The Association of Grain Yield and Agronomical Traits with Genes of Plant Height, Photoperiod Sensitivity and Plastid Glutamine Synthetase in Winter Bread Wheat (Triticum aestivum L.) Collection
Abstract
1. Introduction
2. Results
2.1. Sequence Comparison and PCR Marker Development
2.2. One-Way Analysis
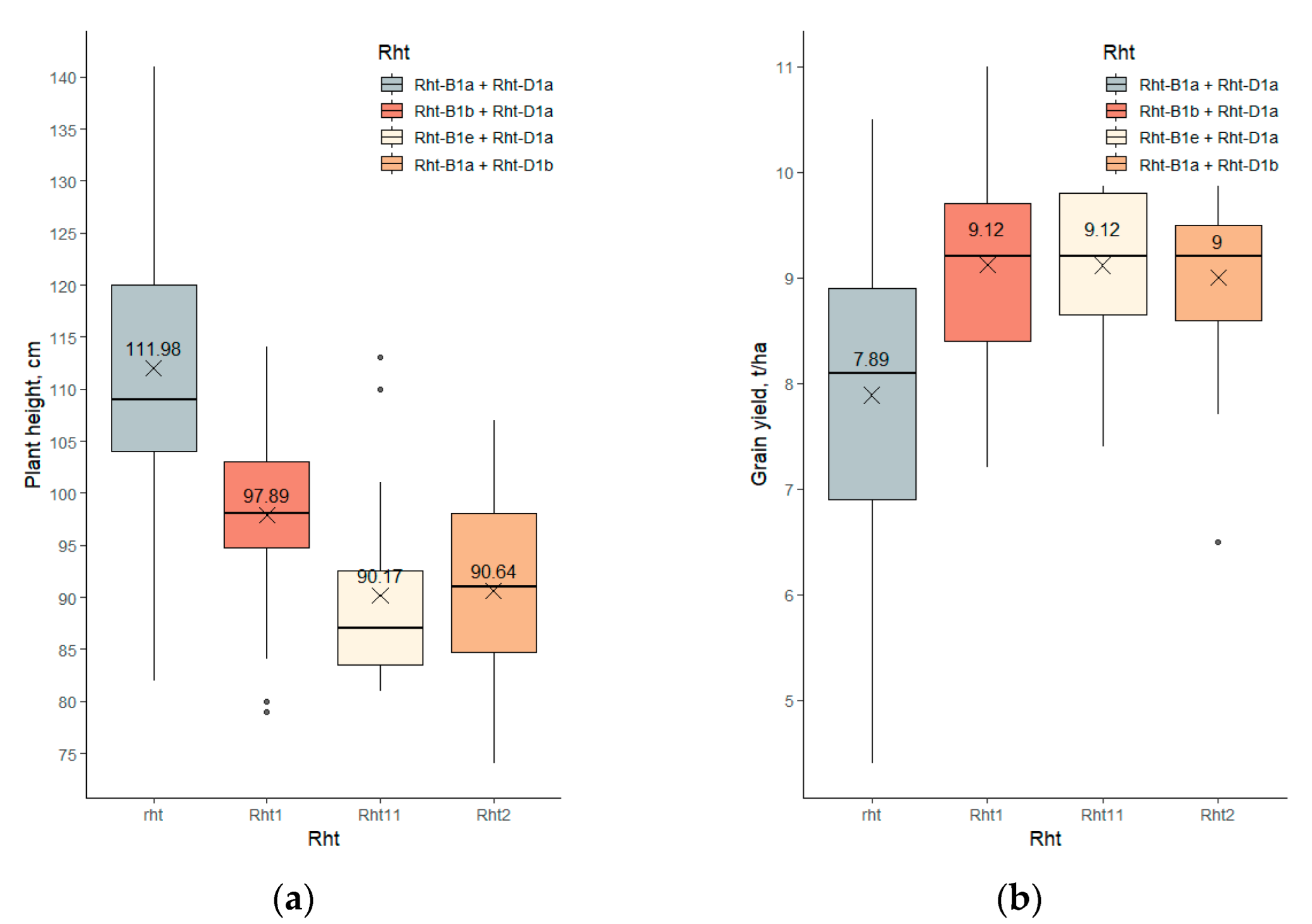
2.2.1. Rht-1 Effects
2.2.2. Ppd-D1 Effects
2.2.3. GS2-A1 Effects
2.3. Multifactor Analysis
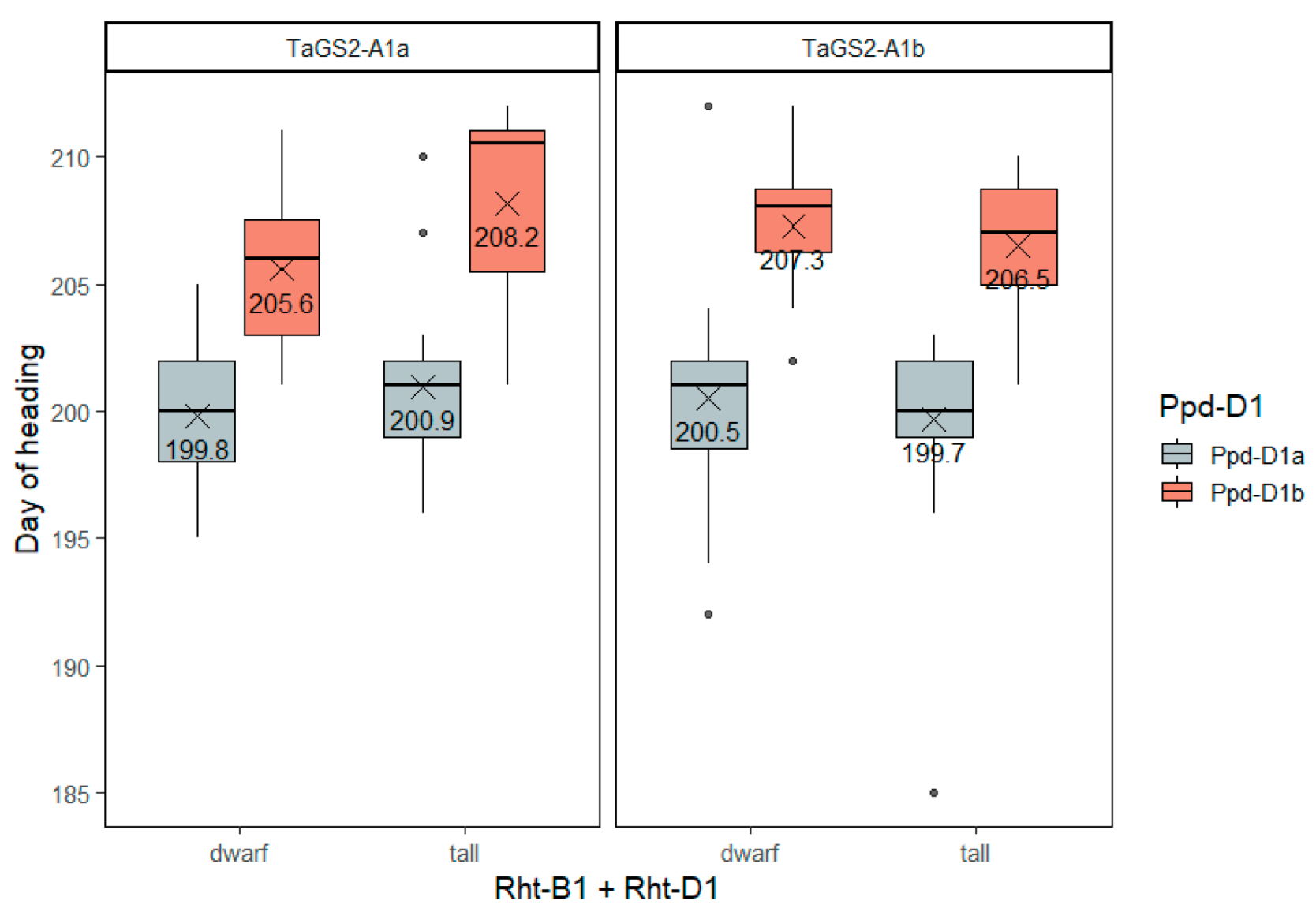
2.3.1. Heading Date
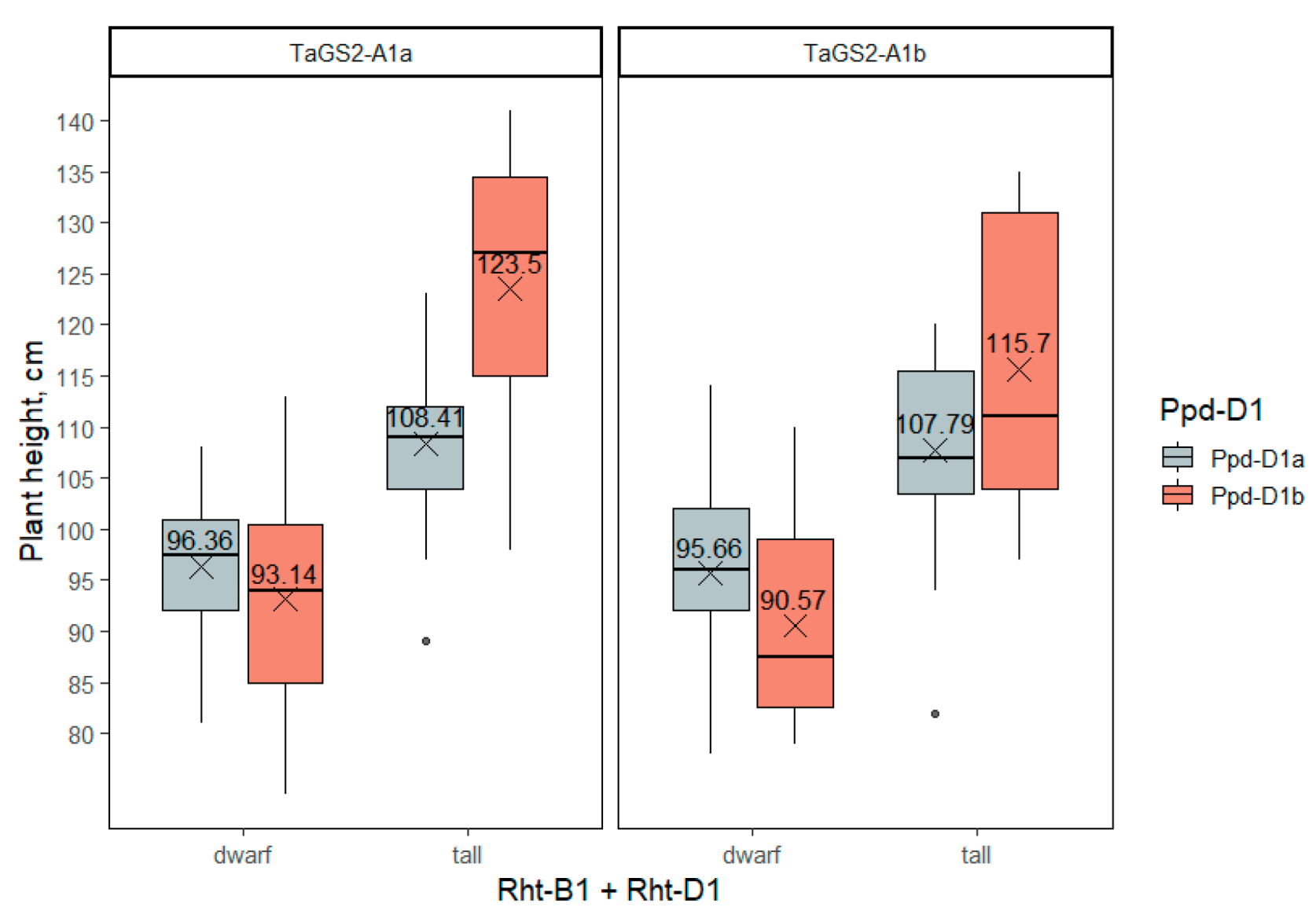
2.3.2. Plant Height
2.3.3. Grain Yield
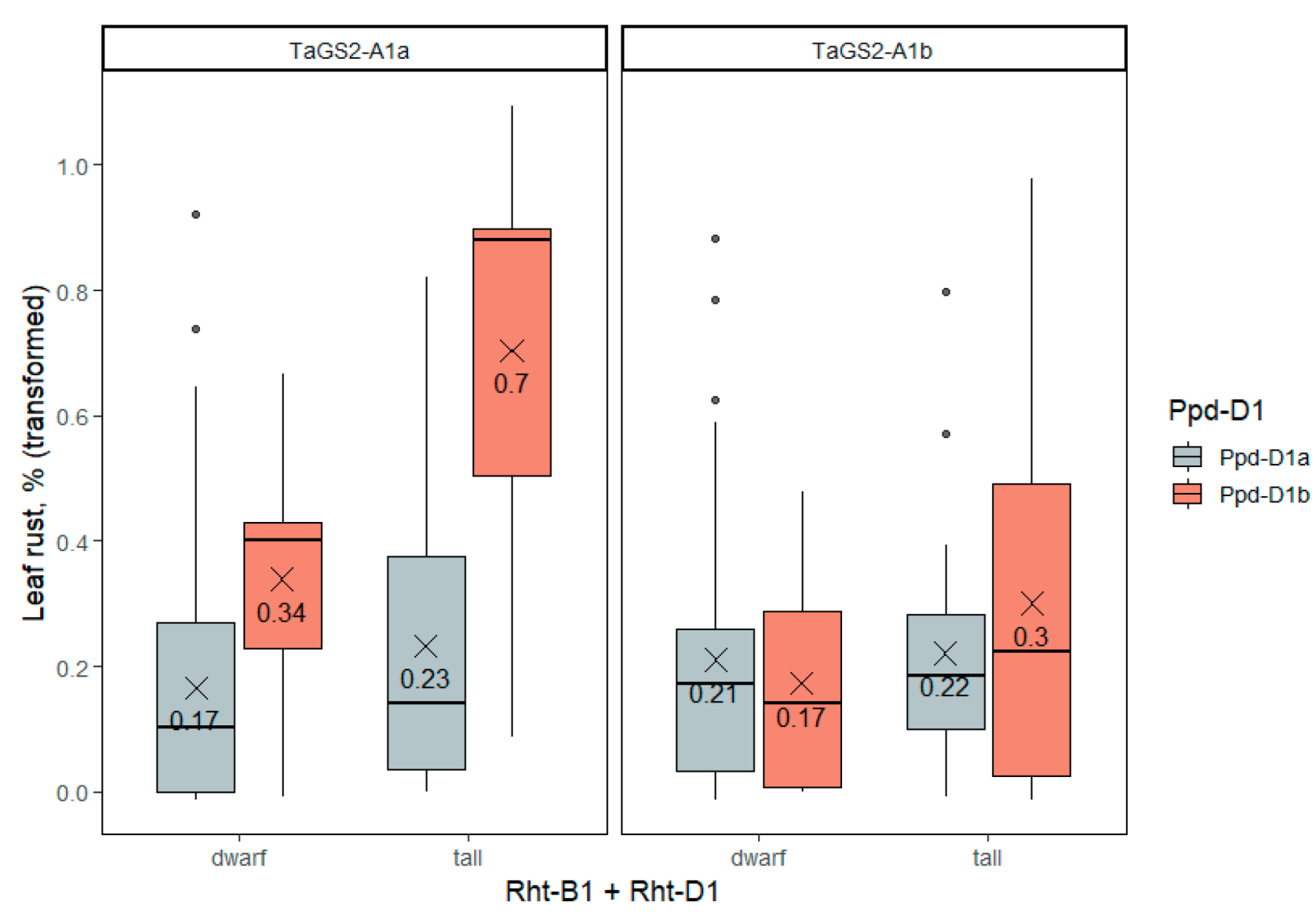
2.3.4. Leaf Rust
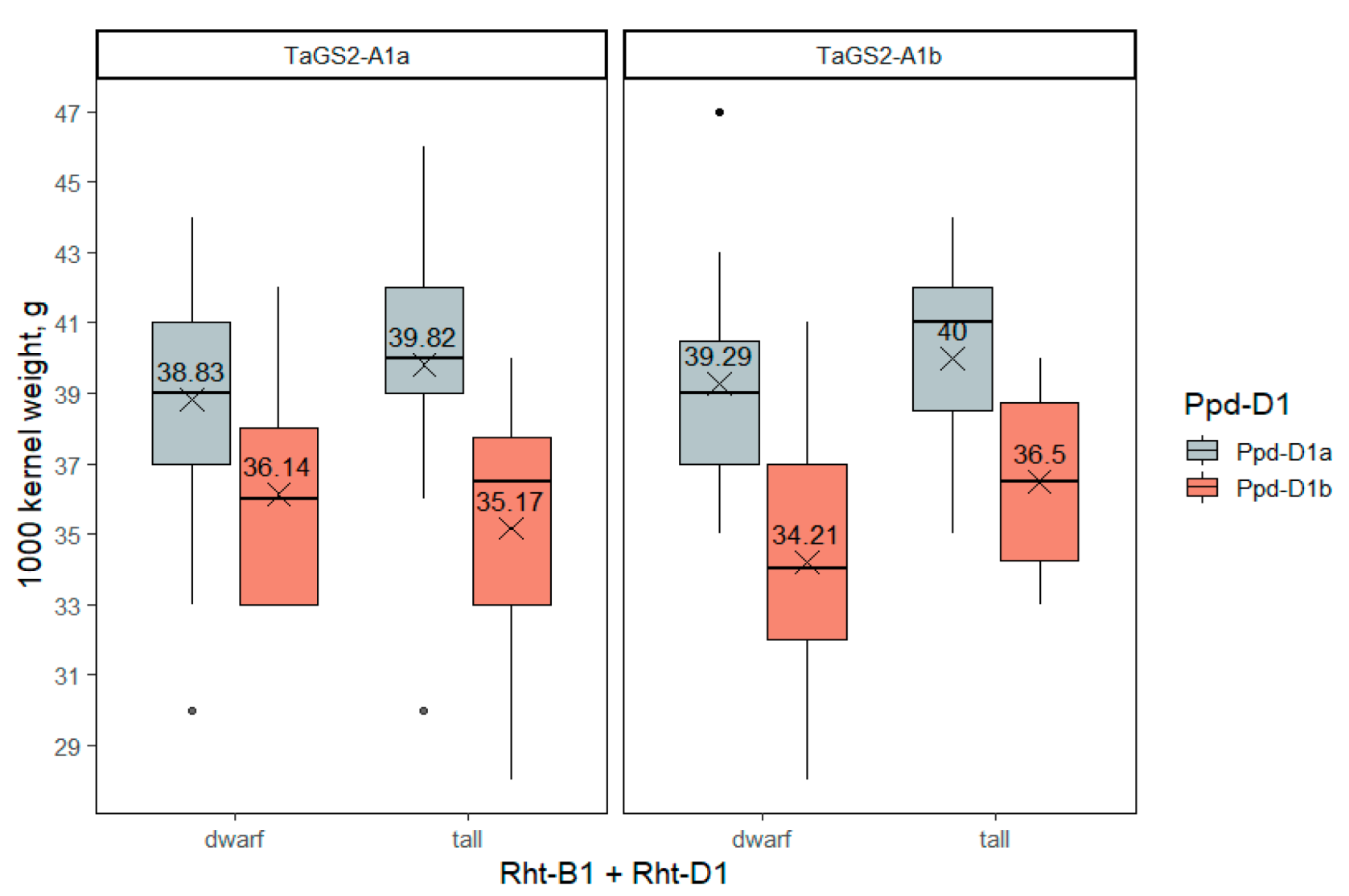
2.3.5. The 1000-Kernel Weight
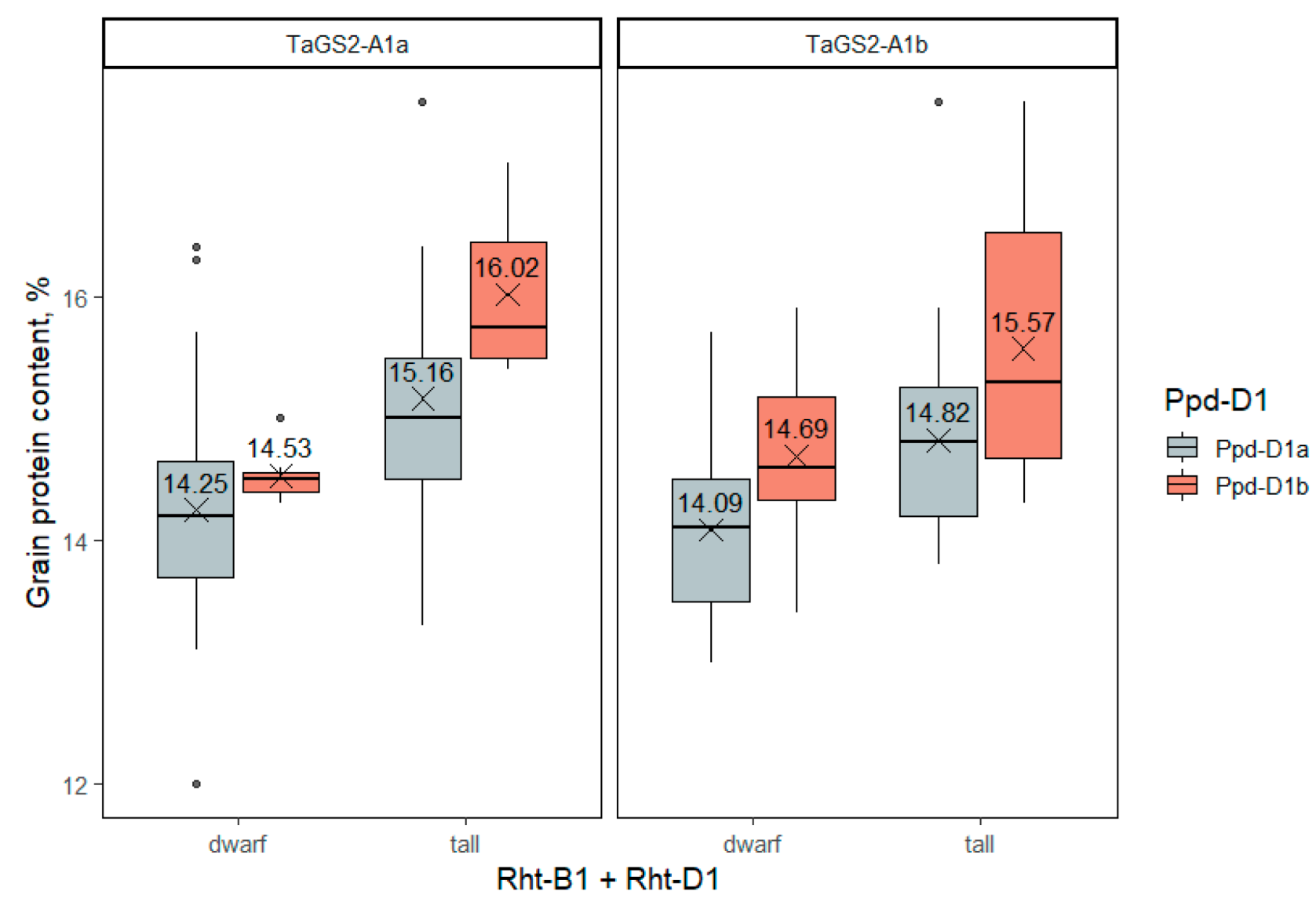
2.3.6. Grain Protein Content
2.3.7. Grain Protein Yield
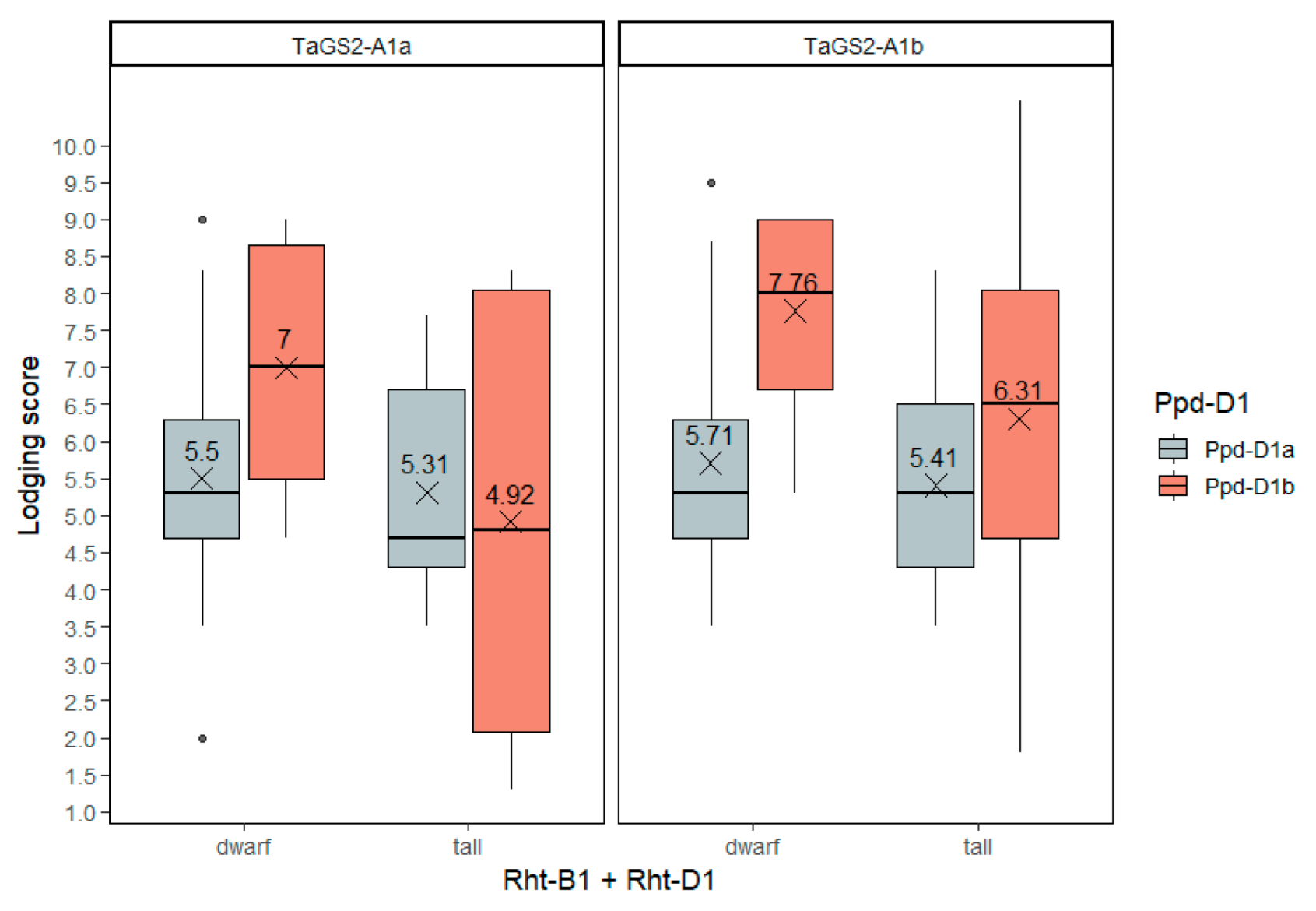
2.3.8. Lodging Resistance
3. Discussion
4. Materials and Methods
4.1. Plant Material and Phenotyping
4.2. STS Marker Development for the TaGS2-A1 Gene
4.3. DNA Extraction and PCR-Markers
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grote, U.; Fasse, A.; Nguyen, T.T.; Erenstein, O. Food Security and the Dynamics of Wheat and Maize Value Chains in Africa and Asia. Front. Sustain. Food Syst. 2021, 4, 617009. [Google Scholar] [CrossRef]
- Flintham, J.E.; Börner, A.; Worland, A.J.; Gale, M.D. Optimizing Wheat Grain Yield: Effects of Rht (Gibberellin-Insensitive) Dwarfing Genes. J. Agric. Sci. 1997, 128, 11–25. [Google Scholar] [CrossRef]
- Hayat, H.; Mason, R.E.; Lozada, D.N.; Acuna, A.; Holder, A.; Larkin, D.; Winn, Z.; Murray, J.; Murphy, J.P.; Moon, D.E.; et al. Effects of Allelic Variation at Rht-B1 and Rht-D1 on Grain Yield and Agronomic Traits of Southern US Soft Red Winter Wheat. Euphytica 2019, 215, 172. [Google Scholar] [CrossRef]
- Jatayev, S.; Sukhikh, I.; Vavilova, V.; Smolenskaya, S.E.; Goncharov, N.P.; Kurishbayev, A.; Zotova, L.; Absattarova, A.; Serikbay, D.; Hu, Y.-G.; et al. Green Revolution “Stumbles” in a Dry Environment: Dwarf Wheat with Rht Genes Fails to Produce Higher Grain Yield than Taller Plants under Drought. Plant Cell Environ. 2020, 43, 2355–2364. [Google Scholar] [CrossRef]
- Peng, J.; Richards, D.E.; Hartley, N.M.; Murphy, G.P.; Devos, K.M.; Flintham, J.E.; Beales, J.; Fish, L.J.; Worland, A.J.; Pelica, F.; et al. “Green Revolution” Genes Encode Mutant Gibberellin Response Modulators. Nature 1999, 400, 256–261. [Google Scholar] [CrossRef]
- Wu, J.; Kong, X.; Wan, J.; Liu, X.; Zhang, X.; Guo, X.; Zhou, R.; Zhao, G.; Jing, R.; Fu, X.; et al. Dominant and Pleiotropic Effects of a GAI Gene in Wheat Results from a Lack of Interaction between DELLA and GID1. Plant Physiol. 2011, 157, 2120–2130. [Google Scholar] [CrossRef]
- Wilhelm, E.P.; Boulton, M.I.; Al-Kaff, N.; Balfourier, F.; Bordes, J.; Greenland, A.J.; Powell, W.; Mackay, I.J. Rht-1 and Ppd-D1 Associations with Height, GA Sensitivity, and Days to Heading in a Worldwide Bread Wheat Collection. Theor. Appl. Genet. 2013, 126, 2233–2243. [Google Scholar] [CrossRef]
- Divashuk, M.G.; Vasilyev, A.V.; Bespalova, L.A.; Karlov, G.I. Identity of the Rht-11 and Rht-B1e Reduced Plant Height Genes. Russ. J. Genet. 2012, 48, 761–763. [Google Scholar] [CrossRef]
- Bazhenov, M.S.; Divashuk, M.G.; Amagai, Y.; Watanabe, N.; Karlov, G.I. Isolation of the Dwarfing Rht-B1p (Rht17) Gene from Wheat and the Development of an Allele-Specific PCR Marker. Mol. Breed. 2015, 35, 213. [Google Scholar] [CrossRef]
- Börner, A.; Worland, A.J.; Plaschke, J.; Schumann, E.; Law, C.N. Pleiotropic Effects of Genes for Reduced Height (Rht) and Day-Length Insensitivity (Ppd) on Yield and Its Components for Wheat Grown in Middle Europe. Plant Breed. 1993, 111, 204–216. [Google Scholar] [CrossRef]
- Allan, R.E. Agronomic Comparisons among Wheat Lines Nearly Isogenic for Three Reduced-Height Genes1. Crop Sci. 1986, 26, 707–710. [Google Scholar] [CrossRef]
- Khadka, K.; Kaviani, M.; Raizada, M.N.; Navabi, A. Phenotyping and Identification of Reduced Height (Rht) Alleles (Rht-B1b and Rht-D1b) in a Nepali Spring Wheat (Triticum aestivum L.) Diversity Panel to Enable Seedling Vigor Selection. Agronomy 2021, 11, 2412. [Google Scholar] [CrossRef]
- McClung, A.M.; Cantrell, R.G.; Quick, J.S.; Gregory, R.S. Influence of the Rht1 Semidwarf Gene on Yield, Yield Components, and Grain Protein in Durum Wheat1. Crop Sci. 1986, 26, 1095–1099. [Google Scholar] [CrossRef]
- Sri, S.; Gosman, N.; Steed, A.; Hollins, T.; Bayles, R.; Jennings, P.; Nicholson, P. Semi-Dwarfing Rht-B1 and Rht-D1 Loci of Wheat Differ Significantly in Their Influence on Resistance to Fusarium Head Blight. TAG Theor. Appl. Genet. 2008, 118, 695–702. [Google Scholar] [CrossRef]
- Li, S.; Tian, Y.; Wu, K.; Ye, Y.; Yu, J.; Zhang, J.; Liu, Q.; Hu, M.; Li, H.; Tong, Y.; et al. Modulating Plant Growth–Metabolism Coordination for Sustainable Agriculture. Nature 2018, 560, 595–600. [Google Scholar] [CrossRef]
- Miedaner, T.; Herter, C.P.; Ebmeyer, E.; Kollers, S.; Korzun, V. Use of Non-Adapted Quantitative Trait Loci for Increasing Fusarium Head Blight Resistance for Breeding Semi-Dwarf Wheat. Plant Breed. 2019, 138, 140–147. [Google Scholar] [CrossRef]
- Tian, X.; Xia, X.; Xu, D.; Liu, Y.; Xie, L.; Hassan, M.A.; Song, J.; Li, F.; Wang, D.; Zhang, Y.; et al. Rht24b, an Ancient Variation of TaGA2ox-A9, Reduces Plant Height without Yield Penalty in Wheat. New Phytol. 2022, 233, 738–750. [Google Scholar] [CrossRef]
- Mo, Y.; Vanzetti, L.S.; Hale, I.; Spagnolo, E.J.; Guidobaldi, F.; Al-Oboudi, J.; Odle, N.; Pearce, S.; Helguera, M.; Dubcovsky, J. Identification and Characterization of Rht25, a Locus on Chromosome Arm 6AS Affecting Wheat Plant Height, Heading Time, and Spike Development. Theor. Appl. Genet. 2018, 131, 2021–2035. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.H.; Rebetzke, G.J.; Azanza, F.; Richards, R.A.; Spielmeyer, W. Molecular Mapping of Gibberellin-Responsive Dwarfing Genes in Bread Wheat. Theor. Appl. Genet. 2005, 111, 423–430. [Google Scholar] [CrossRef]
- Guo, Z.; Song, Y.; Zhou, R.; Ren, Z.; Jia, J. Discovery, Evaluation and Distribution of Haplotypes of the Wheat Ppd-D1 Gene. New Phytol. 2010, 185, 841–851. [Google Scholar] [CrossRef]
- Wordland, A.J. The Importance of Italian Wheats to Worldwide Varietal Improvement [Triticum aestivum L.]. J. Genet. Breed. 1999, 53, 165–171. [Google Scholar]
- Worland, A.J.; Börner, A.; Korzun, V.; Li, W.M.; Petrovíc, S.; Sayers, E.J. The Influence of Photoperiod Genes on the Adaptability of European Winter Wheats. Euphytica 1998, 100, 385–394. [Google Scholar] [CrossRef]
- Brasier, K.G.; Tamang, B.G.; Carpenter, N.R.; Fukao, T.; Reiter, M.S.; Pitman, R.M.; Sneller, C.H.; Thomason, W.E.; Griffey, C.A. Photoperiod Response Gene Ppd-D1 Affects Nitrogen Use Efficiency in Soft Red Winter Wheat. Crop Sci. 2018, 58, 2593–2606. [Google Scholar] [CrossRef]
- Beales, J.; Turner, A.; Griffiths, S.; Snape, J.W.; Laurie, D.A. A Pseudo-Response Regulator Is Misexpressed in the Photoperiod Insensitive Ppd-D1a Mutant of Wheat (Triticum aestivum L.). Theor. Appl. Genet. 2007, 115, 721–733. [Google Scholar] [CrossRef]
- Gooding, M.J.; Addisu, M.; Uppal, R.K.; Snape, J.W.; Jones, H.E. Effect of Wheat Dwarfing Genes on Nitrogen-Use Efficiency. J. Agric. Sci. 2012, 150, 3–22. [Google Scholar] [CrossRef]
- Wang, D.; Xu, Z.; Zhao, J.; Wang, Y.; Yu, Z. Excessive Nitrogen Application Decreases Grain Yield and Increases Nitrogen Loss in a Wheat–Soil System. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2011, 61, 681–692. [Google Scholar] [CrossRef]
- Taira, M.; Valtersson, U.; Burkhardt, B.; Ludwig, R.A. Arabidopsis Thaliana GLN2-Encoded Glutamine Synthetase Is Dual Targeted to Leaf Mitochondria and Chloroplasts. Plant Cell 2004, 16, 2048–2058. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-P.; Zhao, X.-Q.; He, X.; Zhao, G.-Y.; Li, B.; Liu, D.-C.; Zhang, A.-M.; Zhang, X.-Y.; Tong, Y.-P.; Li, Z.-S. Haplotype Analysis of the Genes Encoding Glutamine Synthetase Plastic Isoforms and Their Association with Nitrogen-Use- and Yield-Related Traits in Bread Wheat. New Phytol. 2011, 189, 449–458. [Google Scholar] [CrossRef]
- Hirel, B.; Tétu, T.; Lea, P.J.; Dubois, F. Improving Nitrogen Use Efficiency in Crops for Sustainable Agriculture. Sustainability 2011, 3, 1452–1485. [Google Scholar] [CrossRef]
- Habash, D.Z.; Massiah, A.J.; Rong, H.L.; Wallsgrove, R.M.; Leigh, R.A. The Role of Cytosolic Glutamine Synthetase in Wheat. Ann. Appl. Biol. 2001, 138, 83–89. [Google Scholar] [CrossRef]
- Marino, D.; Cañas, R.A.; Betti, M. Is Plastidic Glutamine Synthetase Essential for C3 Plants? A Tale of Photorespiratory Mutants, Ammonium Tolerance and Conifers. New Phytol. 2022, 234, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Lee, J.; Kichey, T.; Gerentes, D.; Zivy, M.; Tatout, C.; Dubois, F.; Balliau, T.; Valot, B.; Davanture, M.; et al. Two Cytosolic Glutamine Synthetase Isoforms of Maize Are Specifically Involved in the Control of Grain Production. Plant Cell 2006, 18, 3252–3274. [Google Scholar] [CrossRef] [PubMed]
- Nigro, D.; Fortunato, S.; Giove, S.L.; Mangini, G.; Yacoubi, I.; Simeone, R.; Blanco, A.; Gadaleta, A. Allelic Variants of Glutamine Synthetase and Glutamate Synthase Genes in a Collection of Durum Wheat and Association with Grain Protein Content. Diversity 2017, 9, 52. [Google Scholar] [CrossRef]
- Hu, M.; Zhao, X.; Liu, Q.; Hong, X.; Zhang, W.; Zhang, Y.; Sun, L.; Li, H.; Tong, Y. Transgenic Expression of Plastidic Glutamine Synthetase Increases Nitrogen Uptake and Yield in Wheat. Plant Biotechnol. J. 2018, 16, 1858–1867. [Google Scholar] [CrossRef] [PubMed]
- Bazhenov, M.S.; Chernook, A.G.; Bespalova, L.A.; Gritsay, T.I.; Polevikova, N.A.; Karlov, G.I.; Nazarova, L.A.; Divashuk, M.G. Alleles of the GRF3-2A Gene in Wheat and Their Agronomic Value. Int. J. Mol. Sci. 2021, 22, 12376. [Google Scholar] [CrossRef]
- Walkowiak, S.; Gao, L.; Monat, C.; Haberer, G.; Kassa, M.T.; Brinton, J.; Ramirez-Gonzalez, R.H.; Kolodziej, M.C.; Delorean, E.; Thambugala, D.; et al. Multiple Wheat Genomes Reveal Global Variation in Modern Breeding. Nature 2020, 588, 277–283. [Google Scholar] [CrossRef]
- Achilli, A.L.; Roncallo, P.F.; Larsen, A.O.; Dreisigacker, S.; Echenique, V. Population Structure, Allelic Variation at Rht-B1 and Ppd-A1 Loci and Its Effects on Agronomic Traits in Argentinian Durum Wheat. Sci. Rep. 2022, 12, 9629. [Google Scholar] [CrossRef]
- Wang, J.; Guan, Y.; Wu, L.; Guan, X.; Cai, W.; Huang, J.; Dong, W.; Zhang, B. Changing Lengths of the Four Seasons by Global Warming. Geophys. Res. Lett. 2021, 48, e2020GL091753. [Google Scholar] [CrossRef]
- Simón, M.R.; Worland, A.J.; Struik, P.C. Influence of Plant Height and Heading Date on the Expression of the Resistance to Septoria Tritici Blotch in Near Isogenic Lines of Wheat. Crop Sci. 2004, 44, 2078–2085. [Google Scholar] [CrossRef]
- Singh, R.P.; Huerta-Espino, J.; Bhavani, S.; Herrera-Foessel, S.A.; Singh, D.; Singh, P.K.; Velu, G.; Mason, R.E.; Jin, Y.; Njau, P.; et al. Race Non-Specific Resistance to Rust Diseases in CIMMYT Spring Wheats. Euphytica 2011, 179, 175–186. [Google Scholar] [CrossRef]
- Shah, L.; Yahya, M.; Shah, S.M.A.; Nadeem, M.; Ali, A.; Ali, A.; Wang, J.; Riaz, M.W.; Rehman, S.; Wu, W.; et al. Improving Lodging Resistance: Using Wheat and Rice as Classical Examples. Int. J. Mol. Sci. 2019, 20, 4211. [Google Scholar] [CrossRef]
- Alaux, M.; Rogers, J.; Letellier, T.; Flores, R.; Alfama, F.; Pommier, C.; Mohellibi, N.; Durand, S.; Kimmel, E.; Michotey, C.; et al. Linking the International Wheat Genome Sequencing Consortium Bread Wheat Reference Genome Sequence to Wheat Genetic and Phenomic Data. Genome Biol. 2018, 19, 111. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and Applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, K.B.; Nicholas, H.B. GeneDoc: A Tool for Editing and Annotating Multiple Sequence Alignments. Embnew. News 1997, 4, 14. [Google Scholar]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A Tool to Design Target-Specific Primers for Polymerase Chain Reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Doyle, P.J. DNA Protocols for Plants. In Molecular Techniques in Taxonomy; Hewitt, G.M., Johnston, A.W.B., Young, J.P.W., Eds.; NATO ASI Series; Springer: Berlin/Heidelberg, Germany, 1991; pp. 283–293. ISBN 978-3-642-83964-1. [Google Scholar]
- Ellis, M.; Spielmeyer, W.; Gale, K.; Rebetzke, G.; Richards, R. “Perfect” Markers for the Rht-B1b and Rht-D1b Dwarfing Genes in Wheat. Theor. Appl. Genet. 2002, 105, 1038–1042. [Google Scholar] [CrossRef]
- Pearce, S.; Saville, R.; Vaughan, S.P.; Chandler, P.M.; Wilhelm, E.P.; Sparks, C.A.; Al-Kaff, N.; Korolev, A.; Boulton, M.I.; Phillips, A.L.; et al. Molecular Characterization of Rht-1 Dwarfing Genes in Hexaploid Wheat. Plant Physiol. 2011, 157, 1820–1831. [Google Scholar] [CrossRef]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for Association Mapping of Complex Traits in Diverse Samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef]
- Cramer, A.O.J.; van Ravenzwaaij, D.; Matzke, D.; Steingroever, H.; Wetzels, R.; Grasman, R.P.P.P.; Waldorp, L.J.; Wagenmakers, E.-J. Hidden Multiplicity in Exploratory Multiway ANOVA: Prevalence and Remedies. Psychon. Bull. Rev. 2016, 23, 640–647. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2; Springer: New York, NY, USA, 2009; ISBN 978-0-387-98140-6. [Google Scholar]
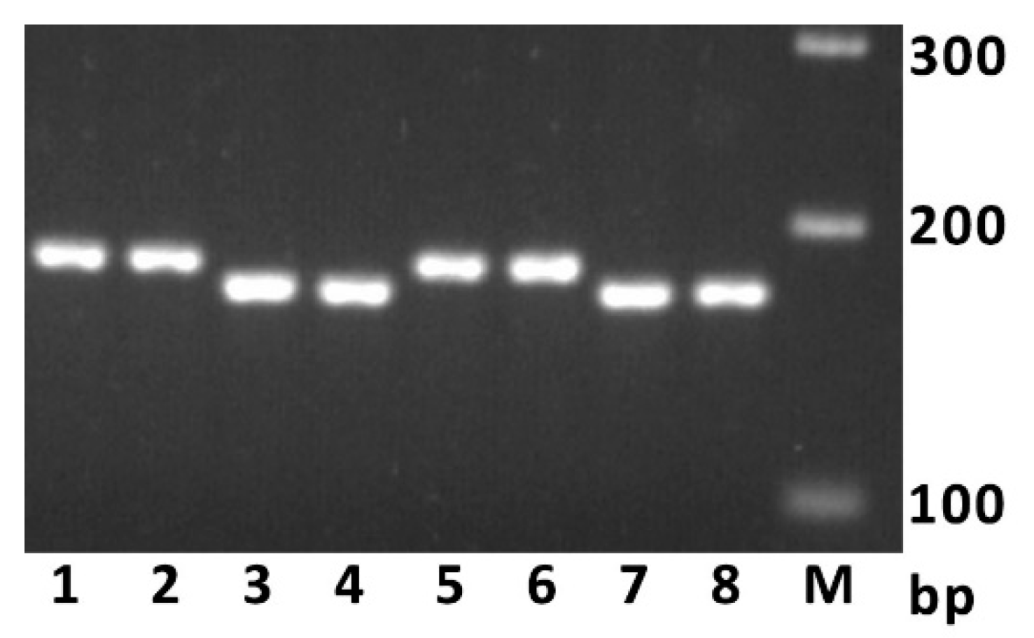


| Name | Sequence (5′->3′) | Alleles Detected, Product Length |
|---|---|---|
| BF | GGTAGGGAGGCGAGAGGCGAG | (Used with MR1, WR1, MR3, WR3) |
| MR1 | CATCCCCATGGCCATCTCGAGCTA | Rht-B1b, 237 bp |
| WR1 | CATCCCCATGGCCATCTCGAGCTG | Rht-B1a (not Rht-B1b) *, 237 bp |
| MR3 | GGCCATCTCCAGCTGCTCCAGCTA | Rht-B1e, 228 bp |
| WR3 | GGCCATCTCCAGCTGCTCCAGCTT | Rht-B1a (not Rht-B1e) *, 228 bp |
| DF | CGCGCAATTATTGGCCAGAGATAG | Rht-D1b, 254 bp |
| MR2 | CCCCATGGCCATCTCGAGCTGCTA | |
| DF2 | GGCAAGCAAAAGCTTCGCG | Rht-D1a (not Rht-D1b) *, 264 bp |
| WR2 | GGCCATCTCGAGCTGCAC | |
| Ppd-D1_F | ACGCCTCCCACTACACTG | Ppd-D1a, 288 bp; Ppd-D1b, 414 bp |
| Ppd-D1_R1 | GTTGGTTCAAACAGAGAGC | |
| Ppd-D1_R2 | CACTGGTGGTAGCTGAGATT | |
| pGS2-A1-F | GGCCTCCGCTCCCATAAATATAA | TaGS2-A1a, 172 bp; TaGS2-A1b, 182 bp |
| pGS2-A1-R | AACGCAACAGAGATTGAAGAAGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazhenov, M.S.; Bespalova, L.A.; Kocheshkova, A.A.; Chernook, A.G.; Puzyrnaya, O.Y.; Agaeva, E.V.; Nikitina, E.A.; Igonin, V.N.; Bazhenova, S.S.; Vertikova, E.A.; et al. The Association of Grain Yield and Agronomical Traits with Genes of Plant Height, Photoperiod Sensitivity and Plastid Glutamine Synthetase in Winter Bread Wheat (Triticum aestivum L.) Collection. Int. J. Mol. Sci. 2022, 23, 11402. https://doi.org/10.3390/ijms231911402
Bazhenov MS, Bespalova LA, Kocheshkova AA, Chernook AG, Puzyrnaya OY, Agaeva EV, Nikitina EA, Igonin VN, Bazhenova SS, Vertikova EA, et al. The Association of Grain Yield and Agronomical Traits with Genes of Plant Height, Photoperiod Sensitivity and Plastid Glutamine Synthetase in Winter Bread Wheat (Triticum aestivum L.) Collection. International Journal of Molecular Sciences. 2022; 23(19):11402. https://doi.org/10.3390/ijms231911402
Chicago/Turabian StyleBazhenov, Mikhail S., Ludmila A. Bespalova, Alina A. Kocheshkova, Anastasiya G. Chernook, Olga Y. Puzyrnaya, Elena V. Agaeva, Ekaterina A. Nikitina, Vladimir N. Igonin, Svetlana S. Bazhenova, Elena A. Vertikova, and et al. 2022. "The Association of Grain Yield and Agronomical Traits with Genes of Plant Height, Photoperiod Sensitivity and Plastid Glutamine Synthetase in Winter Bread Wheat (Triticum aestivum L.) Collection" International Journal of Molecular Sciences 23, no. 19: 11402. https://doi.org/10.3390/ijms231911402
APA StyleBazhenov, M. S., Bespalova, L. A., Kocheshkova, A. A., Chernook, A. G., Puzyrnaya, O. Y., Agaeva, E. V., Nikitina, E. A., Igonin, V. N., Bazhenova, S. S., Vertikova, E. A., Kharchenko, P. N., Karlov, G. I., & Divashuk, M. G. (2022). The Association of Grain Yield and Agronomical Traits with Genes of Plant Height, Photoperiod Sensitivity and Plastid Glutamine Synthetase in Winter Bread Wheat (Triticum aestivum L.) Collection. International Journal of Molecular Sciences, 23(19), 11402. https://doi.org/10.3390/ijms231911402





