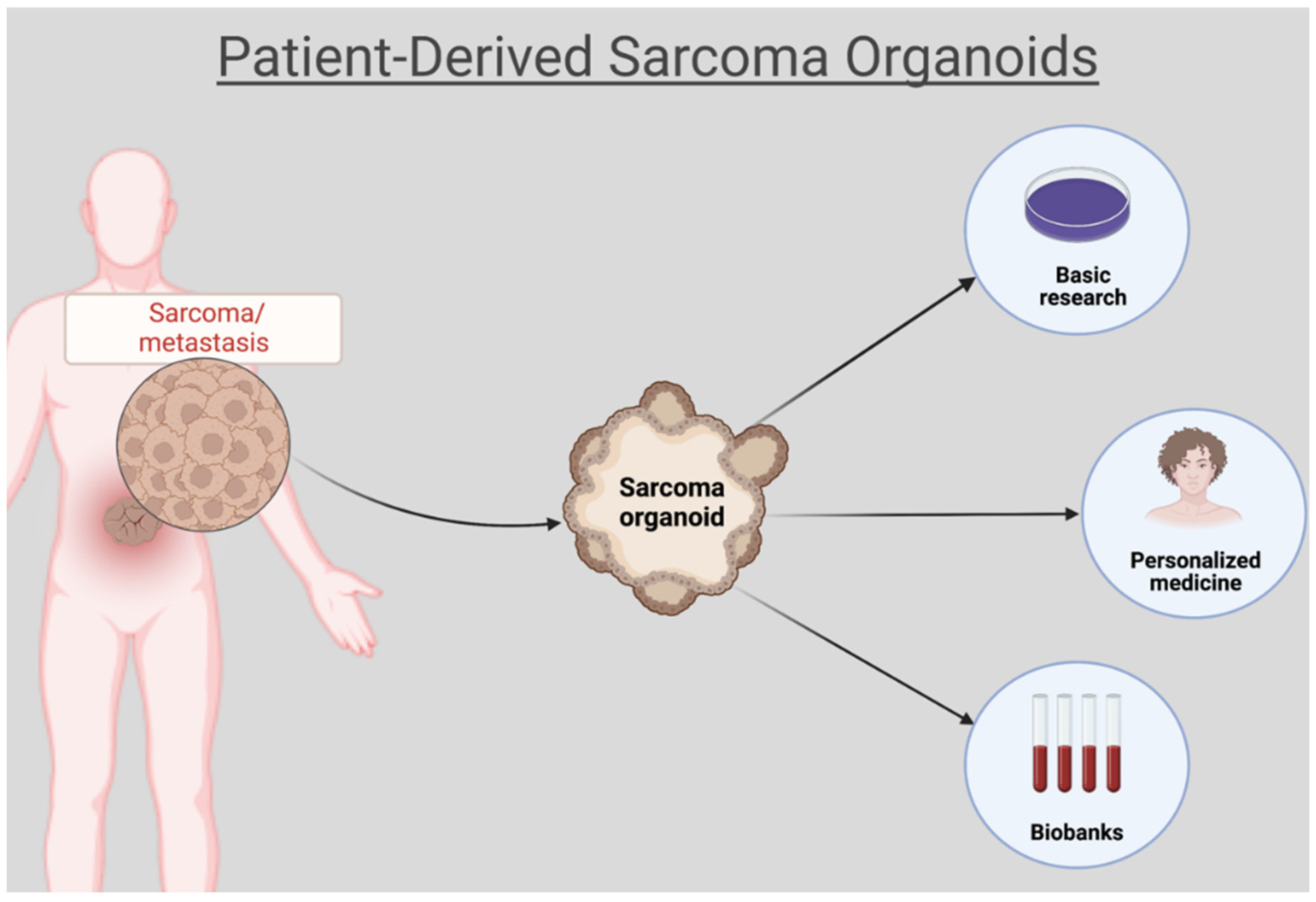
Organoids: A New Chapter in Sarcoma Diagnosis and Treatment
Abstract
1. Introduction
2. Soft Tissue Sarcoma
2.1. Rhabdomyosarcoma
2.2. Non-Rhabdomyosarcoma
3. Primary Bone Sarcoma
3.1. Osteosarcoma
3.2. Chondrosarcoma
3.3. Ewing Sarcoma
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hui, J.Y. Epidemiology and Etiology of Sarcomas. Surg. Clin. N. Am. 2016, 96, 901–914. [Google Scholar] [CrossRef]
- Skubitz, K.M.; D’Adamo, D.R. Sarcoma. Mayo. Clin. Proc. 2007, 82, 1409–1432. [Google Scholar] [CrossRef] [PubMed]
- Lupo, P.J.; Spector, L.G.; O’Brien, S.; Schiffman, J.D.; Hettmer, S. Epidemiology of Bone and Soft Tissue Sarcomas. In Sarcomas of Bone and Soft Tissues in Children and Adolescents; Arnd, C.A.S., Ed.; Pediatric Oncology; Springer: Cham, Switzerland, 2020. [Google Scholar]
- American Cancer Society. Key Statistics for Soft Tissue Sarcomas. Available online: https://www.cancer.org/cancer/soft-tissue-sarcoma/about/key-statistics.html (accessed on 17 June 2022).
- American Cancer Society. Key Statistics about Bone Cancer. Available online: https://www.cancer.org/cancer/bone-cancer/about/key-statistics.html (accessed on 17 June 2022).
- American Cancer Society. Signs and Symptoms of Soft Tissue Sarcomas. Available online: https://www.cancer.org/cancer/soft-tissue-sarcoma/detection-diagnosis-staging/signs-symptoms.html (accessed on 17 June 2022).
- American Cancer Society. Survival Rates for Soft Tissue Sarcoma. Available online: https://www.cancer.org/cancer/soft-tissue-sarcoma/detection-diagnosis-staging/survival-rates.html (accessed on 17 June 2022).
- American Cancer Society. Signs and Symptoms of Bone Cancer. Available online: https://www.cancer.org/cancer/bone-cancer/detection-diagnosis-staging/signs-symptoms.html (accessed on 17 June 2022).
- American Cancer Society. Can Bone Cancer Be Found Early? Available online: https://www.cancer.org/cancer/bone-cancer/detection-diagnosis-staging/detection.html (accessed on 17 June 2022).
- American Cancer Society. Survival Rates for Osteosarcoma. Available online: https://www.cancer.org/cancer/osteosarcoma/detection-diagnosis-staging/survival-rates.html (accessed on 17 June 2022).
- American Cancer Society. Survival Rates for Ewing Tumors. Available online: https://www.cancer.org/cancer/ewing-tumor/detection-diagnosis-staging/survival-rates.html (accessed on 17 June 2022).
- American Cancer Society. Survival Rates for Bone Cancer. Available online: https://www.cancer.org/cancer/bone-cancer/detection-diagnosis-staging/survival-statistics.html (accessed on 17 June 2022).
- American Cancer Society. Tests for Soft Tissue Sarcomas. Available online: https://www.cancer.org/cancer/soft-tissue-sarcoma/detection-diagnosis-staging/how-diagnosed.html (accessed on 17 June 2022).
- American Cancer Society. Tests for Bone Cancer. Available online: https://www.cancer.org/cancer/bone-cancer/detection-diagnosis-staging/how-diagnosed.html (accessed on 17 June 2022).
- American Cancer Society. Treatment of Soft Tissue Sarcomas, by Stage. Available online: https://www.cancer.org/cancer/soft-tissue-sarcoma/treating/by-stage.html (accessed on 17 June 2022).
- American Cancer Society. Treating Specific Types of Bone Cancer. Available online: https://www.cancer.org/cancer/bone-cancer/treating/treating-specific-bone-cancers.html (accessed on 17 June 2022).
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef]
- Huch, M.; Koo, B.K. Modeling mouse and human development using organoid cultures. Development 2015, 142, 3113–3125. [Google Scholar] [CrossRef] [PubMed]
- Corro, C.; Novellasdemunt, L.; Li, V.S.W. A brief history of organoids. Am. J. Physiol. Cell Physiol. 2020, 319, C151–C165. [Google Scholar] [CrossRef]
- Orkin, R.W.; Gehron, P.; McGoodwin, E.B.; Martin, G.R.; Valentine, T.; Swarm, R. A murine tumor producing a matrix of basement membrane. J. Exp. Med. 1977, 145, 204–220. [Google Scholar] [CrossRef]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef]
- Lehmann, R.; Lee, C.M.; Shugart, E.C.; Benedetti, M.; Charo, R.A.; Gartner, Z.; Hogan, B.; Knoblich, J.; Nelson, C.M.; Wilson, K.M. Human organoids: A new dimension in cell biology. Mol. Biol. Cell 2019, 30, 1129–1137. [Google Scholar] [CrossRef]
- Azar, J.; Bahmad, H.F.; Daher, D.; Moubarak, M.M.; Hadadeh, O.; Monzer, A.; Al Bitar, S.; Jamal, M.; Al-Sayegh, M.; Abou-Kheir, W. The Use of Stem Cell-Derived Organoids in Disease Modeling: An Update. Int. J. Mol. Sci. 2021, 22, 7667. [Google Scholar] [CrossRef]
- Drost, J.; Clevers, H. Organoids in cancer research. Nat. Rev. Cancer 2018, 18, 407–418. [Google Scholar] [CrossRef]
- Gatzweiler, C.; Ridinger, J.; Herter, S.; Gerloff, X.F.; ElHarouni, D.; Berker, Y.; Imle, R.; Schmitt, L.; Kreth, S.; Stainczyk, S.; et al. Functional Therapeutic Target Validation Using Pediatric Zebrafish Xenograft Models. Cancers 2022, 14, 849. [Google Scholar] [CrossRef]
- Meister, M.T.; Groot Koerkamp, M.J.A.; de Souza, T.; Breunis, W.B.; Frazer-Mendelewska, E.; Brok, M.; DeMartino, J.; Manders, F.; Calandrini, C.; Kerstens, H.H.D.; et al. Mesenchymal tumor organoid models recapitulate rhabdomyosarcoma subtypes. bioRxiv 2022. [Google Scholar] [CrossRef]
- Gaebler, M.; Silvestri, A.; Reichardt, P.; Wardelmann, E.; Gambara, G.; Haybaeck, J.; Stroebel, P.; Niethard, M.; Erdmann, G.; Regenbrecht, C.R. Abstract 469: Patient-derived sarcoma models: First results from the SARQMA study. Cancer Res. 2019, 79, 469. [Google Scholar] [CrossRef]
- Boulay, G.; Cironi, L.; Garcia, S.P.; Rengarajan, S.; Xing, Y.H.; Lee, L.; Awad, M.E.; Naigles, B.; Iyer, S.; Broye, L.C.; et al. The chromatin landscape of primary synovial sarcoma organoids is linked to specific epigenetic mechanisms and dependencies. Life Sci. Alliance 2021, 4, 808. [Google Scholar] [CrossRef] [PubMed]
- Maloney, E.; Clark, C.; Sivakumar, H.; Yoo, K.; Aleman, J.; Rajan, S.A.P.; Forsythe, S.; Mazzocchi, A.; Laxton, A.W.; Tatter, S.B.; et al. Immersion Bioprinting of Tumor Organoids in Multi-Well Plates for Increasing Chemotherapy Screening Throughput. Micromachines 2020, 11, 208. [Google Scholar] [CrossRef] [PubMed]
- Maru, Y.; Tanaka, N.; Tatsumi, Y.; Nakamura, Y.; Itami, M.; Hippo, Y. Kras activation in endometrial organoids drives cellular transformation and epithelial-mesenchymal transition. Oncogenesis 2021, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Maru, Y.; Tanaka, N.; Tatsumi, Y.; Nakamura, Y.; Yao, R.; Noda, T.; Itami, M.; Hippo, Y. Probing the tumorigenic potential of genetic interactions reconstituted in murine fallopian tube organoids. J. Pathol. 2021, 255, 177–189. [Google Scholar] [CrossRef] [PubMed]
- McCorkle, J.R.; Burgess, B.T.; McDowell, A.B.; DeJohn, J.; DeSimone, C.P.; Ueland, F.R.; Kolesar, J.M.; Gorski, J.W. Abstract B22: Development of the first ovarian carcinosarcoma patient-derived xenograft and tissue organoid model to predict clinical response to chemotherapy. Clin. Cancer Res. 2020, 26, B22. [Google Scholar] [CrossRef]
- Wang, J.; Aldahamsheh, O.; Ferrena, A.; Singh, S.; Singla, A.; Borjihan, H.; Yaguare, S.; Viscarret, V.; Tingling, J.; Zi, X.; et al. Abstract 2008: Targeting SKP2 by p27 Inhibits stemness and prolong the survival in osteosarcoma. Cancer Res. 2021, 81, 2008. [Google Scholar] [CrossRef]
- Subramaniam, D.; Angulo, P.; Ponnurangam, S.; Dandawate, P.; Ramamoorthy, P.; Srinivasan, P.; Iwakuma, T.; Weir, S.J.; Chastain, K.; Anant, S. Suppressing STAT5 signaling affects osteosarcoma growth and stemness. Cell Death Dis. 2020, 11, 149. [Google Scholar] [CrossRef]
- Johansson, S. Patient-Derived Organoid Culture for 3D Culture of Colorectal Cancer, Renal Cancer and Osteosarcoma; Uppsala University: Uppsala, Sweden, 2019. [Google Scholar]
- He, A.; Huang, Y.; Cheng, W.; Zhang, D.; He, W.; Bai, Y.; Gu, C.; Ma, Z.; He, Z.; Si, G.; et al. Organoid culture system for patient-derived lung metastatic osteosarcoma. Med. Oncol. 2020, 37, 105. [Google Scholar] [CrossRef] [PubMed]
- Veys, C.; Benmoussa, A.; Contentin, R.; Duchemin, A.; Brotin, E.; Lafont, J.E.; Saintigny, Y.; Poulain, L.; Denoyelle, C.; Demoor, M.; et al. Tumor Suppressive Role of miR-342-5p in Human Chondrosarcoma Cells and 3D Organoids. Int. J. Mol. Sci. 2021, 22, 5590. [Google Scholar] [CrossRef] [PubMed]
- Maurer, L.M.; Venier, R.M.; Julian, C.; Bailey, K.M. Abstract A14: Exploiting DNA damage repair defects to enhance PD-L1 expression in Ewing sarcoma. Cancer Res. 2020, 80, A14. [Google Scholar] [CrossRef]
- Maurer, L.M.; Daley, J.D.; Mukherjee, E.; Venier, R.M.; Julian, C.M.; Bailey, N.G.; Jacobs, M.F.; Kumar-Sinha, C.; Raphael, H.; Periyapatna, N.; et al. BRCA1-Associated RING Domain-1 (BARD1) Loss and GBP1 Expression Enhance Sensitivity to DNA Damage in Ewing Sarcoma. Cancer Res. Commun. 2022, 2, 220–232. [Google Scholar] [CrossRef]
- Komatsu, A.; Matsumoto, K.; Yoshimatsu, Y.; Sin, Y.; Kubota, A.; Saito, T.; Mizumoto, A.; Ohashi, S.; Muto, M.; Noguchi, R.; et al. The CAM Model for CIC-DUX4 Sarcoma and Its Potential Use for Precision Medicine. Cells 2021, 10, 2613. [Google Scholar] [CrossRef]
- Kim, J.; Koo, B.K.; Knoblich, J.A. Human organoids: Model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 571–584. [Google Scholar] [CrossRef]
- Shankaran, A.; Prasad, K.; Chaudhari, S.; Brand, A.; Satyamoorthy, K. Advances in development and application of human organoids. 3 Biotech 2021, 11, 257. [Google Scholar] [CrossRef]
- Clinton, J.; McWilliams-Koeppen, P. Initiation, Expansion, and Cryopreservation of Human Primary Tissue-Derived Normal and Diseased Organoids in Embedded Three-Dimensional Culture. Curr. Protoc. Cell Biol. 2019, 82, e66. [Google Scholar] [CrossRef]
- Fisher, R.; Pusztai, L.; Swanton, C. Cancer heterogeneity: Implications for targeted therapeutics. Br. J. Cancer 2013, 108, 479–485. [Google Scholar] [CrossRef]
- Colella, G.; Fazioli, F.; Gallo, M.; De Chiara, A.; Apice, G.; Ruosi, C.; Cimmino, A.; de Nigris, F. Sarcoma Spheroids and Organoids-Promising Tools in the Era of Personalized Medicine. Int. J. Mol. Sci. 2018, 19, 615. [Google Scholar] [CrossRef]
- Kondo, T. Current status and perspectives of patient-derived rare cancer models. Hum. Cell 2020, 33, 919–929. [Google Scholar] [CrossRef]
- Zanoni, M.; Cortesi, M.; Zamagni, A.; Arienti, C.; Pignatta, S.; Tesei, A. Modeling neoplastic disease with spheroids and organoids. J. Hematol. Oncol. 2020, 13, 97. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef]
- Kapalczynska, M.; Kolenda, T.; Przybyla, W.; Zajaczkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Blizniak, R.; Luczewski, L.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Gunti, S.; Hoke, A.T.K.; Vu, K.P.; London, N.R., Jr. Organoid and Spheroid Tumor Models: Techniques and Applications. Cancers 2021, 13, 874. [Google Scholar] [CrossRef]
- Sbaraglia, M.; Bellan, E.; Dei Tos, A.P. The 2020 WHO Classification of Soft Tissue Tumours: News and perspectives. Pathologica 2021, 113, 70–84. [Google Scholar] [CrossRef]
- Shariati, L.; Esmaeili, Y.; Haghjooy Javanmard, S.; Bidram, E.; Amini, A. Organoid technology: Current standing and future perspectives. Stem Cells 2021, 39, 1625–1649. [Google Scholar] [CrossRef]
| STS Type | Organoids | Culture Conditions | Main Results | References |
|---|---|---|---|---|
| Embryonal rhabdomyosarcoma | Patient-derived long-term organoid-like cultures |
|
| [25] |
| Rhabdomyosarcoma | Patient-derived rhabdomyosarcoma organoids |
|
| [26] |
| Myxoid liposarcoma, undifferentiated pleomorphic sarcoma, biphasic synovial sarcoma | Patient-derived non-rhabdomyosarcoma organoids |
|
| [27] |
| Synovial sarcoma | Synovial sarcoma and Ewing sarcoma patient-derived tumor organoids |
|
| [28] |
| Skin fibrosarcoma | Patient-derived skin fibrosarcoma organoids |
|
| [29] |
| Uterine carcinosarcoma | Murine endometrial organoids |
|
| [30] |
| Ovarian carcinosarcoma | Murine fallopian tube organoids |
|
| [31] |
| Ovarian carcinosarcoma | Ovarian carcinosarcoma organoid cell lines |
|
| [32] |
| PBS Type | Organoids | Culture Conditions | Main Results | References |
|---|---|---|---|---|
| Osteosarcoma | Osteosarcoma organoid culture |
|
| [33] |
| Osteosarcoma | Multicell-type lung organoid model |
|
| [34] |
| Osteosarcoma | Patient-derived osteosarcoma organoids |
|
| [35] |
| Osteosarcoma | Patient-derived lung metastatic osteosarcoma organoids |
|
| [36] |
| Chondrosarcoma | Three- dimensional chondrosarcoma organoid model |
|
| [37] |
| Ewing sarcoma | Patient-derived Ewing-sarcoma organoids |
|
| [38] |
| Ewing sarcoma | Patient-derived Ewing-sarcoma organoids |
|
| [39] |
| Ewing-like small round cell sarcoma | Tumor organoids from chorioallantoic membrane tumor |
|
| [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Psilopatis, I.; Kokkali, S.; Palamaris, K.; Digklia, A.; Vrettou, K.; Theocharis, S. Organoids: A New Chapter in Sarcoma Diagnosis and Treatment. Int. J. Mol. Sci. 2022, 23, 11271. https://doi.org/10.3390/ijms231911271
Psilopatis I, Kokkali S, Palamaris K, Digklia A, Vrettou K, Theocharis S. Organoids: A New Chapter in Sarcoma Diagnosis and Treatment. International Journal of Molecular Sciences. 2022; 23(19):11271. https://doi.org/10.3390/ijms231911271
Chicago/Turabian StylePsilopatis, Iason, Stefania Kokkali, Kostas Palamaris, Antonia Digklia, Kleio Vrettou, and Stamatios Theocharis. 2022. "Organoids: A New Chapter in Sarcoma Diagnosis and Treatment" International Journal of Molecular Sciences 23, no. 19: 11271. https://doi.org/10.3390/ijms231911271
APA StylePsilopatis, I., Kokkali, S., Palamaris, K., Digklia, A., Vrettou, K., & Theocharis, S. (2022). Organoids: A New Chapter in Sarcoma Diagnosis and Treatment. International Journal of Molecular Sciences, 23(19), 11271. https://doi.org/10.3390/ijms231911271







