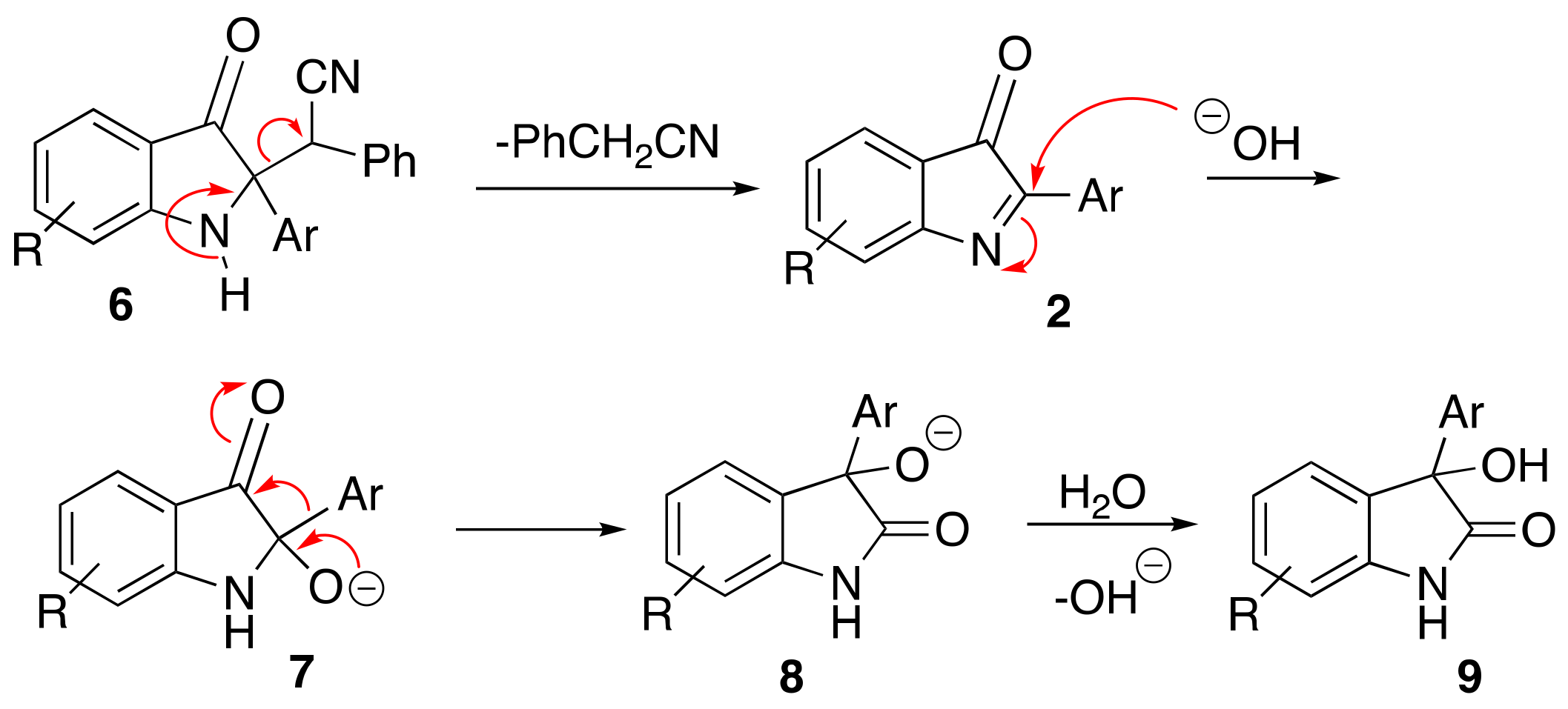
3. Methods and Materials
General
NMR spectra,
1H and
13C were measured in solutions of CDCl
3 or DMSO-
d6 on a Bruker AVANCE-III HD instrument (at 400.40 or 100.61 MHz, respectively). Residual solvent signals were used as internal standards, in DMSO-
d6 (2.50 ppm for
1H, and 40.45 ppm for
13C nuclei) or in CDCl
3 (7.26 ppm for
1H, and 77.16 ppm for
13C nuclei). High-resolution mass spectra were registered with a Bruker Maxis spectrometer (electrospray ionization, in MeCN solution, using HCO
2Na–HCO
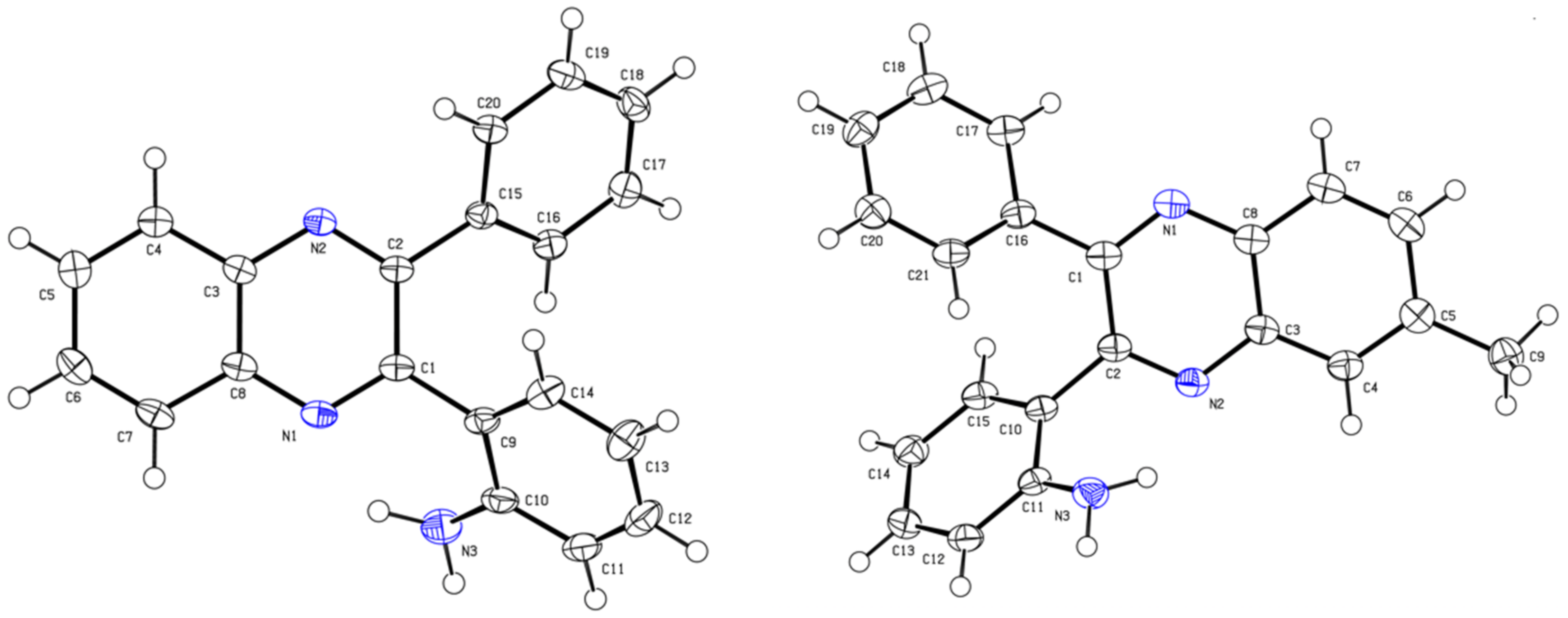
2H for calibration). Single crystal X-ray diffraction analysis of compounds
4aa (C
20H
15N
3) and
4′ba (C
21H
17N
3) was performed on an automatic four-circle diffractometer Agilent Super Nova, Dual, Cu at zero, Atlas S2 at 100(2) K. See
Supplementary Materials for NMR (
Figures S1–S25), HRMS (
Figures S26–S37) spectral charts and X-ray analysis data (
Figures S38 and S39 and Tables S1–S14). IR spectra were measured on FT-IR spectrometer Shimadzu IR Affinity-1S equipped with an ATR sampling module. Melting points were measured with a Stuart SMP30 apparatus. MW-assisted reactions were conducted in G10 and G30 vials using an Anton Paar Monowave 300 reactor with automatic temperature control. Reaction progress and purity of isolated compounds were controlled by TLC on ALUGRAM Xtra SIL G UV 254 plates. Column chromatography was performed with Macherey Nagel Silica gel 60 (particle size: 0.063–0.2 mm). 2-Phenyl-3
H-indol-3-one (
2) [
24], 2,4’-diaryl-4’
H-spiro[indole- 3,5’-isoxazole] (
5a) [
21], 4’-phenyl-2-(
p-tolyl)-4’
H-spiro[indole-3,5’-isoxazole] (
5b) [
21], 2-(4-methoxyphenyl)-4’-phenyl-4’
H-spiro[indole-3,5’-isoxazole] (
5c) [
2]
, 2-(naphthalen-2- yl)-4’-phenyl-4’
H-spiro[indole-3,5’-isoxazole] (
5d) [
21], 4’-phenyl-2-(5,6,7,8-tetrahydronaphthalen-2-yl)-4’
H-spiro[indole-3,5’-isoxazole] (
5e) [
21], 2-(2,3-dihydrobenzo[
b][
1,
4]dioxin-6-yl)-4’-phenyl-4’
H-spiro[indole-3,5’-isoxazole] (
5f) [
21], 2-methyl-4’-phenyl-4’
H- spiro[indole-3,5’-isoxazole] (
5g) [
21], 4’-phenyl-2-(thiophen-2-yl)-4’
H-spiro[indole- 3,5’-isoxazole] (
5h) [
21], 5-isopropyl-2,4’-diphenyl-4’
H-spiro[indole-3,5’-isoxazole] (
5i) [
21], 2-(3-oxo-2-phenylindolin-2-yl)-2-phenylacetonitrile (
6a) [
21], 2-(3-oxo-2-(
p-tolyl)indolin-2-yl)-2-phenylacetonitrile (
6b) [
21], 2-(2-(4-methoxyphenyl)-3-oxoindolin-2-yl)- 2-phenylacetonitrile (
6c) [
21], 2-(2-(naphthalen-2-yl)-3-oxoindolin-2-yl)-2-phenylacetonitrile (
6d) [
21], 2-(3-oxo-2-(5,6,7,8-tetrahydronaphthalen-2-yl)indolin-2-yl)-2-phenylacetonitrile (
6e) [
21], 2-(2-(2,3-dihydrobenzo[
b][
1,
4]dioxin-6-yl)-3-oxoindolin-2-yl)-2- phenylacetonitrile (
6f) [
21], 2-(2-methyl-3-oxoindolin-2-yl)-2-phenylacetonitrile (
6g) [
21], 2-(3-oxo-2-(thiophen-2-yl)indolin-2-yl)-2-phenylacetonitrile (
6h) [
21]
, 2-(5-isopropyl-3- oxo-2-phenylindolin-2-yl)-2-phenylacetonitrile (
6i) [
21] were synthesized according to procedures published in our recent reports and were identical to those were described. All other reagents and solvents were purchased from commercial vendors and used as received.
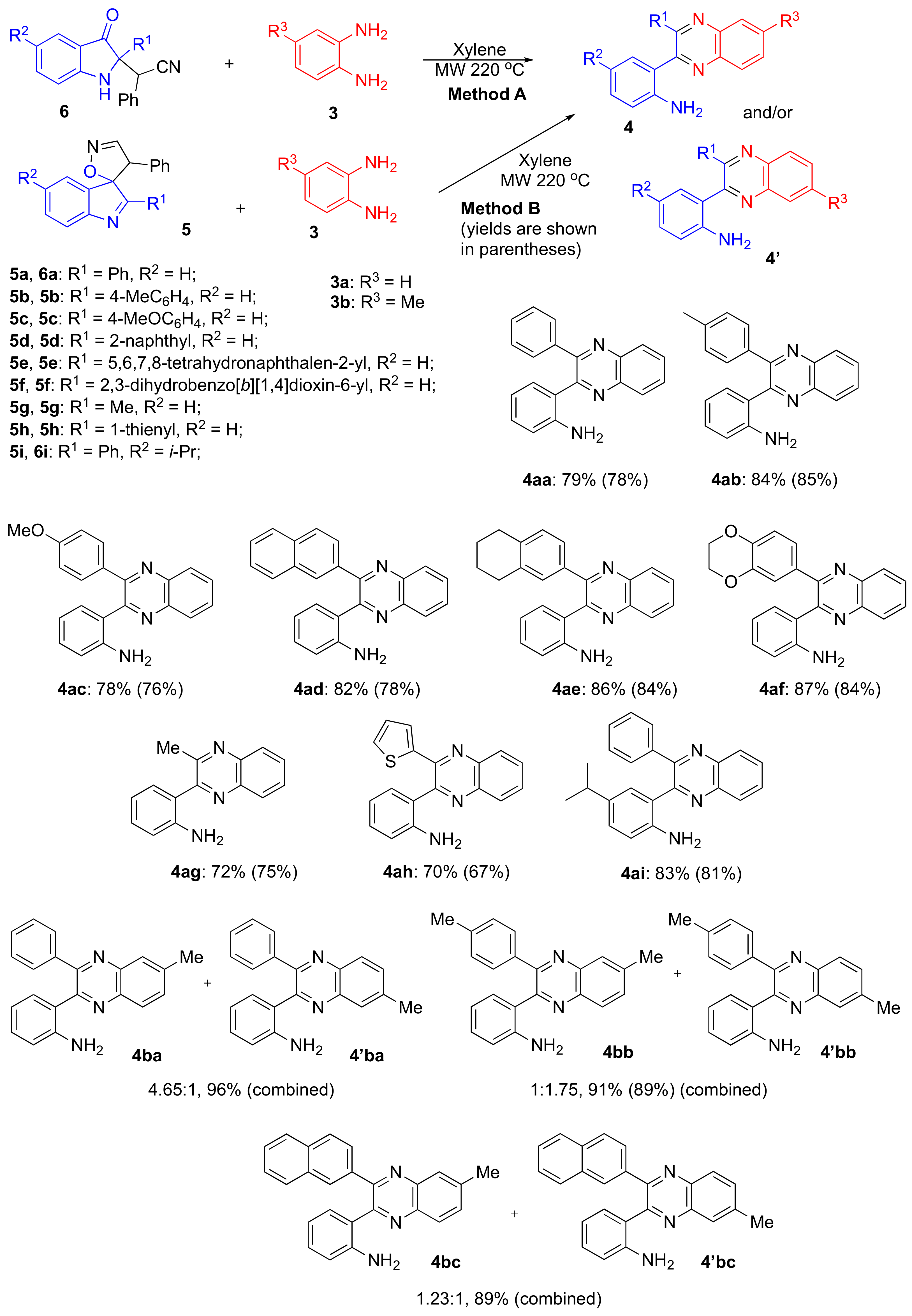
Method A for preparation of quinoxalines 4:
2-(3-Oxo-2-phenylindolin-2-yl)-2-phenylacetonitrile (6) (1.00 mmol) in 2 mL of xylene and 1,2-phenylenediamine (3) (216 mg, 2.00 mmol) were charged in a G10 vial. The vial was sealed and heated in the microwave apparatus at 220 °C for 1 h. After completion of the reaction vial was opened and the reaction mixture concentrated in vacuo. The crude material was purified by column chromatography (EtOAc/Hexane, 1:3, v/v).
Method B for preparation of quinoxalines 4:
2,4’-Diaryl-4’H-spiro[indole-3,5’-isoxazole] (5) (1.00 mmol) in 2 mL of xylene and 1,2-phenylenediamine (3) (216 mg, 2.00 mmol) were charged in a G10 vial. The vial was sealed and heated in the microwave apparatus at 220 °C for 1 h. After completion of the reaction, the vial was opened, and the reaction mixture was concentrated in vacuo. The crude material was purified by column chromatography (EtOAc/Hexane, 1:3, v/v).
Method C for preparation of quinoxalines 4:
2-Phenyl-3
H-indol-3-one (
2) [
1] (207 mg, 1.00 mmol) in 2 mL of xylene and 1,2-phenylenediamine (216 mg, 2.00 mmol) were charged in a G10 vial. The vial was sealed, and heated in the microwave apparatus at 220 °C for 15 min. After completion of the reaction, the vial was opened, and the reaction mixture was concentrated in vacuo. The crude material was purified by column chromatography (EtOAc/Hexane, 1:3,
v/
v).
Method D for preparation of quinoxalines 4 (Scale-up procedure):
2-(3-Oxo-2-phenylindolin-2-yl)-2-phenylacetonitrile (6) (1.62 g, 5.00 mmol) in 10 mL of xylene and 1,2-phenylenediamine (3) (1.08 g, 10.00 mmol) were charged in a G30 vial. The vial was sealed and heated in the microwave apparatus at 220 °C for 1 h. After completion of reaction, the vial was opened, and the reaction mixture was concentrated in vacuo. The crude material was purified by column chromatography (EtOAc/Hexane, 1:3, v/v).
2-(3-Phenylquinoxalin-2-yl)aniline (
4aa): This compound was prepared via
Method A employing 2-(3-oxo-2-phenylindolin-2-yl)-2-phenylacetonitrile (
6a) [
21] (324 mg, 1.00 mmol) and 1,2-phenylenediamine (216 mg, 2.00 mmol) in a yield of 234 mg (0.79 mmol, 79%), or via
Method B employing 2,4’-diaryl-4’
H-spiro[indole-3,5’-isoxazole] (
5a) [
2] (324 mg, 1.00 mmol) and 1,2-phenylenediamine (216 mg, 2.00 mmol) in a yield of 217 mg (0.73 mmol, 73%), or via
Method C employing 2-phenyl-3
H-indol-3-one (
2) [
1] (207 mg, 1 mmol) and 1,2-phenylenediamine (216 mg, 2.00 mmol) in a yield of 267 mg (0.9 mmol, 90%). The scale-up procedure employing
Method D afforded the same product in a yield of 1.098 g (3.70 mmol, 74%). Purification was performed by column chromatography (EtOAc/Hexane = 1:3). The titled compound was obtained as yellow solid, m.p. 149.7–151.0 °C, R
f 0.26 (EtOAc/Hexane = 1:3).
1H NMR (400 MHz, CDCl
3) δ 8.23–8.14 (m, 1H), 8.14–8.06 (m, 1H), 7.83–7.72 (m, 2H), 7.64–7.53 (m, 2H), 7.40–7.28 (m, 3H), 7.17–7.08 (m, 1H), 6.89–6.77 (m, 2H), 6.55 (t,
J = 7.5 Hz, 1H), 4.63 (br. s, 2H);
13C{
1H} NMR (101 MHz, CDCl
3) δ 154.4, 153.1, 145.5, 141.2, 140.5, 139.0, 131.9, 130.1 (3C), 129.7 (2C), 129.4, 129.0, 128.8, 128.4 (2C), 123.3, 118.2, 117.1; FTIR,
vmax: 3454, 3345, 3063, 1620, 1489, 1464, 1441, 1403, 1350, 1307 cm
−1; HRMS (ESI TOF)
m/
z: calc’d for C
20H
15N
3Na [M + Na]
+: 320.1158, found 320.1167 (-2.9 ppm).
2-(3-(p-Tolyl)quinoxalin-2-yl)aniline (
4ab): This compound was prepared via
Method A employing 2-(3-oxo-2-(
p-tolyl)indolin-2-yl)-2-phenylacetonitrile (
6b) [
21] (338 mg, 1.00 mmol) and 1,2-phenylenediamine (216 mg, 2.00 mmol) in a yield of 260 mg (0.84 mmol, 84%). Alternatively, this compound was prepared via
Method B employing 4’-phenyl-2-(
p-tolyl)-4’
H-spiro[indole-3,5’-isoxazole] (
5b) [
21] (338 mg, 1.00 mmol) and 1,2-phenylenediamine (216 mg, 2.00 mmol) in a yield of 264 mg (0.85 mmol, 85%). Purification was performed by column chromatography (EtOAc/Hexane = 1:3). The titled compound was obtained as yellow solid, m.p. 136.3–137.9 °C, R
f 0.29 (EtOAc/Hexane = 1:3).
1H NMR (400 MHz, CDCl
3) δ 8.17 (d,
J = 9.9 Hz, 1H), 8.10 (d,
J = 7.1 Hz, 1H), 7.81–7.70 (m, 2H), 7.49 (d,
J = 8.1 Hz, 2H), 7.13 (d,
J = 8.1 Hz, 3H), 6.90 (d,
J = 7.7 Hz, 1H), 6.81 (d,
J = 8.1 Hz, 1H), 6.58 (t,
J = 7.5 Hz, 1H), 4.59 (br. s, 2H), 2.35 (s, 3H);
13C{
1H} NMR (101 MHz, CDCl
3) δ 154.4, 153.0, 145.4, 141.2, 140.4, 139.0, 136.1, 131.8, 130.0 (2C), 129.9, 129.6 (2C), 129.3, 129.1 (2C), 128.7, 123.6, 118.2, 117.1, 21.5; FTIR,
vmax: 3461, 3348, 3030, 1615, 1498, 1445, 1393, 1342, 1307, 1221 cm
−1; HRMS (ESI TOF)
m/
z: calc’d for C
21H
18N
3 [M + H]
+: 312.1495, found 312.1498 (−1.0 ppm).
2-(3-(4-Methoxyphenyl)quinoxalin-2-yl)aniline (
4ac): This compound was prepared via
Method A employing 2-(2-(4-methoxyphenyl)-3-oxoindolin-2-yl)-2-phenylacetonitrile (
6c) [
21] (354 mg, 1.00 mmol) and 1,2-phenylenediamine (216 mg, 2.00 mmol) in a yield of 255 mg (0.78 mmol, 78%). Alternatively, this compound was prepared via
Method B employing 2-(4-methoxyphenyl)-4’-phenyl-4’
H-spiro[indole-3,5’-isoxazole] (
5c) [
21] (354 mg, 1.00 mmol) and 1,2-phenylenediamine (216 mg, 2.00 mmol) in a yield of 249 mg (0.76 mmol, 76%). Purification was performed by column chromatography (Benzene/EA, 10:1,
v/
v). The titled compound was obtained as yellow solid, m.p. 215–217 °C; R
f 0.4 (Benzene/EA, 10:1,
v/
v).
1H NMR (400 MHz, CDCl
3) δ 8.19–8.11 (m, 1H), 8.12–8.06 (m, 1H), 7.79–7.71 (m, 2H), 7.55 (d,
J = 8.6 Hz, 2H), 7.14 (t,
J = 7.3 Hz, 1H), 6.91 (d,
J = 7.5 Hz, 1H), 6.83 (dd,
J = 12.8, 8.4 Hz, 3H), 6.60 (t,
J = 7.5 Hz, 1H), 4.56 (s, 2H), 3.81 (s, 3H);
13C{
1H} NMR (101 MHz, CDCl
3) δ 160.3, 153.9, 152.9, 145.3, 141.2, 140.3, 131.7, 131.3, 131.2 (2C), 130.1, 130.0, 129.8, 129.2, 128.7, 123.6, 118.3, 117.1, 113.8 (2C), 55.4; FTIR,
vmax: 3451, 3241, 1679, 1611, 1556, 1537, 1456, 1418, 1271, 1231 cm
−1; HRMS (ESI TOF) m/z: calc’d for C
21H
18N
3O [M + H]
+: 328.1444, found 328.1439 (1.7 ppm).
2-(3-(Naphthalen-2-yl)quinoxalin-2-yl)aniline (
4ad): This compound was prepared via
Method A employing 2-(2-(naphthalen-2-yl)-3-oxoindolin-2-yl)-2-phenylacetonitrile (
6d) [
21] (374 mg, 1.00 mmol) and 1,2-phenylenediamine (216 mg, 2.00 mmol) in a yield of 284 mg (0.82 mmol, 82%). Alternatively, this compound was prepared via
Method B employing 2-(naphthalen-2-yl)-4’-phenyl-4’
H-spiro[indole-3,5’-isoxazole] (
5d) [
21] (374 mg, 1.00 mmol) and 1,2-phenylenediamine (216 mg, 2.00 mmol) in a yield of 271 mg (0.78 mmol, 78%). Purification was performed by column chromatography (EtOAc/Hexane = 1:3). The titled compound was obtained as yellow solid, m.p. 181.0–182.0 °C, R
f 0.23 (EtOAc/Hexane = 1:3).
1H NMR (400 MHz, CDCl
3) δ 8.27–8.17 (m, 2H), 8.17–8.09 (m, 1H), 7.86–7.77 (m, 4H), 7.75 (d,
J = 8.6 Hz, 1H), 7.60 (d,
J = 8.6 Hz, 1H), 7.54–7.43 (m, 2H), 7.17–7.07 (m, 1H), 6.89 (d,
J = 7.8 Hz, 1H), 6.82 (d,
J = 7.0 Hz, 1H), 6.49 (t,
J = 6.9 Hz, 1H), 4.67 (br. S, 2H);
13C{
1H} NMR (101 MHz, CDCl
3) δ 154.2, 153.2, 145.5, 141.3, 140.6, 136.5, 133.4, 133.3, 131.9, 130.2 (3C), 129.6, 129.4, 128.9, 128.8, 127.8 (2C), 126.9 (2C), 126.3, 123.3, 118.3, 117.1; FTIR,
vmax: 3331, 1773, 1635, 1562, 1509, 1478, 1456, 1398, 1342, 1305, 1242 cm
−1; HRMS (ESI TOF) m/z: calc’d for C
24H
18N
3 [M + H]
+: 348.1495, found 348.1501 (−1.6 ppm).
2-(3-(5,6,7,8-Tetrahydronaphthalen-2-yl)uinoxaline-2-yl)aniline (
4ae): This compound was prepared via
Method A employing 2-(3-oxo-2-(5,6,7,8-tetrahydronaphthalen- 2-yl)indolin-2-yl)-2-phenylacetonitrile (
6e) [
21] (378 mg, 1.00 mmol) and 1,2-phenylenediamine (216 mg, 2.00 mmol) in a yield of 302 mg (0.86 mmol, 86%). Alternatively, this compound was prepared via
Method B employing 4′-phenyl-2-(5,6,7,8-tetrahydronaphthalen-2-yl)-4′
H-spiro[indole-3,5′-isoxazole] (
5e) [
21] (378 mg, 1.00 mmol) and 1,2-phenylenediamine (216 mg, 2.00 mmol) in a yield of 295 mg (0.84 mmol, 84%). Purification was performed by column chromatography (EtOAc/Hexane = 1:3). The titled compound was obtained as a brown oil, R
f 0.31 (EtOAc/Hexane = 1:3).
1H NMR (400 MHz, CDCl
3) δ 8.21–8.13 (m, 1H), 8.13–8.05 (m, 1H), 7.81–7.69 (m, 2H), 7.40 (s, 1H), 7.21–7.10 (m, 2H), 6.98–6.90 (m, 2H), 6.81 (d,
J = 6.8 Hz, 1H), 6.60 (t,
J = 6.9 Hz, 1H), 4.58 (br. s, 2H), 2.74 (d,
J = 3.3 Hz, 4H), 1.78 (quint,
J = 3.5 Hz, 4H);
13C{
1H} NMR (101 MHz, CDCl
3) δ 154.5, 153.1, 145.3, 141.3, 140.4, 138.4, 137.3, 136.0, 131.8, 130.3, 130.0 (2C), 129.8, 129.3, 128.9, 128.7, 126.8, 123.7, 118.2, 117.0, 29.5, 29.4, 23.2 (2C); FTIR,
vmax: 3457, 3358, 3060, 2232, 1617, 1498, 1448, 1343, 1307, 1249 cm
−1; HRMS (ESI TOF)
m/
z: calc’d for C
24H
22N
3 [M + H]
+: 352.1808, found 352.1817 (−2.5 ppm).
2-(3-(2,3-Dihydrobenzo[b][
1,
4]
dioxin-6-yl)quinoxalin-2-yl)aniline (
4af): This compound was prepared via
Method A employing 2-(2-(2,3-dihydrobenzo[
b][
1,
4]dioxin-6-yl)- 3-oxoindolin-2-yl)-2-phenylacetonitrile (
6f) [
21] (382 mg, 1.00 mmol) and 1,2-phenylenediamine (216 mg, 2.00 mmol) in a yield of 308 mg (0.87 mmol, 87%). Alternatively, this compound was prepared via
Method B employing 2-(2,3-dihydrobenzo[
b][
1,
4]dioxin-6-yl)-4’-phenyl-4’
H-spiro[indole-3,5’-isoxazole] (
5f) [
21] (382 mg, 1.00 mmol) and 1,2-phenylenediamine (216 mg, 2.00 mmol) in a yield of 298 mg (0.84 mmol, 84%). Purification was performed by column chromatography (EtOAc/Hexane = 1:2). The titled compound was obtained as brown oil, R
f 0.31 (EtOAc/Hexane = 1:2).
1H NMR (400 MHz, DMSO-
d6) δ 8.14–8.04 (m, 2H), 7.88–7.78 (m, 2H), 7.13–7.04 (m, 2H), 7.01 (d,
J = 10.8 Hz, 1H), 6.87 (d,
J = 7.5 Hz, 1H), 6.79–6.70 (m, 2H), 6.49 (t,
J = 7.5 Hz, 1H), 5.20 (br. s, 2H), 4.22 (d,
J = 6.6 Hz, 4H);
13C{
1H} NMR (101 MHz, DMSO-
d6) δ 152.9 (2C), 146.3, 144.2, 142.8, 140.6, 140.2, 131.8, 130.5, 130.1, 129.8, 129.5, 128.8, 128.7, 123.2, 122.6, 118.2, 116.5, 115.7, 115.5, 64.3, 64.0; FTIR,
vmax: 3447, 3338, 3235, 1634, 1622, 1587, 1509, 1496, 1456, 1418, 1396, 1343, 1299, 1279, 1259 cm
−1; HRMS (ESI TOF) m/z: calc’d for C
22H
18N
3O
2 [M + H]
+: 356.1394, found 356.1387 (1.9 ppm).
2-(3-Methylquinoxalin-2-yl)aniline (
4ag): This compound was prepared via
Method A employing 2-(2-methyl-3-oxoindolin-2-yl)-2-phenylacetonitrile (
6g) [
21] (262 mg, 1.00 mmol) and 1,2-phenylenediamine (216 mg, 2.00 mmol) in a yield of 170 mg (0.72 mmol, 72%). Alternatively, this compound was prepared via
Method B employing 2-methyl-4’-phenyl-4’
H-spiro[indole-3,5’-isoxazole] (
5g) [
21] (262 mg, 1.00 mmol) and 1,2-phenylenediamine (216 mg, 2.00 mmol) in a yield of 176 mg (0.75 mmol, 75%). Purification was performed by column chromatography (EtOAc/Hexane = 1:1). The titled compound was obtained as a yellow oil, R
f 0.43 (EtOAc/Hexane = 1:1).
1H NMR (400 MHz, DMSO-
d6) δ 8.04 (d,
J = 1.9 Hz, 2H), 7.85–7.73 (m, 2H), 7.17 (t,
J = 7.6 Hz, 1H), 7.11 (d,
J = 7.6 Hz, 1H), 6.81 (d,
J = 8.1 Hz, 1H), 6.67 (t,
J = 7.4 Hz, 1H), 5.11 (br. s, 2H), 2.57 (s, 3H);
13C{
1H} NMR (101 MHz, DMSO-
d6) δ 154.5, 153.9, 146.2, 140.8, 140.6, 129.8, 129.6 (2C), 129.0, 128.8, 128.1, 123.0, 115.9, 115.4, 22.9; FTIR,
vmax: 3437, 3312, 3189, 1642, 1602, 1569, 1499, 1486, 1456, 1393, 1372, 1340, 1307 cm
−1; HRMS (ESI TOF)
m/
z: calc’d for C
15H
14N
3 [M + H]
+: 236.1182, found 236.1187 (−2.2 ppm).
2-(3-(Thiophen-2-yl)quinoxalin-2-yl)aniline (
4ah): This compound was prepared via
Method A employing 2-(3-oxo-2-(thiophen-2-yl)indolin-2-yl)-2-phenylacetonitrile (
6h) [
21] (330 mg, 1.00 mmol) and 1,2-phenylenediamine (216 mg, 2.00 mmol) in a yield of 212 mg (0.70 mmol, 70%). Alternatively, this compound was prepared via
Method B employing 4’-phenyl-2-(thiophen-2-yl)-4’
H-spiro[indole-3,5’-isoxazole] (
5h) [
21] (330 mg, 1.00 mmol) and 1,2-phenylenediamine (216 mg, 2.00 mmol) in a yield of 203 mg (0.67 mmol, 67%). Purification was performed by column chromatography (Benzene). The titled compound was obtained as green-yellow solid, m.p. 131–132 °C; R
f 0.3 (Benzene).
1H NMR (400 MHz, CDCl
3) δ 8.14–8.10 (m, 1H), 8.10–8.04 (m, 1H), 7.80–7.74 (m, 1H), 7.74–7.69 (m, 1H), 7.41 (dd,
J = 5.0, 1.1 Hz, 1H), 7.33–7.27 (m, 1H), 7.20 (dd,
J = 7.6, 1.4 Hz, 1H), 6.96 (dd,
J = 3.8, 1.1 Hz, 1H), 6.91 (dd,
J = 5.0, 3.8 Hz, 1H), 6.89–6.81 (m, 2H), 4.03 (br. s, 2H);
13C{
1H} NMR (101 MHz, CDCl
3) δ 151.1, 147.7, 144.8, 142.6, 141.3, 140.5, 130.7, 130.7, 130.5, 129.8, 129.8, 129.0 (2C), 128.9, 128.2, 124.2, 119.0, 117.0; FTIR,
vmax: 3331, 3063, 1779, 1610, 1471, 1435, 1305, 1242 cm
−1; HRMS (ESI TOF) m/z: calc’d for C
18H
14N
3S [M + H]
+: 304.0903, found 304.0904 (−0.3 ppm).
4-Isopropyl-2-(3-phenylquinoxalin-2-yl)aniline (
4ai): This compound was prepared via
Method A employing 2-(5-isopropyl-3-oxo-2-phenylindolin-2-yl)-2-phenylacetonitrile (
6i) [
21] (366 mg, 1.00 mmol) and 1,2-phenylenediamine (216 mg, 2.00 mmol) in a yield of 281 mg (0.83 mmol, 83%). Alternatively, this compound was prepared via
Method B employing 5-isopropyl-2,4’-diphenyl-4’
H-spiro[indole-3,5’-isoxazole] (
5i) [
21] (366 mg, 1.00 mmol) and 1,2-phenylenediamine (216 mg, 2.00 mmol) in a yield of 275 mg (0.81 mmol, 81%). Purification was performed by column chromatography (EtOAc/Hexane = 1:2). The titled compound was obtained as yellow oil, R
f 0.47 (EtOAc/Hexane = 1:2).
1H NMR (400 MHz, CDCl
3) δ 1H NMR (400 MHz, CDCl
3) δ 8.22–8.14 (m, 1H), 8.14–8.06 (m, 1H), 7.81–7.71 (m, 2H), 7.55 (s, 2H), 7.33 (s, 3H), 6.96 (d,
J = 8.2 Hz, 1H), 6.75 (d,
J = 8.3 Hz, 1H), 6.67 (s, 1H), 4.71 (br. s, 2H), 2.61–2.46 (m, 1H), 0.87 (d,
J = 7.0 Hz, 6H);
13C{
1H} NMR (101 MHz, CDCl
3) δ 154.7, 153.5, 143.5, 141.0, 140.4, 139.4, 138.3, 130.1, 130.0 (2C), 129.7 (2C), 129.3, 128.7 (2C), 128.4, 128.3 (2C), 122.4, 117.1, 33.0, 23.9 (2C); FTIR,
vmax: 3444, 3348, 3053, 2954, 2868, 1897, 1624, 1501, 1425, 1400, 1343, 1290, 1221, 1194 cm
−1; HRMS (ESI TOF) m/z: calc’d for C
23H
22N
3 [M + H]
+: 340.1808, found 340.1816 (−2.2 ppm).
2-(6-Methyl-3-phenylquinoxalin-2-yl)aniline (
4ba)
and 2-(7-Methyl-3-phenylquinoxalin-2-yl)aniline (
4′ba): This compounds was prepared via
Method A employing 2-(3-oxo-2-phenylindolin-2-yl)-2-phenylacetonitrile (
6a) [
21] (324 mg, 1.00 mmol) and 4-methyl-1,2-phenylenediamine (244 mg, 2.00 mmol) in a yield of 300 mg (0.96 mmol,
4ba/4′ba = 4.65:1.0, total yield 96%). Purification was performed by column chromatography (EtOAc/Hexane = 1:3). The titled compounds were obtained as yellow solid, m.p. 156.3–159.2 °C, R
f 0.29 (EtOAc/Hexane = 1:3). Major isomer (
4ba):
1H NMR (400 MHz, CDCl
3) δ 8.06 (d,
J = 8.6 Hz, 1H), 7.88 (s, 1H), 7.65–7.53 (m, 3H), 7.33 (s, 3H), 7.12 (t,
J = 7.7 Hz, 1H), 6.88–6.77 (m, 2H), 6.55 (t,
J = 7.5 Hz, 1H), 4.61 (br. s, 2H), 2.62 (s, 3H);
13C{
1H} NMR (101 MHz, CDCl
3) δ 153.5, 152.9, 145.5, 140.6, 139.7, 139.2, 132.5, 132.4, 131.9, 130.0, 129.7 (2C), 128.9, 128.8, 128.3 (2C), 127.6, 123.4, 118.1, 117.0, 22.1; Minor isomer (
4′ba):
1H NMR (400 MHz, CDCl
3) δ 7.99 (d,
J = 8.6 Hz, 1H), 7.95 (s, 1H), 7.65–7.53 (m, 3H), 7.33 (s, 3H), 7.12 (t,
J = 7.7 Hz, 1H), 6.88–6.77 (m, 2H), 6.55 (t,
J = 7.5 Hz, 1H), 4.61 (br. s, 2H), 2.62 (s, 3H);
13C{
1H} NMR (101 MHz, CDCl
3) δ 154.3, 152.1, 145.4, 141.3, 140.7 (2C), 139.0, 132.4, 131.9, 129.9, 129.7 (2C), 129.4, 128.5, 128.3 (2C), 128.2, 123.4, 118.2, 117.0, 22.1; FTIR,
vmax: 3421, 3335, 2921, 1632, 1557, 1484, 1446, 1348, 1300, 1250 cm
−1; HRMS (ESI TOF)
m/
z: calc’d for C
21H
18N
3 [M + H]
+: 312.1495, found 312.1495 (0.0 ppm).
2-(6-Methyl-3-(p-tolyl)quinoxalin-2-yl)aniline(4bb)and 2-(7-Methyl-3-(p-tolyl)quinoxalin-2-yl)aniline (
4′bb)
: This compounds were prepared via
Method A employing 2-(3-oxo-2-(
p-tolyl)indolin-2-yl)-2-phenylacetonitrile (
6b) [
21] (338 mg, 1.00 mmol) and 4-methyl-1,2-phenylenediamine (244 mg, 2.00 mmol) in a yield of 296 mg (0.91 mmol,
4bb’:4bb = 1.0:1.75, total yield 91%). Alternatively, these compounds were prepared via
Method B employing 4’-phenyl-2-(
p-tolyl)-
4’H-spiro[indole-3,5’-isoxazole] (
5b) [
21] (338 mg, 1.00 mmol) and 5-methyl-1,2-phenylenediamine (244 mg, 2.00 mmol) in a yield of 289 mg (0.89 mmol, 89%). Purification was performed by column chromatography (EtOAc/Hexane = 1:3). The titled compounds were obtained as yellow solid, m.p. 176.1–179.6 °C, R
f 0.31 (EtOAc/Hexane = 1:3). Major isomer (
4′bb):
1H NMR (400 MHz, CDCl
3) δ 7.98 (d,
J = 8.6 Hz, 1H), 7.94 (s, 1H), 7.63–7.54 (m, 1H), 7.51–7.43 (m, 2H), 7.17–7.08 (m, 3H), 6.88 (d,
J = 7.7 Hz, 1H), 6.80 (d,
J = 6.8 Hz, 1H), 6.58 (t,
J = 7.5 Hz, 1H), 4.56 (br. s, 2H), 2.61 (s, 3H), 2.34 (s, 3H);
13C{
1H} NMR (101 MHz, CDCl
3) δ 154.2, 152.0, 145.3, 141.3, 140.6, 140.4, 138.9, 136.2, 132.2, 131.8, 129.9, 129.6 (2C), 129.1 (2C), 128.3, 128.2, 123.8, 118.2, 117.0, 22.0, 21.5; Minor isomer (
4bb):
1H NMR (400 MHz, CDCl
3) δ 8.05 (d,
J = 8.6 Hz, 1H), 7.86 (s, 1H), 7.63–7.54 (m, 1H), 7.51–7.43 (m, 2H), 7.17–7.08 (m, 3H), 6.88 (d,
J = 7.7 Hz, 1H), 6.80 (d,
J = 6.8 Hz, 1H), 6.58 (t,
J = 7.5 Hz, 1H), 4.56 (br. s, 2H), 2.61 (s, 3H), 2.34 (s, 3H);
13C{
1H} NMR (101 MHz, CDCl
3) δ 153.5, 152.9, 145.4, 140.6, 140.5, 139.7, 138.8, 136.2, 132.4, 131.8, 129.9, 129.6 (2C), 129.1 (2C), 128.9, 127.6, 123.7, 118.2, 117.0, 22.0, 21.5; FTIR,
vmax: 3414, 3318, 3027, 1922, 1617, 1491, 1448, 1342, 1307, 1247 cm
−1; HRMS (ESI TOF) m/z: calc’d for C
22H
19N
3Na [M + Na]
+: 348.1471, found 348.1480 (−2.7 ppm).
2-(7-Methyl-3-(naphthalen-2-yl)quinoxalin-2-yl)aniline (
4′bc)
and 2-(6-Methyl-3- (naphthalen-2-yl)quinoxalin-2-yl)aniline (
4bc)
: This compounds was prepared via
Method A employing 2-(2-(naphthalen-2-yl)-3-oxoindolin-2-yl)-2-phenylacetonitrile (
6c) [
2] (374 mg, 1.00 mmol) and 4-methyl-1,2-phenylenediamine (244 mg, 2.00 mmol) in a yield of 320 mg (0.89 mmol,
4bc/4′bc = 1.23:1.0, total yield 89%). Purification was performed by column chromatography (EtOAc/Hexane = 1:3). The titled compounds were obtained as yellow solid, m.p. 155.9–158.7 °C, R
f 0.26 (EtOAc/Hexane = 1:3). Major isomer (
4bc):
1H NMR (400 MHz, CDCl
3) δ 8.19 (s, 1H), 8.10 (d,
J = 8.4 Hz, 1H), 7.90 (s, 1H), 7.85–7.76 (m, 2H), 7.73 (d,
J = 8.7 Hz, 1H), 7.67–7.54 (m, 2H), 7.54–7.42 (m, 2H), 7.11 (t,
J = 7.7 Hz, 1H), 6.92–6.77 (m, 2H), 6.53–6.44 (m, 1H), 4.66 (br. s, 2H), 2.66–2.60 (m, 3H);
13C{
1H} NMR (101 MHz, CDCl
3) δ 153.3, 153.1, 145.5, 140.7, 140.7, 139.8, 136.7, 133.4 (2C), 132.6, 131.9, 130.1, 129.5, 128.9, 128.8, 127.7 (3C), 127.0, 126.8, 126.3, 123.5, 118.3, 117.1, 22.1; Minor isomer (
4′bc):
1H NMR (400 MHz, CDCl
3) δ 8.19 (s, 1H), 8.02 (d,
J = 8.6 Hz, 1H), 7.99 (s, 1H), 7.85–7.76 (m, 2H), 7.73 (d,
J = 8.7 Hz, 1H), 7.67–7.54 (m, 2H), 7.54–7.42 (m, 2H), 7.11 (t,
J = 7.7 Hz, 1H), 6.92–6.77 (m, 2H), 6.53–6.44 (m, 1H), 4.66 (br. s, 2H), 2.66–2.60 (m, 3H);
13C{
1H} NMR (101 MHz, CDCl
3) δ 154.0, 152.2, 145.4, 141.4, 140.8, 139.1, 136.7, 133.4 (2C), 132.5, 131.9, 130.0, 129.6, 128.8, 128.3, 128.2, 127.7 (2C), 127.0, 126.9, 126.3, 123.5, 118.3, 117.1, 22.1; FTIR,
vmax: 3451, 3378, 1619, 1557, 1489, 1458, 1338, 1304, 1247 cm
−1; HRMS (ESI TOF)
m/
z: calc’d for C
25H
20N
3 [M + H]
+: 362.1652, found 362.1662 (−2.8 ppm).