Role of Demyelination in the Persistence of Neurological and Mental Impairments after COVID-19
Abstract
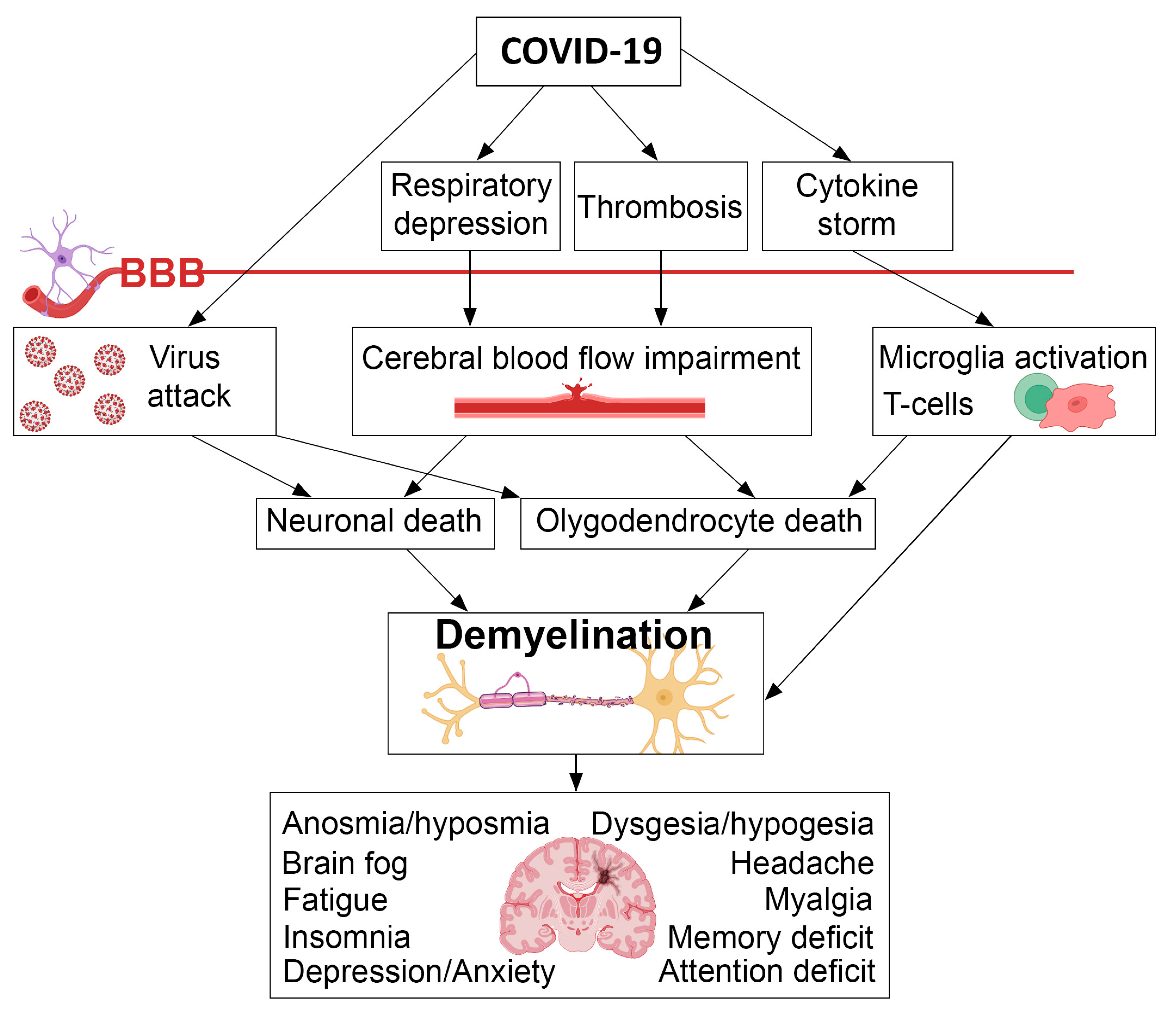
1. Introduction
2. Neurological and Mental Consequences of COVID-19
3. Predictors of Post-COVID Neurological Complications
4. Clinical Significance of Demyelination
4.1. Demyelinating Diseases and Mental Disorders
4.2. Demyelination and Aging
5. Possible Mechanisms of Demyelination Caused by COVID-19
5.1. Inflammation and Autoimmune Response
5.2. Direct Effect of the Virus on Oligodendrocytes
5.3. Cerebral Blood Flow Impairment
6. Demyelination in COVID and Post-COVID Patients
| MRI Technology | Time from Onset | Neurologic Symptoms/Diagnosis | Demyelination/ Sample Size | Demyelination-Related MRI Findings | Reference |
|---|---|---|---|---|---|
| T2-FLAIR, T1w, DTI (FA) | 3 months after COVID-19 | No specific neurological manifestations at the acute stage | 19 mild, 32 severe/82 | No obvious lesions on the conventional MRI, decreases in volume, length, and the mean FA in subcortical WM tracts in severe compared to mild patients, and in mild patients compared to controls | Qin et al. [5] |
| Conventional MRI, 3D T1w, 3D-pcASL, DTI (FA) | 3–10 months after COVID-19 | No specific neurological manifestations at the acute stage | 13 mild, 21 severe/34 | The trends in volume of subcortical nuclei and white matter tracts were different for 3–10 months period in patients with mild and severe COVID-19 | Tian et al. [57] |
| T1w, Gd-T1w FLAIR, DWI, ADC | Acute COVID-19 | Late awakening after withdrawal of sedation, acute neurologic symptoms | 5/73 (7%) | Multiple bilateral WM deep and periventricular, corpus callosum and basal ganglia lesions in patients with severe COVID-19 | Chougar et al. [151] |
| T1w, Gd-T1w T2/FLAIR | Acute COVID-19 | Acute neurologic symptoms during hospital stay | 1/20 (5%) | MS plaque exacerbation | Mahammedi et al. [152] |
| 7/20 (35%) | Nonspecific T2/FLAIR hyperintensity | ||||
| 3/20 (15%) | Subcortical white matter lesions | ||||
| T2w, DWI, FLAIR, SWI | Acute COVID-19 | Agitation, spatial disorientation, seizure/Subacute encephalopathy | 4/21 (19%) | Multifocal laminar cortical brain lesions detected by FLAIR hyperintensity | Anzalone et al. [154] |
| DWI, SWI, T2w, FLAIR, | Acute COVID-19 | Abnormal mental status/ Thromboembolism, microbleeds, arterial microvascular thrombosis | 4/* | Mild FLAIR hyperintensity along some cortical regions | Nicholson et al. [155] |
| T1W, Gd-T1w, DWI, gradient-echo T2, SWI, FLAIR | Acute COVID-19 | Alteration of consciousness, pathological wakefulness, confusion, agitation | 11/37 (30%) | Non-confluent multifocal WM hyperintense lesions on FLAIR with variable enhancement | Kremer et al. [156] |
| T2W, DWI, FLAIR | Acute COVID-19 | Diminished mental status/Diffuse leukoencephalopathy | 10/27 (37%) | Abnormal T2 hyperintensities bilateral deep and subcortical WM | Radmanesh et al. [157] |
| FLAIR, SWI, DWI, MRA | Acute COVID-19 | De novo acute neurologic symptoms/Encephalopathy (74%), acute necrotizing encephalopathy (7%), and vasculopathy (19%). | 6/27 (22%) | FLAIR hyperintensities in deep WM, the corpus callosum, and the basal ganglia | Scullen et al. [158] |
| SWI, DWI, Gd- DSC-PWI, ASL-PWI, T2w, Gd-T2w, FLAIR, Gd- FLAIR, T1w/TSE, Gd-T1w/TSE, T1w/GRE IR, Gd-T1w/GRE IR MRA | Acute COVID-19 and follow up | Acute neurologic symptoms during hospital stay/leukoencephalopathy, encephalopathy, hypoxic/metabolic changes, encephalitis | 23/41 (53%) | Confluent, symmetric, periventricular juxtacortical WM lesions, changes in cerebellar peduncles, corpus callosum, olfactory bulbs and tracts | Klironomos et al. [159] |
| FLAIR, SWI, DWI, MRA | Long COVID-19 | Smell and taste dysfunction, vertigo, headache, dizziness, fatigue | 16 mild, 23 moderate/39 (100%) | Hyperintense lesions on FLAIR, microhemorrhage on SWI | Marcic et al. [160] |
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Zanin, L.; Saraceno, G.; Panciani, P.P.; Renisi, G.; Signorini, L.; Migliorati, K.; Fontanella, M.M. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir. 2020, 162, 1491–1494. [Google Scholar] [CrossRef] [PubMed]
- Conklin, J.; Frosch, M.P.; Mukerji, S.S.; Rapalino, O.; Maher, M.D.; Schaefer, P.W.; Lev, M.H.; Gonzalez, R.G.; Das, S.; Champion, S.N.; et al. Susceptibility-weighted imaging reveals cerebral microvascular injury in severe COVID-19. J. Neurol. Sci. 2021, 421, 117308. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Patel, S.; Gentleman, S.; Matthews, P.M.; Floor, T.; Danes, B. Neurological Consequences of COVID-19 Infection. arXiv 2021, arXiv:2106.05388. [Google Scholar]
- Qin, Y.; Wu, J.; Chen, T.; Li, J.; Zhang, G.; Wu, D.; Zhou, Y.; Zheng, N.; Cai, A.; Ning, Q.; et al. Long-term microstructure and cerebral blood flow changes in patients recovered from COVID-19 without neurological manifestations. J. Clin. Investig. 2021, 131, e147329. [Google Scholar] [CrossRef]
- Najt, P.; Richards, H.L.; Fortune, D.G. Brain imaging in patients with COVID-19: A systematic review. Brain, Behav. Immun.-Health 2021, 16, 100290. [Google Scholar] [CrossRef]
- de Erausquin, G.A.; Snyder, H.; Carrillo, M.; Hosseini, A.A.; Brugha, T.S.; Seshadri, S. The chronic neuropsychiatric sequelae of COVID-19: The need for a prospective study of viral impact on brain functioning. Alzheimer’s Dement. 2021, 17, 1056–1065. [Google Scholar] [CrossRef]
- Richter, A.G.; Shields, A.M.; Karim, A.; Birch, D.; Faustini, S.E.; Steadman, L.; Ward, K.; Plant, T.; Reynolds, G.; Veenith, T.; et al. Establishing the prevalence of common tissue-specific autoantibodies following severe acute respiratory syndrome coronavirus 2 infection. Clin. Exp. Immunol. 2021, 205, 99–105. [Google Scholar] [CrossRef]
- Tremblay, M.E.; Madore, C.; Bordeleau, M.; Tian, L.; Verkhratsky, A. Neuropathobiology of COVID-19: The Role for Glia. Front. Cell. Neurosci. 2020, 14, 592214. [Google Scholar] [CrossRef]
- Moonis, G.; Filippi, C.G.; Kirsch, C.F.E.; Mohan, S.; Stein, E.G.; Hirsch, J.A.; Mahajan, A. The Spectrum of Neuroimaging Findings on CT and MRI in Adults with COVID-19. Am. J. Roentgenol. 2021, 217, 959–974. [Google Scholar] [CrossRef]
- Ladecola, C.; Anrather, J.; Kamel, H. Effects of COVID-19 on the Nervous System. Cell 2020, 183, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Mahalakshmi, A.M.; Ray, B.; Tuladhar, S.; Bhat, A.; Paneyala, S.; Patteswari, D.; Sakharkar, M.K.; Hamdan, H.; Ojcius, D.M.; Bolla, S.R.; et al. Does COVID-19 contribute to development of neurological disease? Immun. Inflamm. Dis. 2021, 9, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Shabani, Z. Demyelination as a result of an immune response in patients with COVID-19. Acta Neurol. Belg. 2021, 121, 859–866. [Google Scholar] [CrossRef]
- Bougakov, D.; Podell, K.; Goldberg, E. Multiple Neuroinvasive Pathways in COVID-19 The Current COVID-19 Pandemic. Mol. Neurobiol. 2021, 58, 564–575. [Google Scholar] [CrossRef]
- Crunfli, F.; Carregari, V.C.; Veras, F.P.; Vendramini, P.H.; Valença, G.F.; Saraiva, A.; Marcelo, L.; Brandão-teles, C.; Silva, G.; Reis-de-oliveira, G.; et al. SARS-CoV-2 infects brain astrocytes of COVID-19 patients and impairs neuronal viability. MedRxiv 2021. [Google Scholar] [CrossRef]
- Abdullah, F.; Myers, J.; Basu, D.; Tintinger, G.; Ueckermann, V.; Mathebula, M.; Ramlall, R.; Spoor, S.; de Villiers, T.; Van der Walt, Z.; et al. Decreased severity of disease during the first global omicron variant covid-19 outbreak in a large hospital in tshwane, south africa. Int. J. Infect. Dis. 2022, 116, 38–42. [Google Scholar] [CrossRef]
- Nyberg, T.; Ferguson, N.M.; Nash, S.G.; Webster, H.H.; Flaxman, S.; Andrews, N.; Hinsley, W.; Bernal, J.L.; Kall, M.; Bhatt, S.; et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: A cohort study. Lancet 2022, 399, 1303–1312. [Google Scholar] [CrossRef]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef]
- World Health Organization (WHO). A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus; World Health Organization (WHO), WHO Press: Geneva, Switzerland, 2021. [Google Scholar]
- Stefanou, M.I.; Palaiodimou, L.; Bakola, E.; Smyrnis, N.; Papadopoulou, M.; Paraskevas, G.P.; Rizos, E.; Boutati, E.; Grigoriadis, N.; Krogias, C.; et al. Neurological manifestations of long-COVID syndrome: A narrative review. Ther. Adv. Chronic Dis. 2022, 13, 20406223221076890. [Google Scholar] [CrossRef]
- Graham, E.L.; Clark, J.R.; Orban, Z.S.; Lim, P.H.; Szymanski, A.L.; Taylor, C.; DiBiase, R.M.; Jia, D.T.; Balabanov, R.; Ho, S.U.; et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized COVID-19 “long haulers”. Ann. Clin. Transl. Neurol. 2021, 8, 1073–1085. [Google Scholar] [CrossRef]
- Varatharaj, A.; Thomas, N.; Ellul, M.A.; Davies, N.W.S.; Pollak, T.A.; Tenorio, E.L.; Sultan, M.; Easton, A.; Breen, G.; Zandi, M.; et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: A UK-wide surveillance study. Lancet Psychiatry 2020, 7, 875–882. [Google Scholar] [CrossRef]
- Miskowiak, K.W.; Johnsen, S.; Sattler, S.M.; Nielsen, S.; Kunalan, K.; Rungby, J.; Lapperre, T.; Porsberg, C.M. Cognitive impairments four months after COVID-19 hospital discharge: Pattern, severity and association with illness variables. Eur. Neuropsychopharmacol. 2021, 46, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.J.; Sarangi, A.; Bangash, F. Neurological sequelae of COVID-19: A review. Egypt. J. Neurol. Psychiatry Neurosurg. 2021, 57, 122. [Google Scholar] [CrossRef] [PubMed]
- Efstathiou, V.; Stefanou, M.-I.; Demetriou, M.; Siafakas, N.; Makris, M.; Tsivgoulis, G.; Zoumpourlis, V.; Kympouropoulos, S.; Tsoporis, J.; Spandidos, D.; et al. Long COVID and neuropsychiatric manifestations . Exp. Ther. Med. 2022, 23, 363. [Google Scholar] [CrossRef] [PubMed]
- Daroische, R.; Hemminghyth, M.S.; Eilertsen, T.H.; Breitve, M.H.; Chwiszczuk, L.J. Cognitive Impairment After COVID-19—A Review on Objective Test Data. Front. Neurol. 2021, 12, 699582. [Google Scholar] [CrossRef]
- Woo, M.S.; Malsy, J.; Pöttgen, J.; Seddiq Zai, S.; Ufer, F.; Hadjilaou, A.; Schmiedel, S.; Addo, M.M.; Gerloff, C.; Heesen, C.; et al. Frequent neurocognitive deficits after recovery from mild COVID-19. Brain Commun. 2020, 2, fcaa205. [Google Scholar] [CrossRef]
- Bhola, S.; Trisal, J.; Thakur, V.; Kaur, P.; Kulshrestha, S.; Bhatia, S.K.; Kumar, P. Neurological toll of COVID-19. Neurol. Sci. 2022, 43, 2171–2186. [Google Scholar] [CrossRef]
- Magdy, R.; Hussein, M. Could SARS-CoV-2 Infection Be a Novel Risk Factor for Multiple Sclerosis? Neuroimmunomodulation 2022. [Google Scholar] [CrossRef]
- Fernández-Castañeda, A.; Lu, P.; Geraghty, A.C.; Song, E.; Lee, M.-H.; Wood, J.; O’Dea, M.R.; Dutton, S.; Shamardani, K.; Nwangwu, K.; et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell 2022, 185, 2452–2468. [Google Scholar] [CrossRef]
- Miskin, D. COVID-19-associated CNS Demyelinating Diseases Let us know how access to this document benefits you. Thomas Jefferson Univ. Dep. Neurosci. Fac. Pap. 2020, Paper 48. Available online: https://jdc.jefferson.edu/cgi/viewcontent.cgi?article=1048&context=department_neuroscience (accessed on 20 September 2022).
- Zoghi, A.; Ramezani, M.; Roozbeh, M.; Darazam, I.A.; Sahraian, M.A. A case of possible atypical demyelinating event of the central nervous system following COVID-19. Mult. Scler. Relat. Disord. 2020, 44, 102324. [Google Scholar] [CrossRef] [PubMed]
- Karapanayiotides, T.; Geka, E.; Prassopoulos, P.; Koutroulou, I.; Kollaras, P.; Kiourtzieva, E.; Pourzitaki, C.; Veroniki, F.; Sintila, S.A.; Astreinidis, A.; et al. Concentric demyelination pattern in COVID-19-associated acute haemorrhagic leukoencephalitis: A lurking catastrophe? Brain 2021, 143, e100. [Google Scholar] [CrossRef]
- Khandelwal, K.; Puranik, M.; Gupta, V.; Khandelwal, G.; Dave, P.K.; Hirve, M. COVID-19 associated acute demyelination masquerading as stroke: A case report. Egypt. J. Radiol. Nucl. Med. 2021, 52, 32. [Google Scholar] [CrossRef]
- Lima, M.; Aloizou, A.M.; Siokas, V.; Bakirtzis, C.; Liampas, I.; Tsouris, Z.; Bogdanos, D.P.; Baloyannis, S.J.; Dardiotis, E. Coronaviruses and their relationship with multiple sclerosis: Is the prevalence of multiple sclerosis going to increase after the Covid-19 pandemia? Rev. Neurosci. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ismail, I.I.; Salama, S. Association of CNS Demyelination and COVID-19 Infection: An Updated Systematic Review; Springer: Berlin/Heidelberg, Germany, 2022; Volume 269, ISBN 0123456789. [Google Scholar]
- Bracaglia, M.; Naldi, I.; Govoni, A.; Brillanti Ventura, D.; De Massis, P. Acute inflammatory demyelinating polyneuritis in association with an asymptomatic infection by SARS-CoV-2. J. Neurol. 2020, 267, 3166–3168. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, F.; Shen, Y.; Zhang, X.; Cen, Y.; Wang, B.; Zhao, S.; Zhou, Y.; Hu, B.; Wang, M.; et al. Symptoms and Health Outcomes among Survivors of COVID-19 Infection 1 Year after Discharge from Hospitals in Wuhan, China. JAMA Netw. Open 2021, 4, e2127403. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Alonso-Lana, S.; Marquié, M.; Ruiz, A.; Boada, M. Cognitive and Neuropsychiatric Manifestations of COVID-19 and Effects on Elderly Individuals With Dementia. Front. Aging Neurosci. 2020, 12, 588872. [Google Scholar] [CrossRef]
- Sharun, K.; Dhama, K.; Pawde, A.M.; Gortázar, C.; Tiwari, R.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J.; de la Fuente, J.; Michalak, I.; Attia, Y.A. SARS-CoV-2 in animals: Potential for unknown reservoir hosts and public health implications. Vet. Q. 2021, 41, 181–201. [Google Scholar] [CrossRef]
- Pilotto, A.; Cristillo, V.; Cotti Piccinelli, S.; Zoppi, N.; Bonzi, G.; Sattin, D.; Schiavolin, S.; Raggi, A.; Canale, A.; Gipponi, S.; et al. Long-term neurological manifestations of COVID-19: Prevalence and predictive factors. Neurol. Sci. 2021, 42, 4903–4907. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef] [PubMed]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2020, 101, 93–135. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Zheng, B.; Daines, L. Long-Term Sequelae of COVID-19: A Systematic Review and Meta-Analysis of One-Year Follow-Up Studies on. Pathogens 2022, 11, 269. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, F.M.; Lam, K.; Sounderajah, V.; Clarke, J.M.; Ashrafian, H.; Darzi, A. Characteristics and predictors of acute and chronic post-COVID syndrome: A systematic review and meta-analysis. eClinicalMedicine 2021, 36, 100899. [Google Scholar] [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Bahmer, T.; Borzikowsky, C.; Lieb, W.; Horn, A.; Krist, L.; Fricke, J.; Scheibenbogen, C.; Rabe, K.F.; Maetzler, W.; Maetzler, C.; et al. Severity, predictors and clinical correlates of post-COVID syndrome (PCS) in Germany: A prospective, multi-centre, population-based cohort study. eClinicalMedicine 2022, 51, 101549. [Google Scholar] [CrossRef]
- Asadi-Pooya, A.A.; Akbari, A.; Emami, A.; Lotfi, M.; Rostamihosseinkhani, M.; Nemati, H.; Barzegar, Z.; Kabiri, M.; Zeraatpisheh, Z.; Farjoud-Kouhanjani, M.; et al. Risk factors associated with long covid syndrome: A retrospective study. Iran. J. Med. Sci. 2021, 46, 428–436. [Google Scholar] [CrossRef]
- Chan Sui Ko, A.; Candellier, A.; Mercier, M.; Joseph, C.; Schmit, J.L.; Lanoix, J.P.; Andrejak, C. Number of initial symptoms is more related to long COVID-19 than acute severity of infection: A prospective cohort of hospitalized patients. Int. J. Infect. Dis. 2022, 118, 220–223. [Google Scholar] [CrossRef]
- Tavares-Júnior, J.W.L.; de Souza, A.C.C.; Borges, J.W.P.; Oliveira, D.N.; Siqueira-Neto, J.I.; Sobreira-Neto, M.A.; Braga-Neto, P. COVID-19 associated cognitive impairment: A systematic review. Cortex 2022, 152, 77–97. [Google Scholar] [CrossRef]
- Light, S.N. The Combined Use of Neuropsychiatric and Neuropsychological Assessment Tools to Make a Differential Dementia Diagnosis in the Presence of “long-Haul” COVID-19. Case Rep. Neurol. 2022, 14, 130–148. [Google Scholar] [CrossRef]
- Rolland, Y.; Baziard, M. Coronavirus Disease-2019 in Older People with Cognitive Impairment. Clin. Geriatr. Med. 2022, 38, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Hariyanto, T.I.; Putri, C.; Arisa, J.; Situmeang, R.F.V.; Kurniawan, A. Dementia and outcomes from coronavirus disease 2019 (COVID-19) pneumonia: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2021, 93, 104299. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.L.; Pilling, L.C.; Atkins, J.L.; Masoli, J.A.H.; Delgado, J.; Kuchel, G.A.; Melzer, D.; Newman, A.B. ApoE e4e4 Genotype and Mortality with COVID-19 in UK Biobank. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2020, 75, 1801–1803. [Google Scholar] [CrossRef] [PubMed]
- Pop-Vicas, A.; Osman, F.; Tsaras, G.; Seigworth, C.; Munoz-Price, L.S.; Safdar, N. Predictors of Persistent Symptoms after SARS-CoV-2 Infection among Healthcare Workers: Results of a Multi-site Survey. Infect. Control Hosp. Epidemiol. 2022, 2, 1–4. [Google Scholar] [CrossRef]
- Tian, T.; Wu, J.; Chen, T.; Li, J.; Yan, S.; Zhou, Y.; Peng, X.; Li, Y.; Zheng, N.; Cai, A.; et al. Long-term follow-up of dynamic brain changes in patients recovered from COVID-19 without neurological manifestations. JCI Insight 2022, 7, e155827. [Google Scholar] [CrossRef]
- Ogier, M.; Andéol, G.; Sagui, E.; Dal Bo, G. How to detect and track chronic neurologic sequelae of COVID-19? Use of auditory brainstem responses and neuroimaging for long-term patient follow-up. Brain Behav. Immun.-Health 2020, 5, 100081. [Google Scholar] [CrossRef]
- Papuc, E.; Rejdak, K. The role of myelin damage in Alzheimer’s disease pathology. Arch. Med. Sci. 2020, 16, 345–351. [Google Scholar] [CrossRef]
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015, 15, 545–558. [Google Scholar] [CrossRef]
- Lyon-Caen, O.; Jouvent, R.; Hauser, S.; Chaunu, M.P.; Benoit, N.; Widlöcher, D.; Lhermitte, F. Cognitive Function in Recent-Onset Demyelinating Diseases. Arch. Neurol. 1986, 43, 1138–1141. [Google Scholar] [CrossRef]
- Chiaravalloti, N.D.; DeLuca, J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008, 7, 1139–1151. [Google Scholar] [CrossRef]
- Campanholo, K.R.; Pitombeira, M.S.; Rimkus, C.M.; Mendes, M.F.; Apóstolos-Pereira, S.L.; Busatto Filho, G.; Callegaro, D.; Buchpiguel, C.A.; Duran, F.L.S.; De Paula Faria, D. Myelin imaging measures as predictors of cognitive impairment in MS patients: A hybrid PET-MRI study. Mult. Scler. Relat. Disord. 2022, 57, 103331. [Google Scholar] [CrossRef] [PubMed]
- Bouhrara, M.; Reiter, D.A.; Bergeron, C.M.; Zukley, L.M.; Ferrucci, L.; Resnick, S.M.; Spencer, R.G. Evidence of demyelination in mild cognitive impairment and dementia using a direct and specific magnetic resonance imaging measure of myelin content. Alzheimer’s Dement. 2018, 14, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Xu, G.; Wang, J.; Yin, N.; Meng, N. Clinical and MRI predictors of cognitive decline in patients with relapsing-remitting multiple sclerosis: A 2-year longitudinal study. Mult. Scler. Relat. Disord. 2022, 65, 103838. [Google Scholar] [CrossRef]
- van Schependom, J.; Nagels, G. Targeting cognitive impairment in multiple sclerosis-the road toward an imaging-based biomarker. Front. Neurosci. 2017, 11, 2012–2017. [Google Scholar] [CrossRef]
- Benedict, R.H.B.; Weinstock-Guttman, B.; Fishman, I.; Sharma, J.; Tjoa, C.W.; Bakshi, R. Prediction of Neuropsychological Impairment in Multiple Sclerosis. Arch. Neurol. 2004, 61, 226. [Google Scholar] [CrossRef]
- Hulst, H.E.; Steenwijk, M.D.; Versteeg, A.; Pouwels, P.J.W.; Vrenken, H.; Uitdehaag, B.M.J.; Polman, C.H.; Geurts, J.J.G.; Barkhof, F. Cognitive impairment in MS: Impact of white matter integrity, gray matter volume, and lesions. Neurology 2013, 80, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Rocca, M.A.; Amato, M.P.; De Stefano, N.; Enzinger, C.; Geurts, J.J.; Penner, I.K.; Rovira, A.; Sumowski, J.F.; Valsasina, P.; Filippi, M. Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol. 2015, 14, 302–317. [Google Scholar] [CrossRef]
- Bishop, N.A.; Lu, T.; Yankner, B.A. Neural mechanisms of ageing and cognitive decline. Nature 2010, 464, 529–535. [Google Scholar] [CrossRef]
- Ge, Y.; Grossman, R.I.; Babb, J.S.; Rabin, M.L.; Mannon, L.J.; Kolson, D.L. Age-related total gray matter and white matter changes in normal adult brain. Part II: Quantitative magnetization transfer ratio histogram analysis. Am. J. Neuroradiol. 2002, 23, 1334–1341. [Google Scholar]
- Lockhart, S.N. Structural Imaging Measures of Brain Aging. Neuropsychol. Rev. 2014, 24, 271–289. [Google Scholar] [CrossRef]
- DeCarli, C.; Massaro, J.; Harvey, D.; Hald, J.; Tullberg, M.; Au, R.; Beiser, A.; D’Agostino, R.; Wolf, P.A. Measures of brain morphology and infarction in the framingham heart study: Establishing what is normal. Neurobiol. Aging 2005, 26, 491–510. [Google Scholar] [CrossRef] [PubMed]
- Pakkenberg, B.; Gundersen, H.J.G. Neocortical Neuron Number in Humans: Effect of Sex and Age. J. Comp. Neurol. 1997, 384, 312–320. [Google Scholar] [CrossRef]
- Freeman, S.H.; Kandel, R.; Cruz, L.; Rozkalne, A.; Newell, K.; Frosch, M.P.; Hedley-whyte, E.T.; Joseph, J.; Lipsitz, L.; Hyman, B.T. Preservation of Neuronal Number Despite Age-Related Cortical Brain Atrophy in Elderly Subjects Without Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2008, 67, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Mark, J.; Paul, D.; Dorothy, G.; Juan, C. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer ’ s disease. Lancet 1994, 344, 769–772. [Google Scholar]
- Gunning-Dixon, F.M.; Brickman, A.M.; Cheng, J.C.; Alexopoulos, G.S. Aging of cerebral white matter: A review of MRI findings. Int. J. Geriatr. Psychiatry 2009, 24, 109–117. [Google Scholar] [CrossRef]
- Peters, A. The Effects of Normal Aging on Nerve Fibers and Neuroglia in the Central Nervous System. J. Neurocytol. 2002, 31, 581–593. [Google Scholar] [CrossRef]
- Park, D.C.; Polk, T.A.; Mikels, J.A.; Taylor, S.F.; Marshuetz, C. Cerebral aging: Integration of brain and behavioral models of cognitive function. Dialogues Clin. Neurosci. 2001, 3, 151–165. [Google Scholar] [CrossRef]
- Rivera, A.D.; Chacon-De-La-Rocha, I.; Pieropan, F.; Papanikolau, M.; Azim, K.; Butt, A.M. Keeping the ageing brain wired: A role for purine signalling in regulating cellular metabolism in oligodendrocyte progenitors. Pflügers Arch.-Eur. J. Physiol. 2021, 473, 775–783. [Google Scholar] [CrossRef]
- Rivera, A.D.; Butt, A.M.; Azim, K. Resolving the age-related decline in central nervous system myelin turnover and drug discovery for oligodendroglial rejuvenation. Neural Regen. Res. 2022, 17, 2677–2678. [Google Scholar] [CrossRef]
- Peters, A. The effects of normal aging on myelinated nerve fibers in monkey central nervous system. Front. Neuroanat. 2009, 3, 1–10. [Google Scholar] [CrossRef]
- O’Sullivan, M.; Jones, D.K.; Summers, P.E.; Morris, R.G.; Williams, S.C.R.; Markus, H.S. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology 2001, 57, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Dean, D.C.; Hurley, S.A.; Kecskemeti, S.R.; O’Grady, J.P.; Canda, C.; Davenport-Sis, N.J.; Carlsson, C.M.; Zetterberg, H.; Blennow, K.; Asthana, S.; et al. Association of amyloid pathology with myelin alteration in preclinical Alzheimer disease. JAMA Neurol. 2017, 74, 41–49. [Google Scholar] [CrossRef]
- Arshad, M.; Stanley, J.A.; Raz, N. Adult age differences in subcortical myelin content are consistent with protracted myelination and unrelated to diffusion tensor imaging indices. Neuroimage 2016, 143, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Denissen, S.; Engemann, D.A.; De Cock, A.; Costers, L.; Baijot, J.; Laton, J.; Penner, I.K.; Grothe, M.; Kirsch, M.; D’hooghe, M.B.; et al. Brain age as a surrogate marker for cognitive performance in multiple sclerosis. Eur. J. Neurol. 2022, 29, 3039–3049. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, T.; van der Meer, D.; Doan, N.T.; Schwarz, E.; Lund, M.J.; Agartz, I.; Alnæs, D.; Barch, D.M.; Baur-Streubel, R.; Bertolino, A.; et al. Common brain disorders are associated with heritable patterns of apparent aging of the brain. Nat. Neurosci. 2019, 22, 1617–1623. [Google Scholar] [CrossRef]
- Høgestøl, E.A.; Kaufmann, T.; Nygaard, G.O.; Beyer, M.K.; Sowa, P.; Nordvik, J.E.; Kolskår, K.; Richard, G.; Andreassen, O.A.; Harbo, H.F.; et al. Cross-sectional and longitudinal MRI brain scans reveal accelerated brain aging in multiple sclerosis. Front. Neurol. 2019, 10, 450. [Google Scholar] [CrossRef]
- Cole, J.H.; Raffel, J.; Friede, T.; Eshaghi, A.; Brownlee, W.J.; Chard, D.; De Stefano, N.; Enzinger, C.; Pirpamer, L.; Filippi, M.; et al. Longitudinal Assessment of Multiple Sclerosis with the Brain-Age Paradigm. Ann. Neurol. 2020, 88, 93–105. [Google Scholar] [CrossRef]
- Krupa, K.; Bekiesinska-Figatowska, M. Congenital and acquired abnormalities of the corpus callosum: A pictorial essay. Biomed Res. Int. 2013, 2013, 265619. [Google Scholar] [CrossRef]
- Corrigan, N.M.; Yarnykh, V.L.; Hippe, D.S.; Owen, J.P.; Huber, E.; Zhao, T.C.; Kuhl, P.K. Myelin development in cerebral gray and white matter during adolescence and late childhood. Neuroimage 2021, 227, 117678. [Google Scholar] [CrossRef]
- Fumagalli, M.; Lecca, D.; Coppolino, G.T.; Parravicini, C.; Abbracchio, M.P.; Fumagalli, M.; Lecca, D.; Coppolino, G.T.; Parravicini, C.; Abbracchio, M.P. Pharmacological Properties and Biological Functions of the GPR17 Receptor, a Potential Target for Neuro-Regenerative Medicine. Adv. Exp. Med. Biol. 2017, 19, 169–192. [Google Scholar] [CrossRef]
- Butt, A.M.; Papanikolaou, M.; Rivera, A. Physiology of oligodendroglia. Adv. Exp. Med. Biol. 2019, 1775, 117–128. [Google Scholar] [CrossRef]
- Coppolino, G.T.; Marangon, D.; Negri, C.; Menichetti, G.; Fumagalli, M.; Gelosa, P.; Dimou, L.; Furlan, R.; Lecca, D.; Abbracchio, M.P. Differential local tissue permissiveness influences the final fate of GPR17-expressing oligodendrocyte precursors in two distinct models of demyelination. Glia 2018, 66, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Viganò, F.; Schneider, S.; Cimino, M.; Bonfanti, E.; Gelosa, P.; Sironi, L.; Abbracchio, M.P.; Dimou, L. GPR17 expressing NG2-Glia: Oligodendrocyte progenitors serving as a reserve pool after injury. Glia 2016, 64, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Rivera, A.D.; Pieropan, F.; Chacon-De-La-Rocha, I.; Lecca, D.; Abbracchio, M.P.; Azim, K.; Butt, A.M. Functional genomic analyses highlight a shift in Gpr17-regulated cellular processes in oligodendrocyte progenitor cells and underlying myelin dysregulation in the aged mouse cerebrum. Aging Cell 2021, 20, e13335. [Google Scholar] [CrossRef] [PubMed]
- Neumann, B.; Baror, R.; Zhao, C.; Segel, M.; Dietmann, S.; Rawji, K.S.; Foerster, S.; McClain, C.R.; Chalut, K.; van Wijngaarden, P.; et al. Metformin Restores CNS Remyelination Capacity by Rejuvenating Aged Stem Cells. Cell Stem Cell 2019, 25, 473–485.e8. [Google Scholar] [CrossRef]
- Bartzokis, G. Age-related myelin breakdown: A developmental model of cognitive decline and Alzheimer’s disease. Neurobiol. Aging 2004, 25, 5–18. [Google Scholar] [CrossRef]
- Nasrabady, S.E.; Rizvi, B.; Goldman, J.E.; Brickman, A.M. White matter changes in Alzheimer’s disease: A focus on myelin and oligodendrocytes. Acta Neuropathol. Commun. 2018, 6, 22. [Google Scholar] [CrossRef]
- Sanai, S.A.; Saini, V.; Benedict, R.H.B.; Zivadinov, R.; Teter, B.E.; Ramanathan, M.; Weinstock-Guttman, B. Aging and multiple sclerosis. Mult. Scler. 2016, 22, 717–725. [Google Scholar] [CrossRef]
- Love, S. Demyelinating diseases. J. Clin. Pathol. 2006, 59, 1151–1159. [Google Scholar] [CrossRef]
- van Langelaar, J.; Rijvers, L.; Smolders, J.; van Luijn, M.M. B and T Cells Driving Multiple Sclerosis: Identity, Mechanisms and Potential Triggers. Front. Immunol. 2020, 11, 760. [Google Scholar] [CrossRef]
- Jones, C.T. Childhood autoimmune neurologic diseases of the central nervous system. Neurol. Clin. 2003, 21, 745–764. [Google Scholar] [CrossRef]
- Pivneva, T.A. Mechanisms underlying the process of demyelination in multiple sclerosis. Neurophysiology 2009, 41, 365–373. [Google Scholar] [CrossRef]
- Dhib-Jalbut, S. Pathogenesis of myelin/oligodendrocyte damage in multiple sclerosis. Neurology 2007, 68, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Kuerten, S.; Lanz, T.V.; Lingampalli, N.; Lahey, L.J.; Kleinschnitz, C.; Mäurer, M.; Schroeter, M.; Braune, S.; Ziemssen, T.; Ho, P.P.; et al. Autoantibodies against central nervous system antigens in a subset of B cell-dominant multiple sclerosis patients. Proc. Natl. Acad. Sci. USA 2020, 117, 21512–21518. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Ma, Q.; Li, C.; Liu, R.; Zhao, L.; Wang, W.; Zhang, P.; Liu, X.; Gao, G.; Liu, F.; et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microbes Infect. 2020, 9, 1123–1130. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Rana, V.; Parama, D.; Banik, K.; Girisa, S.; Henamayee, S.; Thakur, K.K.; Dutta, U.; Garodia, P.; Gupta, S.C.; et al. COVID-19, cytokines, inflammation, and spices: How are they related? Life Sci. 2021, 284, 119201. [Google Scholar] [CrossRef]
- Sriwastava, S.; Tandon, M.; Podury, S.; Prasad, A.; Wen, S.; Guthrie, G.; Kakara, M.; Jaiswal, S.; Subedi, R.; Elkhooly, M.; et al. COVID-19 and neuroinflammation: A literature review of relevant neuroimaging and CSF markers in central nervous system inflammatory disorders from SARS-COV2. J. Neurol. 2021, 268, 4448–4478. [Google Scholar] [CrossRef]
- Banks, W. Blood-Brain Barrier Transport of Cytokines: A Mechanism for Neuropathology. Curr. Pharm. Des. 2005, 11, 973–984. [Google Scholar] [CrossRef]
- Matschke, J.; Lütgehetmann, M.; Hagel, C.; Sperhake, J.P.; Schröder, A.S.; Edler, C.; Mushumba, H.; Fitzek, A.; Allweiss, L.; Dandri, M.; et al. Neuropathology of patients with COVID-19 in Germany: A post-mortem case series. Lancet Neurol. 2020, 19, 919–929. [Google Scholar] [CrossRef]
- Göbel, K.; Ruck, T.; Meuth, S.G. Cytokine signaling in multiple sclerosis: Lost in translation. Mult. Scler. J. 2018, 24, 432–439. [Google Scholar] [CrossRef]
- Wang, K.; Song, F.; Fernandez-Escobar, A.; Luo, G.; Wang, J.-H.; Sun, Y. The Properties of Cytokines in Multiple Sclerosis: Pros and Cons. Am. J. Med. Sci. 2018, 356, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Zhang, W.; Roehrl, V.B.; Roehrl, M.W.; Roehrl, M.H. An Autoantigen-ome from HS-Sultan B-Lymphoblasts Offers a Molecular Map for Investigating Autoimmune Sequelae of COVID-19. bioRxiv 2021. [Google Scholar] [CrossRef]
- Winchester, N.; Calabrese, C.; Calabrese, L.H. The intersection of COVID-19 and autoimmunity: What is our current understanding? Pathog. Immun. 2021, 6, 31–54. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Huang, Y.H.; Chuang, Y.H.; Peng, C.M.; Wang, L.C.; Lin, Y.T.; Chiang, B.L. Autoantibodies against human epithelial cells and endothelial cells after severe acute respiratory syndrome (SARS)-associated coronavirus infection. J. Med. Virol. 2005, 77, 1–7. [Google Scholar] [CrossRef]
- Ehrenfeld, M.; Tincani, A.; Andreoli, L.; Cattalini, M.; Greenbaum, A. Covid-19 and autoimmunity Michael. Autoimmun. Rev. J. 2020, 19, 102597. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Estes, S.K.; Ali, R.A.; Gandhi, A.A.; Yalavarthi, S.; Shi, H.; Sule, G.; Gockman, K.; Madison, J.A.; Zuo, M.; et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci. Transl. Med. 2020, 12, eabd3876. [Google Scholar] [CrossRef]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.-H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef]
- Gazzaruso, C.; Carlo Stella, N.; Mariani, G.; Nai, C.; Coppola, A.; Naldani, D.; Gallotti, P. High prevalence of antinuclear antibodies and lupus anticoagulant in patients hospitalized for SARS-CoV2 pneumonia. Clin. Rheumatol. 2020, 39, 2095–2097. [Google Scholar] [CrossRef]
- Wang, E.Y.; Mao, T.; Klein, J.; Dai, Y.; Huck, J.D.; Jaycox, J.R.; Liu, F.; Zhou, T.; Israelow, B.; Wong, P.; et al. Diverse functional autoantibodies in patients with COVID-19. Nature 2021, 595, 283–288. [Google Scholar] [CrossRef]
- Johnsson, M.; Asztely, F.; Hejnebo, S.; Axelsson, M.; Malmeström, C.; Olausson, T.; Lycke, J. SARS-COV-2 a trigger of myelin oligodendrocyte glycoprotein-associated disorder. Ann. Clin. Transl. Neurol. 2022, 9, 1296–1301. [Google Scholar] [CrossRef]
- Sanna, G.B.M.C.M.K.M.H.G. Central nervous system involvement in the antiphospholipid (Hughes) syndrome. Rheumatology 2003, 42, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Kent, M.; Alvarez, F.; Vogt, E.; Fyffe, R.; Ng, A.K.; Rote, N. Monoclonal antiphosphatidylserine antibodies react directly with feline and murine central nervous system. J. Rheumatol. 1997, 24, 1725–1733. [Google Scholar] [PubMed]
- Santos, M.; de Carvalho, J.; Brotto, M.; Bonfa, E.; Rocha, F. Peripheral neuropathy in patients with primary antiphospholipid (Hughes’) syndrome. Lupus 2010, 19, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.W.X.; Fong, S.W.; Young, B.E.; Chan, Y.H.; Lee, B.; Amrun, S.N.; Chee, R.S.; Yeo, N.K.; Tambyah, P.; Pada, S.; et al. Persistent Symptoms and Association With Inflammatory Cytokine Signatures in Recovered Coronavirus Disease 2019 Patients. Open Forum Infect Dis. 2021, 8, ofab156. [Google Scholar] [CrossRef]
- Eijlers, A.J.C.; Meijer, K.A.; Van Geest, Q.; Geurts, J.J.G.; Schoonheim, M.M. Determinants of cognitive impairment in patients with multiple sclerosis with and without atrophy. Radiology 2018, 288, 544–551. [Google Scholar] [CrossRef]
- Harbo, H.F.; Gold, R.; Tintora, M. Sex and gender issues in multiple sclerosis. Ther. Adv. Neurol. Disord. 2013, 6, 237–248. [Google Scholar] [CrossRef]
- Shcherbak, S.G.; Anisenkova, A.Y.; Mosenko, S.V.; Glotov, O.S.; Chernov, A.N.; Apalko, S.V.; Urazov, S.P.; Garbuzov, E.Y.; Khobotnikov, D.N.; Klitsenko, O.A.; et al. Basic Predictive Risk Factors for Cytokine Storms in COVID-19 Patients. Front. Immunol. 2021, 12, 745515. [Google Scholar] [CrossRef]
- Bigdelou, B.; Sepand, M.R.; Najafikhoshnoo, S.; Negrete, J.A.T.; Sharaf, M.; Ho, J.Q.; Sullivan, I.; Chauhan, P.; Etter, M.; Shekarian, T.; et al. COVID-19 and Preexisting Comorbidities: Risks, Synergies, and Clinical Outcomes. Front. Immunol. 2022, 13, 2077. [Google Scholar] [CrossRef]
- Liu, D.; Yuan, X.; Gao, F.; Zhao, B.; Ding, L.; Huan, M.; Liu, C.; Jiang, L. High Number and Specific Comorbidities Could Impact the Immune Response in COVID-19 Patients. Front. Immunol. 2022, 13, 899930. [Google Scholar] [CrossRef]
- Kennedy, P.G.E. Viral encephalitis: Causes, differential diagnosis, and management. Neurol. Pract. 2004, 75, i10–i15. [Google Scholar] [CrossRef]
- Thurnher, M.M.; Thurnher, S.A.; Mühlbauer, B.; Hainfellner, J.A.; Steuer, A.; Fleischmann, D.; Trattnig, S.; Budka, H.; Schindler, E. Progressive multifocal leukoencephalopathy in AIDS: Initial and follow-up CT and MRI. Neuroradiology 1997, 39, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Sweet, T.M.; Del Valle, L.; Khalili, K. Molecular biology and immunoregulation of human neurotropic JC virus in CNS. J. Cell. Physiol. 2002, 191, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, L.; Bao, L.; Liu, J.; Zhu, H.; Lv, Q.; Liu, R.; Chen, W.; Tong, W.; Wei, Q.; et al. SARS-CoV-2 crosses the blood–brain barrier accompanied with basement membrane disruption without tight junctions alteration. Signal Transduct. Target. Ther. 2021, 6, 337. [Google Scholar] [CrossRef]
- Pan, R.; Zhang, Q.; Anthony, S.M.; Zhou, Y.; Zou, X.; Cassell, M.; Perlman, S. Oligodendrocytes that survive acute coronavirus infection induce prolonged inflammatory responses in the CNS. Proc. Natl. Acad. Sci. USA 2020, 117, 15902–15910. [Google Scholar] [CrossRef] [PubMed]
- Pantoni, L.; Garcia, J.H.; Gutierrez, J.A. Cerebral white matter is highly vulnerable to ischemia. Stroke 1996, 27, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Remyelination is critical for white matter stroke recovery. Eur. J. Biomed. Res. 2015, 1, 18–22. [Google Scholar] [CrossRef]
- Khodanovich, M.Y.; Kisel, A.A.; Akulov, A.E.; Atochin, D.N.; Kudabaeva, M.S.; Glazacheva, V.Y.; Svetlik, M.V.; Medvednikova, Y.A.; Mustafina, L.R.; Yarnykh, V.L. Quantitative assessment of demyelination in ischemic stroke in vivo using macromolecular proton fraction mapping. J. Cereb. Blood Flow Metab. 2018, 38, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Dewar, D.; Underhill, S.M.; Goldberg, M.P. Oligodendrocytes and ischemic brain injury. J. Cereb. Blood Flow Metab. 2003, 23, 263–274. [Google Scholar] [CrossRef]
- Jing, L.; He, Q.; Zhang, J.Z.; Andy Li, P. Temporal profile of astrocytes and changes of oligodendrocyte-based myelin following middle cerebral artery occlusion in diabetic and non-diabetic rats. Int. J. Biol. Sci. 2013, 9, 190–199. [Google Scholar] [CrossRef]
- Khodanovich, M.Y.; Gubskiy, I.L.; Kudabaeva, M.S.; Namestnikova, D.D.; Kisel, A.A.; Anan’ina, T.V.; Tumentceva, Y.A.; Mustafina, L.R.; Yarnykh, V.L. Long-term monitoring of chronic demyelination and remyelination in a rat ischemic stroke model using macromolecular proton fraction mapping. J. Cereb. Blood Flow Metab. 2021, 41, 2856–2869. [Google Scholar] [CrossRef]
- Etherton, M.R.; Wu, O.; Giese, A.; Lauer, A.; Boulouis, G.; Mills, B.; Cloonan, L.; Donahue, K.L.; Copen, W.; Schaefer, P.; et al. White Matter Integrity and Early Outcomes After Acute Ischemic Stroke. Transl. Stroke Res. 2019, 10, 630–638. [Google Scholar] [CrossRef]
- Ginsberg, M.D.; Hedley-Whyte, E.T.; Richardson, E.P. Hypoxic-Ischemic Leukoencephalopathy in Man. Arch. Neurol. 1976, 33, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Anan’ina, T.; Kisel, A.; Kudabaeva, M.; Chernysheva, G.; Smolyakova, V.; Usov, K.; Krutenkova, E.; Plotnikov, M.; Khodanovich, M. Neurodegeneration, Myelin Loss and Glial Response in the Three-Vessel Global Ischemia Model in Rat. Int. J. Mol. Sci. 2020, 21, 6246. [Google Scholar] [CrossRef] [PubMed]
- Brun, G.; Hak, J.F.; Coze, S.; Kaphan, E.; Carvelli, J.; Girard, N.; Stellmann, J.P. COVID-19-White matter and globus pallidum lesions: Demyelination or small-vessel vasculitis? Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, 4–6. [Google Scholar] [CrossRef]
- Belani, P.; Schefflein, J.; Kihira, S.; Rigney, B.; Delman, B.N.; Mahmoudi, K.; Mocco, J.; Majidi, S.; Yeckley, J.; Aggarwal, A.; et al. COVID-19 Is an independent risk factor for acute ischemic stroke. Am. J. Neuroradiol. 2020, 41, 1361–1364. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, M.; El-Hajj Sleiman, J.; Beauchemin, P.; Rangachari, M. SARS-CoV-2 and Multiple Sclerosis: Potential for Disease Exacerbation. Front. Immunol. 2022, 13, 871276. [Google Scholar] [CrossRef]
- Novi, G.; Rossi, T.; Pedemonte, E.; Saitta, L.; Rolla, C.; Roccatagliata, L.; Inglese, M.; Farinini, D. Acute disseminated encephalomyelitis after SARS-CoV-2 infection. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, 4–6. [Google Scholar] [CrossRef]
- Abdi, S.; Ghorbani, A.; Fatehi, F. The association of SARS-CoV-2 infection and acute disseminated encephalomyelitis without prominent clinical pulmonary symptoms. J. Neurol. Sci. 2020, 416, 117001. [Google Scholar] [CrossRef]
- Chougar, L.; Shor, N.; Weiss, N.; Galanaud, D.; Leclercq, D.; Mathon, B.; Belkacem, S.; Ströer, S.; Burrel, S.; Boutolleau, D.; et al. Retrospective observational study of brain MRI findings in patients with acute SARS-CoV-2 infection and neurologic manifestations. Radiology 2020, 297, E313–E323. [Google Scholar] [CrossRef]
- Mahammedi, A.; Saba, L.; Vagal, A.; Leali, M.; Rossi, A.; Gaskill, M.; Sengupta, S.; Zhang, B.; Carriero, A.; Bachir, S.; et al. Imaging of neurologic disease in hospitalized patients with COVID-19: An italian multicenter retrospective observational study. Radiology 2020, 297, E270–E273. [Google Scholar] [CrossRef]
- Moriguchi, T.; Harii, N.; Goto, J.; Harada, D.; Sugawara, H.; Takamino, J.; Ueno, M.; Sakata, H.; Kondo, K.; Myose, N.; et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020, 94, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Anzalone, N.; Castellano, A.; Scotti, R.; Scandroglio, A.M.; Filippi, M.; Ciceri, F.; Tresoldi, M.; Falini, A. Multifocal laminar cortical brain lesions: A consistent MRI finding in neuro-COVID-19 patients. J. Neurol. 2020, 267, 2806–2809. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, P.; Alshafai, L.; Krings, T. Neuroimaging findings in patients with COVID-19. Am. J. Neuroradiol. 2020, 41, 1380–1383. [Google Scholar] [CrossRef]
- Kremer, S.; Lersy, F.; de Sèze, J.; Ferré, J.-C.; Maamar, A.; Nicol, C.-B.; Collange, O.; Bonneville, F.; Adam, G.; Martin-Blondel, G.; et al. Brain MRI Findings in Severe COVID-19: A Retrospective Observational Study. Radiology 2020, 297, E242–E251. [Google Scholar] [CrossRef] [PubMed]
- Radmanesh, A.; Derman, A.; Lui, Y.W.; Raz, E.; Loh, J.P.; Hagiwara, M.; Borja, M.J.; Zan, E.; Fatterpekar, G.M. COVID-19–associated Diffuse Leukoencephalopathy and Microhemorrhages. Radiology 2020, 297, E223–E227. [Google Scholar] [CrossRef]
- Scullen, T.; Keen, J.; Mathkour, M.; Dumont, A.S.; Kahn, L. Coronavirus 2019 (COVID-19)eAssociated Encephalopathies and Cerebrovascular Disease: The New Orleans Experience. World Neurosurg. 2020, 141, e437–e446. [Google Scholar] [CrossRef] [PubMed]
- Klironomos, S.; Tzortzakakis, A.; Kits, A.; Öhberg, C.; Kollia, E.; Ahoromazdae, A.; Almqvist, H.; Aspelin, Å.; Martin, H.; Ouellette, R.; et al. Nervous system involvement in coronavirus disease 2019: Results from a retrospective consecutive neuroimaging cohort. Radiology 2020, 297, E324–E334. [Google Scholar] [CrossRef]
- Marcic, L.; Marcic, M.; Kojundzic, S.L.; Marcic, B.; Capkun, V.; Vukojevic, K. Personalized approach to patient with mri brain changes after sars-cov-2 infection. J. Pers. Med. 2021, 11, 442. [Google Scholar] [CrossRef]
- Song, S.K.; Sun, S.W.; Ramsbottom, M.J.; Chang, C.; Russell, J.; Cross, A.H. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 2002, 17, 1429–1436. [Google Scholar] [CrossRef]
- Whittall, K.P.; MacKay, A.L.; Graeb, D.A.; Nugent, R.A.; Li, D.K.B.; Paty, D.W. In vivo measurement of T2 distributions and water contents in normal human brain. Magn. Reson. Med. 1997, 37, 34–43. [Google Scholar] [CrossRef]
- Deoni, S.C.L.L.; Rutt, B.K.; Arun, T.; Pierpaoli, C.; Jones, D.K. Gleaning multicomponent T1 and T2 information from steady-state imaging data. Magn. Reson. Med. 2008, 60, 1372–1387. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.; Kim, D.H.; Du, Y.P. In vivo multi-slice mapping of myelin water content using T2* decay. Neuroimage 2010, 52, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Varma, G.; Duhamel, G.; De Bazelaire, C.; Alsop, D.C. Magnetization transfer from inhomogeneously broadened lines: A potential marker for myelin. Magn. Reson. Med. 2015, 73, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Varma, G.; Girard, O.M.; Prevost, V.H.; Grant, A.K.; Duhamel, G.; Alsop, D.C. Interpretation of magnetization transfer from inhomogeneously broadened lines (ihMT) in tissues as a dipolar order effect within motion restricted molecules. J. Magn. Reson. 2015, 260, 67–76. [Google Scholar] [CrossRef]
- Prevost, V.H.; Girard, O.M.; Mchinda, S.; Varma, G.; Alsop, D.C.; Duhamel, G. Optimization of inhomogeneous magnetization transfer (ihMT) MRI contrast for preclinical studies using dipolar relaxation time (T1D) filtering. NMR Biomed. 2017, 30, e3706. [Google Scholar] [CrossRef]
- Dula, A.N.; Gochberg, D.F.; Valentine, H.L.; Valentine, W.M.; Does, M.D. Multiexponential T2, magnetization transfer, and quantitative histology in white matter tracts of rat spinal cord. Magn. Reson. Med. 2010, 63, 902–909. [Google Scholar] [CrossRef]
- Janve, V.A.; Zu, Z.; Yao, S.Y.; Li, K.; Zhang, F.L.; Wilson, K.J.; Ou, X.; Does, M.D.; Subramaniam, S.; Gochberg, D.F. The radial diffusivity and magnetization transfer pool size ratio are sensitive markers for demyelination in a rat model of type III multiple sclerosis (MS) lesions. Neuroimage 2013, 74, 298–305. [Google Scholar] [CrossRef]
- Samsonov, A.; Alexander, A.L.; Mossahebi, P.; Wu, Y.C.; Duncan, I.D.; Field, A.S. Quantitative MR imaging of two-pool magnetization transfer model parameters in myelin mutant shaking pup. Neuroimage 2012, 62, 1390–1398. [Google Scholar] [CrossRef]
- Underhill, H.R.; Rostomily, R.C.; Mikheev, A.M.; Yuan, C.; Yarnykh, V.L. Fast bound pool fraction imaging of the in vivo rat brain: Association with myelin content and validation in the C6 glioma model. Neuroimage 2011, 54, 2052–2065. [Google Scholar] [CrossRef]
- Odrobina, E.E.; Lam, T.Y.J.; Pun, T.; Midha, R.; Stanisz, G.J. MR properties of excised neural tissue following experimentally induced demyelination. NMR Biomed. 2005, 18, 277–284. [Google Scholar] [CrossRef]
- Ou, X.; Sun, S.W.; Liang, H.F.; Song, S.K.; Gochberg, D.F. The MT pool size ratio and the DTI radial diffusivity may reflect the myelination in shiverer and control mice. NMR Biomed. 2009, 22, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Sun, S.W.; Liang, H.F.; Song, S.K.; Gochberg, D.F. Quantitative magnetization transfer measured pool-size ratio reflects optic nerve myelin content in ex vivo mice. Magn. Reson. Med. 2009, 61, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Rausch, M.; Tofts, P.; Lervik, P.; Walmsley, A.; Mir, A.; Schubart, A.; Seabrook, T. Characterization of white matter damage in animal models of multiple sclerosis by magnetization transfer ratio and quantitative mapping of the apparent bound proton fraction f*. Mult. Scler. 2009, 15, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Schmierer, K.; Tozer, D.J.; Scaravilli, F.; Altmann, D.R.; Barker, G.J.; Tofts, P.S.; Miller, D.H. Quantitative magnetization transfer imaging in postmortem multiple sclerosis brain. J. Magn. Reson. Imaging 2007, 26, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Thiessen, J.D.; Zhang, Y.; Zhang, H.; Wang, L.; Buist, R.; Del Bigio, M.R.; Kong, J.; Li, X.M.; Martin, M. Quantitative MRI and ultrastructural examination of the cuprizone mouse model of demyelination. NMR Biomed. 2013, 26, 1562–1581. [Google Scholar] [CrossRef] [PubMed]
- Naumova, A.V.; Akulov, A.E.; Khodanovich, M.Y.; Yarnykh, V.L. High-resolution three-dimensional macromolecular proton fraction mapping for quantitative neuroanatomical imaging of the rodent brain in ultra-high magnetic fields. Neuroimage 2017, 147, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Khodanovich, M.Y.; Pishchelko, A.O.; Glazacheva, V.Y.; Pan, E.S.; Akulov, A.E.; Svetlik, M.V.; Tyumentseva, Y.A.; Anan’ina, T.V. Yarnykh Vasily Leonidovich Quantitative Imaging of White and Gray Matter Remyelination in the Cuprizone Demyelination Model Using the Macromolecular Proton Fraction. Cells 2019, 8, 1204. [Google Scholar] [CrossRef]
- Khodanovich, M.Y.Y.; Sorokina, I.V.V.; Glazacheva, V.Y.Y.; Akulov, A.E.E.; Nemirovich-Danchenko, N.M.M.; Romashchenko, A.V.V.; Tolstikova, T.G.G.; Mustafina, L.R.R.; Yarnykh, V.L.L. Histological validation of fast macromolecular proton fraction mapping as a quantitative myelin imaging method in the cuprizone demyelination model. Sci. Rep. 2017, 7, srep46686. [Google Scholar] [CrossRef]
- Yarnykh, V.L.; Krutenkova, E.P.; Aitmagambetova, G.; Henson, L.K.J.; Piedmont, H.; Repovic, P.; Mayadev, A.; Qian, P.; Gangadharan, B. Iron-insensitive quantitative assessment of subcortical gray matter demyelination in multiple sclerosis using macromolecular proton fraction. Am. J. Neuroradiol. 2018, 39, 618–625. [Google Scholar] [CrossRef]
- Yarnykh, V.L.L.; Bowen, J.D.D.; Samsonov, A.; Repovic, P.; Mayadev, A.; Qian, P.; Gangadharan, B.; Keogh, B.P.P.; Maravilla, K.R.R.; Henson, L.K.J.; et al. Fast Whole-Brain Three-dimensional Macromolecular Proton Fraction Mapping in Multiple Sclerosis. Radiology 2014, 274, 210–220. [Google Scholar] [CrossRef]
- Petrie, E.C.; Cross, D.J.; Yarnykh, V.L.; Richards, T.; Martin, N.M.; Pagulayan, K.; Hoff, D.; Hart, K.; Mayer, C.; Tarabochia, M.; et al. Neuroimaging, Behavioral, and Psychological Sequelae of Repetitive Combined Blast/Impact Mild Traumatic Brain Injury in Iraq and Afghanistan War Veterans. J. Neurotrauma 2014, 31, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, L.P.; Yarnykh, V.L.; Parshukova, D.A.; Kornetova, E.G.; Semke, A.V.; Usova, A.V.; Pishchelko, A.O.; Khodanovich, M.Y.; Ivanova, S.A. Global hypomyelination of the brain white and gray matter in schizophrenia: Quantitative imaging using macromolecular proton fraction. Transl. Psychiatry 2021, 11, 365. [Google Scholar] [CrossRef] [PubMed]
- Korostyshevskaya, A.M.; Savelov, A.A.; Papusha, L.I.; Druy, A.E.; Yarnykh, V.L. Congenital medulloblastoma: Fetal and postnatal longitudinal observation with quantitative MRI. Clin. Imaging 2018, 52, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Yarnykh, V.L.L.; Prihod’ko, I.Y.Y.; Savelov, A.A.A.; Korostyshevskaya, A.M.M. Quantitative assessment of normal fetal brain myelination using fast macromolecular proton fraction mapping. Am. J. Neuroradiol. 2018, 39, 1341–1348. [Google Scholar] [CrossRef]
- Korostyshevskaya, A.M.M.; Prihod’ko, I.Y.Y.; Savelov, A.A.A.; Yarnykh, V.L.L. Direct comparison between apparent diffusion coefficient and macromolecular proton fraction as quantitative biomarkers of the human fetal brain maturation. J. Magn. Reson. Imaging 2019, 50, 52–61. [Google Scholar] [CrossRef]
| Study Parameters | Huang et al. [39] | Zhang et al. [38] | Graham et al. [21] | Pilotto et al. [42] | Woo et al. [27] |
|---|---|---|---|---|---|
| Time from onset, months | 6.2 (5.8–6.6) | 12.0 (11.9–12.4) | 5.3 (3.4–6.4) | 6 | 2.8 (0.7–3.5) |
| Sample size | 1733 | 2433 | 100 | 165 | 18 |
| Male/female (%) | 52/48 | 49.5/50.5 | 70/30 | 69.7/30.3 | 56/44 |
| Age, years | 57.0 (47.0–65.0) | 60.0 (49.0–68.0) | 43.2 (11.3) | 64.8 ± 12.6 | 42.2 (14.3) |
| Mild/severe COVID-19 (%) | 25/75 | 72.1/27.9 | 100/0 | 34.5/65.5 ** | 100/0 |
| Neurological symptoms | |||||
| “Brain fog” | N/R | N/R | 81% | N/R | N/R |
| Headache | 2% | 2.3% | 68% | 9.7% | N/R |
| Myalgia | 2% | 7.9% | 55% | 29.6% | N/R |
| Fatigue | 63% | 27.7% | 85% | 34% | 16.7% |
| Anosmia/hyposmia | 11% | 1.3% | 55% | 18% | N/R |
| Dysgeusia/hypogeusia | 7% | 1.4% | 59% | 18% | N/R |
| Mental disorders | |||||
| Insomnia | 26% | N/R | 33% | 30.8% | N/R |
| Depression/Anxiety | 23% | 10.4% | 47% | 26.7% | 11.1% * |
| Memory deficit | N/R | N/R | 32% | 31% | 44.4% |
| Attention deficit | N/R | N/R | 27% | 31% | 50% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khodanovich, M.Y.; Kamaeva, D.A.; Naumova, A.V. Role of Demyelination in the Persistence of Neurological and Mental Impairments after COVID-19. Int. J. Mol. Sci. 2022, 23, 11291. https://doi.org/10.3390/ijms231911291
Khodanovich MY, Kamaeva DA, Naumova AV. Role of Demyelination in the Persistence of Neurological and Mental Impairments after COVID-19. International Journal of Molecular Sciences. 2022; 23(19):11291. https://doi.org/10.3390/ijms231911291
Chicago/Turabian StyleKhodanovich, Marina Y., Daria A. Kamaeva, and Anna V. Naumova. 2022. "Role of Demyelination in the Persistence of Neurological and Mental Impairments after COVID-19" International Journal of Molecular Sciences 23, no. 19: 11291. https://doi.org/10.3390/ijms231911291
APA StyleKhodanovich, M. Y., Kamaeva, D. A., & Naumova, A. V. (2022). Role of Demyelination in the Persistence of Neurological and Mental Impairments after COVID-19. International Journal of Molecular Sciences, 23(19), 11291. https://doi.org/10.3390/ijms231911291






