Targeting Signaling Pathway Downstream of RIG-I/MAVS in the CNS Stimulates Production of Endogenous Type I IFN and Suppresses EAE
Abstract
1. Introduction
2. Results
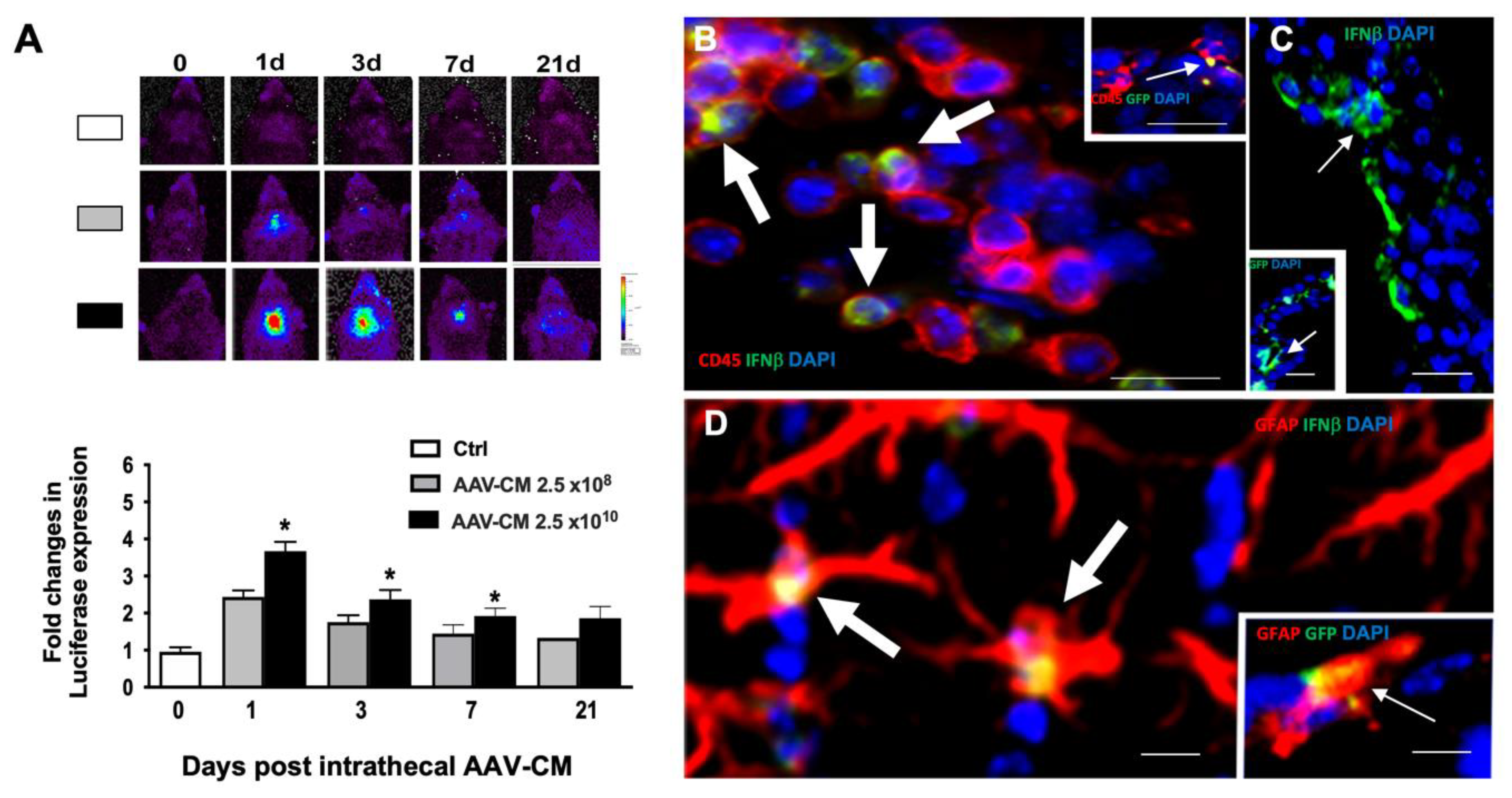
2.1. Intrathecal AAV-CM Induced IFN Beta Response in the CNS
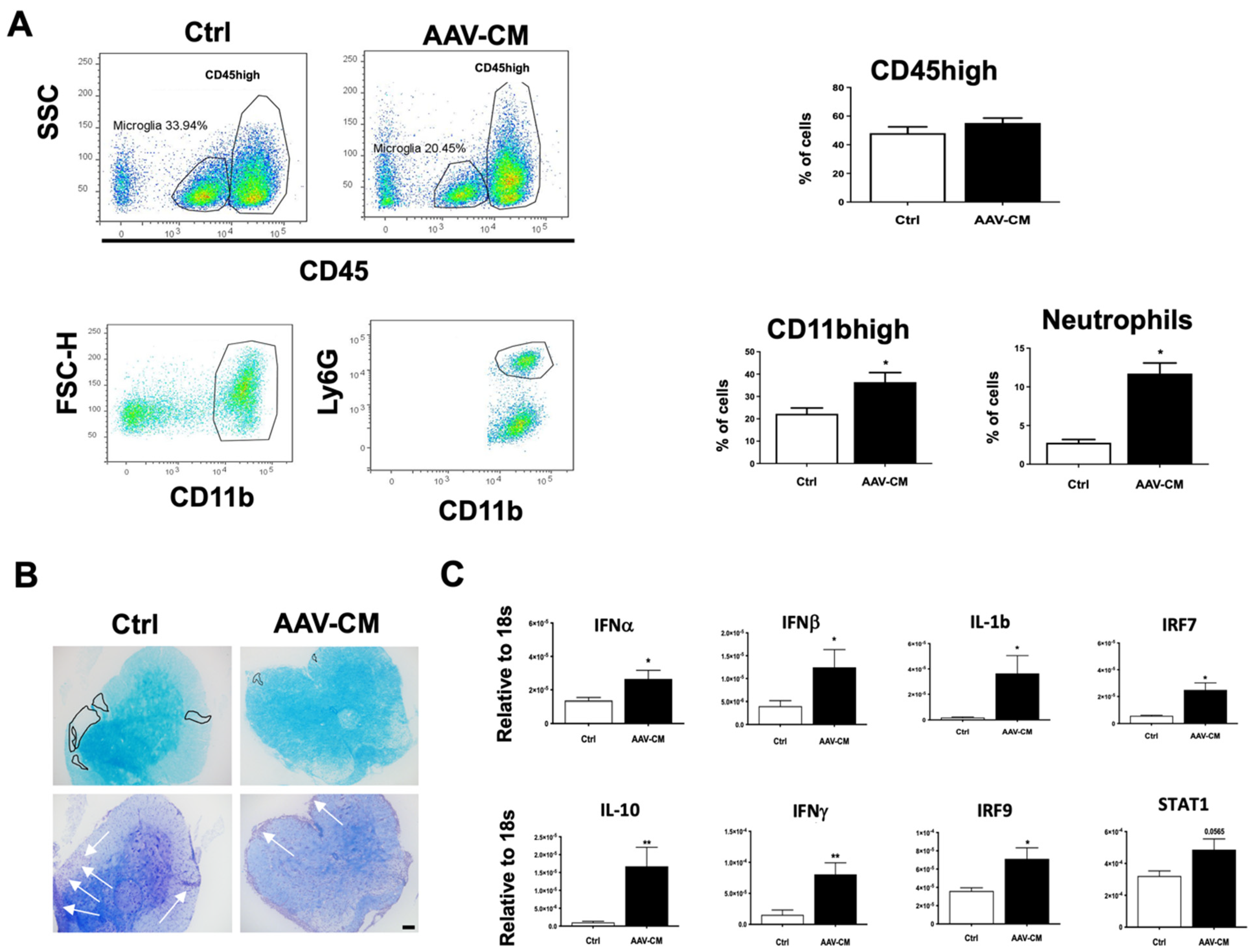
2.2. Intrathecal AAV-CM Treatment Enhanced CNS Recruitment of Myeloid Cells
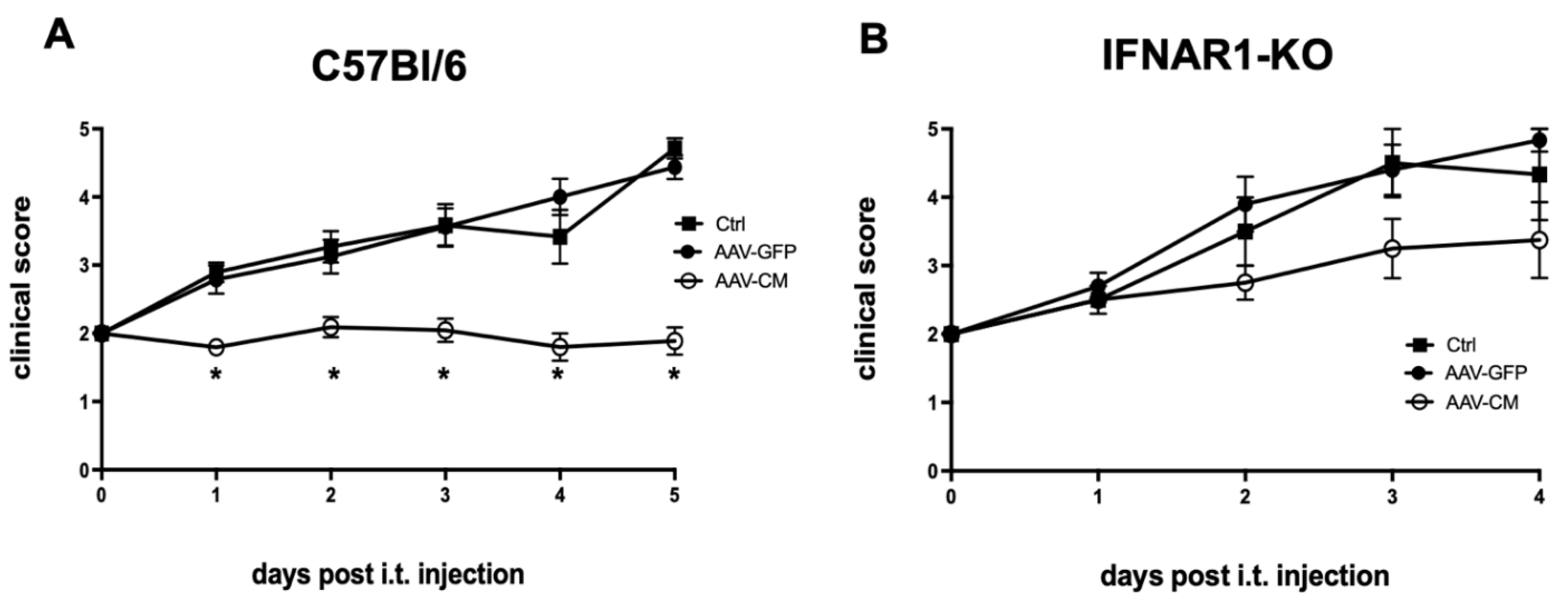
2.3. Intrathecal AAV-CM Suppressed EAE in an IFNAR-Dependent Manner
2.4. Intrathecal AAV-CM Altered Inflammatory Programs in the CNS of Mice with EAE
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. EAE Induction
4.3. Intrathecal Injection
4.4. In Vivo Imaging
4.5. Tissue Processing
4.6. Flow Cytometry
4.7. Histology
4.8. RNA Isolation and RT-qPCR
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Owens, T.; Khorooshi, R.; Wlodarczyk, A.; Asgari, N. Interferons in the Central Nervous System: A Few Instruments Play Many Tunes: Glial Interferons. Glia 2014, 62, 339–355. [Google Scholar] [CrossRef] [PubMed]
- Kigerl, K.A.; de Rivero Vaccari, J.P.; Dietrich, W.D.; Popovich, P.G.; Keane, R.W. Pattern Recognition Receptors and Central Nervous System Repair. Exp. Neurol. 2014, 258, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Owens, T.; Benmamar-Badel, A.; Wlodarczyk, A.; Marczynska, J.; Mørch, M.T.; Dubik, M.; Arengoth, D.S.; Asgari, N.; Webster, G.; Khorooshi, R. Protective Roles for Myeloid Cells in Neuroinflammation. Scand. J. Immunol. 2020, 92, e12963. [Google Scholar] [CrossRef] [PubMed]
- Khorooshi, R.; Marczynska, J.; Dieu, R.S.; Wais, V.; Hansen, C.R.; Kavan, S.; Thomassen, M.; Burton, M.; Kruse, T.; Webster, G.A.; et al. Innate Signaling within the Central Nervous System Recruits Protective Neutrophils. Acta Neuropathol. Commun. 2020, 8, 2. [Google Scholar] [CrossRef]
- Khorooshi, R.; Mørch, M.T.; Holm, T.H.; Berg, C.T.; Dieu, R.T.; Dræby, D.; Issazadeh-Navikas, S.; Weiss, S.; Lienenklaus, S.; Owens, T. Induction of Endogenous Type I Interferon within the Central Nervous System Plays a Protective Role in Experimental Autoimmune Encephalomyelitis. Acta Neuropathol. 2015, 130, 107–118. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Toll-like Receptor and RIG-1-like Receptor Signaling. Ann. N. Y. Acad. Sci. 2008, 1143, 1–20. [Google Scholar] [CrossRef]
- Wu, B.; Hur, S. How RIG-I like Receptors Activate MAVS. Curr. Opin. Virol. 2015, 12, 91–98. [Google Scholar] [CrossRef]
- Dann, A.; Poeck, H.; Croxford, A.L.; Gaupp, S.; Kierdorf, K.; Knust, M.; Pfeifer, D.; Maihoefer, C.; Endres, S.; Kalinke, U.; et al. Cytosolic RIG-I–like Helicases Act as Negative Regulators of Sterile Inflammation in the CNS. Nat. Neurosci. 2012, 15, 98–106. [Google Scholar] [CrossRef]
- Nistal-Villán, E.; Rodríguez-García, E.; Di Scala, M.; Ferrero-Laborda, R.; Olagüe, C.; Vales, Á.; Carte-Abad, B.; Crespo, I.; García-Sastre, A.; Prieto, J.; et al. A RIG-I 2CARD-MAVS200 Chimeric Protein Reconstitutes IFN-β Induction and Antiviral Response in Models Deficient in Type I IFN Response. J. Innate Immun. 2015, 7, 466–481. [Google Scholar] [CrossRef]
- Lienenklaus, S.; Cornitescu, M.; Ziętara, N.; Łyszkiewicz, M.; Gekara, N.; Jabłońska, J.; Edenhofer, F.; Rajewsky, K.; Bruder, D.; Hafner, M.; et al. Novel Reporter Mouse Reveals Constitutive and Inflammatory Expression of IFN-β In Vivo. J. Immunol. 2009, 183, 3229–3236. [Google Scholar] [CrossRef]
- Scheu, S.; Dresing, P.; Locksley, R.M. Visualization of IFNβ Production by Plasmacytoid versus Conventional Dendritic Cells under Specific Stimulation Conditions in Vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 20416–20421. [Google Scholar] [CrossRef] [PubMed]
- Sprokholt, J.K.; Kaptein, T.M.; van Hamme, J.L.; Overmars, R.J.; Gringhuis, S.I.; Geijtenbeek, T.B.H. RIG-I-like Receptor Activation by Dengue Virus Drives Follicular T Helper Cell Formation and Antibody Production. PLoS Pathog. 2017, 13, e1006738. [Google Scholar] [CrossRef] [PubMed]
- Dieu, R.S.; Wais, V.; Sørensen, M.Z.; Marczynska, J.; Dubik, M.; Kavan, S.; Thomassen, M.; Burton, M.; Kruse, T.; Khorooshi, R.; et al. Central Nervous System-Endogenous TLR7 and TLR9 Induce Different Immune Responses and Effects on Experimental Autoimmune Encephalomyelitis. Front. Neurosci. 2021, 15, 685645. [Google Scholar] [CrossRef]
- Aschauer, D.F.; Kreuz, S.; Rumpel, S. Analysis of Transduction Efficiency, Tropism and Axonal Transport of AAV Serotypes 1, 2, 5, 6, 8 and 9 in the Mouse Brain. PLoS ONE 2013, 8, e76310. [Google Scholar] [CrossRef] [PubMed]
- Pignataro, D.; Sucunza, D.; Vanrell, L.; Lopez-Franco, E.; Dopeso-Reyes, I.G.; Vales, A.; Hommel, M.; Rico, A.J.; Lanciego, J.L.; Gonzalez-Aseguinolaza, G. Adeno-Associated Viral Vectors Serotype 8 for Cell-Specific Delivery of Therapeutic Genes in the Central Nervous System. Front. Neuroanat. 2017, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Bettelli, E.; Das, M.P.; Howard, E.D.; Weiner, H.L.; Sobel, R.A.; Kuchroo, V.K. IL-10 Is Critical in the Regulation of Autoimmune Encephalomyelitis as Demonstrated by Studies of IL-10- and IL-4-Deficient and Transgenic Mice. J. Immunol. 1998, 161, 3299–3306. [Google Scholar] [PubMed]
- Melero-Jerez, C.; Suardíaz, M.; Lebrón-Galán, R.; Marín-Bañasco, C.; Oliver-Martos, B.; Machín-Díaz, I.; Fernández, Ó.; de Castro, F.; Clemente, D. The Presence and Suppressive Activity of Myeloid-Derived Suppressor Cells Are Potentiated after Interferon-β Treatment in a Murine Model of Multiple Sclerosis. Neurobiol. Dis. 2019, 127, 13–31. [Google Scholar] [CrossRef]
- Hunter, S.F.; Miller, D.J.; Rodriguez, M. Monoclonal Remyelination-Promoting Natural Autoantibody SCH 94.03: Pharmacokinetics and in Vivo Targets within Demyelinated Spinal Cord in a Mouse Model of Multiple Sclerosis. J. Neurol. Sci. 1997, 150, 103–113. [Google Scholar] [CrossRef]
- Thio, C.L.-P.; Lai, A.C.-Y.; Chi, P.-Y.; Webster, G.; Chang, Y.-J. Toll-like Receptor 9–Dependent Interferon Production Prevents Group 2 Innate Lymphoid Cell–Driven Airway Hyperreactivity. J. Allergy Clin. Immunol. 2019, 144, 682–697.e9. [Google Scholar] [CrossRef]
- Ottum, P.A.; Arellano, G.; Reyes, L.I.; Iruretagoyena, M.; Naves, R. Opposing Roles of Interferon-Gamma on Cells of the Central Nervous System in Autoimmune Neuroinflammation. Front. Immunol. 2015, 6, 539. [Google Scholar] [CrossRef]
- Iyer, S.S.; Cheng, G. Role of Interleukin 10 Transcriptional Regulation in Inflammation and Autoimmune Disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef]
- Peiseler, M.; Kubes, P. More Friend than Foe: The Emerging Role of Neutrophils in Tissue Repair. J. Clin. Investig. 2019, 129, 2629–2639. [Google Scholar] [CrossRef] [PubMed]
- Brownell, J.; Bruckner, J.; Wagoner, J.; Thomas, E.; Loo, Y.-M.; Gale, M.; Liang, T.J.; Polyak, S.J. Direct, Interferon-Independent Activation of the CXCL10 Promoter by NF-ΚB and Interferon Regulatory Factor 3 during Hepatitis C Virus Infection. J. Virol. 2014, 88, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Seth, R.B.; Sun, L.; Ea, C.-K.; Chen, Z.J. Identification and Characterization of MAVS, a Mitochondrial Antiviral Signaling Protein That Activates NF-ΚB and IRF3. Cell 2005, 122, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Hilliard, B.; Samoilova, E.B.; Liu, T.S.; Rostami, A.; Chen, Y. Experimental Autoimmune Encephalomyelitis in NF-Kappa B-Deficient Mice:Roles of NF-Kappa B in the Activation and Differentiation of Autoreactive T Cells. J. Immunol. 1999, 163, 2937–2943. [Google Scholar]
- Yue, Y.; Stone, S.; Lin, W. Role of Nuclear Factor ΚB in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis. Neural. Regen. Res. 2018, 13, 1507. [Google Scholar] [CrossRef]
- Narumi, S.; Kaburaki, T.; Yoneyama, H.; Iwamura, H.; Kobayashi, Y.; Matsushima, K. Neutralization of IFN-Inducible Protein 10/CXCL10 Exacerbates Experimental Autoimmune Encephalomyelitis. Eur. J. Immunol. 2002, 32, 1784–1791. [Google Scholar] [CrossRef]
- Klein, R.S. Regulation of Neuroinflammation: The Role of CXCL10 in Lymphocyte Infiltration during Autoimmune Encephalomyelitis. J. Cell. Biochem. 2004, 92, 213–222. [Google Scholar] [CrossRef]
- Paul, A.; Tang, T.H.; Ng, S.K. Interferon Regulatory Factor 9 Structure and Regulation. Front. Immunol. 2018, 9, 1831. [Google Scholar] [CrossRef]
- Dubik, M.; Marczynska, J.; Mørch, M.T.; Webster, G.; Jensen, K.N.; Wlodarczyk, A.; Khorooshi, R.; Owens, T. Innate Signaling in the CNS Prevents Demyelination in a Focal EAE Model. Front. Neurosci. 2021, 15, 682451. [Google Scholar] [CrossRef]
- Berg, C.T.; Khorooshi, R.; Asgari, N.; Owens, T. Influence of Type I IFN Signaling on Anti-MOG Antibody-Mediated Demyelination. J. Neuroinflamm. 2017, 14, 127. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kronborg Hansen, A.K.; Dubik, M.; Marczynska, J.; Ojha, B.; Nistal-Villán, E.; González Aseguinolaza, G.; Arengoth, D.S.; Owens, T.; Khorooshi, R. Targeting Signaling Pathway Downstream of RIG-I/MAVS in the CNS Stimulates Production of Endogenous Type I IFN and Suppresses EAE. Int. J. Mol. Sci. 2022, 23, 11292. https://doi.org/10.3390/ijms231911292
Kronborg Hansen AK, Dubik M, Marczynska J, Ojha B, Nistal-Villán E, González Aseguinolaza G, Arengoth DS, Owens T, Khorooshi R. Targeting Signaling Pathway Downstream of RIG-I/MAVS in the CNS Stimulates Production of Endogenous Type I IFN and Suppresses EAE. International Journal of Molecular Sciences. 2022; 23(19):11292. https://doi.org/10.3390/ijms231911292
Chicago/Turabian StyleKronborg Hansen, Anne K., Magdalena Dubik, Joanna Marczynska, Bhavya Ojha, Estanislao Nistal-Villán, Gloria González Aseguinolaza, Dina S. Arengoth, Trevor Owens, and Reza Khorooshi. 2022. "Targeting Signaling Pathway Downstream of RIG-I/MAVS in the CNS Stimulates Production of Endogenous Type I IFN and Suppresses EAE" International Journal of Molecular Sciences 23, no. 19: 11292. https://doi.org/10.3390/ijms231911292
APA StyleKronborg Hansen, A. K., Dubik, M., Marczynska, J., Ojha, B., Nistal-Villán, E., González Aseguinolaza, G., Arengoth, D. S., Owens, T., & Khorooshi, R. (2022). Targeting Signaling Pathway Downstream of RIG-I/MAVS in the CNS Stimulates Production of Endogenous Type I IFN and Suppresses EAE. International Journal of Molecular Sciences, 23(19), 11292. https://doi.org/10.3390/ijms231911292






