Evaluation of Circulating MicroRNAs and Adipokines in Breast Cancer Survivors with Arm Lymphedema
Abstract
1. Introduction
2. Results
2.1. Characteristics of Study Participants
2.2. Circulating miRNAs Are Associated with Lymphedema Development in Breast Cancer Survivors
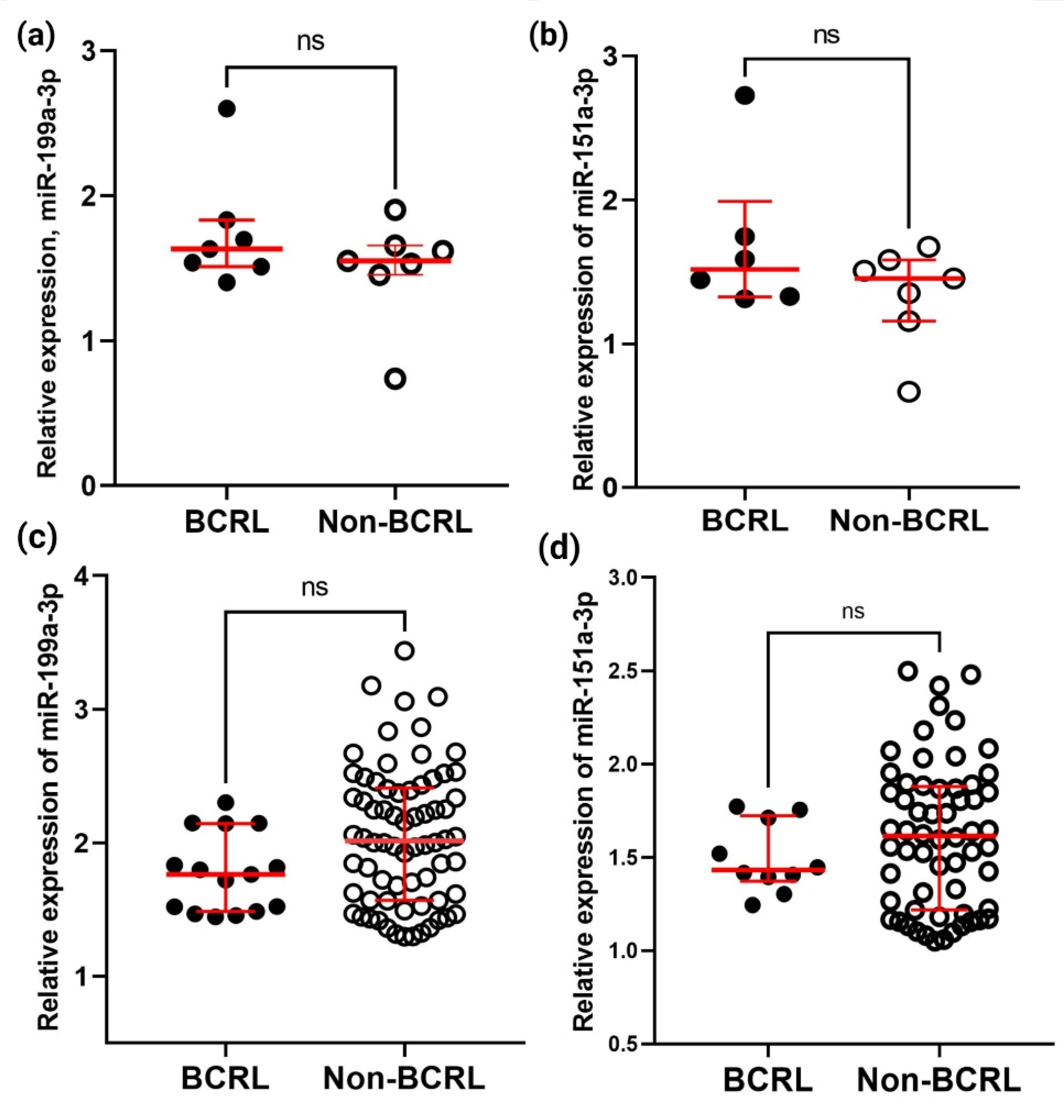
2.3. Verification and Validation of the Differential Expression of miR-199a-3p and miR-151a-3p in BCRL by qPCR
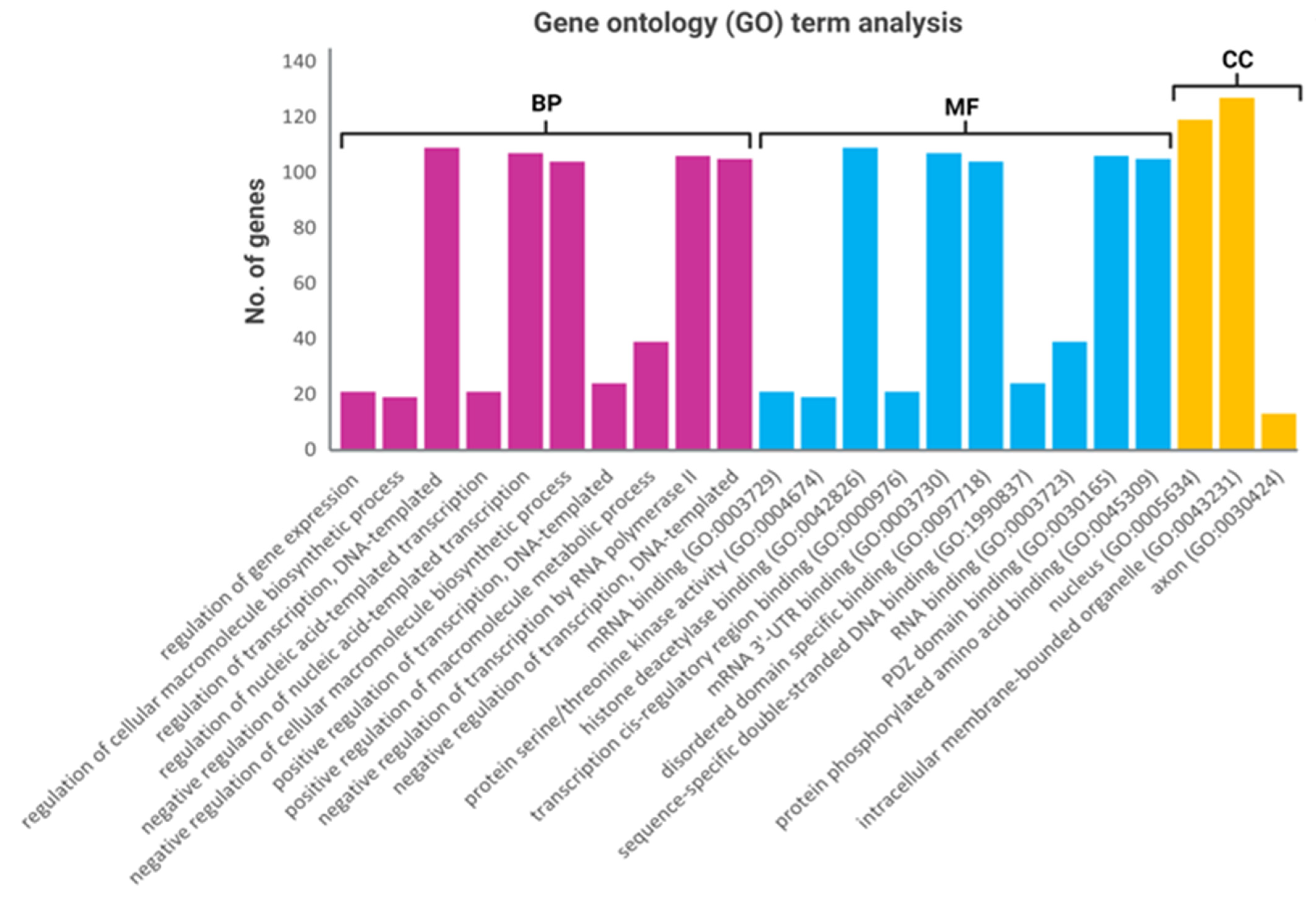
2.4. Functional Annotation Analysis of the Predicted Target Genes
2.5. Circulating Leptin, Adiponectin, and Adiponectin/Leptin Ratio
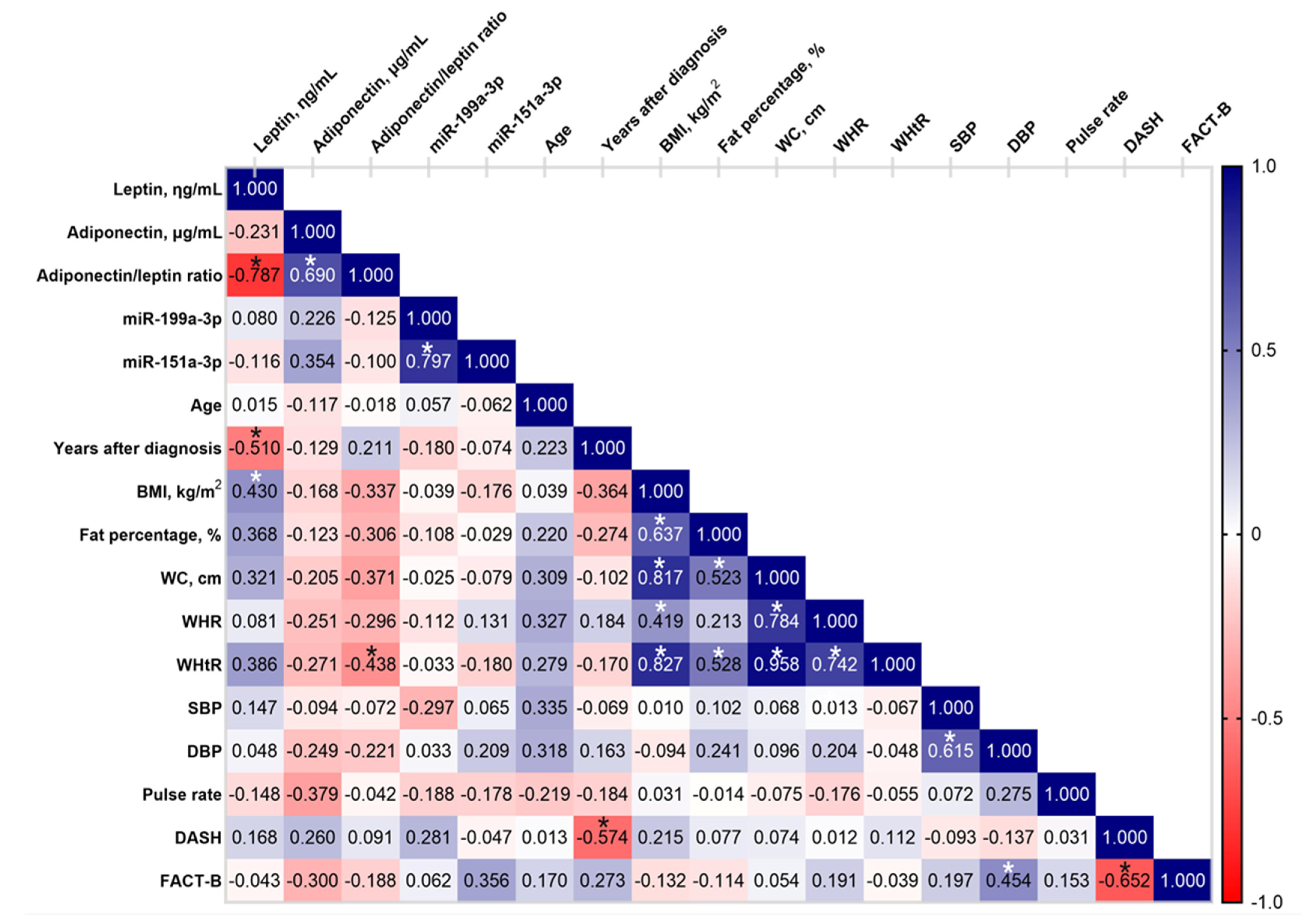
2.6. Correlation Analysis of Circulating Adipokines with Relevant Characteristics in the BCRL Cohort
3. Discussion
4. Materials and Methods
4.1. Study Participants and Data Collection
4.2. RNA Extraction
4.3. miRNA Sequencing (Library Preparation, Pre-Processing, and Data Analysis)
4.4. Validation of MicroRNA Using RT-qPCR
4.5. MicroRNA Target Prediction, Gene Ontology, and Pathway Analysis
4.6. Measurement Using Adipokines Levels in ELISA
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef]
- National Cancer Registry Department, National Cancer Institute. Malaysian Study on Cancer Survival (MySCan); Ministry of Health Malaysia: Putrajaya, Malaysia, 2018. [Google Scholar]
- Brunelle, C.L.; Roberts, S.A.; Horick, N.K.; Gillespie, T.C.; Jacobs, J.M.; Daniell, K.M.; Naoum, G.E.; Taghian, A.G. Integrating Symptoms Into the Diagnostic Criteria for Breast Cancer–Related Lymphedema: Applying Results From a Prospective Surveillance Program. Phys. Ther. 2020, 100, 2186–2197. [Google Scholar] [CrossRef]
- Zou, L.; Liu, F.H.; Shen, P.P.; Hu, Y.; Liu, X.Q.; Xu, Y.Y.; Pen, Q.L.; Wang, B.; Zhu, Y.Q.; Tian, Y. The incidence and risk factors of related lymphedema for breast cancer survivors post-operation: A 2-year follow-up prospective cohort study. Breast Cancer 2018, 25, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Byun, H.K.; Chang, J.S.; Im, S.H.; Kirova, Y.M.; Arsene-Henry, A.; Choi, S.H.; Cho, Y.U.; Park, H.S.; Kim, J.Y.; Suh, C.-O.; et al. Risk of Lymphedema Following Contemporary Treatment for Breast Cancer: An Analysis of 7617 Consecutive Patients From a Multidisciplinary Perspective. Ann. Surg. 2021, 274, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Jaimez, P.; Armora Verdú, M.; Forero, C.G.; Álvarez Salazar, S.; Fuster Linares, P.; Monforte-Royo, C.; Masia, J. Breast cancer-related lymphoedema: Risk factors and prediction model. J. Adv. Nurs. 2022, 78, 765–775. [Google Scholar] [CrossRef]
- Navickas, R.; Gal, D.; Laucevičius, A.; Taparauskaitė, A.; Zdanytė, M.; Holvoet, P. Identifying circulating microRNAs as biomarkers of cardiovascular disease: A systematic review. Cardiovasc. Res. 2016, 111, 322–337. [Google Scholar] [CrossRef]
- Swarbrick, S.; Wragg, N.; Ghosh, S.; Stolzing, A. Systematic Review of miRNA as Biomarkers in Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 6156–6167. [Google Scholar] [CrossRef]
- Wiedrick, J.T.; Phillips, J.I.; Lusardi, T.A.; McFarland, T.J.; Lind, B.; Sandau, U.S.; Harrington, C.A.; Lapidus, J.A.; Galasko, D.R.; Quinn, J.F.; et al. Validation of MicroRNA Biomarkers for Alzheimer’s Disease in Human Cerebrospinal Fluid. J. Alzheimers Dis. 2019, 67, 875–891. [Google Scholar] [CrossRef] [PubMed]
- Filipów, S.; Łaczmański, Ł. Blood Circulating miRNAs as Cancer Biomarkers for Diagnosis and Surgical Treatment Response. Front. Genet. 2019, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- Ghantous, C.M.; Kamareddine, L.; Farhat, R.; Zouein, F.A.; Mondello, S.; Kobeissy, F.; Zeidan, A. Advances in Cardiovascular Biomarker Discovery. Biomedicines 2020, 8, 552. [Google Scholar] [CrossRef] [PubMed]
- Siasos, G.; Bletsa, E.; Stampouloglou, P.K.; Oikonomou, E.; Tsigkou, V.; Paschou, S.A.; Vlasis, K.; Marinos, G.; Vavuranakis, M.; Stefanadis, C.; et al. MicroRNAs in cardiovascular disease. Hell. J. Cardiol. 2020, 61, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Yusof, K.M.; Groen, K.; Rosli, R.; Avery-Kiejda, K.A. Crosstalk Between microRNAs and the Pathological Features of Secondary Lymphedema. Front. Cell Dev. Biol. 2021, 9, 732415. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, S. MicroRNAs in fibrosis: Opportunities and challenges. Arthritis Res. Ther. 2016, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Yusof, K.M.; Avery-Kiejda, K.A.; Ahmad Suhaimi, S.; Ahmad Zamri, N.; Rusli, M.E.F.; Mahmud, R.; Saini, S.M.; Abdul Wahhab Ibraheem, S.; Abdullah, M.; Rosli, R. Assessment of Potential Risk Factors and Skin Ultrasound Presentation Associated with Breast Cancer-Related Lymphedema in Long-Term Breast Cancer Survivors. Diagnostics 2021, 11, 1303. [Google Scholar] [CrossRef] [PubMed]
- Doruk Analan, P.; Kaya, E. Is There a Relationship Between Insulin Resistance and Breast Cancer-Related Lymphedema? A Preliminary Study. Lymphat. Res. Biol. 2022, 20, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Kim, J.; Lee, M.-J.; Roche, L.; Yang, N.L.; Tsao, P.S.; Rockson, S.G. Prospective Transcriptomic Pathway Analysis of Human Lymphatic Vascular Insufficiency: Identification and Validation of a Circulating Biomarker Panel. PLoS ONE 2012, 7, e52021. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.B. The complex role of adipokines in obesity, inflammation, and autoimmunity. Clin. Sci. 2021, 135, 731–752. [Google Scholar] [CrossRef]
- Sitar-Taut, A.-V.; Coste, S.C.; Tarmure, S.; Orasan, O.H.; Fodor, A.; Negrean, V.; Pop, D.; Zdrenghea, D.; Login, C.; Tiperciuc, B.; et al. Diabetes and Obesity-Cumulative or Complementary Effects On Adipokines, Inflammation, and Insulin Resistance. J. Clin. Med. 2020, 9, 2767. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Ramírez, B.; Becerril, S.; Salvador, J.; Colina, I.; Gómez-Ambrosi, J. Adiponectin-leptin Ratio is a Functional Biomarker of Adipose Tissue Inflammation. Nutrients 2019, 11, 454. [Google Scholar] [CrossRef] [PubMed]
- Coriddi, M.; Dayan, J.; Sobti, N.; Nash, D.; Goldberg, J.; Klassen, A.; Pusic, A.; Mehrara, B. Systematic Review of Patient-Reported Outcomes following Surgical Treatment of Lymphedema. Cancers 2020, 12, 565. [Google Scholar] [CrossRef] [PubMed]
- Azhar, S.H.; Lim, H.Y.; Tan, B.-K.; Angeli, V. The Unresolved Pathophysiology of Lymphedema. Front. Physiol. 2020, 11, 137. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, M.; Lopez, G.; Michelotti, A.; Venetis, K.; Sajjadi, E.; De Mattos-Arruda, L.; Ghidini, M.; Runza, L.; de Sire, A.; Boldorini, R.; et al. Integrating Biological Advances into the Clinical Management of Breast Cancer Related Lymphedema. Front. Oncol. 2020, 10, 422. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.; Levenhagen, K.; Ryans, K.; Perdomo, M.; Gilchrist, L. Interventions for Breast Cancer-Related Lymphedema: Clinical Practice Guideline From the Academy of Oncologic Physical Therapy of APTA. Phys. Ther. 2020, 100, 1163–1179. [Google Scholar] [CrossRef] [PubMed]
- Longhurst, E.; Dylke, E.S.; Kilbreath, S.L. Use of compression garments by women with lymphoedema secondary to breast cancer treatment. Support Care Cancer 2018, 26, 2625–2632. [Google Scholar] [CrossRef] [PubMed]
- Koc, M.; Wald, M.; Varaliová, Z.; Ondrůjová, B.; Čížková, T.; Brychta, M.; Kračmerová, J.; Beranová, L.; Pala, J.; Šrámková, V.; et al. Lymphedema alters lipolytic, lipogenic, immune and angiogenic properties of adipose tissue: A hypothesis-generating study in breast cancer survivors. Sci. Rep. 2021, 11, 8171. [Google Scholar] [CrossRef]
- Åkerman, L.; Casas, R.; Ludvigsson, J.; Tavira, B.; Skoglund, C. Serum miRNA levels are related to glucose homeostasis and islet autoantibodies in children with high risk for type 1 diabetes. PLoS ONE 2018, 13, e0191067. [Google Scholar] [CrossRef]
- Wang, R.; Hong, J.; Cao, Y.; Shi, J.; Gu, W.; Ning, G.; Zhang, Y.; Wang, W. Elevated circulating microRNA-122 is associated with obesity and insulin resistance in young adults. Eur. J. Endocrinol. 2015, 172, 291–300. [Google Scholar] [CrossRef]
- Kim, H.; Bae, Y.U.; Lee, H.; Kim, H.; Jeon, J.S.; Noh, H.; Han, D.C.; Byun, D.W.; Kim, S.H.; Park, H.K.; et al. Effect of diabetes on exosomal miRNA profile in patients with obesity. BMJ Open Diabetes Res. Care 2020, 8, e001403. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Tang, Q. Reduced expression of microRNA-199a-3p is associated with vascular endothelial cell injury induced by type 2 diabetes mellitus. Exp. Ther. Med. 2018, 16, 3639–3645. [Google Scholar] [CrossRef]
- Ahmed, U.; Ashfaq, U.A.; Qasim, M.; Ahmad, I.; Ahmad, H.U.; Tariq, M.; Masoud, M.S.; Khaliq, S. Dysregulation of circulating miRNAs promotes the pathogenesis of diabetes-induced cardiomyopathy. PLoS ONE 2021, 16, e0250773. [Google Scholar] [CrossRef]
- Kataru, R.P.; Wiser, I.; Baik, J.E.; Park, H.J.; Rehal, S.; Shin, J.Y.; Mehrara, B.J. Fibrosis and secondary lymphedema: Chicken or egg? Transl. Res. 2019, 209, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Hirakawa, S.; Suzuki, M.; Sakabe, J.-i.; Ogawa, M.; Yamamoto, S.; Hiraide, T.; Sasaki, T.; Yamamoto, N.; Inuzuka, K.; et al. Potential role of transforming growth factor-beta 1/Smad signaling in secondary lymphedema after cancer surgery. Cancer Sci. 2020, 111, 2620–2634. [Google Scholar] [CrossRef] [PubMed]
- Zampell, J.C.; Elhadad, S.; Avraham, T.; Weitman, E.; Aschen, S.; Yan, A.; Mehrara, B.J. Toll-like receptor deficiency worsens inflammation and lymphedema after lymphatic injury. Am. J. Physiol. Cell Physiol. 2012, 302, C709–C719. [Google Scholar] [CrossRef]
- Yang, R.; Xu, X.; Li, H.; Chen, J.; Xiang, X.; Dong, Z.; Zhang, D. p53 induces miR199a-3p to suppress SOCS7 for STAT3 activation and renal fibrosis in UUO. Sci. Rep. 2017, 7, 43409. [Google Scholar] [CrossRef]
- Ghadge, A.A.; Khaire, A.A. Leptin as a predictive marker for metabolic syndrome. Cytokine 2019, 121, 154735. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-a.; Montagnani, M.; Koh, K.K.; Quon, M.J. Reciprocal Relationships Between Insulin Resistance and Endothelial Dysfunction. Circulation 2006, 113, 1888–1904. [Google Scholar] [CrossRef]
- Koh, K.K.; Park, S.M.; Quon, M.J. Leptin and cardiovascular disease: Response to therapeutic interventions. Circulation 2008, 117, 3238–3249. [Google Scholar] [CrossRef] [PubMed]
- Scallan, J.P.; Hill, M.A.; Davis, M.J. Lymphatic vascular integrity is disrupted in type 2 diabetes due to impaired nitric oxide signalling. Cardiovasc. Res. 2015, 107, 89–97. [Google Scholar] [CrossRef]
- Poetsch, M.S.; Strano, A.; Guan, K. Role of Leptin in Cardiovascular Diseases. Front. Endocrinol. 2020, 11, 354. [Google Scholar] [CrossRef]
- Sato, A.; Kamekura, R.; Kawata, K.; Kawada, M.; Jitsukawa, S.; Yamashita, K.; Sato, N.; Himi, T.; Ichimiya, S. Novel Mechanisms of Compromised Lymphatic Endothelial Cell Homeostasis in Obesity: The Role of Leptin in Lymphatic Endothelial Cell Tube Formation and Proliferation. PLoS ONE 2016, 11, e0158408. [Google Scholar] [CrossRef]
- Norden, P.R.; Kume, T. The Role of Lymphatic Vascular Function in Metabolic Disorders. Front. Physiol. 2020, 11, 404. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Zawieja, S.; Wang, W.; Zawieja, D.C.; Muthuchamy, M. Lymphatic system: A vital link between metabolic syndrome and inflammation. Ann. N. Y. Acad. Sci. 2010, 1207 (Suppl. 1), E94–E102. [Google Scholar] [CrossRef] [PubMed]
- Cuzzone, D.A.; Weitman, E.S.; Albano, N.J.; Ghanta, S.; Savetsky, I.L.; Gardenier, J.C.; Joseph, W.J.; Torrisi, J.S.; Bromberg, J.F.; Olszewski, W.L.; et al. IL-6 regulates adipose deposition and homeostasis in lymphedema. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1426–H1434. [Google Scholar] [CrossRef] [PubMed]
- Djap, H.S.; Sutrisna, B.; Soewondo, P.; Djuwita, R.; Timotius, K.H.; Trihono; Sharif, S.; Tjang, Y.S. Waist to height ratio (0.5) as a predictor for prediabetes and type 2 diabetes in Indonesia. IOP Conf. Ser. Mater. Sci. Eng. 2018, 434, 012311. [Google Scholar] [CrossRef]
- Bohr, A.D.; Laurson, K.; McQueen, M.B. A novel cutoff for the waist-to-height ratio predicting metabolic syndrome in young American adults. BMC Public Health 2016, 16, 295. [Google Scholar] [CrossRef]
- Rodea-Montero, E.R.; Evia-Viscarra, M.L.; Apolinar-Jiménez, E. Waist-to-Height Ratio Is a Better Anthropometric Index than Waist Circumference and BMI in Predicting Metabolic Syndrome among Obese Mexican Adolescents. Int. J. Endocrinol. 2014, 2014, 195407. [Google Scholar] [CrossRef]
- Md Yusof, K.; Mahmud, R.; Abdullah, M.; Avery-Kiejda, K.A.; Rosli, R. Cross-Cultural Adaptation of the Functional Assessment of Cancer Therapy-Breast (FACT-B) in Malaysian Breast Cancer Survivors. Asian Pac. J. Cancer Prev. 2021, 22, 1055–1061. [Google Scholar] [CrossRef]
- Fusaro, V.A.; Patil, P.; Gafni, E.; Wall, D.P.; Tonellato, P.J. Biomedical Cloud Computing With Amazon Web Services. PLOS Comput. Biol. 2011, 7, e1002147. [Google Scholar] [CrossRef]
- Kozomara, A.; Griffiths-Jones, S. miRBase: Annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014, 42, D68–D73. [Google Scholar] [CrossRef]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- McDermott, A.M.; Kerin, M.J.; Miller, N. Identification and Validation of miRNAs as Endogenous Controls for RQ-PCR in Blood Specimens for Breast Cancer Studies. PLoS ONE 2014, 8, e83718. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X. miRDB: An online database for prediction of functional microRNA targets. Nucleic Acids Res. 2019, 48, D127–D131. [Google Scholar] [CrossRef]
- Tokar, T.; Pastrello, C.; Rossos, A.E.M.; Abovsky, M.; Hauschild, A.C.; Tsay, M.; Lu, R.; Jurisica, I. mirDIP 4.1-integrative database of human microRNA target predictions. Nucleic Acids Res. 2018, 46, D360–D370. [Google Scholar] [CrossRef]
- Xie, Z.; Bailey, A.; Kuleshov, M.V.; Clarke, D.J.B.; Evangelista, J.E.; Jenkins, S.L.; Lachmann, A.; Wojciechowicz, M.L.; Kropiwnicki, E.; Jagodnik, K.M.; et al. Gene Set Knowledge Discovery with Enrichr. Curr. Protoc. 2021, 1, e90. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef]
- McDuff, S.G.R.; Mina, A.I.; Brunelle, C.L.; Salama, L.; Warren, L.E.G.; Abouegylah, M.; Swaroop, M.; Skolny, M.N.; Asdourian, M.; Gillespie, T.; et al. Timing of Lymphedema after Treatment for Breast Cancer: When Are Patients Most at Risk? Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 62–70. [Google Scholar] [CrossRef]
- Sanders, K.A.; Benton, M.C.; Lea, R.A.; Maltby, V.E.; Agland, S.; Griffin, N.; Scott, R.J.; Tajouri, L.; Lechner-Scott, J. Next-generation sequencing reveals broad down-regulation of microRNAs in secondary progressive multiple sclerosis CD4+ T cells. Clin. Epigenet. 2016, 8, 87. [Google Scholar] [CrossRef]
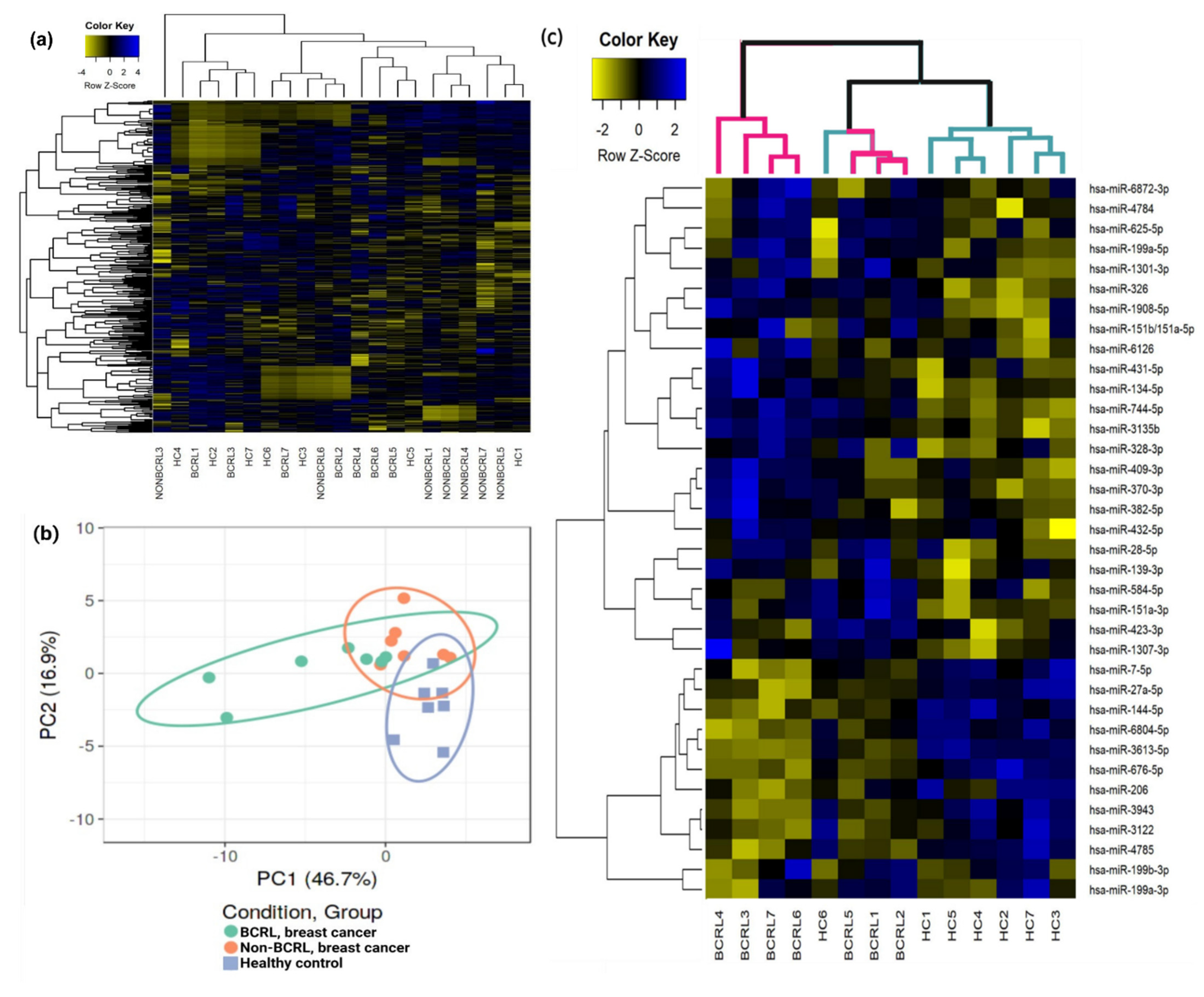

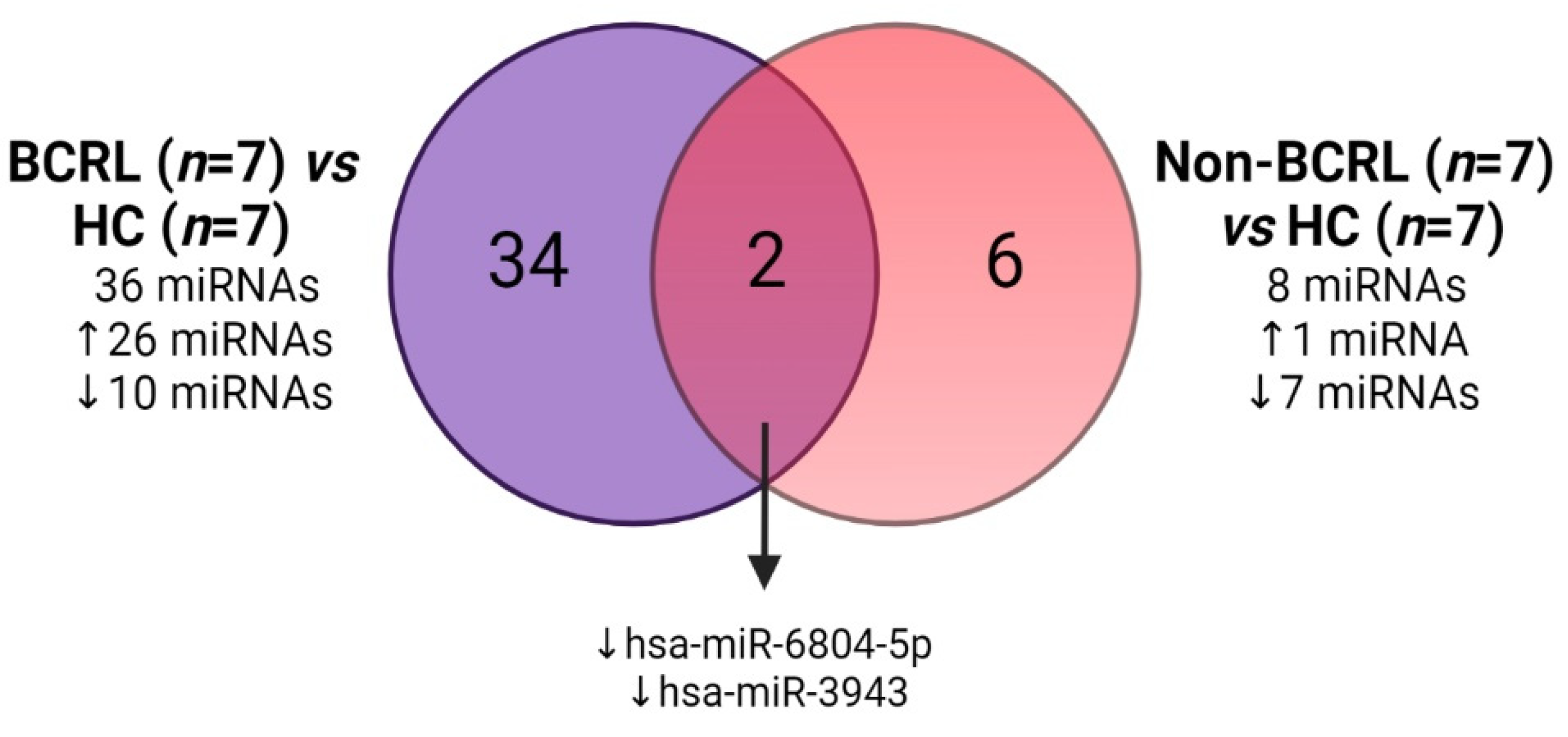


| Characteristics | BCRL (n = 23) | Non-BCRL (n = 90) | OR [95% C.I.] | p-Value |
|---|---|---|---|---|
| Age, years a | 52.43 ± 7.70 | 51.61 ± 8.51 | 0.674 | |
| Years after diagnosis b | 4.00 (4.00) | 4.00 (5.00) | 0.528 | |
| BMI, kg/m2 b | 29.30 (8.97) | 26.71 (7.64) | 0.073 | |
| Fat percentage, % b | 36.30 (9.50) | 37.40 (7.30) | 0.404 | |
| Waist circumference, cm b | 98.50 (18.00) | 89.60 (15.95) | 0.049 * | |
| Waist-to-hip ratio a | 0.91 ± 0.07 | 0.88 ± 0.06 | 0.076 | |
| Waist-to-height ratio b | 0.62 (0.15) | 0.57 (0.10) | 0.049 * | |
| Systolic blood pressure, mm/Hg b, n = 110 | 132.50 (28.25) | 125.50 (22.75) | 0.167 | |
| Diastolic blood pressure, mm/Hg b, n = 110 | 79.00 (18.75) | 79.00 (13.75) | 0.616 | |
| Heart rate, bpm b | 81.50 (20.75) | 80.50 (15.50) | 0.473 | |
| Arm disability scores (DASH) b | 40.00 (28.33) | 17.37 (21.67) | <0.001 ϕ | |
| QoL scores (FACT-B) a | 107.76 ± 20.50 | 114.59 ± 17.00 | 0.103 | |
| Co-morbidities | ||||
| Hypertension c | 10 (43.47%) | 15 (16.67%) | 3.85 [1.42–10.39] | 0.006 † |
| Diabetes mellitus c | 7 (30.43 %) | 10 (11.11%) | 3.50 [1.16–10.56] | 0.021 * |
| miRNA | Average Read Counts | p-Value | FDR-adj p-Value | Log2 FC | FC | |
|---|---|---|---|---|---|---|
| BCRL | HC | |||||
| hsa-miR-3613-5p | 113.182 | 369.874 | 3.83 × 10−8 | 1.54 × 10−5 | −1.708 | 0.306 |
| hsa-miR-144-5p | 57.294 | 173.178 | 6.96 × 10−5 | 4.66 × 10−3 | −1.596 | 0.331 |
| hsa-miR-206 | 9.864 | 29.701 | 3.32 × 10−3 | 3.81 × 10−2 | −1.590 | 0.332 |
| hsa-miR-676-5p | 3.988 | 10.401 | 6.60 × 10−4 | 1.56 × 10−2 | −1.383 | 0.383 |
| hsa-miR-6804-5p | 11.032 | 28.050 | 2.66 × 10−4 | 1.07 × 10−2 | −1.346 | 0.393 |
| hsa-miR-3122 | 8.868 | 21.873 | 5.50 × 10−3 | 4.91 × 10−2 | −1.302 | 0.405 |
| hsa-miR-3943 | 8.738 | 21.506 | 1.39 × 10−4 | 7.05 × 10−3 | −1.299 | 0.406 |
| hsa-miR-4785 | 4.925 | 10.897 | 4.45 × 10−3 | 4.59 × 10−2 | −1.146 | 0.452 |
| hsa-miR-7-5p | 64.671 | 137.452 | 8.82 × 10−4 | 1.77 × 10−2 | −1.088 | 0.471 |
| hsa-miR-27a-5p | 12.987 | 26.987 | 2.30 × 10−3 | 2.99 × 10−2 | −1.055 | 0.481 |
| hsa-miR-423-3p | 602.444 | 294.668 | 4.36 × 10−4 | 1.46 × 10−2 | 1.032 | 2.044 |
| hsa-miR-199b-3p | 402.345 | 194.458 | 2.18 × 10−3 | 2.92 × 10−2 | 1.049 | 2.069 |
| hsa-miR-584-5p | 899.623 | 424.343 | 6.30 × 10−4 | 1.56 × 10−2 | 1.084 | 2.120 |
| hsa-miR-1908-5p | 60.460 | 28.355 | 4.76 × 10−3 | 4.73 × 10−2 | 1.092 | 2.132 |
| hsa-miR-199a-3p | 2601.828 | 1168.249 | 5.40 × 10−3 | 4.91 × 10−2 | 1.155 | 2.227 |
| hsa-miR-139-3p | 49.942 | 22.281 | 1.99 × 10−3 | 2.86 × 10−2 | 1.164 | 2.241 |
| hsa-miR-625-5p | 76.687 | 33.298 | 9.70 × 10−4 | 1.86 × 10−2 | 1.204 | 2.303 |
| hsa-miR-28-5p | 27.377 | 11.869 | 3.22 × 10−3 | 3.81 × 10−2 | 1.206 | 2.307 |
| hsa-miR-1301-3p | 51.522 | 21.774 | 6.71 × 10−4 | 1.56 × 10−2 | 1.243 | 2.366 |
| hsa-miR-328-3p | 124.036 | 52.264 | 1.12 × 10−3 | 2.05 × 10−2 | 1.247 | 2.373 |
| hsa-miR-1307-3p | 309.259 | 129.059 | 1.46 × 10−4 | 7.05 × 10−3 | 1.261 | 2.396 |
| hsa-miR-151b/151a-5p | 217.733 | 89.353 | 6.80 × 10−4 | 1.56 × 10−2 | 1.285 | 2.437 |
| hsa-miR-326 | 50.869 | 20.848 | 1.98 × 10−3 | 2.86 × 10−2 | 1.287 | 2.440 |
| hsa-miR-744-5p | 248.133 | 101.532 | 4.87 × 10−4 | 1.50 × 10−2 | 1.289 | 2.444 |
| hsa-miR-6126 | 121.629 | 46.948 | 1.59 × 10−3 | 2.62 × 10−2 | 1.373 | 2.591 |
| hsa-miR-6872-3p | 45.793 | 17.625 | 5.05 × 10−3 | 4.83 × 10−2 | 1.377 | 2.598 |
| hsa-miR-151a-3p | 2350.841 | 847.293 | 2.59 × 10−7 | 5.20 × 10−5 | 1.472 | 2.775 |
| hsa-miR-370-3p | 109.725 | 37.192 | 1.79 × 10−3 | 2.76 × 10−2 | 1.561 | 2.950 |
| hsa-miR-382-5p | 324.453 | 102.653 | 2.75 × 10−3 | 3.45 × 10−2 | 1.660 | 3.161 |
| hsa-miR-199a-5p | 77.776 | 22.796 | 7.49 × 10−6 | 1.00 × 10−3 | 1.771 | 3.412 |
| hsa-miR-409-3p | 251.053 | 72.922 | 1.25 × 10−3 | 2.18 × 10−2 | 1.784 | 3.443 |
| hsa-miR-134-5p | 119.347 | 33.867 | 6.99 × 10−4 | 1.56 × 10−2 | 1.817 | 3.524 |
| hsa-miR-432-5p | 521.532 | 132.257 | 7.92 × 10−4 | 1.68 × 10−2 | 1.979 | 3.943 |
| hsa-miR-3135b | 234.283 | 59.139 | 3.64 × 10−5 | 3.00 × 10−3 | 1.986 | 3.962 |
| hsa-miR-4784 | 145.818 | 36.234 | 3.73 × 10−5 | 3.00 × 10−3 | 2.009 | 4.024 |
| hsa-miR-431-5p | 205.717 | 39.445 | 1.58 × 10−4 | 7.05 × 10−3 | 2.383 | 5.215 |
| miRNA | Average Read Counts | p-Value | FDR-adj p-Value | Log2 FC | FC | |
|---|---|---|---|---|---|---|
| Non-BCRL | HC | |||||
| hsa-miR-3147 | 8.299 | 24.182 | 3.46 × 10−5 | 9.63 × 10−3 | −1.543 | 0.343 |
| hsa-miR-92b-5p | 16.151 | 41.999 | 5.89 × 10−5 | 9.63 × 10−3 | −1.379 | 0.385 |
| hsa-miR-6722-3p | 14.501 | 33.790 | 3.42 × 10−4 | 2.79 × 10−2 | −1.220 | 0.429 |
| hsa-miR-3943 | 9.417 | 21.506 | 2.13 × 10−4 | 2.33 × 10−2 | −1.191 | 0.438 |
| hsa-miR-149-3p | 11.406 | 26.042 | 6.01 × 10−4 | 3.93 × 10−2 | −1.191 | 0.438 |
| hsa-miR-6804-5p | 12.564 | 28.050 | 1.11 × 10−3 | 4.52 × 10−2 | −1.159 | 0.448 |
| hsa-miR-604 | 10.165 | 21.617 | 9.02 × 10−4 | 4.35 × 10−2 | −1.089 | 0.470 |
| hsa-miR-941 | 76.161 | 27.029 | 9.31 × 10−4 | 4.35 × 10−2 | 1.495 | 2.818 |
| Pathway | No. of Overlapping Genes | Adjusted p-Value | Genes |
|---|---|---|---|
| PI3K-Akt signalling pathway | 24 | 1.37 × 10−5 | YWHAE;ITGA3;INSR;ITGA1;FN1;PTEN;PPP2R2A;TSC1;LPAR4;FGF2;THBS1;MTOR;GHR;FGF7;PPP2R5E;ERBB4;AKT3;DDIT4;CHAD;ITGA8;ITGB8;PKN2;ITGA6;TP53 |
| MAPK signalling pathway | 19 | 2.78 × 10−4 | MAP3K2;DUSP5;INSR;NLK;FGF2;RPS6KA3;RPS6KA6;FGF7;RPS6KA5;ERBB4;TAOK1;AKT3;GNA12;TAB2;TP53;LAMTOR3;CRK;MAP3K4;MAP3K5 |
| Regulation of actin cytoskeleton | 16 | 2.78 × 10−4 | ITGA3;ITGA1;FN1;LPAR4;FGF2;MYLK4;GNA13;FGF7;CFL2;GNA12;ITGA8;ITGB8;ITGA6;CRK;PFN2;PAK4 |
| Central carbon metabolism in cancer | 8 | 2.72 × 10−3 | PDHA1;AKT3;PTEN;TP53;HIF1A;HK2;MTOR;GLS |
| Sphingolipid signalling pathway | 10 | 3.70 × 10−3 | GNA13;PPP2R5E;PRKCE;AKT3;GNA12;PTEN;PPP2R2A;PLCB1;TP53;MAP3K5 |
| Focal adhesion | 13 | 4.19 × 10−3 | ITGA3;ITGA1;FN1;PTEN;THBS1;MYLK4;AKT3;CHAD;ITGA8;ITGB8;ITGA6;CRK;PAK4 |
| ECM−receptor interaction | 8 | 6.54 × 10−3 | ITGA3;ITGA1;CHAD;ITGA8;FN1;ITGB8;ITGA6;THBS1 |
| MicroRNAs in cancer | 16 | 6.54 × 10−3 | PRKCE;DNMT3A;PTEN;HMGA2;THBS1;MTOR;GLS;FOXP1;RPS6KA5;MMP16;DDIT4;TIMP3;ZFPM2;TP53;CRK;PAK4 |
| Dilated cardiomyopathy | 8 | 9.34 × 10−3 | ITGA3;ITGA1;ITGA8;ATP2A2;ITGB8;ITGA6;ADRB1;SLC8A1 |
| Phospholipase D signalling pathway | 10 | 9.34 × 10−3 | GRM3;GNA13;INSR;AKT3;GNA12;TSC1;LPAR4;PLCB1;MTOR;RAPGEF4 |
| mTOR signalling pathway | 10 | 1.00 × 10−2 | RPS6KA3;RPS6KA6;INSR;AKT3;DDIT4;PTEN;TSC1;LAMTOR3;LPIN2;MTOR |
| Insulin resistance | 8 | 1.38 × 10−2 | RPS6KA3;RPS6KA6;PRKCE;INSR;AKT3;PTEN;PTPRF;MTOR |
| cGMP-PKG signalling | 10 | 1.47 × 10−2 | GNA13;PRKCE;INSR;AKT3;GNA12;ATP2A2;ADRB1;PLCB1;SLC8A1;MYLK4 |
| Hyperthrophic cardiomyopathy | 7 | 1.73 × 10−2 | ITGA3;ITGA1;ITGA8;ATP2A2;ITGB8;ITGA6;SLC8A1 |
| TGF-beta signalling pathway | 7 | 1.84 × 10−2 | GREM1;SMAD2;TGIF2;ACVR1C;THBS1;ACVR2B;ACVR2A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusof, K.M.; Groen, K.; Rosli, R.; Abdullah, M.; Mahmud, R.; Avery-Kiejda, K.A. Evaluation of Circulating MicroRNAs and Adipokines in Breast Cancer Survivors with Arm Lymphedema. Int. J. Mol. Sci. 2022, 23, 11359. https://doi.org/10.3390/ijms231911359
Yusof KM, Groen K, Rosli R, Abdullah M, Mahmud R, Avery-Kiejda KA. Evaluation of Circulating MicroRNAs and Adipokines in Breast Cancer Survivors with Arm Lymphedema. International Journal of Molecular Sciences. 2022; 23(19):11359. https://doi.org/10.3390/ijms231911359
Chicago/Turabian StyleYusof, Khairunnisa’ Md, Kira Groen, Rozita Rosli, Maha Abdullah, Rozi Mahmud, and Kelly A. Avery-Kiejda. 2022. "Evaluation of Circulating MicroRNAs and Adipokines in Breast Cancer Survivors with Arm Lymphedema" International Journal of Molecular Sciences 23, no. 19: 11359. https://doi.org/10.3390/ijms231911359
APA StyleYusof, K. M., Groen, K., Rosli, R., Abdullah, M., Mahmud, R., & Avery-Kiejda, K. A. (2022). Evaluation of Circulating MicroRNAs and Adipokines in Breast Cancer Survivors with Arm Lymphedema. International Journal of Molecular Sciences, 23(19), 11359. https://doi.org/10.3390/ijms231911359






