Abstract
Obesity is a risk factor for knee osteoarthritis (KOA). Neuromedin U (NMU) and NMU receptors (NMUR1 and NMUR2) are associated with obesity-related disorders and found in mast cells (MCs), which are elevated in osteoarthritis. However, NMU/NMUR expression was not examined in the synovial membrane (SM) or synovial MCs of obese osteoarthritis patients. We compared expression of NMU, NMUR1, NMUR2, and the mast cell (MC) marker, CPA3, in the SM of KOA patients categorized as normal weight (NW; BMI < 25 kg/m2, n = 79), overweight (OW; BMI ≥ 25 and <30 kg/m2, n = 87), and obese (OB; ≥30 kg/m2, n = 40). To study NMU/NMUR expression in MCs, we compared the MC-rich fraction (MC-RF), CD88(+) MC-RF, and CD88(−) MC-RF, extracted using magnetic isolation, with the MC-poor fraction (MC-PF). While NMU and NMUR2 expression were comparable, NMUR1 was significantly elevated in OW and OB compared to NW. Moreover, CPA3 levels were significantly greater in OB than NW. NMUR1 and CPA3 expression were significantly higher in both the CD88(+) and CD88(−) MC-RF than MC-PF. Therefore, NMUR1 expression was elevated in the SM of OB KOA patients, and its expression was found in MCs. Further investigation to analyze the NMU/NMUR1 pathway in MC may provide a link between obesity and KOA pathology.
1. Introduction
Research suggests that being obese is associated with an elevated prevalence and occurrence of osteoarthritis (OA) in both weight-bearing and non-weight-bearing joints [1,2]. Such evidence implies that factors other than mechanical loading are involved in the link between obesity and OA. These factors could additionally play a role in OA pathology. To date, however, these factors and their related mechanisms remain to be elucidated.
According to several studies, synovial neuropeptides may contribute to OA pathology [3,4,5,6,7]. Neuromedin U (NMU) is one such example. This bioactive peptide was initially extracted from the spinal cord of pigs [8] and forms part of the NMU system, which also comprises the NMU receptors, NMUR1 and NMUR2. Together, these components of the NMU system play a role in multiple physiological functions such as inflammation, stress responses, circadian rhythmicity, and feeding behavior [9,10,11,12,13]. Experiments using genetic ablation or overexpression of NMU have demonstrated the presence of crosstalk between the NMU system and obesity-related pathological factors [14,15]. NMU was also shown to promote autoantibody-mediated arthritis in mice [16]. To the contrary, NMU was reported to be a suppressor of this pathology in a murine collagen-induced arthritis (CIA) model [17]. However, expression levels of NMU/NMURs were not examined in the synovium of obese OA patients.
NMU mRNA was observed in antigen-presenting cells, such as monocytes and dendritic cells. Meanwhile, NMUR1 mRNA is present in T cells, natural killer cells, eosinophils, and mast cells (MCs) [10,12,13,18]. In addition to being found in the synovial membrane (SM), MCs are elevated in patients with rheumatoid arthritis (RA) [19,20,21] and OA [22,23,24], suggesting that MCs could constitute a crucial component of the mechanism underlying both acute and chronic inflammation. More recent studies have demonstrated a potential association between MCs and knee OA (KOA) severity [25,26]. Given that we also previously reported increased MC marker expression in obese KOA patients [6,27,28], we hypothesized that MCs may contribute to the NMU/NMURs system in the osteoarthritic synovium of obese individuals.
Complement receptors (CRs) play an important role in innate immune defense and local inflammation [29]. Complement component 5a (C5a) and C5a receptor (CD88) signaling play an important role in MC activation via granulation [30]. A previous study reported that C5a-receptor (CD88)-positive MCs exist in the SM of OA and rheumatoid arthritis and that the number of these cells was increased in RA [31]. In addition, increased C5a was found in the serum of obese children [32]. Therefore, investigation of NMU/NMURs expression in MC, particularly CD88(+) MC, may be important in obese KOA pathology.
Here, we studied the expression of NMU, NMUR1, NMUR2, and the MC marker, CPA3, in the synovium of obese OA patients and examined whether NMU/NMUR expression is found in synovial CD88(+) and CD88(−) MCs subsets.
2. Results
2.1. Patient Features According to BMI
The patients’ clinical features are presented in Table 1. Those categorized as overweight (OW) and obese (OB) were significantly younger than those in the normal-weight (NW) group (p = 0.023 and p = 0.010, respectively). In contrast, the percentage of patients with Kellgren and Lawrence grade 2, 3, and 4 (p = 0.675) KOA was not different among the BMI groups.

Table 1.
Patients’ clinical characteristics by body mass index group.
2.2. Synovial Membrane Levels of NMU/NMURs and CPA3 by BMI
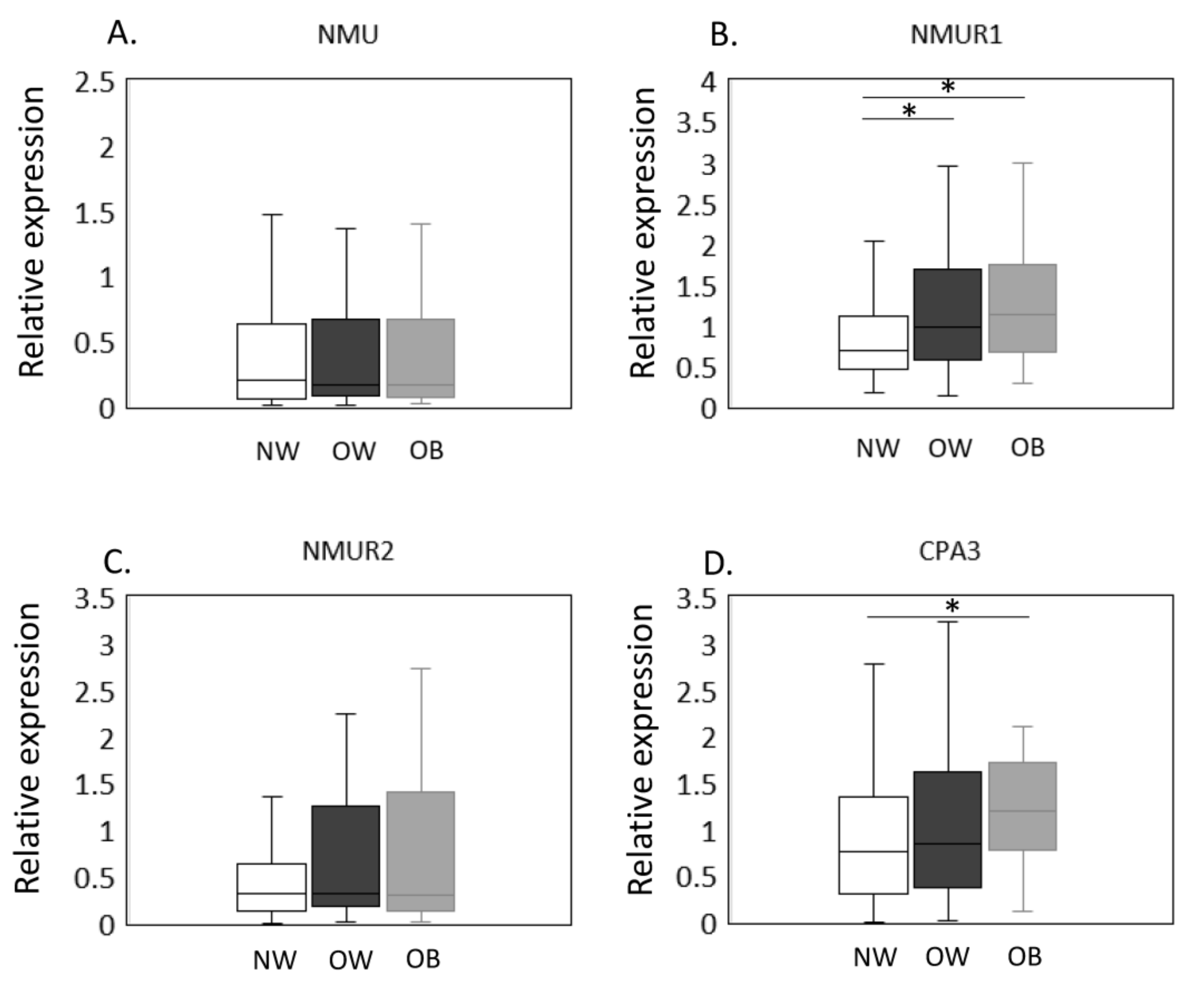
The expression of NMU/NMURs and CPA3 mRNA in NW, OW, and OB groups was estimated by qPCR (Figure 1A–D). No significant difference was observed in NMU (p = 0.948, Figure 1A) expression among the BMI groups. In contrast, NMUR1 levels were significantly greater in OW and OB than NW individuals (OW, p = 0.023; OB, p = 0.010, Figure 1B). NMUR2, however, was comparable across BMI groups (p = 0.327, Figure 1C). Meanwhile, CPA3 expression was significantly elevated in OB compared to NW patients (p = 0.020; Figure 1D) but was not different between NW and OW patients (p = 0.872, Figure 1D).
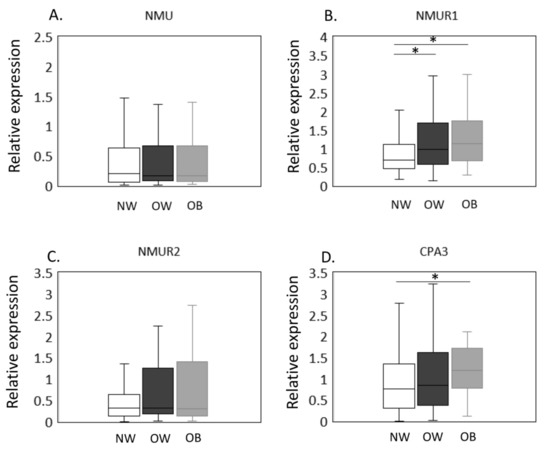
Figure 1.
Expression of CPA3 and NMU/NMURs in the synovial membrane of normal-weight, overweight, and obese groups. The expression of NMU/NMURs and CPA3 mRNA in NW, OW, and OB groups was estimated by qPCR (A–D). NMU (A), NMUR1 (B), NMUR2 (C), and CPA3 (D) mRNA expression in the synovial membrane of normal-weight (NW, n = 79), overweight (OW, n = 87), and obese (OB, n = 40) patients with knee osteoarthritis. Gene expression is presented in box and whisker plots, showing the median, 25th, and 75th percentiles and range. * p < 0.05.
2.3. Comparison of NMU/NMUR and CPA3 Expression between NW and OB Groups in a Propensity Score-Matched Cohort
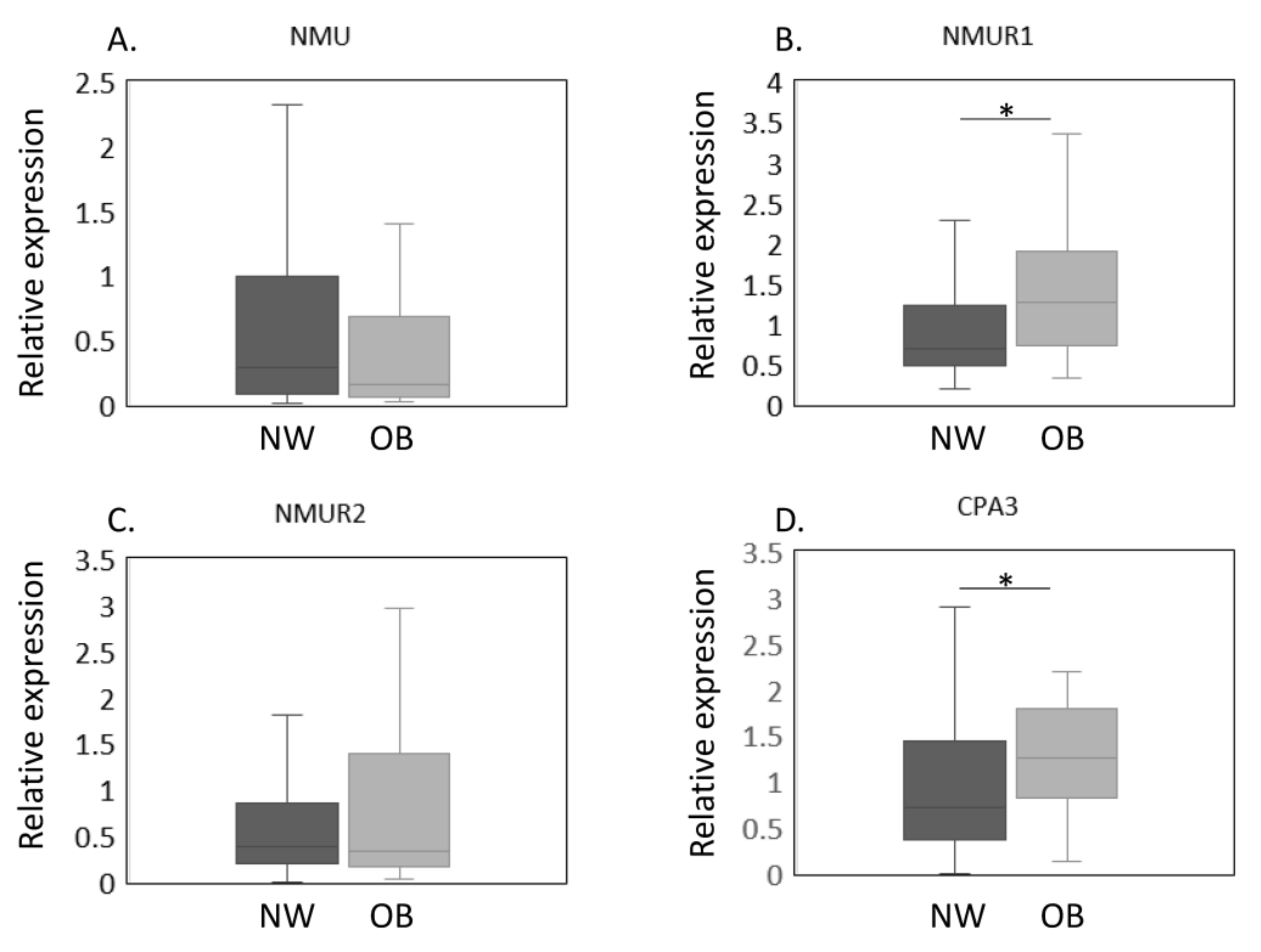
The expression of NMU/NMURs and CPA3 mRNA in NW and OB groups in a propensity score-matched cohort was estimated by qPCR (Figure 2A–D). Our results thus far showed that OB patients had higher NMUR1 and CPA3 expression and were significantly younger than NW patients. To eliminate the effect of age on gene expression, we conducted a propensity score analysis to create a matched cohort. The patients’ clinical characteristics after the propensity score analysis are shown in Table 2. Both age and the proportion with patients with Kellgren and Lawrence grade 2–4 were similar between NW and OB patients (age, p = 0.429; proportion with Kellgren and Lawrence grade 2–4, p = 0.432). Similarly, no significant differences were noted in NMU levels (p = 0.448, Figure 2A). In contrast, NMUR1 expression was significantly elevated in OB compared with NW patients (p = 0.012, Figure 2B). While NMUR2 expression did not differ among the BMI groups (p = 0.905, Figure 2C), CPA3 levels were significantly greater in the OB than in the NW group (p = 0.023, Figure 2D).
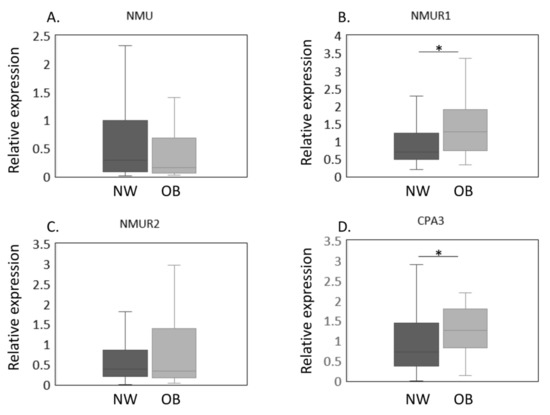
Figure 2.
Expression of NMU/NMURs and CPA3 in normal-weight and obese groups after propensity score matching. The expression of NMU/NMURs and CPA3 mRNA in normal-weight (NW, n = 38) and obese (OB, n = 38) groups in a propensity score-matched cohort was estimated by qPCR (A–D). The expression of NMU (A), NMUR1 (B), NMUR2 (C), CPA3 (D) mRNA in the synovial membrane of NW and OB patients with knee osteoarthritis after propensity score matching. Gene expressions are presented in box and whisker plots, showing the median, 25th, and 75th percentiles and range. * p < 0.05.

Table 2.
Clinical characteristics after propensity score analysis.
2.4. Expression of Synovial NMU/NMURs and CPA3 in Non-MC and MC Fractions
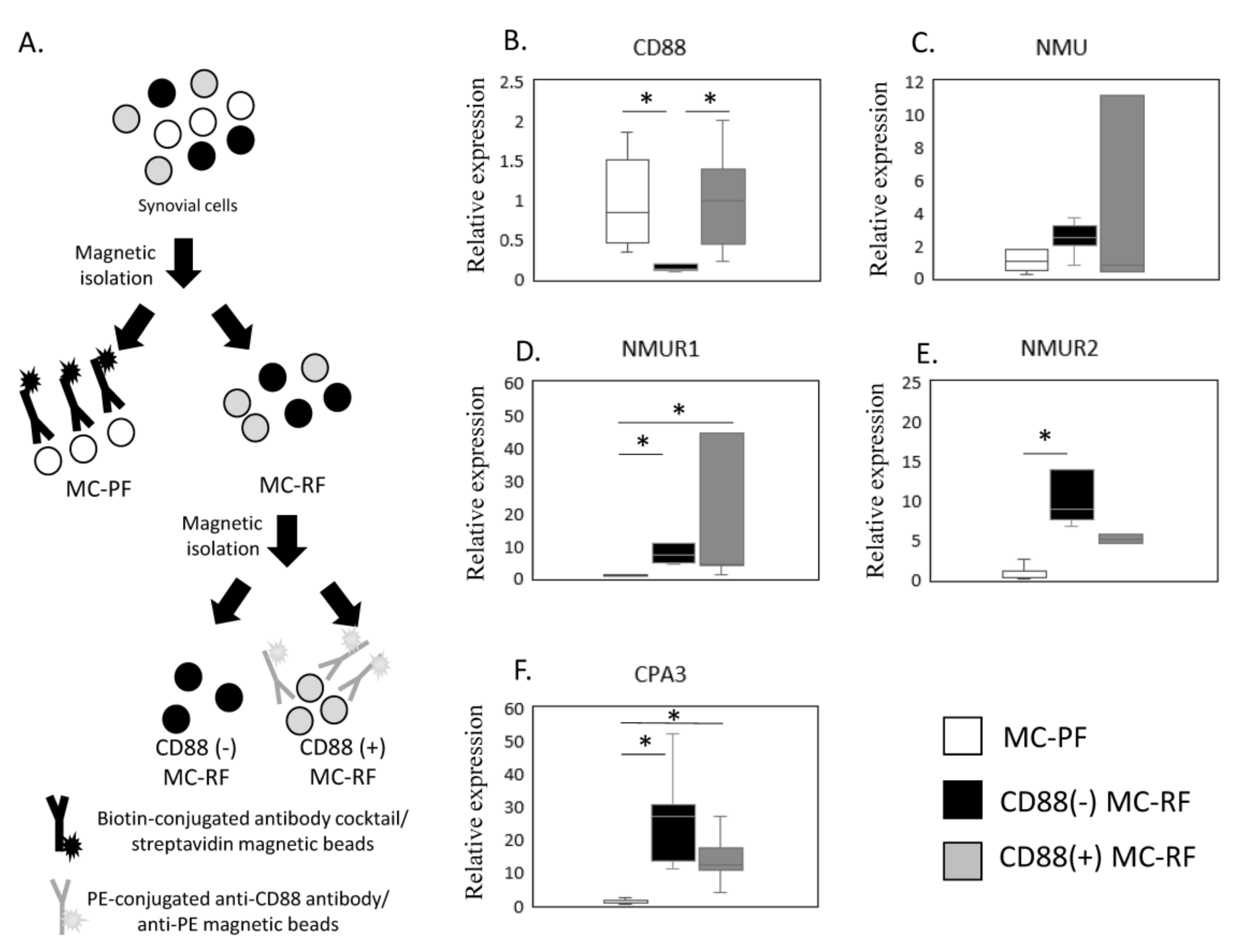
The expression of NMU/NMURs and CPA3 mRNA in non-MC and CD88(−) and CD88(+) MC fractions was estimated by qPCR (Figure 3A–F). Given that NMUR1 and CPA3 were elevated in the SM of OB patients, we next examined NMU/NMURs expression in MCs by comparing the CD88(−) MC-rich fraction (MC-RF) (Figure 3A) and CD88(+) MC-RF with the MC-poor fraction (MC-PF), which we isolated from 5 SM samples from obese KOA patients (Figure 3B–F). We confirmed that CD88(+) MC-RF showed significantly higher expression of CD88 than the CD88(−) MC-RF (p = 0.011, Figure 3B). While there were no differences in NMU expression among the fractions (p = 0.247, Figure 3C), both CD88(−) MC-RF and CD88(+) MC-RF expressed significantly higher levels of NMUR1 (p = 0.011 and p = 0.027, respectively, Figure 3D) than the MC-PF. Additionally, NMUR2 levels were significantly greater in the CD88(−) MC-RF than the MC-PF (p = 0.004, Figure 3E). Both CD88(−) and CD88(+) MC-RFs expressed significantly higher levels of CPA3 (p = 0.011 and p = 0.027, respectively, Figure 3F) than the MC-PF.
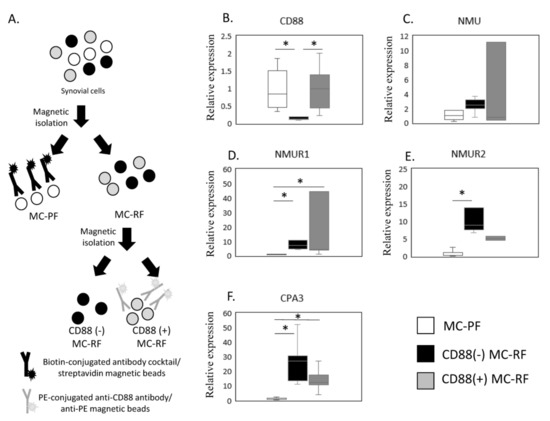
Figure 3.
Expression of NMU/NMURs and CPA3. (A) Schematic showing the process used to isolate the mast cell (MC)-poor fraction (MC-PF; THY-1+, CD3+, CD14+, or CD19+CD235+) and CD88(−) and CD88(+) MC-rich fractions (MC-RFs; THY-1-CD3-CD14-CD19-CD235-). MC-RF and MC-PF were magnetically separated from other synovium-derived cells using biotin-conjugated antibody cocktails and streptavidin beads. Subsequently, the MC-RF was further divided into CD88(−) and CD88(+) MC-RFs using PE-conjugated anti-CD88 antibody and anti-PE beads. The expression of NMU/NMURs and CPA3 mRNA in non-MC and CD88(−) and CD88(+) MC fractions was estimated by qPCR (B–F). CD88 (B), NMU (C), NMUR1 (D), NMUR2(E), and CPA3 (F) expression levels in MC-PF and CD88(−) and CD88(+) MC-RF derived from the synovial membrane of obese KOA patients (n = 5). * p < 0.05.
3. Discussion
This study in patients with KOA found that NMUR1 expression was elevated in the SM of OB compared to NW individuals. Further, NMUR1 expression was detected in MC fractions, suggesting that MCs may contribute to the NMU/NMUR system in the osteoarthritic synovium of obese patients.
According to a recent report, low-grade inflammation, or metainflammation, plays a role in the pathogenesis of obesity and obesity-related diseases [33]. OB and OW individuals with KOA exhibit greater synovial inflammation than NW individuals with KOA [34]. Evidence suggests that NMU plays a role in inflammatory conditions. For example, NMU-deficient mice show lower levels of interleukin (IL)-6 secretion from macrophages [13,35]. Additionally, interaction between NMU and NMUR1 was shown to induce the release of IL-4, -5, -6, -10, and -13 in mouse helper T cell lines [36]. We found that mRNA levels of NUMR1 were increased in the SM of OW and OB patients, suggesting that increased NUMR1 expression in the SM may contribute to obesity-related synovial pathology. To the contrary, NMU was shown to suppress autoantibody-mediated arthritis in a murine collagen-induced arthritis model. NMU-23 administration induced the expansion of innate lymphoid cells and elevated eosinophil, IL-4, IL-5, and IL-13 expression in the joint of CIA mice [17]. As MCs contribute to arthritis by cytokine secretion, a further investigation of the proteins secreted by NMU-stimulated MCs may reveal the association between obese and KOA pathology.
A previous study reported that, compared to NW individuals, OW and OB patients undergo TKA at a younger age [6]. Consistent with this, we also noted that those in the OB group were significantly younger than those in the NW group. Furthermore, almost all synovial samples were obtained from patients with late-stage OA undergoing TKA. Given that an individual’s gene expression profile can change with age, we performed a propensity score analysis to eliminate the effect of age on gene expression and found that similar to before propensity score matching, NUMR1 and CPA3 expression were significantly higher than in OB than NW patients. Therefore, our findings suggest that elevated NMUR1 and CPA3 expression is associated with obesity but not age.
Many immune cell types engaged in obese OA pathology are present in the SM [37]. NMU and NMUR1 expression were observed in several immune cell types [9,10,13,18,36], including antigen-presenting cells such as monocytes and dendritic cells for NMU [13,36], and T cells, natural killer cells, eosinophils, and MCs for NMUR1 [9,10,13,18]. As increased levels of the MC marker, CPA3, were observed in the SM of obese KOA patients, we investigated the expression of NMU/NMURs in MCs. Our results suggest that NMUR1 is expressed in synovial MCs and that elevated NMUR1 may reflect an increase in MCs in the SM. Furthermore, given that previous studies have identified CD88(+) and CD88(−) MC subsets in SM, skin, and lung [31,38,39], and elevated levels of CD88(+) MC in the SM of individuals with RA compared to OA [31], we additionally compared NMU/NMUR in CD88(+) and CD88(−) MC-RFs. Both CD88(+) and CD88(−) MC-RFs isolated from the SM of KOA patients showed high expression of CPA3 and NMUR1. According to a prior study in mice, activation of the NMU/NMUR1 pathway in MCs results in degranulation and neutrophil infiltration [40]. Additionally, MCs and their degranulation products were observed in the SM and synovial fluid of KOA patients [26]. Moreover, the number of synovial MCs correlates with the KOA patients’ synovitis score [25]. As CD88 expression in MCs contributes to granulation [30], this evidence suggests that NMU/NMUR1-mediated activation of MCs, particularly CD88(+) MC subsets, may contribute to the synovial pathology in obese KOA patients.
While NMUR1 expression was predominantly reported in peripheral tissues, NMUR2 was mostly observed in the central nervous system (CNS) [10,41,42,43,44,45,46,47,48], specifically in the hypothalamus, hippocampus, and spinal cord [43,44]. A previous study reporting NMUR2 expression in astrocytes and microglia in the mouse hippocampus suggested that NMU could regulate inflammation in the CNS. However, there are also some reports of NMUR2 expression in peripheral tissues, including in the gastrointestinal and genitourinary tracts, with high concentrations observed in the testis [47,49]. In the present study, we noted NMUR2 mRNA in the SM, with particularly high levels in CD88(−) MCs. However, as NMUR2 did not differ among the BMI groups, we propose that NMUR2 may play a limited role in obesity-related synovial pathology.
There were several limitations in this study. First, we showed that MC fractions highly expressed NMUR1 compared to the non-MC fraction. However, MC-PF isolated by magnetic beads contained a mixed cell population, including fibroblasts, macrophages, B cells, and T cells. The comparison of NMUR1 expression in MC and particular cell types requires isolation using a cell sorter. Second, the role of NMU/NMUR1 on synovial MCs remains unclear. Finally, the reason NMUR2 expression was detected mainly in the CD88(−) MCs remains unclear.
4. Materials and Methods
4.1. Patients and Methods
All participants received total knee arthroplasty (TKA) at our hospital, during which time SM samples were extracted. In total, we extracted 206 SM samples from female patients with radiographic KOA. We promptly froze a small portion of each sample in liquid nitrogen and stored it at −80 °C in preparation for RNA extraction. Samples extracted from five obese KOA patients were used to examine NMU/NMUR expression in MCs.
The study protocol was approved by our institutional Ethics Review Board (approval number: KMEO B19–259). Written informed consent was obtained from all subjects for participation and the extraction of their synovial tissue one day before TKA. This study complied with the principles of the Declaration of Helsinki.
We grouped the patients according to the World Health Organization’s body mass index (BMI) definitions as follows: normal-weight (BMI < 25 kg/m2, n = 79), overweight (BMI ≥ 25 and <30 kg/m2, n = 87), and obese (≥30 kg/m2, n = 40). Expression levels of NMU/NMURs and the MC marker, CPA3, in the SM determined using real-time PCR were compared between pairs of the BMI groups.
4.2. qPCR
SM samples in TRIzol reagent (Invitrogen, Carlsbad, CA, USA) were homogenized using the polytron homogenizer. The samples were subsequently lysed in 1 mL of TRIzol mixed with 0.2 mL chloroform and vortexed for 30 s before being transferred to a MaXtract high-density tube (Qiagen, Valencia, CA, USA). Following a centrifugation step (12,000 rpm, 5 min), the resulting aqueous phase was mixed with an equal volume of isopropanol containing a precipitation carrier (Ethachinmate; Nippon Gene, Tokyo, Japan). After removing the supernatant, the RNA pellet was rinsed with 75% ethanol and subjected to centrifugation (15,000 rpm, 4 °C, 5 min). After removing the supernatant, the RNA pellet was left to dry before dissolving in RNase-free water. The total RNA concentration was determined with a spectrophotometer (Denovix, Tokyo, Japan). An OD 260/280 ratio greater than 1.8 was used for qPCR analysis. We also confirmed that gel electrophoresis showed clear bands of 28S and 18S. A 1-μg amount of the total RNA was subjected to a cDNA synthesis procedure using Superscript III based on the manufacturer’s protocol (Invitrogen). The qPCR procedure we adopted using SYBR Green is published in detail elsewhere [21,50]. The qPCR primer sequences are provided in Table 3. Gene expression (Gene/GAPDH) was determined using the delta-delta CT method. Relative expression was calculated when the average gene expression (Gene/GAPDH) level in the NW group was 1.

Table 3.
Sequences of primers used in this study.
4.3. Expression of NMU and NMURs in MCs
To evaluate NMU/NMUR expression in MCs, we extracted an MC-rich fraction (MC-RF) and MC-poor fraction (MC-PF) from 5 SM samples from obese KOA patients using magnetic isolation [27,28]. As distinct CD88(+) and CD88(−) MC subsets were previously found in SM samples taken from patients with OA and RA [31], we further divided the MC-RF into CD88(+) and CD88(−) MC-RFs.
To obtain these MC-RFs, fresh SM samples were promptly placed in a collagenase solution overnight for collagenase digestion. The following day, a portion of the cells extracted by this process were used to confirm cell viability by PI staining (cell viability, >90%). The remaining cells were incubated for 30 min at 4 °C with biotin-conjugated anti-THY-1 (synovial fibroblast marker), anti-CD3 (T lymphocyte marker), anti-CD14 (monocyte/macrophage marker), anti-CD19 (B lymphocyte marker), and anti-CD235a (erythroid cell marker) antibodies according to our previous studies [6,27,28]. All biotin-conjugated antibodies were purchased from BioLegend (San Diego, CA, USA). After rinsing twice with PBS, the cells were exposed to streptavidin-conjugated magnetic particles (BD Biosciences, San Jose, CA, USA) and placed into a magnetic separation system (BD Biosciences) for separation into negative (MC-RF; THY-1-, CD3-, CD14-, CD19-, and CD235-) and positive (MC-PF; THY-1+, CD3+, CD14+, CD19+, CD235+) fractions. Subsequently, the MC-RF was reacted with PE-conjugated anti-CD88 antibody (BioLegend) for 30 min at 4 °C. After rinsing twice with PBC, the cells were exposed to anti-PE magnetic particles (BD Biosciences, CA, USA) for separation into a CD88-negative fraction (CD88(−) MC-RF) and positive fraction (CD88(+) MC-RF). The cells in the MC-RF and CD88(−) MC-RF were then subjected to qPCR to examine NMU/NMUR expression. Gene expression (Gene/GAPDH) was determined using the delta-delta CT method. Relative expression was calculated when the average gene expression (Gene/GAPDH) level in MC-PF was 1.
4.4. Statistical Analyses
Statistical analyses were conducted using SPSS 25.0. All data were tested for normality using Shapiro–Wilk’s test. The Kruskal–Wallis test was used to compare gene expression among the three BMI groups. To create a matched cohort of NW and OB OA patients, we calculated each individual’s propensity score based on the baseline clinical variables, age, and proportion with Kellgren and Lawrence grade 2–4. Analysis of categorical variables was performed using Fisher’s exact test; the relationship between NMU/NMURs and CPA3 was determined using Spearman’s correlation coefficient; and comparison of gene expression between MC-RF and MC-PF was conducted using the Mann–Whitney U test. p < 0.05 was indicative of statistical significance.
5. Conclusions
In conclusion, NMUR1 expression was increased in the SM of obese OA patients and its expression was found in MCs. A further investigation to analyze NMU/NMUR1 pathway in MC may provide a link between obesity and KOA pathology.
Author Contributions
Conceptualization, G.I. and K.U.; methodology, K.T. and K.U.; validation, A.T., S.T., Y.O. and K.U.; formal analysis, M.M. and D.I.; investigation, J.A., D.I. and G.I.; resources, S.T., M.M., J.A. and D.I.; data curation, Y.O. and A.T.; writing—original draft preparation, A.T. and K.U.; writing—review and editing, M.T. and K.U.; visualization, A.T. and Y.O.; supervision, K.U.; project administration, K.U.; funding acquisition, S.T. and K.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Grant-in-Aid for Early-Career Scientists, grant number 20K18073, a Grant-in-Aid for Research Activity Start-up grant number 22K20965, and a Kitasato University Research Grant for Young Researchers.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Kitasato University (protocol code, B19-259; Date of approval, 27 January 2020) for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Yuko Onuki for her helpful support during the study. We thank Heidi Tran from DMC Corp. (http://www.dmed.co.jp, accessed on 24 August 2022) for editing a draft of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Grotle, M.; Hagen, K.B.; Natvig, B.; Dahl, F.A.; Kvien, T.K. Obesity and osteoarthritis in knee, hip and/or hand: An epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet. Disord. 2008, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Oliveria, S.A.; Felson, D.T.; Cirillo, P.A.; Reed, J.I.; Walker, A.M. Body weight, body mass index, and incident symptomatic osteoarthritis of the hand, hip, and knee. Epidemiology 1999, 10, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, Y.; Nakasa, T.; Kanemitsu, M.; Nekomoto, A.; Ishikawa, M.; Yimiti, D.; Miyaki, S.; Adachi, N. Therapeutic effect of targeting Substance P on the progression of osteoarthritis. Mod. Rheumatol. 2021, roab089. [Google Scholar] [CrossRef] [PubMed]
- Takano, S.; Uchida, K.; Inoue, G.; Matsumoto, T.; Aikawa, J.; Iwase, D.; Mukai, M.; Miyagi, M.; Takaso, M. Vascular endothelial growth factor expression and their action in the synovial membranes of patients with painful knee osteoarthritis. BMC Musculoskelet. Disord. 2018, 19, 204. [Google Scholar] [CrossRef]
- Takano, S.; Uchida, K.; Inoue, G.; Minatani, A.; Miyagi, M.; Aikawa, J.; Iwase, D.; Onuma, K.; Mukai, M.; Takaso, M. Increase and regulation of synovial calcitonin gene-related peptide expression in patients with painful knee osteoarthritis. J. Pain Res. 2017, 10, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Takano, S.; Inoue, G.; Iwase, D.; Aikawa, J.; Takata, K.; Tazawa, R.; Kawakubo, A.; Sekiguchi, H.; Takaso, M. Increase in mast cell marker expression in the synovium of obese patients with osteoarthritis of the knee. Diabetes Metab. Syndr. Obes. 2019, 12, 377–382. [Google Scholar] [CrossRef]
- Uchida, K.; Takano, S.; Takata, K.; Mukai, M.; Koyama, T.; Ohashi, Y.; Saito, H.; Takaso, M.; Miyagi, M.; Inoue, G. Differential Synovial CGRP/RAMP1 Expression in Men and Women With Knee Osteoarthritis. Cureus 2021, 13, e15483. [Google Scholar] [CrossRef]
- Minamino, N.; Kangawa, K.; Matsuo, H. Neuromedin U-8 and U-25: Novel uterus stimulating and hypertensive peptides identified in porcine spinal cord. Biochem. Biophys. Res. Commun. 1985, 130, 1078–1085. [Google Scholar] [CrossRef]
- Cardoso, V.; Chesne, J.; Ribeiro, H.; Garcia-Cassani, B.; Carvalho, T.; Bouchery, T.; Shah, K.; Barbosa-Morais, N.L.; Harris, N.; Veiga-Fernandes, H. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 2017, 549, 277–281. [Google Scholar] [CrossRef]
- Hedrick, J.A.; Morse, K.; Shan, L.; Qiao, X.; Pang, L.; Wang, S.; Laz, T.; Gustafson, E.L.; Bayne, M.; Monsma, F.J., Jr. Identification of a human gastrointestinal tract and immune system receptor for the peptide neuromedin U. Mol. Pharmacol. 2000, 58, 870–875. [Google Scholar] [CrossRef]
- Martinez, V.G.; O’Driscoll, L. Neuromedin U: A multifunctional neuropeptide with pleiotropic roles. Clin. Chem. 2015, 61, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, M.; Fukuyama, S.; Inoue, H.; Matsumoto, T.; Sato, T.; Tanaka, K.; Kinjyo, I.; Kano, T.; Yoshimura, A.; Kojima, M. The neuropeptide neuromedin U activates eosinophils and is involved in allergen-induced eosinophilia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 290, L971–L977. [Google Scholar] [CrossRef]
- Moriyama, M.; Matsukawa, A.; Kudoh, S.; Takahashi, T.; Sato, T.; Kano, T.; Yoshimura, A.; Kojima, M. The neuropeptide neuromedin U promotes IL-6 production from macrophages and endotoxin shock. Biochem. Biophys. Res. Commun. 2006, 341, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Hanada, R.; Teranishi, H.; Pearson, J.T.; Kurokawa, M.; Hosoda, H.; Fukushima, N.; Fukue, Y.; Serino, R.; Fujihara, H.; Ueta, Y.; et al. Neuromedin U has a novel anorexigenic effect independent of the leptin signaling pathway. Nat. Med. 2004, 10, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, T.J.; Spar, B.D.; Markowitz, L.; Maguire, M.; Golovko, A.; Yang, S.; Farley, C.; Cook, J.A.; Tetzloff, G.; Hoos, L.; et al. Transgenic overexpression of neuromedin U promotes leanness and hypophagia in mice. J. Endocrinol. 2005, 185, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.M.; Auger, J.L.; Gaillard, P.; Weissleder, R.; Wada, E.; Torres, R.; Kojima, M.; Benoist, C.; Mathis, D.; Binstadt, B.A. The neuropeptide neuromedin U promotes autoantibody-mediated arthritis. Arthritis Res. Ther. 2012, 14, R29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qin, Y.; Chen, Z. Neuromedin U Suppresses Collagen-Induced Arthritis through ILC2-Th2 Activation. J. Immunol. Res. 2021, 2021, 5599439. [Google Scholar] [CrossRef]
- Hsu, S.H.; Luo, C.W. Molecular dissection of G protein preference using Gsalpha chimeras reveals novel ligand signaling of GPCRs. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1021–E1029. [Google Scholar] [CrossRef]
- Ramirez, J.; Celis, R.; Usategui, A.; Ruiz-Esquide, V.; Fare, R.; Cuervo, A.; Sanmarti, R.; Pablos, J.L.; Canete, J.D. Immunopathologic characterization of ultrasound-defined synovitis in rheumatoid arthritis patients in clinical remission. Arthritis Res. Ther. 2016, 18, 74. [Google Scholar] [CrossRef]
- Rivellese, F.; Mauro, D.; Nerviani, A.; Pagani, S.; Fossati-Jimack, L.; Messemaker, T.; Kurreeman, F.A.S.; Toes, R.E.M.; Ramming, A.; Rauber, S.; et al. Mast cells in early rheumatoid arthritis associate with disease severity and support B cell autoantibody production. Ann. Rheum. Dis. 2018, 77, 1773–1781. [Google Scholar] [CrossRef]
- Rivellese, F.; Rossi, F.W.; Galdiero, M.R.; Pitzalis, C.; de Paulis, A. Mast Cells in Early Rheumatoid Arthritis. Int. J. Mol. Sci. 2019, 20, 2040. [Google Scholar] [CrossRef] [PubMed]
- Buckley, M.G.; Gallagher, P.J.; Walls, A.F. Mast cell subpopulations in the synovial tissue of patients with osteoarthritis: Selective increase in numbers of tryptase-positive, chymase-negative mast cells. J. Pathol. 1998, 186, 67–74. [Google Scholar] [CrossRef]
- De Lange-Brokaar, B.J.; Kloppenburg, M.; Andersen, S.N.; Dorjee, A.L.; Yusuf, E.; Herb-van Toorn, L.; Kroon, H.M.; Zuurmond, A.M.; Stojanovic-Susulic, V.; Bloem, J.L.; et al. Characterization of synovial mast cells in knee osteoarthritis: Association with clinical parameters. Osteoarthr. Cartil. 2016, 24, 664–671. [Google Scholar] [CrossRef]
- Dean, G.; Hoyland, J.A.; Denton, J.; Donn, R.P.; Freemont, A.J. Mast cells in the synovium and synovial fluid in osteoarthritis. Br. J. Rheumatol. 1993, 32, 671–675. [Google Scholar] [CrossRef]
- Farinelli, L.; Aquili, A.; Mattioli-Belmonte, M.; Manzotti, S.; D’Angelo, F.; Ciccullo, C.; Gigante, A. Synovial mast cells from knee and hip osteoarthritis: Histological study and clinical correlations. J. Exp. Orthop. 2022, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.; Harsulkar, A.; Martson, A.G.; Suutre, S.; Martson, A.; Koks, S. Mast Cells Differentiated in Synovial Fluid and Resident in Osteophytes Exalt the Inflammatory Pathology of Osteoarthritis. Int. J. Mol. Sci. 2022, 23, 541. [Google Scholar] [CrossRef] [PubMed]
- Takata, K.; Uchida, K.; Mukai, M.; Takano, S.; Aikawa, J.; Iwase, D.; Sekiguchi, H.; Miyagi, M.; Inoue, G.; Takaso, M. Increase in Tryptase and Its Role in the Synovial Membrane of Overweight and Obese Patients with Osteoarthritis of the Knee. Diabetes Metab. Syndr. Obes. 2020, 13, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Takata, K.; Uchida, K.; Takano, S.; Mukai, M.; Inoue, G.; Sekiguchi, H.; Aikawa, J.; Miyagi, M.; Iwase, D.; Takaso, M. Possible Regulation of bFGF Expression by Mast Cells in Osteoarthritis Patients with Obesity: A Cross-Sectional Study. Diabetes Metab. Syndr. Obes. 2021, 14, 3291–3297. [Google Scholar] [CrossRef]
- Lubbers, R.; van Essen, M.F.; van Kooten, C.; Trouw, L.A. Production of complement components by cells of the immune system. Clin. Exp. Immunol. 2017, 188, 183–194. [Google Scholar] [CrossRef]
- Yanase, Y.; Matsuo, Y.; Takahagi, S.; Kawaguchi, T.; Uchida, K.; Ishii, K.; Tanaka, A.; Matsubara, D.; Ozawa, K.; Hide, M. Coagulation factors induce human skin mast cell and basophil degranulation via activation of complement 5 and the C5a receptor. J. Allergy Clin. Immunol. 2021, 147, 1101–1104.e7. [Google Scholar] [CrossRef]
- Kiener, H.P.; Baghestanian, M.; Dominkus, M.; Walchshofer, S.; Ghannadan, M.; Willheim, M.; Sillaber, C.; Graninger, W.B.; Smolen, J.S.; Valent, P. Expression of the C5a receptor (CD88) on synovial mast cells in patients with rheumatoid arthritis. Arthritis Rheum. 1998, 41, 233–245. [Google Scholar] [CrossRef]
- Hu, W.; Wang, M.; Yin, C.; Li, S.; Liu, Y.; Xiao, Y. Serum complement factor 5a levels are associated with nonalcoholic fatty liver disease in obese children. Acta Paediatr. 2018, 107, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Bowers, E.; Singer, K. Obesity-induced inflammation: The impact of the hematopoietic stem cell niche. JCI Insight 2021, 6, e145295. [Google Scholar] [CrossRef]
- Kanthawang, T.; Bodden, J.; Joseph, G.B.; Lane, N.E.; Nevitt, M.; McCulloch, C.E.; Link, T.M. Obese and overweight individuals have greater knee synovial inflammation and associated structural and cartilage compositional degeneration: Data from the osteoarthritis initiative. Skelet. Radiol. 2021, 50, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Abbondanzo, S.J.; Manfra, D.J.; Chen, S.C.; Pinzon-Ortiz, M.; Sun, Y.; Phillips, J.E.; Laverty, M.; Vassileva, G.; Hu, W.; Yang, S.; et al. Nmur1-/- mice are not protected from cutaneous inflammation. Biochem. Biophys. Res. Commun. 2009, 378, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.N.; Appelbaum, E.R.; Carpenter, D.C.; Cox, R.F.; Disa, J.; Foley, J.J.; Ghosh, S.K.; Naselsky, D.P.; Pullen, M.A.; Sarau, H.M.; et al. Neuromedin U elicits cytokine release in murine Th2-type T cell clone D10.G4.1. J. Immunol. 2004, 173, 7230–7238. [Google Scholar] [CrossRef]
- Nedunchezhiyan, U.; Varughese, I.; Sun, A.R.; Wu, X.; Crawford, R.; Prasadam, I. Obesity, Inflammation, and Immune System in Osteoarthritis. Front. Immunol. 2022, 13, 907750. [Google Scholar] [CrossRef]
- Ghannadan, M.; Baghestanian, M.; Wimazal, F.; Eisenmenger, M.; Latal, D.; Kargul, G.; Walchshofer, S.; Sillaber, C.; Lechner, K.; Valent, P. Phenotypic characterization of human skin mast cells by combined staining with toluidine blue and CD antibodies. J. Investig. Dermatol. 1998, 111, 689–695. [Google Scholar] [CrossRef]
- Oskeritzian, C.A.; Zhao, W.; Min, H.K.; Xia, H.Z.; Pozez, A.; Kiev, J.; Schwartz, L.B. Surface CD88 functionally distinguishes the MCTC from the MCT type of human lung mast cell. J. Allergy Clin. Immunol. 2005, 115, 1162–1168. [Google Scholar] [CrossRef]
- Moriyama, M.; Sato, T.; Inoue, H.; Fukuyama, S.; Teranishi, H.; Kangawa, K.; Kano, T.; Yoshimura, A.; Kojima, M. The neuropeptide neuromedin U promotes inflammation by direct activation of mast cells. J. Exp. Med. 2005, 202, 217–224. [Google Scholar] [CrossRef]
- Fujii, R.; Hosoya, M.; Fukusumi, S.; Kawamata, Y.; Habata, Y.; Hinuma, S.; Onda, H.; Nishimura, O.; Fujino, M. Identification of neuromedin U as the cognate ligand of the orphan G protein-coupled receptor FM-3. J. Biol. Chem. 2000, 275, 21068–21074. [Google Scholar] [CrossRef] [PubMed]
- Graham, E.S.; Turnbull, Y.; Fotheringham, P.; Nilaweera, K.; Mercer, J.G.; Morgan, P.J.; Barrett, P. Neuromedin U and Neuromedin U receptor-2 expression in the mouse and rat hypothalamus: Effects of nutritional status. J. Neurochem. 2003, 87, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, M.; Moriya, T.; Kawamata, Y.; Ohkubo, S.; Fujii, R.; Matsui, H.; Shintani, Y.; Fukusumi, S.; Habata, Y.; Hinuma, S.; et al. Identification and functional characterization of a novel subtype of neuromedin U receptor. J. Biol. Chem. 2000, 275, 29528–29532. [Google Scholar] [CrossRef] [PubMed]
- Howard, A.D.; Wang, R.; Pong, S.S.; Mellin, T.N.; Strack, A.; Guan, X.M.; Zeng, Z.; Williams, D.L., Jr.; Feighner, S.D.; Nunes, C.N.; et al. Identification of receptors for neuromedin U and its role in feeding. Nature 2000, 406, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Raddatz, R.; Wilson, A.E.; Artymyshyn, R.; Bonini, J.A.; Borowsky, B.; Boteju, L.W.; Zhou, S.; Kouranova, E.V.; Nagorny, R.; Guevarra, M.S.; et al. Identification and characterization of two neuromedin U receptors differentially expressed in peripheral tissues and the central nervous system. J. Biol. Chem. 2000, 275, 32452–32459. [Google Scholar] [CrossRef]
- Szekeres, P.G.; Muir, A.I.; Spinage, L.D.; Miller, J.E.; Butler, S.I.; Smith, A.; Rennie, G.I.; Murdock, P.R.; Fitzgerald, L.R.; Wu, H.; et al. Neuromedin U is a potent agonist at the orphan G protein-coupled receptor FM3. J. Biol. Chem. 2000, 275, 20247–20250. [Google Scholar] [CrossRef]
- Westfall, T.D.; McCafferty, G.P.; Pullen, M.; Gruver, S.; Sulpizio, A.C.; Aiyar, V.N.; Disa, J.; Contino, L.C.; Mannan, I.J.; Hieble, J.P. Characterization of neuromedin U effects in canine smooth muscle. J. Pharmacol. Exp. Ther. 2002, 301, 987–992. [Google Scholar] [CrossRef]
- Yu, X.H.; Cao, C.Q.; Mennicken, F.; Puma, C.; Dray, A.; O’Donnell, D.; Ahmad, S.; Perkins, M. Pro-nociceptive effects of neuromedin U in rat. Neuroscience 2003, 120, 467–474. [Google Scholar] [CrossRef]
- Shan, L.; Qiao, X.; Crona, J.H.; Behan, J.; Wang, S.; Laz, T.; Bayne, M.; Gustafson, E.L.; Monsma, F.J., Jr.; Hedrick, J.A. Identification of a novel neuromedin U receptor subtype expressed in the central nervous system. J. Biol. Chem. 2000, 275, 39482–39486. [Google Scholar] [CrossRef]
- Noordenbos, T.; Yeremenko, N.; Gofita, I.; van de Sande, M.; Tak, P.P.; Canete, J.D.; Baeten, D. Interleukin-17-positive mast cells contribute to synovial inflammation in spondylarthritis. Arthritis Rheum. 2012, 64, 99–109. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).