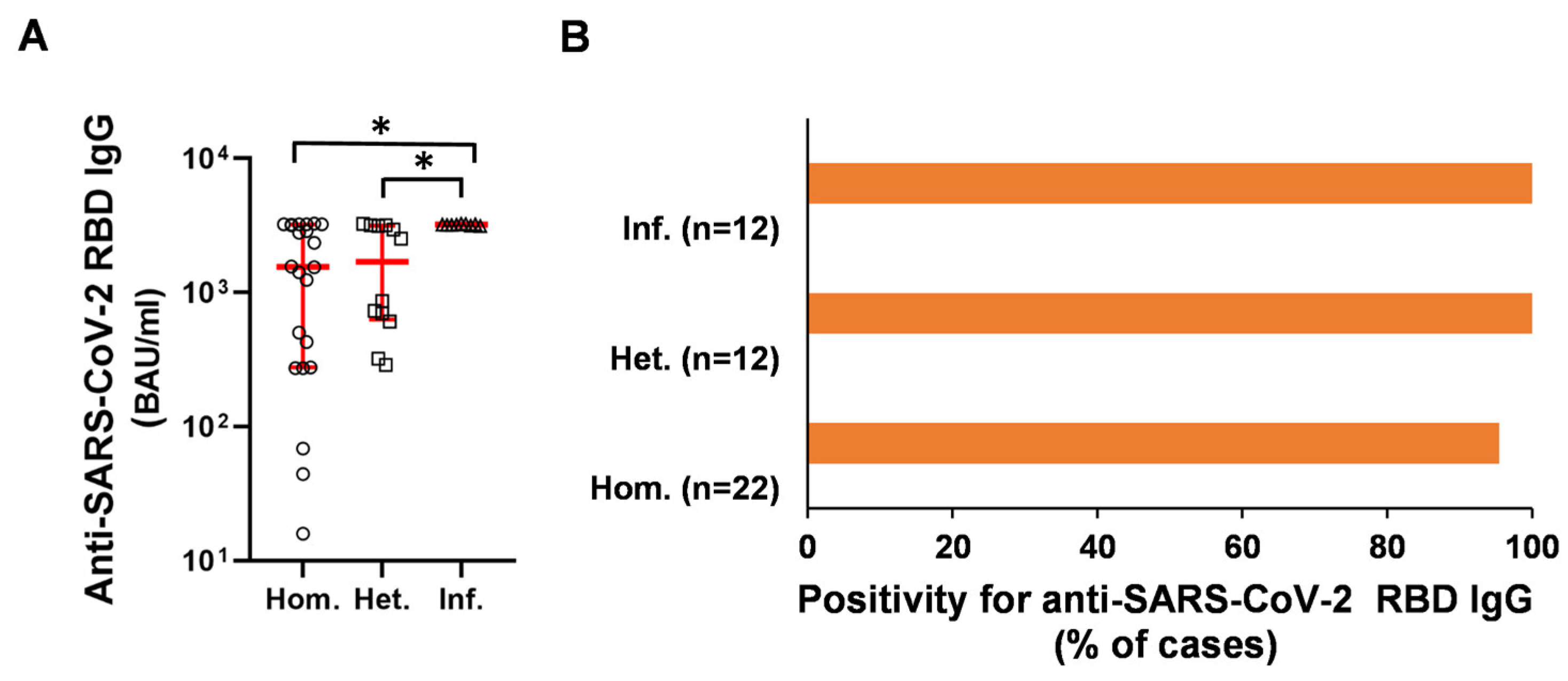
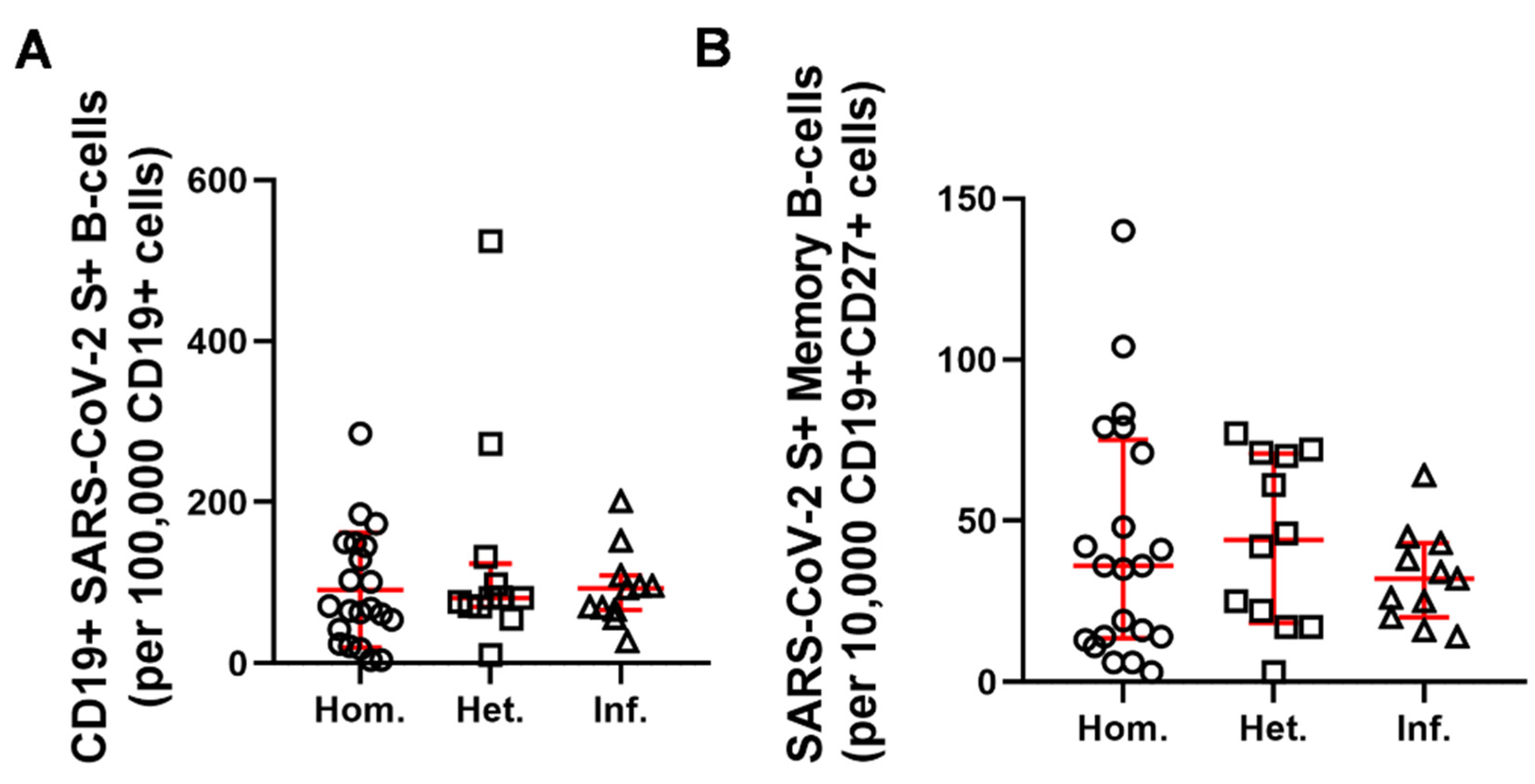
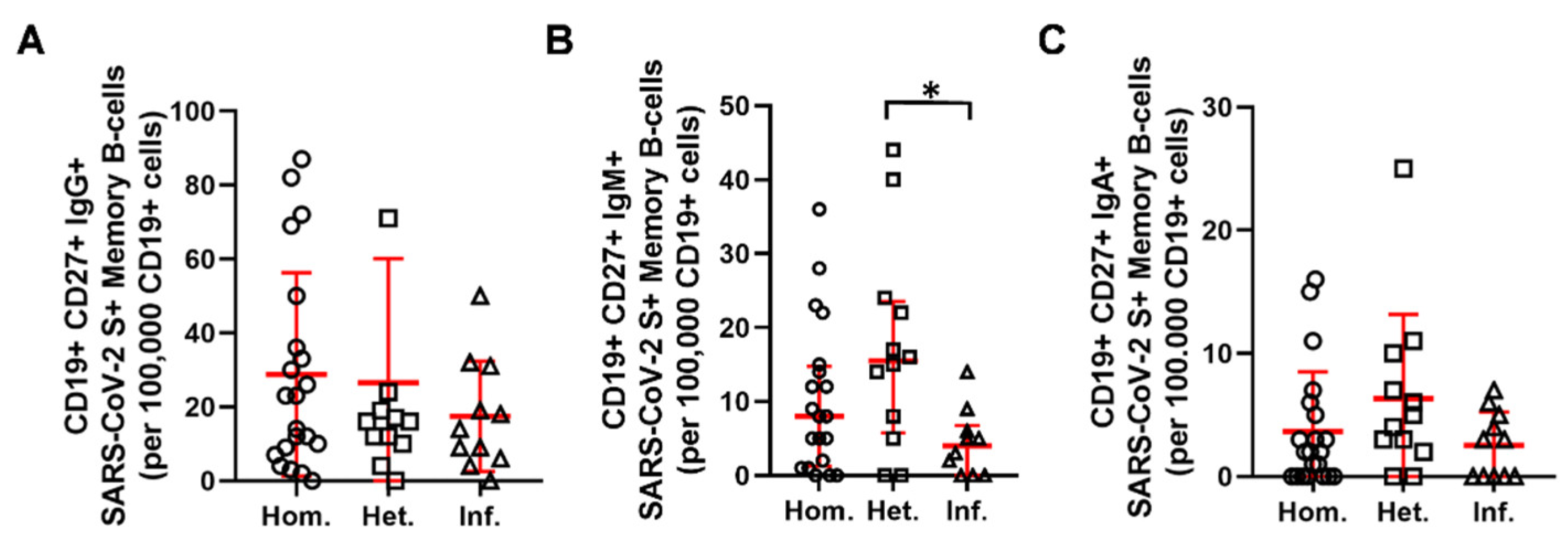
2.2. Humoral and B-Cell Mediated Anti-SARS-CoV-2 Response
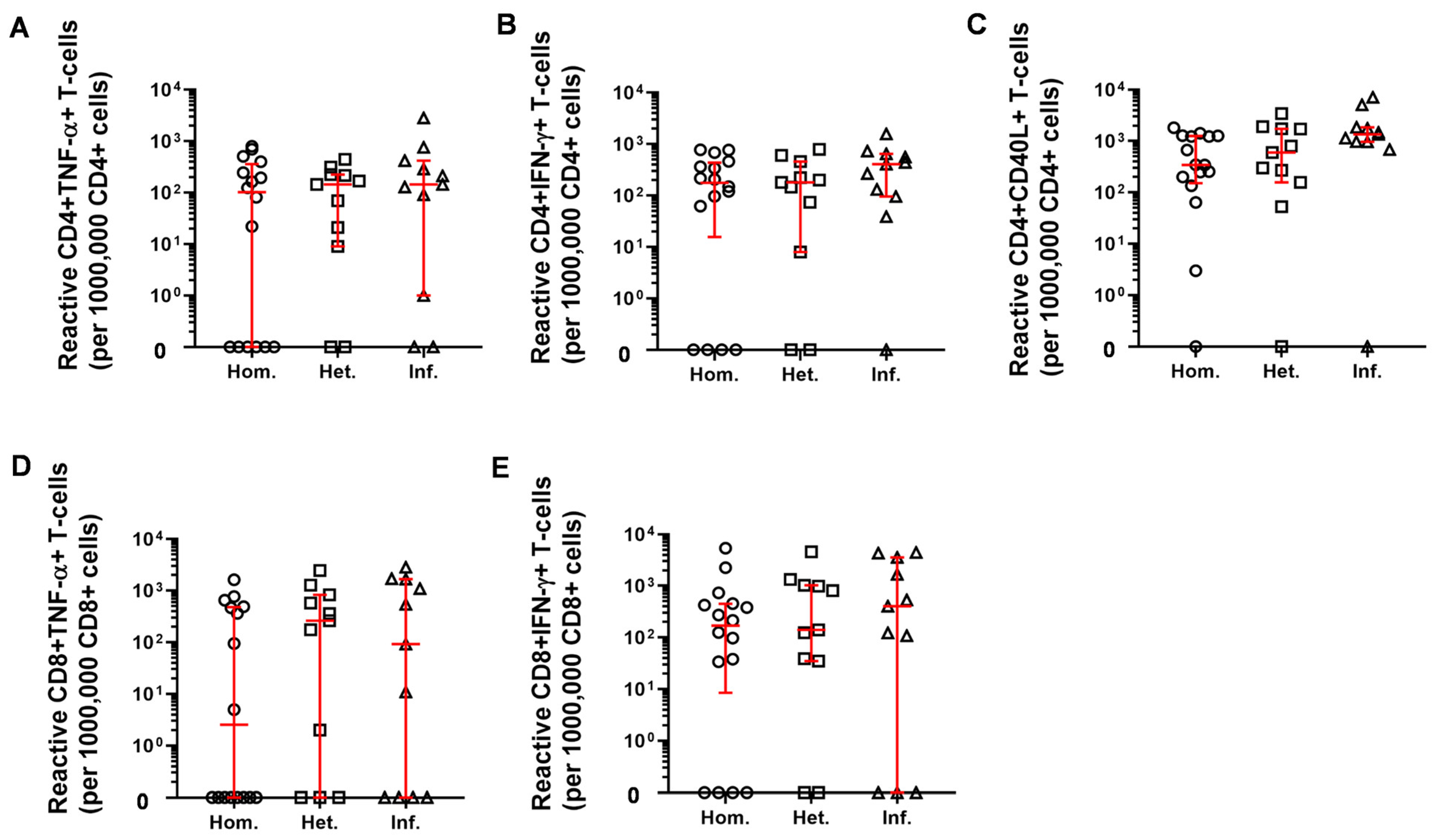
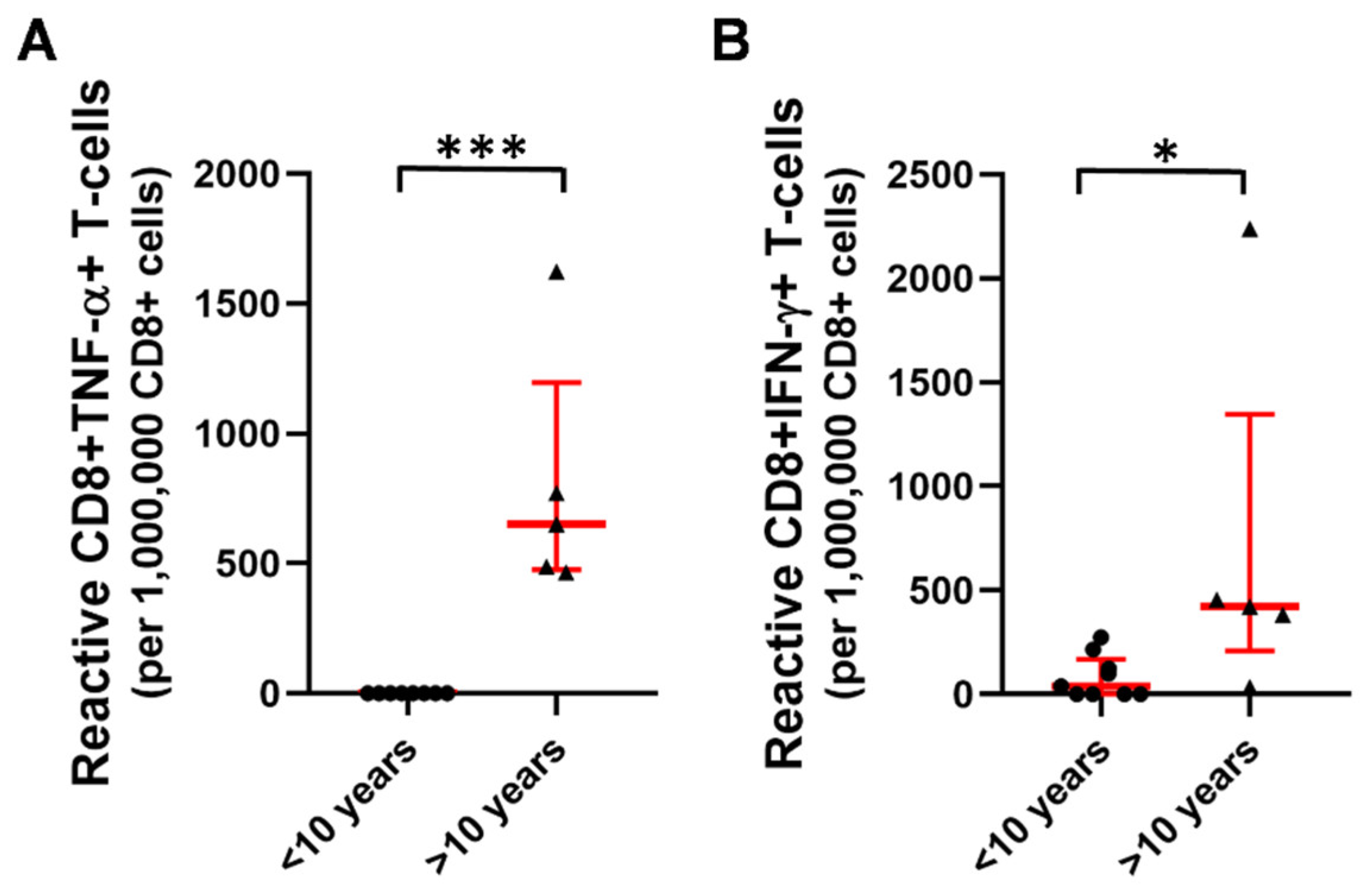
2.3. T-Cell Mediated Anti-SARS-CoV-2 Response
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Study Population
4.3. Study Design
4.4. Measurement of SARS-CoV-2 Specific Antibodies
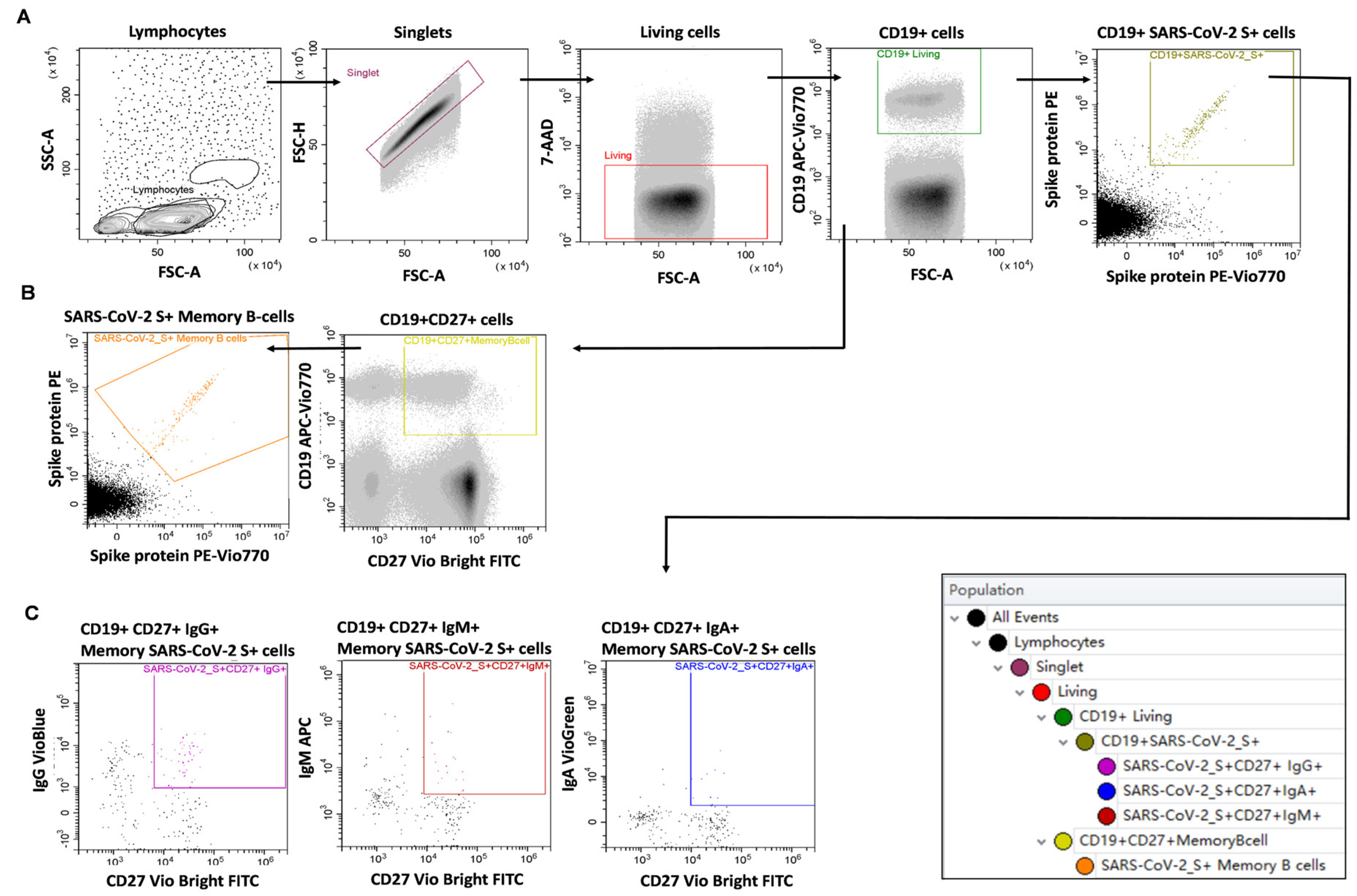
4.5. Measurement of SARS-CoV-2 Specific B-Cell Memory
4.6. Measurement of SARS-CoV-2 Specific T-Cell Mediated Immunity
4.7. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shalash, A.O.; Toth, I.; Skwarczynski, M. The potential of developing a protective peptide-based vaccines against SARS-CoV-2. Drug Dev. Res. 2022, 83, 1251–1256. [Google Scholar] [CrossRef]
- Jena, A.; Mishra, S.; Deepak, P.; Kumar, M.P.; Sharma, A.; Patel, Y.I.; Kennedy, N.A.; Kim, A.H.J.; Sharma, V.; Sebastian, S. Response to SARS-CoV-2 vaccination in immune mediated inflammatory diseases: Systematic review and meta-analysis. Autoimmun Rev. 2022, 21, 102927. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021, 396, 1979–1993. [Google Scholar] [CrossRef]
- Benucci, M.; Damiani, A.; Gobbi, F.L.; Lari, B.; Grossi, V.; Infantino, M.; Manfredi, M. Role of booster with BNT162b2 mRNA in SARS-CoV-2 vaccination in patients with rheumatoid arthritis. Immunol. Res. 2022, 70, 493–500. [Google Scholar] [CrossRef]
- Belleudi, V.; Rosa, A.C.; Poggi, F.R.; Armuzzi, A.; Nicastri, E.; Goletti, D.; Diamanti, A.P.; Davoli, M.; Agabiti, N.; Addis, A. Direct and Indirect Impact of COVID-19 for Patients with Immune-Mediated Inflammatory Diseases: A Retrospective Cohort Study. J. Clin. Med. 2021, 10, 2388. [Google Scholar] [CrossRef]
- Landewe, R.B.; Machado, P.M.; Kroon, F.; Bijlsma, H.W.; Burmester, G.R.; Carmona, L.; Combe, B.; Galli, M.; Gossec, L.; Iagnocco, A.; et al. EULAR provisional recommendations for the management of rheumatic and musculoskeletal diseases in the context of SARS-CoV-2. Ann. Rheum. Dis. 2020, 79, 851–858. [Google Scholar] [CrossRef]
- Fragoulis, G.E.; Karamanakos, A.; Arida, A.; Tektonidou, M.G. Clinical outcomes of breakthrough COVID-19 after booster vaccination in patients with systemic rheumatic diseases. Rmd Open 2022, 8, e002279. [Google Scholar] [CrossRef]
- Fragoulis, G.E.; Bournia, V.K.; Mavrea, E.; Evangelatos, G.; Fragiadaki, K.; Karamanakos, A.; Kravariti, E.; Laskari, K.; Panopoulos, S.; Pappa, M.; et al. COVID-19 vaccine safety and nocebo-prone associated hesitancy in patients with systemic rheumatic diseases: A cross-sectional study. Rheumatol. Int. 2022, 42, 31–39. [Google Scholar] [CrossRef]
- Connolly, C.M.; Ruddy, J.A.; Boyarsky, B.J.; Barbur, I.; Werbel, W.A.; Geetha, D.; Garonzik-Wang, J.M.; Segev, D.L.; Christopher-Stine, L.; Paik, J.J. Disease Flare and Reactogenicity in Patients With Rheumatic and Musculoskeletal Diseases Following Two-Dose SARS-CoV-2 Messenger RNA Vaccination. Arthritis Rheumatol 2022, 74, 28–32. [Google Scholar] [CrossRef]
- Papagoras, C.; Fragoulis, G.E.; Zioga, N.; Simopoulou, T.; Deftereou, K.; Kalavri, E.; Zampeli, E.; Gerolymatou, N.; Kataxaki, E.; Melissaropoulos, K.; et al. Better outcomes of COVID-19 in vaccinated compared to unvaccinated patients with systemic rheumatic diseases. Ann. Rheum. Dis. 2022, 81, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, G.J.; Gemes, N.; Honfi, D.; Szabo, E.; Neuperger, P.; Balog, J.A.; Nagy, L.I.; Szekanecz, Z.; Puskas, L.G.; Toldi, G.; et al. Humoral and Cellular Immunogenicity and Safety of Five Different SARS-CoV-2 Vaccines in Patients With Autoimmune Rheumatic and Musculoskeletal Diseases in Remission or With Low Disease Activity and in Healthy Controls: A Single Center Study. Front. Immunol. 2022, 13, 846248. [Google Scholar] [CrossRef] [PubMed]
- Szekanecz, Z.; Balog, A.; Constantin, T.; Czirjak, L.; Geher, P.; Kovacs, L.; Kumanovics, G.; Nagy, G.; Rakoczi, E.; Szamosi, S.; et al. COVID-19: Autoimmunity, multisystemic inflammation and autoimmune rheumatic patients. Expert Rev. Mol. Med. 2022, 24, e13. [Google Scholar] [CrossRef] [PubMed]
- Vokó, Z.; Kiss, Z.; Surján, G.; Surján, O.; Barcza, Z.; Wittmann, I.; Molnár, G.A.; Nagy, D.; Müller, V.; Bogos, K.; et al. Effectiveness and Waning of Protection with Different SARS-CoV-2 Primary and Booster Vaccines Dur-ing the Delta Pandemic Wave in 2021 in Hungary (HUN-VE 3 Study). Front. Immunol. 2022, 13, 919408. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Haas, E.J.; Milo, R.; Alroy-Preis, S.; Ash, N.; Huppert, A. Waning Immunity after the BNT162b2 Vaccine in Israel. N. Engl. J. Med. 2021, 385, e85. [Google Scholar] [CrossRef]
- Barda, N.; Dagan, N.; Cohen, C.; Hernan, M.A.; Lipsitch, M.; Kohane, I.S.; Reis, B.Y.; Balicer, R.D. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet 2021, 398, 2093–2100. [Google Scholar] [CrossRef]
- Munro, A.P.S.; Janani, L.; Cornelius, V.; Aley, P.K.; Babbage, G.; Baxter, D.; Bula, M.; Cathie, K.; Chatterjee, K.; Dodd, K.; et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): A blinded, multicentre, randomised, controlled, phase 2 trial. Lancet 2021, 398, 2258–2276. [Google Scholar] [CrossRef]
- Chenchula, S.; Karunakaran, P.; Sharma, S.; Chavan, M. Current evidence on efficacy of COVID-19 booster dose vaccination against the Omicron variant: A systematic review. J. Med. Virol. 2022, 94, 2969–2976. [Google Scholar] [CrossRef]
- Bieber, A.; Sagy, I.; Novack, L.; Brikman, S.; Abuhasira, R.; Ayalon, S.; Novofastovski, I.; Abu-Shakra, M.; Mader, R. BNT162b2 mRNA COVID-19 vaccine and booster in patients with autoimmune rheumatic diseases: A national cohort study. Ann. Rheum. Dis. 2022, 81, 1028–1035. [Google Scholar] [CrossRef]
- Beyer, D.K.; Forero, A. Mechanisms of Antiviral Immune Evasion of SARS-CoV-2. J. Mol. Biol. 2022, 434, 167265. [Google Scholar] [CrossRef]
- Gu, W.; Gan, H.; Ma, Y.; Xu, L.; Cheng, Z.J.; Li, B.; Zhang, X.; Jiang, W.; Sun, J.; Sun, B.; et al. The molecular mechanism of SARS-CoV-2 evading host antiviral innate immunity. Virol. J. 2022, 19, 49. [Google Scholar] [CrossRef] [PubMed]
- Pusnik, J.; Richter, E.; Schulte, B.; Dolscheid-Pommerich, R.; Bode, C.; Putensen, C.; Hartmann, G.; Alter, G.; Streeck, H. Memory B cells targeting SARS-CoV-2 spike protein and their dependence on CD4(+) T cell help. Cell Rep. 2021, 35, 109320. [Google Scholar] [CrossRef] [PubMed]
- Dulic, S.; Vasarhelyi, Z.; Sava, F.; Berta, L.; Szalay, B.; Toldi, G.; Kovacs, L.; Balog, A. T-Cell Subsets in Rheumatoid Arthritis Patients on Long-Term Anti-TNF or IL-6 Receptor Blocker Therapy. Mediat. Inflamm. 2017, 2017, 6894374. [Google Scholar] [CrossRef]
- Dulic, S.; Vasarhelyi, Z.; Bajnok, A.; Szalay, B.; Toldi, G.; Kovacs, L.; Balog, A. The Impact of Anti-TNF Therapy on CD4+ and CD8+ Cell Subsets in Ankylosing Spondylitis. Pathobiology 2018, 85, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Dulic, S.; Toldi, G.; Sava, F.; Kovacs, L.; Molnar, T.; Milassin, A.; Farkas, K.; Rutka, M.; Balog, A. Specific T-Cell Subsets Can Predict the Efficacy of Anti-TNF Treatment in Inflammatory Bowel Diseases. Arch. Immunol. Exp. 2020, 68, 12. [Google Scholar] [CrossRef]
- Matula, Z.; Gonczi, M.; Beko, G.; Kadar, B.; Ajzner, E.; Uher, F.; Valyi-Nagy, I. Antibody and T Cell Responses against SARS-CoV-2 Elicited by the Third Dose of BBIBP-CorV (Sinopharm) and BNT162b2 (Pfizer-BioNTech) Vaccines Using a Homologous or Heterologous Booster Vaccination Strategy. Vaccine 2022, 10, 539. [Google Scholar] [CrossRef]
- Meng, H.; Mao, J.; Ye, Q. Booster vaccination strategy: Necessity, immunization objectives, immunization strategy, and safety. J. Med. Virol. 2022, 94, 2369–2375. [Google Scholar] [CrossRef]
- Arbel, R.; Hammerman, A.; Sergienko, R.; Friger, M.; Peretz, A.; Netzer, D.; Yaron, S. BNT162b2 Vaccine Booster and Mortality Due to COVID-19. N. Engl. J. Med. 2021, 385, 2413–2420. [Google Scholar] [CrossRef]
- Manisty, C.; Otter, A.D.; Treibel, T.A.; McKnight, A.; Altmann, D.M.; Brooks, T.; Noursadeghi, M.; Boyton, R.J.; Semper, A.; Moon, J.C. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet 2021, 397, 1057–1058. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef]
- Taylor, W.; Gladman, D.; Helliwell, P.; Marchesoni, A.; Mease, P.; Mielants, H.; Group, C.S. Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Rheum. 2006, 54, 2665–2673. [Google Scholar] [CrossRef] [PubMed]
- Rudwaleit, M.; van der Heijde, D.; Landewe, R.; Listing, J.; Akkoc, N.; Brandt, J.; Braun, J.; Chou, C.T.; Collantes-Estevez, E.; Dougados, M.; et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): Validation and final selection. Ann. Rheum. Dis. 2009, 68, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, M.C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997, 40, 1725. [Google Scholar] [CrossRef] [PubMed]
- Petri, M.; Orbai, A.M.; Alarcon, G.S.; Gordon, C.; Merrill, J.T.; Fortin, P.R.; Bruce, I.N.; Isenberg, D.; Wallace, D.J.; Nived, O.; et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012, 64, 2677–2686. [Google Scholar] [CrossRef]
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013, 65, 1–11. [Google Scholar] [CrossRef]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjogren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017, 69, 35–45. [Google Scholar] [CrossRef]
- International Team for the Revision of the International Criteria for Behçet’s Disease. The International Criteria for Behçet’s Disease (ICBD): A collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J. Eur. Acad Derm. Venereol 2014, 28, 338–347. [Google Scholar] [CrossRef]
- Irsara, C.; Egger, A.E.; Prokop, W.; Nairz, M.; Loacker, L.; Sahanic, S.; Pizzini, A.; Sonnweber, T.; Holzer, B.; Mayer, W.; et al. Clinical validation of the Siemens quantitative SARS-CoV-2 spike IgG assay (sCOVG) reveals improved sensitivity and a good correlation with virus neutralization titers. Clin. Chem. Lab. Med. 2021, 59, 1453–1462. [Google Scholar] [CrossRef]
- Saiag, E.; Goldshmidt, H.; Sprecher, E.; Ben-Ami, R.; Bomze, D. Immunogenicity of a BNT162b2 vaccine booster in health-care workers. Lancet Microbe 2021, 2, e650. [Google Scholar] [CrossRef]


| After the First Vaccine Dose | Number of Cases | After the Second Vaccine Dose | Number of Cases | After the Third Vaccine Dose | Number of Cases | |
|---|---|---|---|---|---|---|
| Local reactions: | Pain | 7 | Pain | 7 | Pain | 9 |
| Erythema | 0 | Erythema | 1 | Erythema | 2 | |
| Swelling | 0 | Swelling | 1 | Swelling | 4 | |
| Pruritus | 0 | Pruritus | 1 | Pruritus | 0 | |
| Tingling | 0 | Tingling | 0 | Tingling | 0 | |
| Systemic reactions: | Fever | 1 | Fever | 1 | Fever | 1 |
| Nausea | 0 | Nausea | 1 | Nausea | 1 | |
| Vomiting | 0 | Vomiting | 0 | Vomiting | 0 | |
| Rhinorrhea | 0 | Rhinorrhea | 0 | Rhinorrhea | 1 | |
| Cough | 0 | Cough | 0 | Cough | 0 | |
| Myalgia | 2 | Myalgia | 3 | Myalgia | 7 | |
| Arthralgia | 0 | Arthralgia | 0 | Arthralgia | 5 | |
| Chills | 1 | Chills | 1 | Chills | 3 | |
| Malaise | 0 | Malaise | 0 | Malaise | 3 | |
| Headache | 2 | Headache | 1 | Headache | 3 | |
| Allergic reaction | 0 | Allergic reaction | 0 | Allergic reaction | 0 | |
| Dizziness | 0 | Dizziness | 0 | Dizziness | 1 | |
| Throat pain | 0 | Throat pain | 0 | Throat pain | 2 | |
| Chest pain or palpitations | 0 | Chest pain or palpitations | 0 | Chest pain or palpitations | 0 | |
| Diarrhea | 0 | Diarrhea | 0 | Diarrhea | 1 | |
| Pruritus | 0 | Pruritus | 0 | Pruritus | 0 | |
| Lack of appetite | 0 | Lack of appetite | 0 | Lack of appetite | 0 | |
| Local lymphadenopathy | 0 | Local lymphadenopathy | 0 | Local lymphadenopathy | 0 | |
| High blood pressure | 1 | High blood pressure | 0 | High blood pressure | 0 | |
| Uveitis | 0 | Uveitis | 0 | Uveitis | 0 | |
| Herpes zoster | 0 | Herpes zoster | 0 | Herpes zoster | 0 | |
| Pericarditis | 0 | Pericarditis | 0 | Pericarditis | 0 | |
| Vaginal bleeding | 0 | Vaginal bleeding | 0 | Vaginal bleeding | 0 |
| Age, (Years) | Female | Disease Duration, (Years) | Therapy | ||||
|---|---|---|---|---|---|---|---|
| GC | cDMARD | bDMARD | JAKi | ||||
| All patients with aiRMDs, n = 46 | 65 ± 10 | 33 (72%) | 9.7 ± 7.2 | 6 | 24 | 20 | 1 |
| Homologous booster vaccination group, n = 22 | 63 ± 10 | 17 (77%) | 8.5 ± 6.2 | 1 | 14 | 12 | 1 |
| RA, n = 12 | 65 ± 10 | 12 (100%) | 8.4 ± 7.2 | 0 | 6 | 8 | 1 |
| PsA, n = 3 | 56 ± 14 | 1 (33%) | 8.7 ± 3.5 | 1 | 2 | 2 | 0 |
| AxSpa, n = 1 | 68 | 0 (0%) | 8 | 0 | 0 | 1 | 0 |
| SLE, n = 1 | 66 | 1 (100%) | 4 | 0 | 1 | 0 | 0 |
| SS, n = 3 | 57 ± 9 | 3 (100%) | 10 ± 8.7 | 0 | 3 | 1 | 0 |
| LVV, n = 1 | 73 | 1 (100%) | 5 | 0 | 1 | 0 | 0 |
| AAV, n = 1 | 69 | 0 (0%) | 9 | 0 | 1 | 0 | 0 |
| Heterologous booster vaccination group, n = 12 | 59 ± 10 | 7 (58%) | 11.2 ± 7.7 | 1 | 10 | 5 | 0 |
| RA, n = 8 | 60 ± 5 | 8 (100%) | 10.9 ± 6.6 | 1 | 6 | 5 | 0 |
| PsA, n = 2 | 56 ± 29 | 1 (50%) | 3.5 ± 0.7 | 0 | 2 | 0 | 0 |
| SLE, n = 1 | 54 | 1 (100%) | 26 | 0 | 1 | 0 | 0 |
| Other vasculitis, n = 1 | 55 | 1 (100%) | 14 | 0 | 1 | 0 | 0 |
| Infection group, n = 12 | 59 ± 9 | 9 (75%) | 10.5 ± 8.6 | 4 | 8 | 6 | 0 |
| RA, n = 5 | 56 ± 11 | 4 (80%) | 13 ± 10.6 | 0 | 4 | 3 | 0 |
| PsA, n = 2 | 55 ± 1 | 1 (50%) | 5 ± 1.4 | 0 | 2 | 2 | 0 |
| SLE, n = 1 | 69 | 1 (100%) | 22 | 1 | 1 | 0 | 0 |
| SS, n = 1 | 59 | 1 (100%) | 14 | 1 | 1 | 0 | 0 |
| LVV, n = 2 | 68 ± 12 | 1 (50%) | 5 ± 5.7 | 1 | 2 | 0 | 0 |
| AAV, n = 1 | 55 | 1 (100%) | 5 | 1 | 0 | 1 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honfi, D.; Gémes, N.; Szabó, E.; Neuperger, P.; Balog, J.Á.; Nagy, L.I.; Toldi, G.; Puskás, L.G.; Szebeni, G.J.; Balog, A. Comparison of Homologous and Heterologous Booster SARS-CoV-2 Vaccination in Autoimmune Rheumatic and Musculoskeletal Patients. Int. J. Mol. Sci. 2022, 23, 11411. https://doi.org/10.3390/ijms231911411
Honfi D, Gémes N, Szabó E, Neuperger P, Balog JÁ, Nagy LI, Toldi G, Puskás LG, Szebeni GJ, Balog A. Comparison of Homologous and Heterologous Booster SARS-CoV-2 Vaccination in Autoimmune Rheumatic and Musculoskeletal Patients. International Journal of Molecular Sciences. 2022; 23(19):11411. https://doi.org/10.3390/ijms231911411
Chicago/Turabian StyleHonfi, Dániel, Nikolett Gémes, Enikő Szabó, Patrícia Neuperger, József Á. Balog, Lajos I. Nagy, Gergely Toldi, László G. Puskás, Gábor J. Szebeni, and Attila Balog. 2022. "Comparison of Homologous and Heterologous Booster SARS-CoV-2 Vaccination in Autoimmune Rheumatic and Musculoskeletal Patients" International Journal of Molecular Sciences 23, no. 19: 11411. https://doi.org/10.3390/ijms231911411
APA StyleHonfi, D., Gémes, N., Szabó, E., Neuperger, P., Balog, J. Á., Nagy, L. I., Toldi, G., Puskás, L. G., Szebeni, G. J., & Balog, A. (2022). Comparison of Homologous and Heterologous Booster SARS-CoV-2 Vaccination in Autoimmune Rheumatic and Musculoskeletal Patients. International Journal of Molecular Sciences, 23(19), 11411. https://doi.org/10.3390/ijms231911411







