Necroptosis Contributes to LPS-Induced Activation of the Hypothalamic-Pituitary-Adrenal Axis in a Piglet Model
Abstract
1. Introduction
2. Results
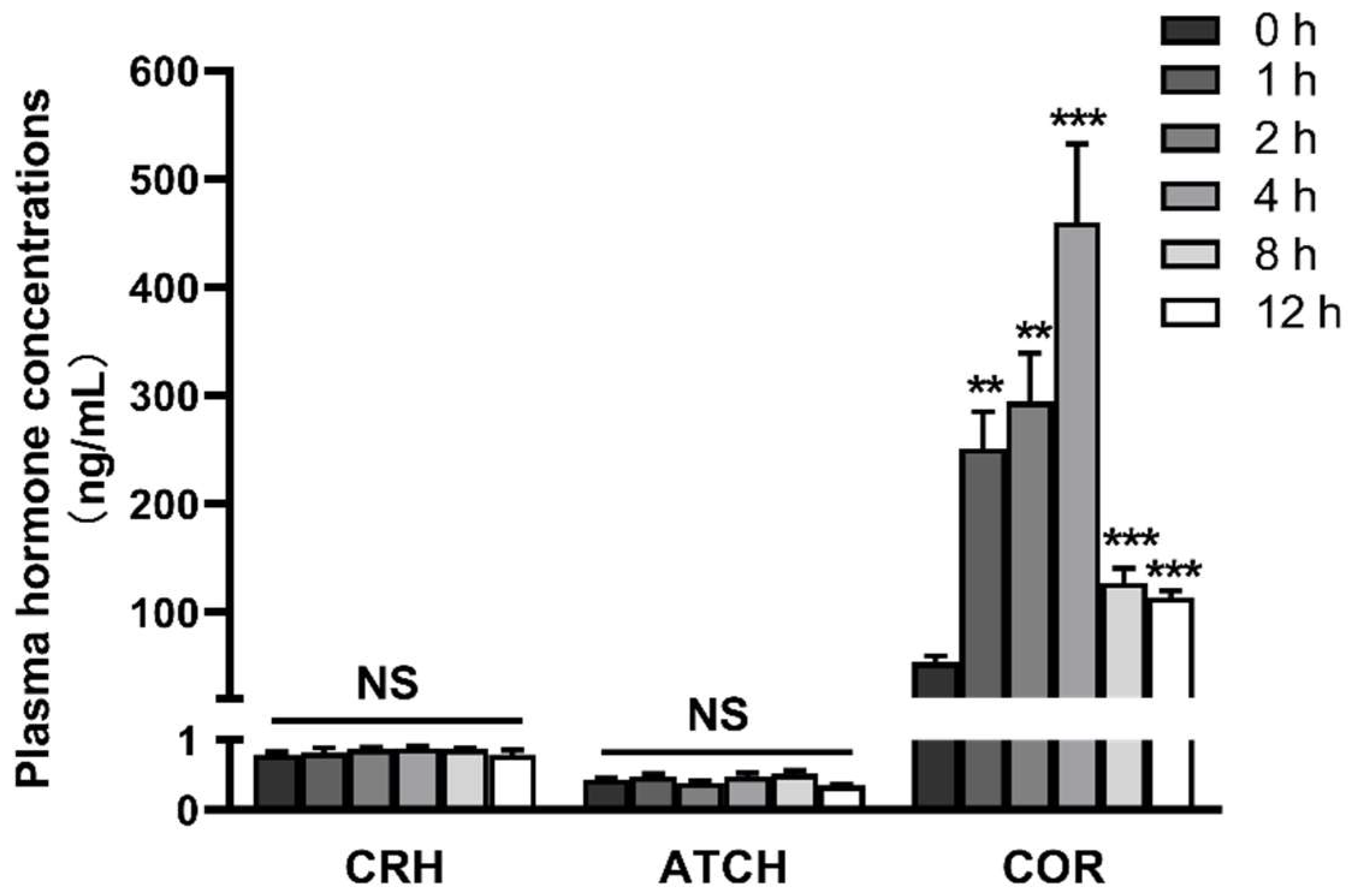
2.1. LPS Induces Activation of the Hypothalamic-Pituitary-Adrenal Axis
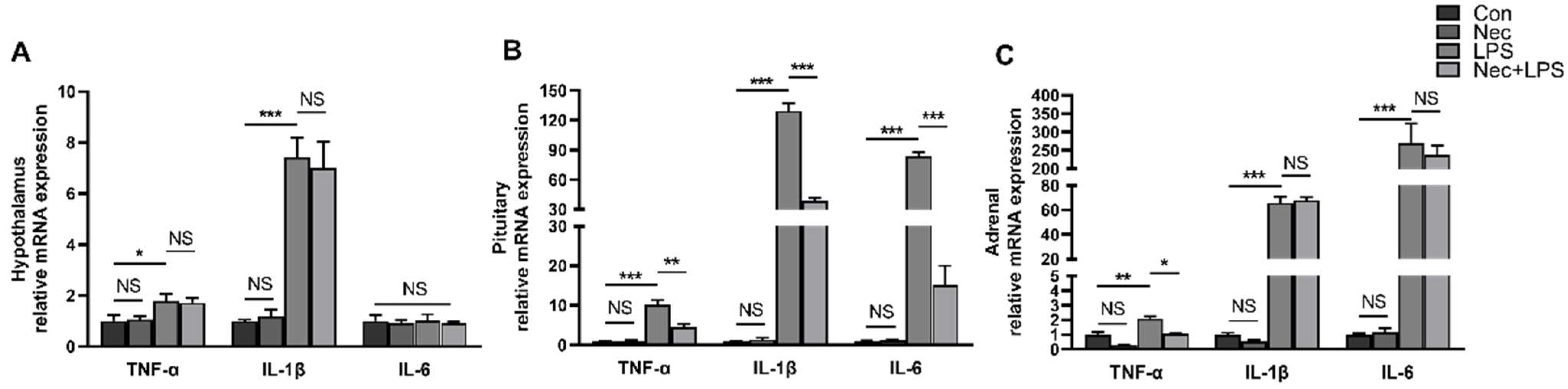
2.2. LPS Induces Dynamic Inflammation of Hypothalamus, Pituitary Gland and Adrenal Gland
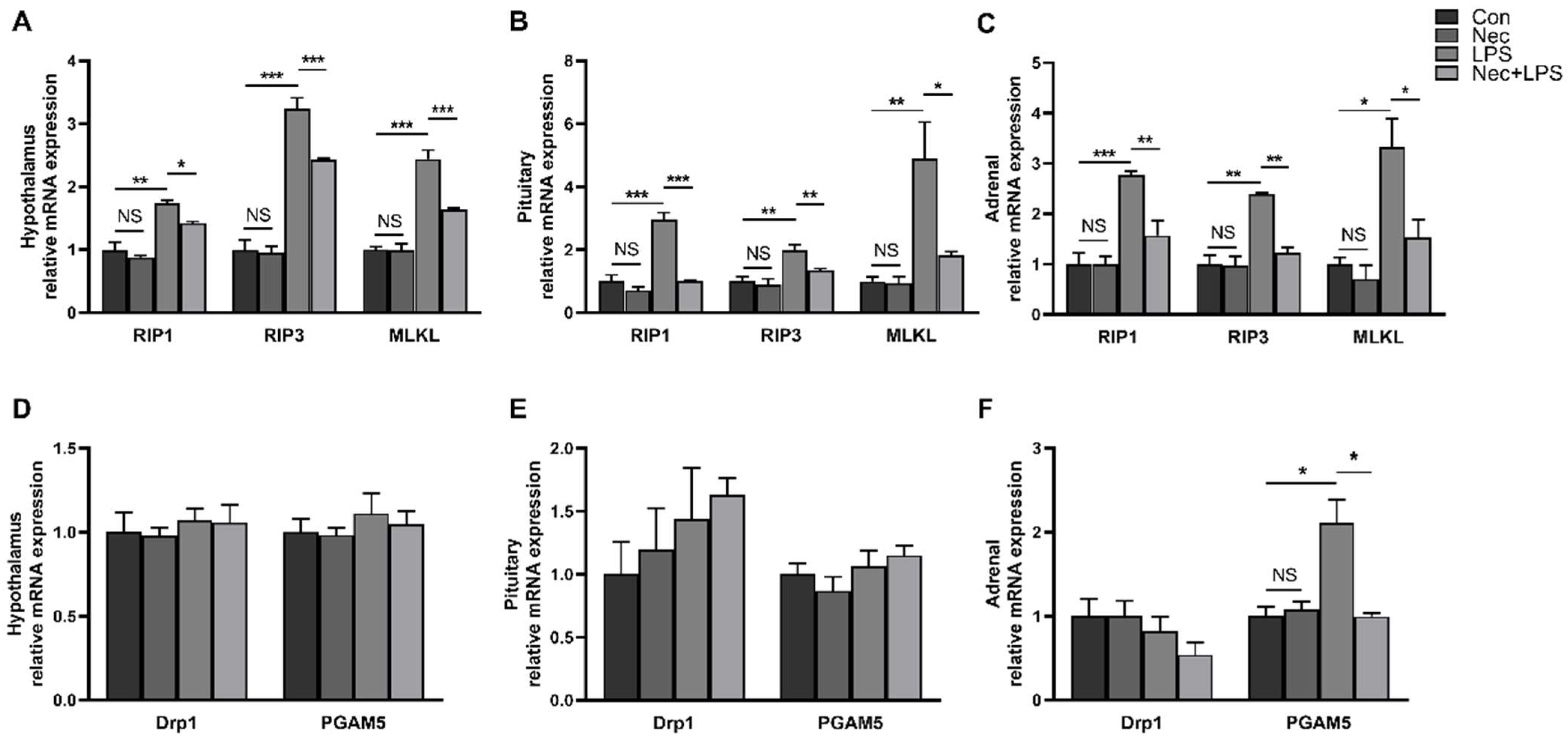
2.3. LPS Induces Dynamic Necroptosis of Hypothalamus, Pituitary Gland and Adrenal Gland
2.4. Nec-1 Inhibits Cell Necroptosis of Hypothalamus, Pituitary Gland and Adrenal Gland
2.5. Nec-1 Alleviates LPS-Induced Inflammation of Pituitary Gland and Adrenal Gland
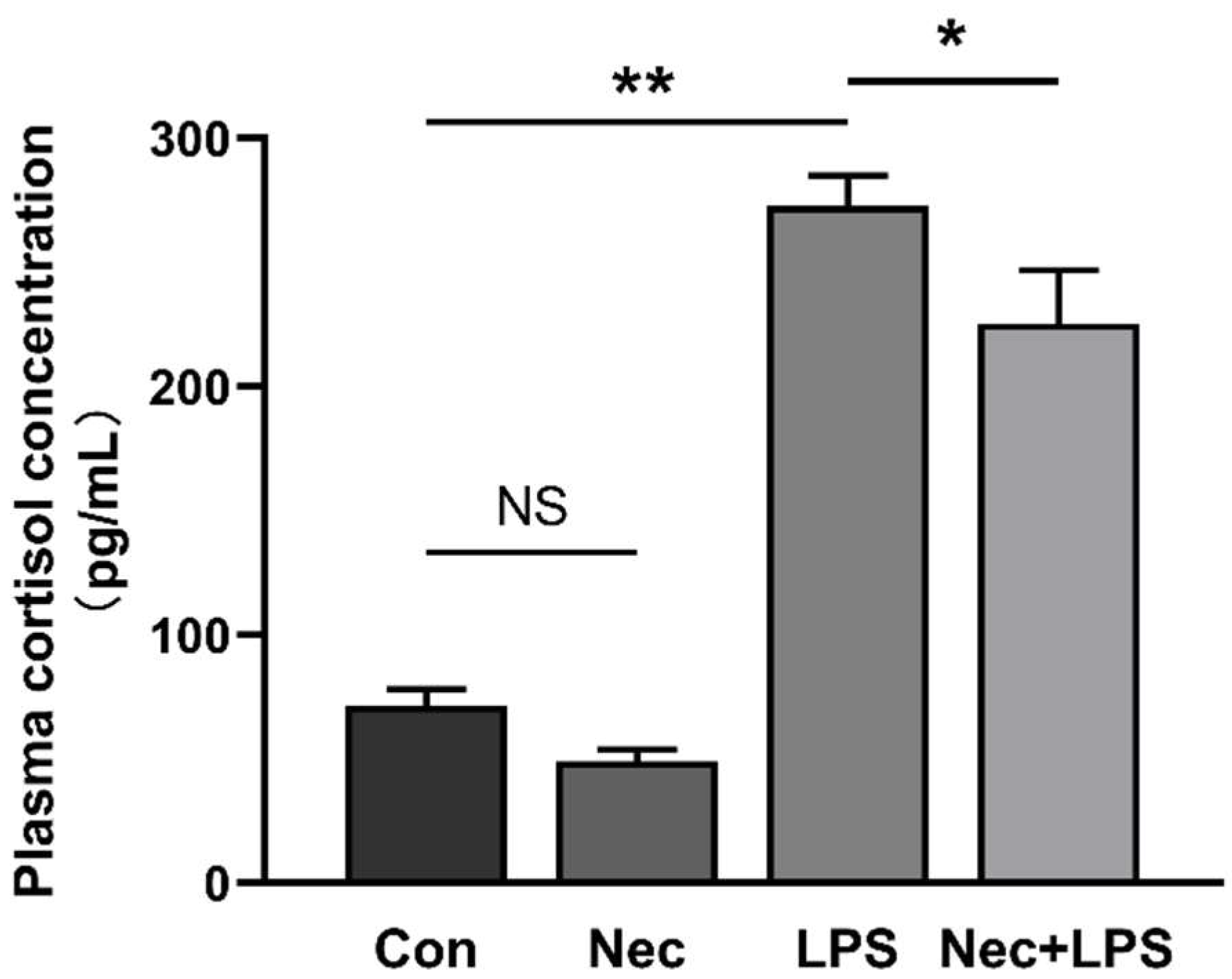
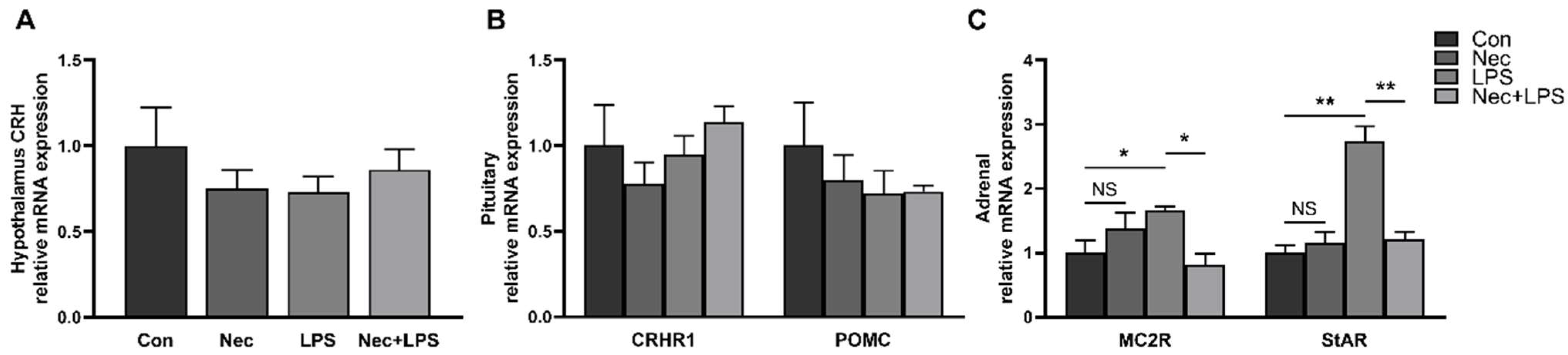
2.6. Inhibition of Necroptosis by Nec-1 Effectively Inhibits LPS-Induced Activation of HPA Axis
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Blood and Tissue Sample Collection
4.4. Measurement of Plasma Hormone Concentrations
4.5. Quantitative Real-Time PCR
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Hotchkiss, R.S.; Karl, I.E. Medical progress: The Pathophysiology and Treatment of Sepsis. N. Engl. J. Med. 2003, 348, 138–150. [Google Scholar] [CrossRef]
- Cavaillon, J.M. Exotoxins and Endotoxins: Inducers of Inflammatory Cytokines. Toxicon 2018, 149, 45–53. [Google Scholar] [CrossRef]
- Haddad, J.J.; Saade, N.E.; Safieh-Garabedian, B. Cytokines and Neuro-Immune-Endocrine Interactions: A Role for the Hypothalamic-Pituitary-Adrenal Revolving Axis. J. Neuroimmunol. 2002, 133, 1–19. [Google Scholar] [CrossRef]
- Leistner, C.; Menke, A. Hypothalamic-Pituitary-Adrenal Axis and Stress. Handb. Clin. Neurol. 2020, 175, 55–64. [Google Scholar]
- Fricker, M.; Tolkovsky, A.M.; Borutaite, V.; Coleman, M.; Brown, G.C. Neuronal Cell Death. Physiol. Rev. 2018, 98, 813–880. [Google Scholar] [CrossRef]
- Gorman, A.M. Neuronal Cell Death in Neurodegenerative Diseases: Recurring Themes Around Protein Handling. J. Cell Mol. Med. 2008, 12, 2263–2280. [Google Scholar] [CrossRef]
- Bredesen, D.E.; Rao, R.V.; Mehlen, P. Cell Death in the Nervous System. Nature 2006, 443, 796–802. [Google Scholar] [CrossRef]
- Arrazola, M.S.; Saquel, C.; Catalan, R.J.; Barrientos, S.A.; Hernandez, D.E.; Martinez, N.W.; Alejandra, C.; Felipe, A.C. Axonal Degeneration Is Mediated by Necroptosis Activation. J. Neurosci. 2019, 39, 3832–3844. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, J.; Geng, R.; Ge, W.; Zhou, Y.; Zhang, C.; Cheng, Y.; Geng, D. The Inhibition of ERK Activation Mediates the Protection of Necrostatin-1 on Glutamate Toxicity in HT-22 Cells. Neurotox Res. 2013, 24, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Yu, Z.; Zhang, Z.; Zhang, J.; Zhang, P.; Li, X.; Li, H.; Shen, H.; Chen, G. RIP3 Participates in Early Brain Injury after Experimental Subarachnoid Hemorrhage in Rats by Inducing Necroptosis. Neurobiol Dis 2019, 129, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Belizario, J.; Vieira-Cordeiro, L.; Enns, S. Necroptotic Cell Death Signaling and Execution Pathway: Lessons from Knockout Mice. Mediat. Inflamm. 2015, 2015, 128076. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, K.; Luz, N.F.; Balaji, S.; Rosa, M.J.D.; O’Donnell, C.L.; Gough, P.J.; Bertin, J.; Welsh, R.M.; Chan, F.K. The Mitochondrial Phosphatase PGAM5 Is Dispensable for Necroptosis but Promotes Inflammasome Activation in Macrophages. J. Immunol. 2016, 196, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jiang, H.; Chen, S.; Du, F.; Wang, X. The Mitochondrial Phosphatase PGAM5 Functions at the Convergence Point of Multiple Necrotic Death Pathways. Cell 2012, 148, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Ofengeim, D.; Najafov, A.; Das, S.; Saberi, S.; Li, Y.; Hitomi, J.; Zhu, H.; Chen, H.; Mayo, L.; et al. RIPK1 Mediates Axonal Degeneration by Promoting Inflammation and Necroptosis in ALS. Science 2016, 353, 603–608. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Li, D.; Wu, J.; Si, W.; Wu, Y. Necrostatin-1 Attenuates Inflammatory Response and Improves Cognitive Function in Chronic Ischemic Stroke Mice. Medicines 2016, 3, 16. [Google Scholar] [CrossRef]
- Degterev, A.; Hitomi, J.; Germscheid, M.; Ch’en, I.L.; Korkina, O.; Teng, X.; Abbott, D.; Cuny, G.D.; Yuan, C.; Wagner, G.; et al. Identification of RIP1 Kinase as a Specific Cellular Target of Necrostatins. Nat. Chem. Biol. 2008, 4, 313–321. [Google Scholar] [CrossRef]
- Dillon, C.P.; Weinlich, R.; Rodriguez, D.A.; Cripps, J.G.; Quarato, G.; Gurung, P.; Verbist, K.C.; Brewer, T.L.; Llambi, F.; Gong, Y.; et al. RIPK1 Blocks Early Postnatal Lethality Mediated by Caspase-8 and RIPK3. Cell 2014, 157, 1189–1202. [Google Scholar] [CrossRef]
- Re, D.B.; Le, V.V.; Yu, C.H.; Amoroso, M.W.; Politi, K.A.; Phani, S.; Ikiz, B.; Hoffmann, L.; Koolen, M.; Nagata, T.; et al. Necroptosis Drives Motor Neuron Death in Models of Both Sporadic and Familial ALS. Neuron 2014, 81, 1001–1008. [Google Scholar] [CrossRef]
- Hartling, C.; Fan, Y.; Weigand, A.; Trilla, I.; Gartner, M.; Bajbouj, M.; Dziobek, I.; Grimm, S. Interaction of HPA Axis Genetics and Early Life Stress Shapes Emotion Recognition in Healthy Adults. Psychoneuroendocrinology 2019, 99, 28–37. [Google Scholar] [CrossRef]
- Kruis, W.; Schiff, M. Combination Treatment with Antibiotics and Glucocorticosteroids for Severe Ischemic Colitis. Digestion 2020, 101, 500–505. [Google Scholar] [CrossRef]
- Xu, X.; Chen, S.K.; Wang, H.B.; Tu, Z.X.; Wang, S.H.; Wang, X.Y.; Zhu, H.; Wang, C.; Zhu, J.; Liu, Y. Medium-Chain TAG Improve Intestinal Integrity by Suppressing Toll-like Receptor 4, Nucleotide-Binding Oligomerisation Domain Proteins and Necroptosis Signalling in Weanling Piglets Challenged with Lipopolysaccharide. Brit. J. Nutr. 2018, 119, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.M.; Tu, Z.X.; Wang, H.B.; Wang, S.H.; Wang, X.Y.; Zhu, H.L.; Andy, C.A.; Liu, Y. Flaxseed Oil Improves Liver Injury and Inhibits Necroptotic and Inflammatory Signaling Pathways Following Lipopolysaccharide Challenge in a Piglet Model. J. Funct. Foods 2018, 46, 482–489. [Google Scholar] [CrossRef]
- Wexler, B.C.; Dolgin, A.E.; Tryczynski, E.W. Effects of a Bacterial Polysaccharide (piromen) on the Pituitary-Adrenal Axis: Adrenal Ascorbic Acid, Cholesterol and Histologic Alterations. Endocrinology 1957, 61, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Beishuizen, A.; Thijs, L.G. Endotoxin and the Hypothalamo-Pituitary-Adrenal (HPA) Axis. J. Endotoxin Res. 2003, 9, 3–24. [Google Scholar]
- Becher, B.; Spath, S.; Goverman, J. Cytokine Networks in Neuroinflammation. Nat. Rev. Immunol. 2017, 17, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Robinson, S.M. Minimal Penetration of Lipopolysaccharide Across the Murine Blood-Brain Barrier. Brain Behav. Immun. 2010, 24, 102–109. [Google Scholar] [CrossRef]
- Pan, W.; Ding, Y.; Yu, Y.; Ohtaki, H.; Nakamachi, T.; Kastin, A.J. Stroke Upregulates TNFalpha Transport Across the Blood-Brain Barrier. Exp. Neurol. 2006, 198, 222–233. [Google Scholar] [CrossRef]
- Qin, L.; Wu, X.; Block, M.L.; Liu, Y.; Breese, G.R.; Hong, J.S.; Knapp, D.J.; Crews, F.T. Systemic LPS Causes Chronic Neuroinflammation and Progressive Neurodegeneration. Glia 2007, 55, 453–462. [Google Scholar] [CrossRef]
- Rivier, C.; Chizzonite, R.; Vale, W. In the Mouse, the Activation of the Hypothalamic-Pituitary-Adrenal Axis by a Lipopolysaccharide (endotoxin) Is Mediated Through Interleukin-1. Endocrinology 1989, 125, 2800–2805. [Google Scholar] [CrossRef]
- Leonard, B.E. Inflammation and Depression: A Causal or Coincidental Link to the Pathophysiology? Acta Neuropsychiatr. 2018, 30, 1–16. [Google Scholar] [CrossRef]
- Lopez, R.B.; Denny, B.T.; Fagundes, C.P. Neural Mechanisms of Emotion Regulation and Their Role in Endocrine and Immune Functioning: A Review with Implications for Treatment of Affective Disorders. Neurosci. Biobehav. Rev. 2018, 95, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Duprez, L.; Takahashi, N.; Hauwermeiren, F.V.; Vandendriessche, B.; Goossens, V.; Berghe, T.V.; Declercq, W.; Libert, C.; Cauwels, A.; Vandenabeele, P. RIP Kinase-Dependent Necrosis Drives Lethal Systemic Inflammatory Response Syndrome. Immunity 2011, 35, 908–918. [Google Scholar] [CrossRef]
- Ofengeim, D.; Mazzitelli, S.; Ito, Y.; DeWitt, J.P.; Mifflin, L.; Zou, C.; Das, S.; Adiconis, X.; Chen, H.; Zhu, H.; et al. RIPK1 Mediates a Disease-Associated Microglial Response in Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2017, 114, E8788–E8797. [Google Scholar] [CrossRef]
- Murakami, Y.; Matsumoto, H.; Roh, M.; Giani, A.; Kataoka, K.; Morizane, Y.; Kayama, M.; Thanos, A.; Nakatake, S.; Notomi, S.; et al. Programmed Necrosis, Not Apoptosis, Is a Key Mediator of Cell Loss and DAMP-Mediated Inflammation in dsRNA-Induced Retinal Degeneration. Cell Death Differ. 2014, 21, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.R.; Kim, J.H.; Park, H.L.; Park, C.K. Ischemia Reperfusion Injury Triggers TNFalpha Induced-Necroptosis in Rat Retina. Curr. Eye Res. 2017, 42, 771–779. [Google Scholar] [CrossRef]
- Caccamo, A.; Branca, C.; Piras, I.S.; Ferreira, E.; Huentelman, M.J.; Liang, W.S.; Readhead, B.; Dudley, J.T.; Spangenberg, E.E.; Green, K.N.; et al. Necroptosis Activation in Alzheimer’s Disease. Nat. Neurosci. 2017, 20, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
- You, Z.; Savitz, S.I.; Yang, J.; Degterev, A.; Yuan, J.; Cuny, G.D.; Moskowitz, M.A.; Whalen, M.J. Necrostatin-1 Reduces Histopathology and Improves Functional Outcome after Controlled Cortical Impact in Mice. J. Cereb. Blood Flow Metab. 2008, 28, 1564–1573. [Google Scholar] [CrossRef]
- Yuan, J.; Amin, P.; Ofengeim, D. Necroptosis and RIPK1-Mediated Neuroinflammation in CNS Diseases. Nat. Rev. Neurosci. 2019, 20, 19–33. [Google Scholar] [CrossRef]
- King, M.D.; Whitaker-Lea, W.A.; Campbell, J.M.; Alleyne, C.H., Jr.; Dhandapani, K.M. Necrostatin-1 Reduces Neurovascular Injury after Intracerebral Hemorrhage. Int. J. Cell Biol. 2014, 2014, 495817. [Google Scholar] [CrossRef]
- Polykratis, A.; Hermance, N.; Zelic, M.; Roderick, J.; Kim, C.; Van, T.M.; Lee, T.H.; Chan, F.K.M.; Pasparakis, M.; Kelliher, M.A. Cutting Edge: RIPK1 Kinase Inactive Mice are Viable and Protected from TNF-Induced Necroptosis in vivo. J. Immunol. 2014, 193, 1539–1543. [Google Scholar] [CrossRef]
- Newton, K.; Dugger, D.L.; Maltzman, A.; Greve, J.M.; Hedehus, M.; Martin-McNulty, B.; Carano, R.A.D.; Cao, T.C.; van Bruggen, N.; Bernstein, L.; et al. RIPK3 Deficiency or Catalytically Inactive RIPK1 Provides Greater Benefit than MLKL Deficiency in Mouse Models of Inflammation and Tissue Injury. Cell Death Differ. 2016, 23, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Tarkowski, E.; Andreasen, N.; Tarkowski, A.; Blennow, K. Intrathecal Inflammation Precedes Development of Alzheimer’s Disease. J. Neurol. Neurosur. Psychiatry 2003, 74, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Bowles, K.R.; Silva, M.C.; Whitney, K.; Bertucci, T.; Berlind, J.E.; Lai, J.D.; Garza, J.C.; Boles, N.C.; Mahali, S.; Strang, K.H.; et al. ELAVL4, Splicing, and Glutamatergic Dysfunction Precede Neuron Loss in MAPT Mutation Cerebral Organoids. Cell 2021, 184, 4547–4563. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.M.; Chen, Q.X.; Chen, Z.B.; Tian, D.F.; Li, M.C.; Wang, J.M.; Wang, L.; Liu, B.H.; Zhang, S.Q.; Li, F.; et al. RIP3 Deficiency Protects Against Traumatic Brain Injury (TBI) Through Suppressing Oxidative Stress, Inflammation and Apoptosis: Dependent on AMPK Pathway. Biochem. Biophys. Res. Commun. 2018, 499, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Ofengeim, D.; Yuan, J.Y. Regulation of RIP1 Kinase Signalling at the Crossroads of Inflammation and Cell Death. Nat. Rev. Mol. Cell Biol. 2013, 14, 727–736. [Google Scholar] [CrossRef]
- Ofengeim, D.; Ito, Y.; Najafov, A.; Zhang, Y.; Shan, B.; DeWitt, J.P.; Ye, J.; Zhang, X.; Chang, A.; Vakifahmetoglu-Norberg, H.; et al. Activation of Necroptosis in Multiple Sclerosis. Cell Rep. 2015, 10, 1836–1849. [Google Scholar] [CrossRef]
- Iannielli, A.; Bido, S.; Folladori, L.; Segnali, A.; Cancellieri, C.; Maresca, A.; Massimino, L.; Rubio, A.; Morabito, G.; Caporali, L.; et al. Pharmacological Inhibition of Necroptosis Protects from Dopaminergic Neuronal Cell Death in Parkinson’s Disease Models. Cell Rep. 2018, 22, 2066–2079. [Google Scholar] [CrossRef]
- Vandenabeele, P.; Grootjans, S.; Callewaert, N.; Takahashi, N. Necrostatin-1 Blocks both RIPK1 and IDO: Consequences for the Study of Cell Death in Experimental Disease Models. Cell Death Differ. 2013, 20, 185–187. [Google Scholar] [CrossRef]
- Wang, Y.X.; Lawson, M.A.; Dantzer, R.; Kelley, K.W. LPS-Induced Indoleamine 2,3-dioxygenase is Regulated in an Interferon-Gamma-Independent Manner by a JNK Signaling Pathway in Primary Murine Microglia. Brain Behav. Immun. 2010, 24, 201–209. [Google Scholar] [CrossRef]
- Zhou, H.R.; Islam, Z.; Pestka, J.J. Kinetics of lipopolysaccharide-induced transcription factor activation inactivation and relation to proinflammatory gene expression in the murine spleen. Toxicol. Appl. Pharmacol. 2003, 187, 147–161. [Google Scholar] [CrossRef]
- Gaines, A.M.; Carroll, J.A.; Yi, G.F.; Allee, G.L.; Zannelli, M.E. Effect of menhaden fish oil supplementation and lipopolysaccharide exposure on nursery pigs II. Effects on the immune axis when fed simple or complex diets containing no spray-dried plasma. Domest. Anim. Endocrinol. 2003, 24, 353–365. [Google Scholar] [CrossRef]
- Liu, Y.L.; Chen, F.; Odle, J.; Lin, X.; Jacobi, S.K.; Zhu, H.L.; Wu, Z.F.; Hou, Y.Q. Fish Oil Enhances Intestinal Integrity and Inhibits TLR4 and NOD2 Signaling Pathways in Weaned Pigs after LPS Challenge. J. Nutr. 2012, 142, 2017–2024. [Google Scholar] [CrossRef] [PubMed]



| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| TNF-α | AAGACACCATGAGCACTGAGA | CGACCAGGAGGAAGGAGAAG |
| IL-1β | GCTAACTACGGTGACAACAATAATG | CTTCTCCACTGCCACGATGA |
| IL-6 | AAGGTGATGCCACCTCAGAC | TCTGCCAGTACCTCCTTGCT |
| RIP1 | ACATCCTGTACGGCAACTCT | CGGGTCCAGGTGTTTATCC |
| RIP3 | CTTGTTGTCTGTCCGTGAGC | GAGGAGGTTGGGCTGTTGA |
| MLKL | TCTCGCTGCTGCTTCA | CTCGCTTGTCTTCCTCTG |
| DRP1 | TGTGGGCTGCAGGTCATTA | TTGCGCTGGGACATTTTAGC |
| PGAM5 | TCTTCATCTGCCACGCCAAT | GGTGATGCTGCCGTTGTTG |
| CRH | CCGCCAGGAGGCACCCGAGAGG | GCCAAACGCACCGTTTCACTTC |
| CRHR1 | CTCATCTCCGCCTTCATCCT | CCAAACCAGCACTTCTCATT |
| POMC | AGTAACTTGCTGGCGTGCAT | GAAGTGGCCCATGACGTACT |
| MC2R | TGTTCCCGCTGATGCTGGTGTT | GGGGTCAGCTGGGCAGAGTGTC |
| StAR | GGAGAGCCGGCAGGAGAATG | CTTCTGCAGGATCTTGATCTTCTTG |
| GAPDH | CGTCCCTGAGACACGATGGT | GCCTTGACTGTGCCGTGGAAT |
| β-actin | TGCGGGACATCAAGGAGAAG | AGTTGAAGGTGGTCTCGTGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, B.; Xu, Q.; Guo, J.; Chen, Q.; Lv, Q.; Xiao, K.; Zhu, H.; Zhao, J.; Liu, Y. Necroptosis Contributes to LPS-Induced Activation of the Hypothalamic-Pituitary-Adrenal Axis in a Piglet Model. Int. J. Mol. Sci. 2022, 23, 11218. https://doi.org/10.3390/ijms231911218
Zhou B, Xu Q, Guo J, Chen Q, Lv Q, Xiao K, Zhu H, Zhao J, Liu Y. Necroptosis Contributes to LPS-Induced Activation of the Hypothalamic-Pituitary-Adrenal Axis in a Piglet Model. International Journal of Molecular Sciences. 2022; 23(19):11218. https://doi.org/10.3390/ijms231911218
Chicago/Turabian StyleZhou, Bei, Qilong Xu, Junjie Guo, Qinliang Chen, Qingqing Lv, Kan Xiao, Huiling Zhu, Jiangchao Zhao, and Yulan Liu. 2022. "Necroptosis Contributes to LPS-Induced Activation of the Hypothalamic-Pituitary-Adrenal Axis in a Piglet Model" International Journal of Molecular Sciences 23, no. 19: 11218. https://doi.org/10.3390/ijms231911218
APA StyleZhou, B., Xu, Q., Guo, J., Chen, Q., Lv, Q., Xiao, K., Zhu, H., Zhao, J., & Liu, Y. (2022). Necroptosis Contributes to LPS-Induced Activation of the Hypothalamic-Pituitary-Adrenal Axis in a Piglet Model. International Journal of Molecular Sciences, 23(19), 11218. https://doi.org/10.3390/ijms231911218







