Keratocyte Differentiation Is Regulated by NF-κB and TGFβ Signaling Crosstalk
Abstract
1. Introduction
2. Results
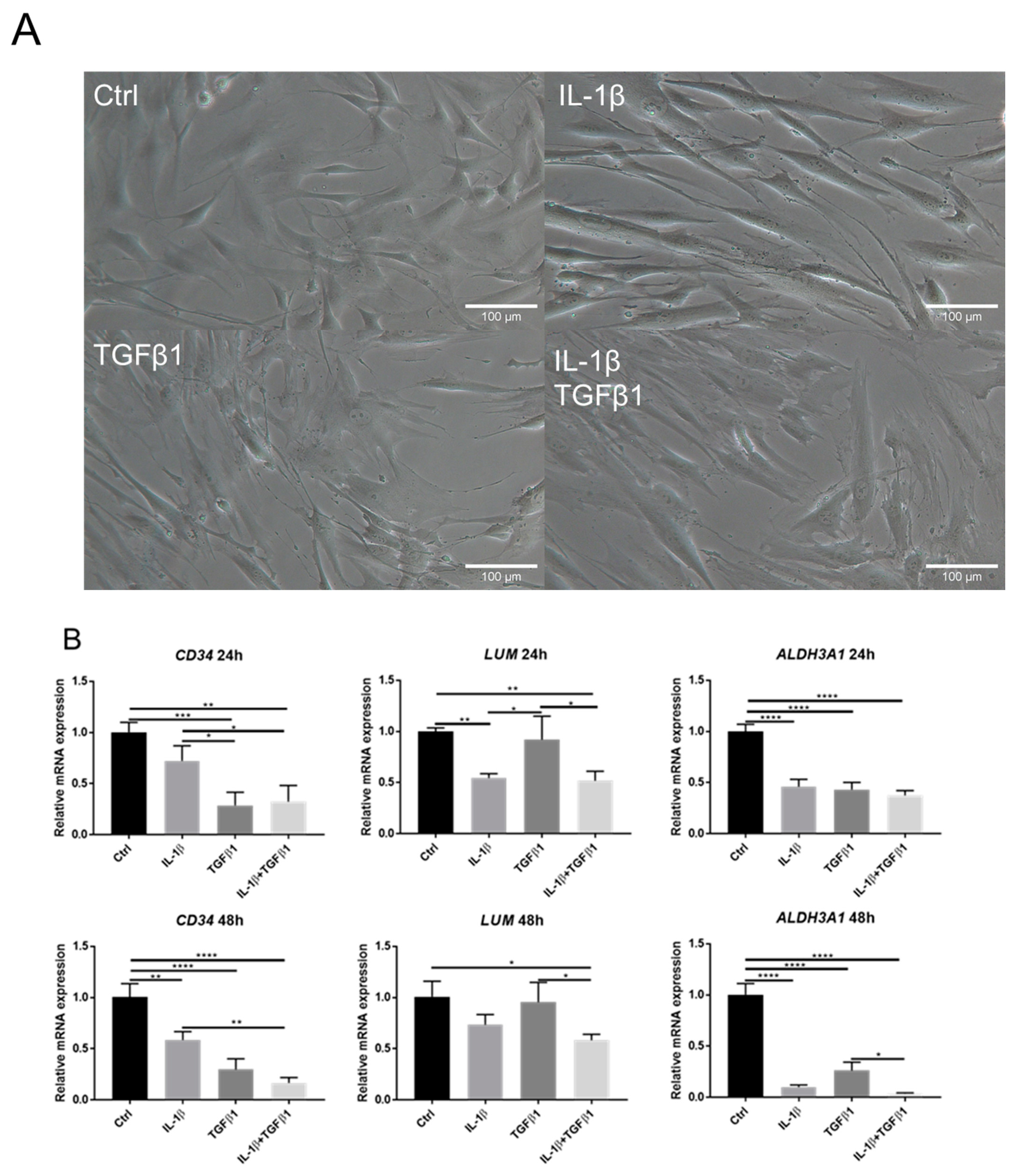
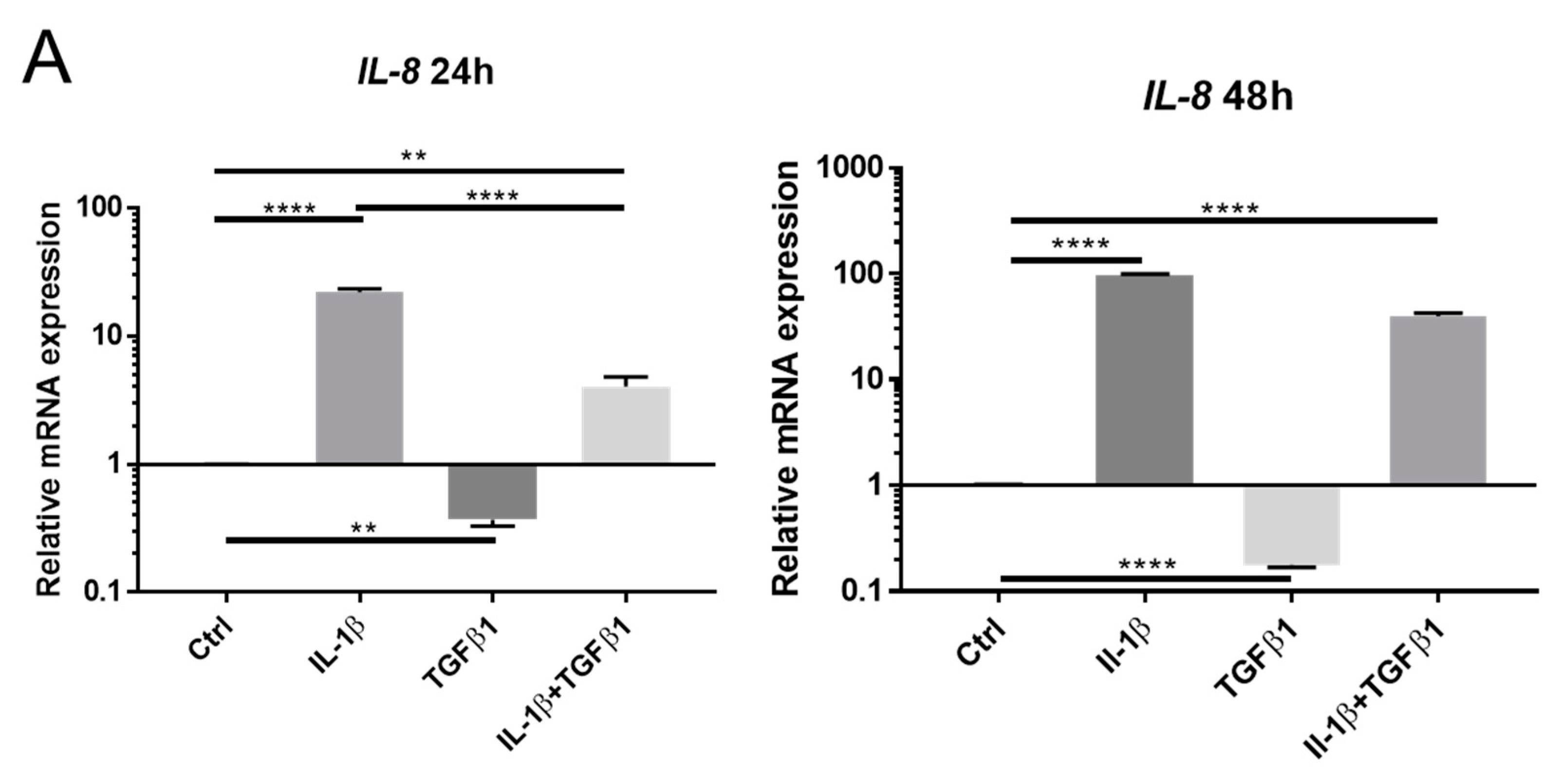
2.1. IL-1β Induces Loss of Keratocyte Properties Independent of TGFβ1
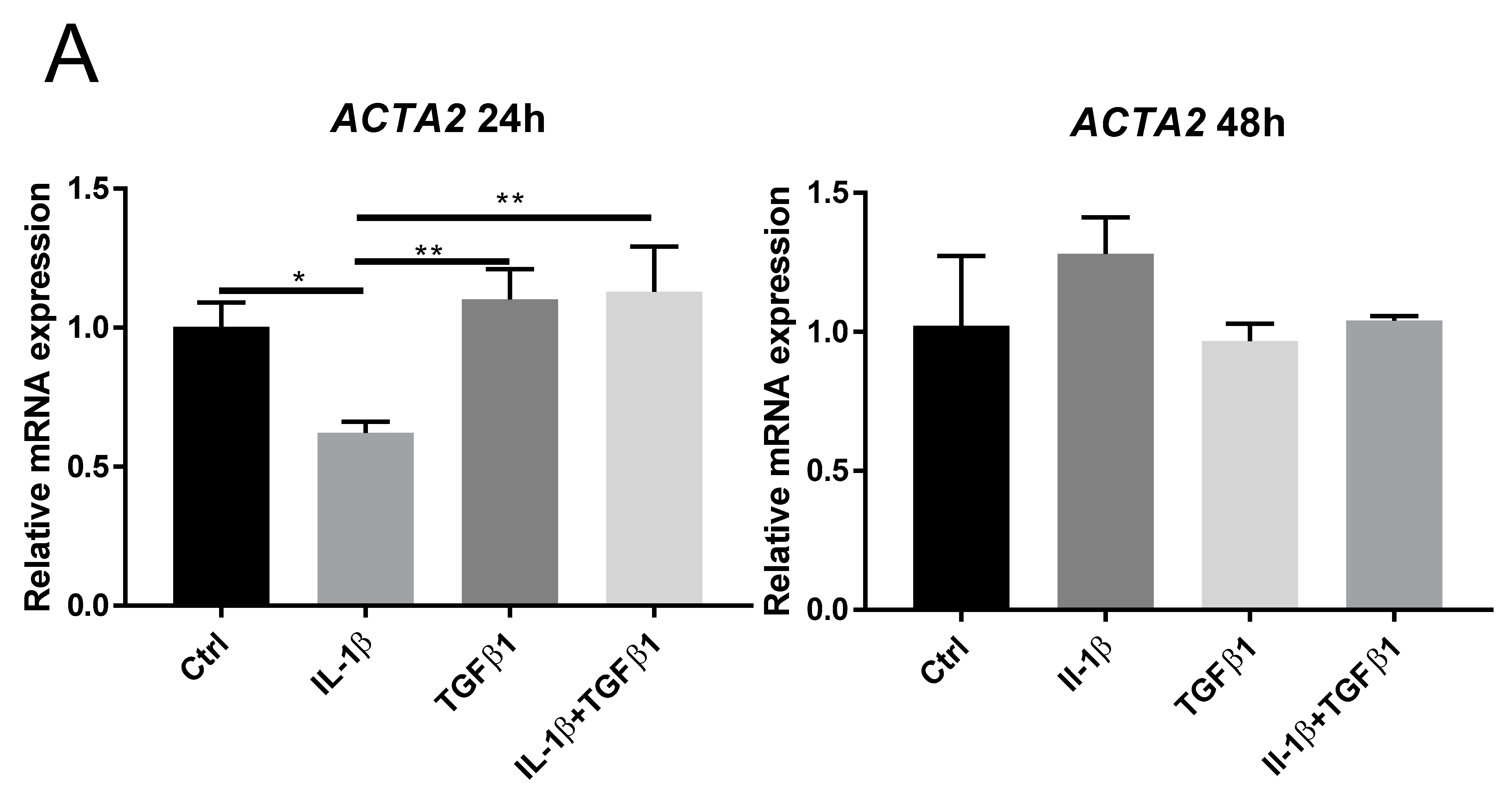
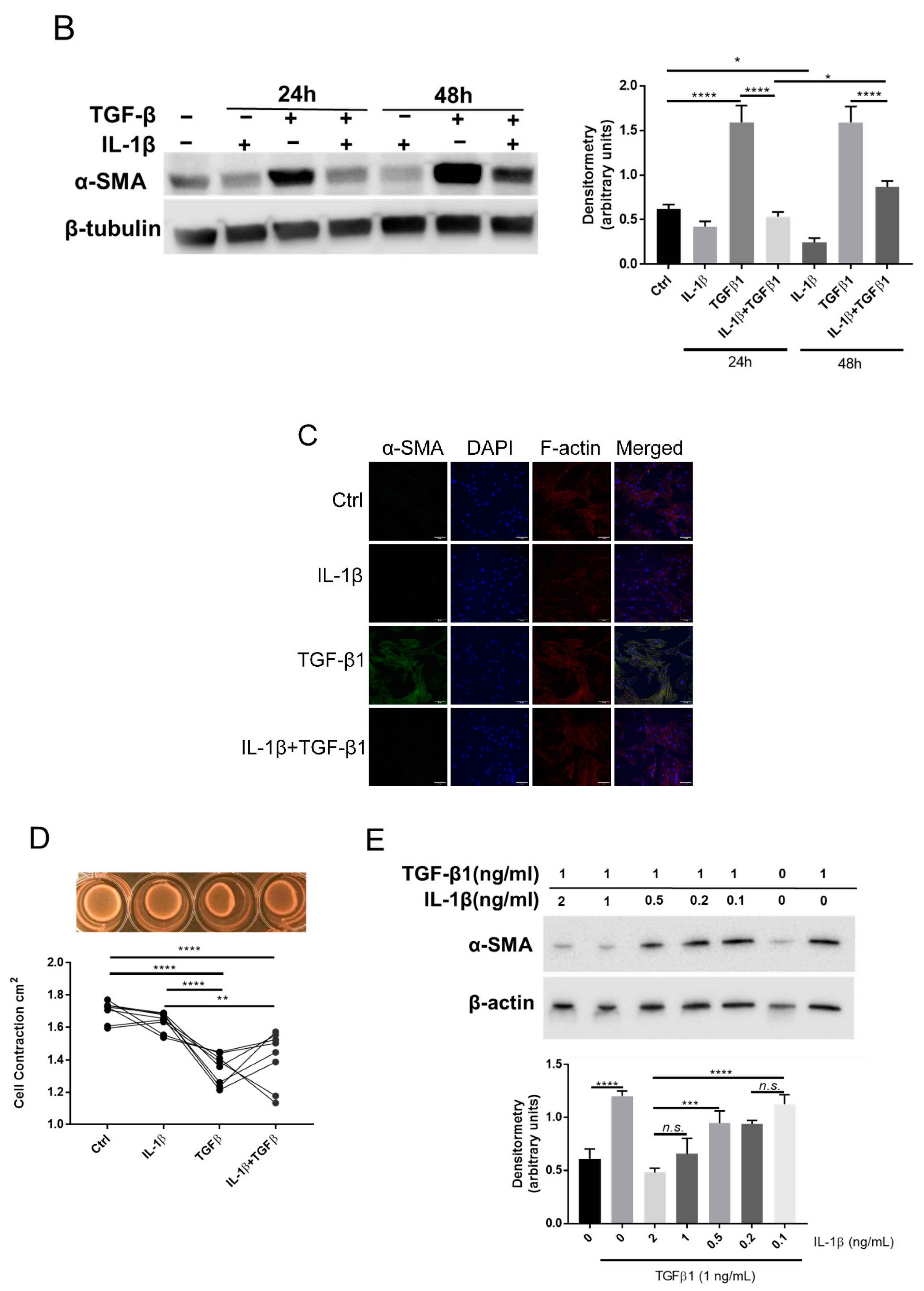
2.2. IL-1β Inhibits TGFβ1 Mediated Myofibroblast Differentiation
2.3. Opposing Effects of IL-1β and TGFβ1 in NF-κB and TGFβ Signaling
2.4. Myofibroblast Differentiation Can Be Regulated by a Selective NF-κB Signaling Inhibitor
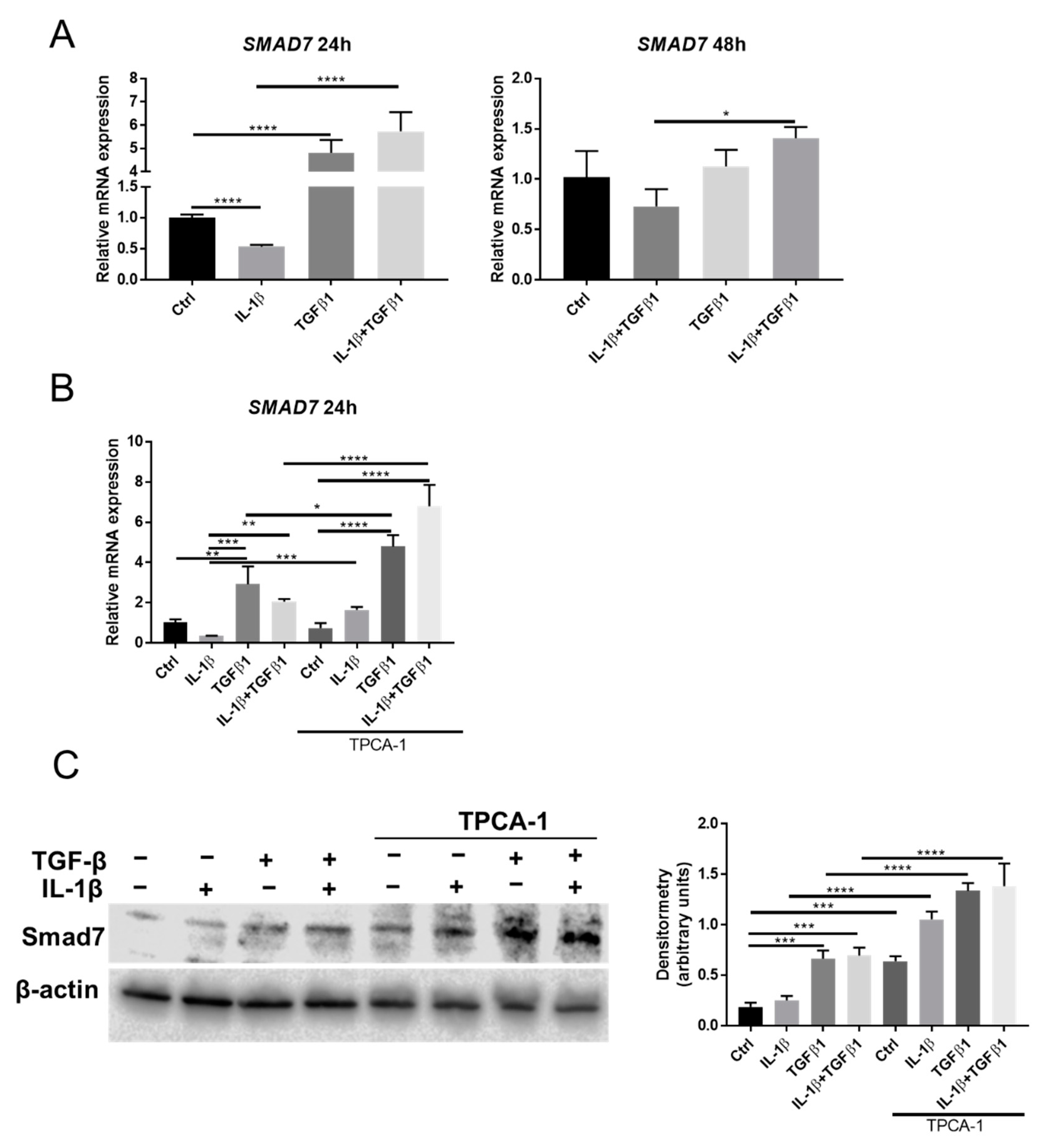
2.5. Smad7 Is Involved in the Crosstalk between NF-κB and TGFβ Signaling
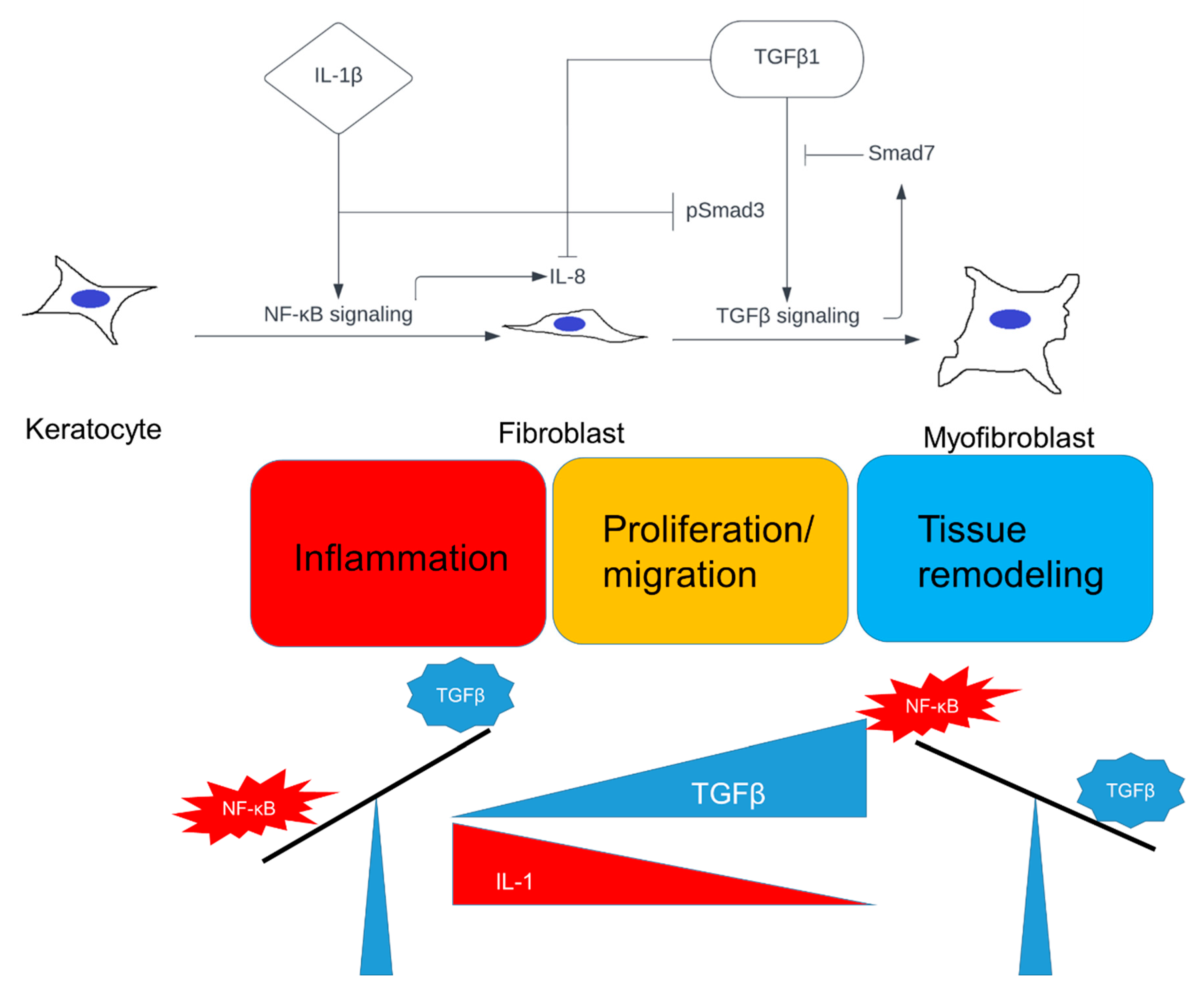
3. Discussion
4. Materials and Methods
4.1. Isolation and Culture of Human Keratocytes
4.2. Reagents for Experiments
4.3. RNA Extraction and qRT-PCR
4.4. Western Blot
4.5. Immunocytochemistry
4.6. Contraction Assay
4.7. Live-Cell Imaging
4.8. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, S.E. Interleukin-1 and Transforming Growth Factor Beta: Commonly Opposing, but Sometimes Supporting, Master Regulators of the Corneal Wound Healing Response to Injury. Investig. Ophthalmol. Vis. Sci. 2021, 62, 8. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.E.; Mohan, R.R.; Mohan, R.R.; Ambrosio, R.; Hong, J.W.; Lee, J.S. The corneal wound healing response: Cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog. Retin. Eye Res. 2001, 20, 625–637. [Google Scholar] [CrossRef]
- Hong, J.W.; Liu, J.J.; Lee, J.S.; Mohan, R.R.; Mohan, R.R.; Woods, D.J.; He, Y.G.; Wilson, S.E. Proinflammatory chemokine induction in keratocytes and inflammatory cell infiltration into the cornea. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2795–2803. [Google Scholar]
- Kaur, H.; Chaurasia, S.S.; Agrawal, V.; Suto, C.; Wilson, S.E. Corneal myofibroblast viability: Opposing effects of IL-1 and TGF beta1. Exp. Eye Res. 2009, 89, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Tandon, A.; Tovey, J.C.; Sharma, A.; Gupta, R.; Mohan, R.R. Role of transforming growth factor Beta in corneal function, biology and pathology. Curr. Mol. Med. 2010, 10, 565–578. [Google Scholar] [CrossRef]
- Jester, J.V.; Ho-Chang, J. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp. Eye Res. 2003, 77, 581–592. [Google Scholar] [CrossRef]
- Saikia, P.; Thangavadivel, S.; Medeiros, C.S.; Lassance, L.; de Oliveira, R.C.; Wilson, S.E. IL-1 and TGF-beta Modulation of Epithelial Basement Membrane Components Perlecan and Nidogen Production by Corneal Stromal Cells. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5589–5598. [Google Scholar] [CrossRef]
- Girard, M.T.; Matsubara, M.; Fini, M.E. Transforming Growth-Factor-Beta and Interleukin-1 Modulate Metalloproteinase Expression by Corneal Stromal Cells. Investig. Ophthalmol. Vis. Sci. 1991, 32, 2441–2454. [Google Scholar]
- Shephard, P.; Martin, G.; Smola-Hess, S.; Brunner, G.; Krieg, T.; Smola, H. Myofibroblast differentiation is induced in keratinocyte-fibroblast co-cultures and is antagonistically regulated by endogenous transforming growth factor-beta and interleukin-1. Am. J. Pathol. 2004, 164, 2055–2066. [Google Scholar] [CrossRef]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte-fibroblast interactions in wound healing. J. Investig. Dermatol. 2007, 127, 998–1008. [Google Scholar] [CrossRef]
- Zhang, H.Y.; GharaeeKermani, M.; Phan, S.H. Regulation of lung fibroblast alpha-smooth muscle actin expression, contractile phenotype, and apoptosis by IL-1 beta. J. Immunol. 1997, 158, 1392–1399. [Google Scholar]
- Ljubimov, A.V.; Saghizadeh, M. Progress in corneal wound healing. Prog. Retin. Eye Res. 2015, 49, 17–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guerriero, E.; Sado, Y.; SundarRaj, N. Rho-mediated regulation of TGF-beta1- and FGF-2-induced activation of corneal stromal keratocytes. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3662–3670. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, J.; Qu, M.; Backman, L.J.; Zhang, A.; Liu, H.; Zhang, X.; Zhou, Q.; Danielson, P. Sustained Release of TPCA-1 from Silk Fibroin Hydrogels Preserves Keratocyte Phenotype and Promotes Corneal Regeneration by Inhibiting Interleukin-1beta Signaling. Adv. Healthc. Mater. 2020, 9, e2000591. [Google Scholar] [CrossRef] [PubMed]
- Saika, S. TGF-beta signal transduction in corneal wound healing as a therapeutic target. Cornea 2004, 23 (Suppl. S8), S25–S30. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, S.; Dong, M.; Li, Y.; Zhou, Q.; Yang, L. The proinflammatory cytokines IL-1beta and TNF-alpha modulate corneal epithelial wound healing through p16(Ink4a) suppressing STAT3 activity. J. Cell. Physiol. 2020, 235, 10081–10093. [Google Scholar] [CrossRef]
- Yan, C.; Gao, N.; Sun, H.; Yin, J.; Lee, P.; Zhou, L.; Fan, X.; Yu, F.S. Targeting Imbalance between IL-1beta and IL-1 Receptor Antagonist Ameliorates Delayed Epithelium Wound Healing in Diabetic Mouse Corneas. Am. J. Pathol. 2016, 186, 1466–1480. [Google Scholar] [CrossRef]
- Osborn, L.; Kunkel, S.; Nabel, G.J. Tumor Necrosis Factor-Alpha and Interleukin-1 Stimulate the Human Immunodeficiency Virus Enhancer by Activation of the Nuclear Factor Kappa-B. Proc. Natl. Acad. Sci. USA 1989, 86, 2336–2340. [Google Scholar] [CrossRef]
- Krasnow, S.W.; Zhang, L.Q.; Leung, K.; Osborn, L.; Kunkel, S.; Nabel, G.J. Tumor-Necrosis-Factor-Alpha, Interleukin-1, and Phorbol-Myristate Acetate Are Independent Activators of Nf-Kappa-B Which Differentially Activate T-Cells. Cytokine 1991, 3, 372–379. [Google Scholar] [CrossRef]
- Croston, G.E.; Cao, Z.D.; Goeddel, D.V. Nf-Kappa-B Activation by Interleukin-1 (Il-1) Requires an Il-1 Receptor-Associated Protein-Kinase Activity. J. Biol. Chem. 1995, 270, 16514–16517. [Google Scholar] [CrossRef]
- Bitzer, M.; von Gersdorff, G.; Liang, D.; Dominguez-Rosales, A.; Beg, A.A.; Rojkind, M.; Bottinger, E.P. A mechanism of suppression of TGF-beta/SMAD signaling by NF-kappa B/RelA. Genes Dev. 2000, 14, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.N.; Chae, K.; Lee, K.; Oh, B.K.; Choi, K.S.; Choi, J.S.; Park, C. Smad 7 expression in human hepatocellular carcinoma; The mechanism of resistance to transforming growth factor-beta. Hepatology 2001, 34, 673a. [Google Scholar]
- Wu, M.F.; Huang, J.H.; Zeng, Q.Y.; Wang, H.W. The role of Smad7 in cutaneous wound healing. Ital. J. Dermatol. Venereol. 2021, 156, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Sloniecka, M.; Le Roux, S.; Zhou, Q.J.; Danielson, P. Substance P Enhances Keratocyte Migration and Neutrophil Recruitment through Interleukin-8. Mol. Pharm. 2016, 89, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Backman, L.J.; Danielson, P. Activation of NF-kappa B signaling via cytosolic mitochondrial RNA sensing in kerotocytes with mitochondrial DNA common deletion. Sci. Rep.-UK 2021, 11, 7360. [Google Scholar] [CrossRef]
- Freudlsperger, C.; Bian, Y.; Contag Wise, S.; Burnett, J.; Coupar, J.; Yang, X.; Chen, Z.; Van Waes, C. TGF-beta and NF-kappaB signal pathway cross-talk is mediated through TAK1 and SMAD7 in a subset of head and neck cancers. Oncogene 2013, 32, 1549–1559. [Google Scholar] [CrossRef]
- de Oliveira, R.C.; Tye, G.; Sampaio, L.P.; Shiju, T.M.; DeDreu, J.; Menko, A.S.; Santhiago, M.R.; Wilson, S.E. TGF beta 1 and TGF beta 2 proteins in corneas with and without stromal fibrosis: Delayed regeneration of apical epithelial growth factor barrier and the epithelial basement membrane in corneas with stromal fibrosis. Exp. Eye Res. 2021, 202, 108325. [Google Scholar] [CrossRef]
- Wilson, S.E.; He, Y.G.; Weng, J.; Li, Q.; McDowall, A.W.; Vital, M.; Chwang, E.L. Epithelial injury induces keratocyte apoptosis: Hypothesized role for the interleukin-1 system in the modulation of corneal tissue organization and wound healing. Exp. Eye Res. 1996, 62, 325–337. [Google Scholar] [CrossRef]
- Mauviel, A.; Lapiere, J.C.; Halcin, C.; Evans, C.H.; Uitto, J. Differential Cytokine Regulation of Type-I and Type-Vii Collagen Gene-Expression in Cultured Human Dermal Fibroblasts. J. Biol. Chem. 1994, 269, 25–28. [Google Scholar] [CrossRef]
- Lopez-Rovira, T.; Chalaux, E.; Rosa, J.L.; Bartrons, R.; Ventura, F. Interaction and functional cooperation of NF-kappa B with Smads—Transcriptional regulation of the junB promoter. J. Biol. Chem. 2000, 275, 28937–28946. [Google Scholar] [CrossRef]
- Descargues, P.; Sil, A.K.; Sano, Y.; Korchynskyi, O.; Han, G.; Owens, P.; Wang, X.J.; Karin, M. IKK alpha is a critical coregulator of a Smad4-independent TGF beta-Smad2/3 signaling pathway that controls keratinocyte differentiation. Proc. Natl. Acad. Sci. USA 2008, 105, 2487–2492. [Google Scholar] [CrossRef] [PubMed]
- Brandl, M.; Seidler, B.; Haller, F.; Adamski, J.; Schmid, R.M.; Saur, D.; Schneider, G. IKK alpha controls canonical TGF beta-SMAD signaling to regulate genes expressing SNAIL and SLUG during EMT in Panc1 cells. J. Cell Sci. 2010, 123, 4231–4239. [Google Scholar] [CrossRef] [PubMed]
- Arsura, M.; Panta, G.R.; Bilyeu, J.D.; Cavin, L.G.; Sovak, M.A.; Oliver, A.A.; Factor, V.; Heuchel, R.; Mercurio, F.; Thorgeirsson, S.S.; et al. Transient activation of NF-kappaB through a TAK1/IKK kinase pathway by TGF-beta1 inhibits AP-1/SMAD signaling and apoptosis: Implications in liver tumor formation. Oncogene 2003, 22, 412–425. [Google Scholar] [CrossRef]
- Gingery, A.; Bradley, E.W.; Pederson, L.; Ruan, M.; Horwood, N.J.; Oursler, M.J. TGF-beta coordinately activates TAK1/MEK/AKT/NFkB and SMAD pathways to promote osteoclast survival. Exp. Cell Res. 2008, 314, 2725–2738. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, G.; Pallone, F.; MacDonald, T.T. Smad7 in TGF-beta-mediated negative regulation of gut inflammation. Trends Immunol. 2004, 25, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.Y.; Hou, C.C.; Wang, W.; Huang, X.R.; Lan, H.Y. Blockade of NFkappaB activation and renal inflammation by ultrasound-mediated gene transfer of Smad7 in rat remnant kidney. Kidney Int. Suppl. 2005, 94, S83–S91. [Google Scholar] [CrossRef] [PubMed]
- Brissoni, B.; Agostini, L.; Kropf, M.; Martinon, F.; Swoboda, V.; Lippens, S.; Everett, H.; Aebi, N.; Janssens, S.; Meylan, E.; et al. Intracellular trafficking of Interleukin-1 receptor I requires Tollip. Curr. Biol. 2006, 16, 2265–2270. [Google Scholar] [CrossRef]
- Burns, K.; Clatworthy, J.; Martin, L.; Martinon, F.; Plumpton, C.; Maschera, B.; Lewis, A.; Ray, K.; Tschopp, J.; Volpe, F. Tollip, a new component of the IL-1RI pathway, links IRAK to the IL-1 receptor. Nat. Cell Biol. 2000, 2, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Didierlaurent, A.; Brissoni, B.; Velin, D.; Aebi, N.; Tardivel, A.; Kaslin, E.; Sirard, J.C.; Angelov, G.; Tschopp, J.; Burns, K. Tollip regulates proinflammatory responses to interleukin-1 and lipopolysaccharide. Mol. Cell. Biol. 2006, 26, 735–742. [Google Scholar] [CrossRef]
- Mendoza, H.; Campbell, D.G.; Burness, K.; Hastie, J.; Ronkina, N.; Shim, J.H.; Arthur, J.S.C.; Davis, R.J.; Gaestel, M.; Johnson, G.L.; et al. Roles for TAB1 in regulating the IL-1-dependent phosphorylation of the TAB3 regulatory subunit and activity of the TAK1 complex. Biochem. J. 2008, 409, 711–722. [Google Scholar] [CrossRef]
- Sun, S.C.; Ganchi, P.A.; Ballard, D.W.; Greene, W.C. Nf-Kappa-B Controls Expression of Inhibitor I-Kappa-B-Alpha—Evidence for an Inducible Autoregulatory Pathway. Science 1993, 259, 1912–1915. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Barrett, T.; Whitmarsh, A.J.; Cavanagh, J.; Sluss, H.K.; Derijard, B.; Davis, R.J. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J 1996, 15, 2760–2770. [Google Scholar] [CrossRef]
- Stramer, B.M.; Zieske, J.D.; Jung, J.C.; Austin, J.S.; Fini, M.E. Molecular mechanisms controlling the fibrotic repair phenotype in cornea: Implications for surgical outcomes. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4237–4246. [Google Scholar] [CrossRef] [PubMed]
- Masur, S.K.; Dewal, H.S.; Dinh, T.T.; Erenburg, I.; Petridou, S. Myofibroblasts differentiate from fibroblasts when plated at low density. Proc. Natl. Acad. Sci. USA 1996, 93, 4219–4223. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Kurosaka, D.; Yoshino, M.; Oshima, T.; Kurosaka, H. Injured corneal epithelial cells promote myodifferentiation of corneal fibroblasts. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2603–2608. [Google Scholar]
- Kaur, H.; Chaurasia, S.S.; de Medeiros, F.W.; Agrawal, V.; Salomao, M.Q.; Singh, N.; Ambati, B.K.; Wilson, S.E. Corneal stroma PDGF blockade and myofibroblast development. Exp. Eye Res. 2009, 88, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, S.S.; Kaur, H.; de Medeiros, F.W.; Smith, S.D.; Wilson, S.E. Dynamics of the expression of intermediate filaments vimentin and desmin during myofibroblast differentiation after corneal injury. Exp. Eye Res. 2009, 89, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Barbosa, F.L.; Torricelli, A.A.M.; Santhiago, M.R.; Wilson, S.E. Transforming growth factor beta and platelet-derived growth factor modulation of myofibroblast development from corneal fibroblasts in vitro. Exp. Eye Res. 2014, 120, 152–160. [Google Scholar] [CrossRef]
- Sloniecka, M.; Le Roux, S.; Boman, P.; Bystrom, B.; Zhou, Q.; Danielson, P. Expression Profiles of Neuropeptides, Neurotransmitters, and Their Receptors in Human Keratocytes In Vitro and In Situ. PLoS ONE 2015, 10, e0134157. [Google Scholar] [CrossRef]
- Jester, J.V.; Huang, J.Y.; Barry-Lane, P.A.; Kao, W.W.Y.; Petroll, W.M.; Cavanagh, H.D. Transforming growth factor(beta)-mediated corneal myofibroblast differentiation requires actin and fibronectin assembly. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1959–1967. [Google Scholar]


| Gene Name | Gene Symbol | Assay ID |
|---|---|---|
| Keratocan | KERA | Hs00559941_m1 |
| Lumican | LUM | Hs00929860_m1 |
| Aldehyde dehydrogenase 3 family member A1 | ALDH3A1 | Hs00964880_m1 |
| CD34 | CD34 | Hs00990732_m1 |
| Alpha 2 smooth muscle actin | ACTA2 | Hs00909449_m1 |
| β-actin | ACTB | 4352667 |
| Antibody | Company | Code | Applications |
|---|---|---|---|
| α-SMA | Abcam | ab5694 | WB |
| NF-κB p65 | Cell Signaling | 8242 | IF |
| Smad7 | R&D systems | MAB2029 | WB |
| Smad3 (phospho S423 + S425) | Abcam | ab52903 | WB |
| Smad2/3 | Cell Signaling | 3102 | WB |
| β-actin | Cell Signaling | 4967 | WB |
| β-tubulin | Abcam | Ab6046 | WB |
| Anti-rabbit Alexa Fluor 555 | Thermo Fisher | A32794 | IF |
| Anti-rabbit IgG HRP-linked | Cell Signaling | 7074 | WB |
| Anti-mouse IgG HRP-linked | Cell Signaling | 7076 | WB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Li, J.; Backman, L.J.; Danielson, P. Keratocyte Differentiation Is Regulated by NF-κB and TGFβ Signaling Crosstalk. Int. J. Mol. Sci. 2022, 23, 11073. https://doi.org/10.3390/ijms231911073
Zhou X, Li J, Backman LJ, Danielson P. Keratocyte Differentiation Is Regulated by NF-κB and TGFβ Signaling Crosstalk. International Journal of Molecular Sciences. 2022; 23(19):11073. https://doi.org/10.3390/ijms231911073
Chicago/Turabian StyleZhou, Xin, Junhong Li, Ludvig J. Backman, and Patrik Danielson. 2022. "Keratocyte Differentiation Is Regulated by NF-κB and TGFβ Signaling Crosstalk" International Journal of Molecular Sciences 23, no. 19: 11073. https://doi.org/10.3390/ijms231911073
APA StyleZhou, X., Li, J., Backman, L. J., & Danielson, P. (2022). Keratocyte Differentiation Is Regulated by NF-κB and TGFβ Signaling Crosstalk. International Journal of Molecular Sciences, 23(19), 11073. https://doi.org/10.3390/ijms231911073






