Clinical Trials of New Drugs for Vascular Cognitive Impairment and Vascular Dementia
Abstract
1. Introduction
1.1. Definitions of Vascular Cognitive Impairment and Vascular Dementia
1.2. Epidemiology of VCI
1.3. Unmet Needs
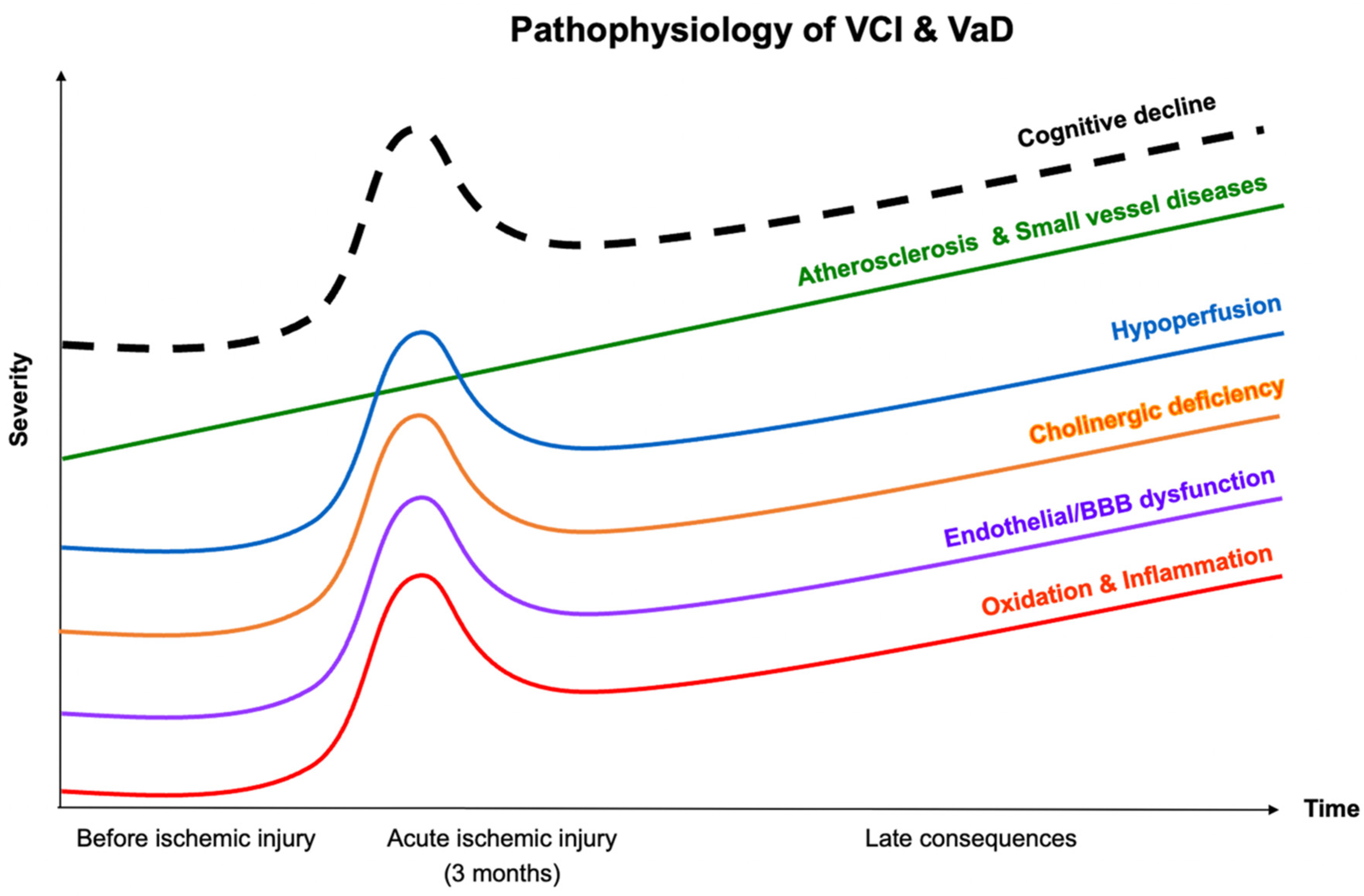
2. Pathophysiology of VCI and VaD
2.1. Atherosclerosis and Cerebral Small-Vessel Diseases
2.2. Hypoperfusion
2.3. Oxidative Stress and Inflammation
2.4. Endothelial Dysfunction and Altered Blood–Brain Barrier (BBB) Permeability
2.5. Cholinergic Hypothesis
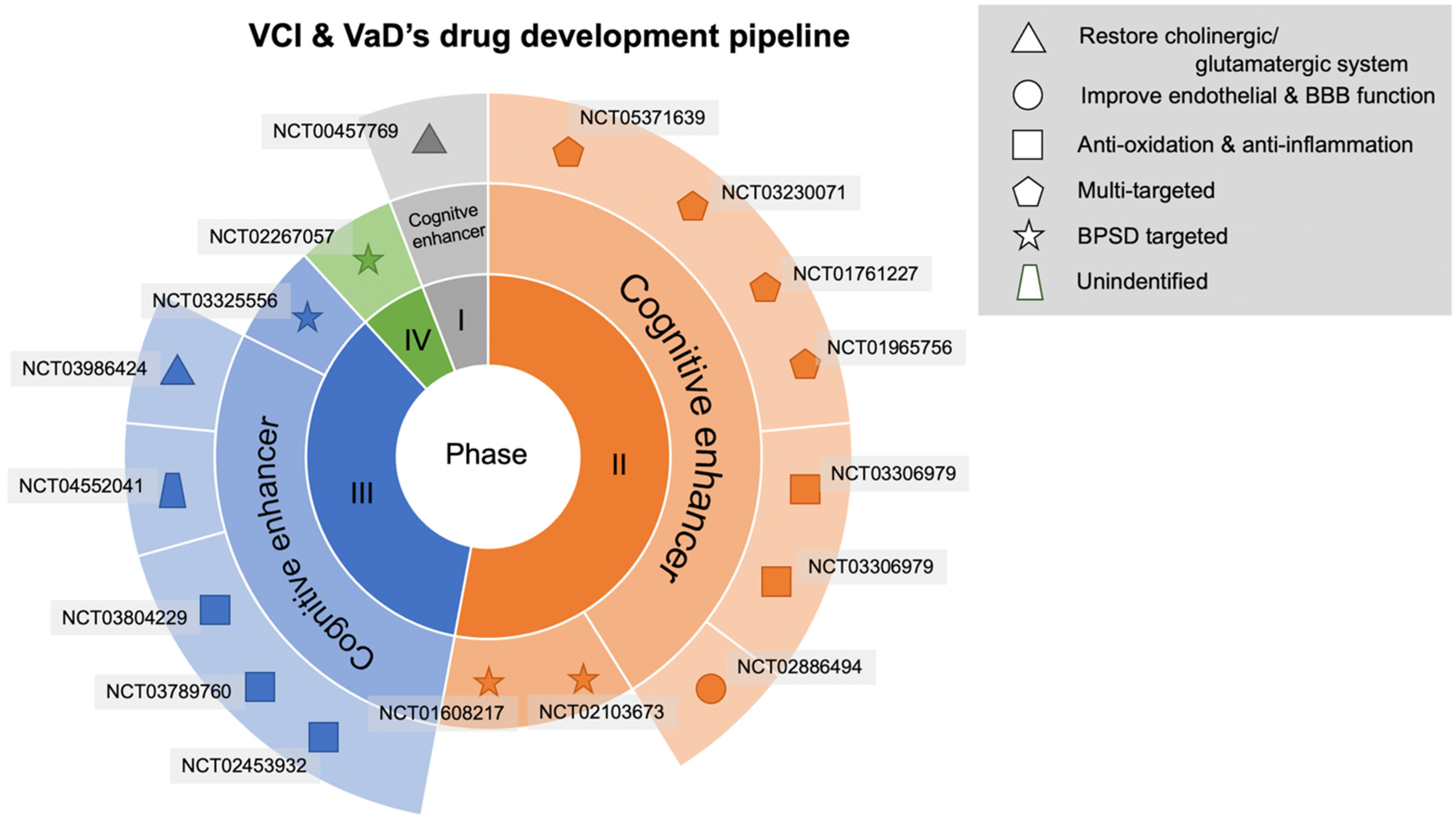
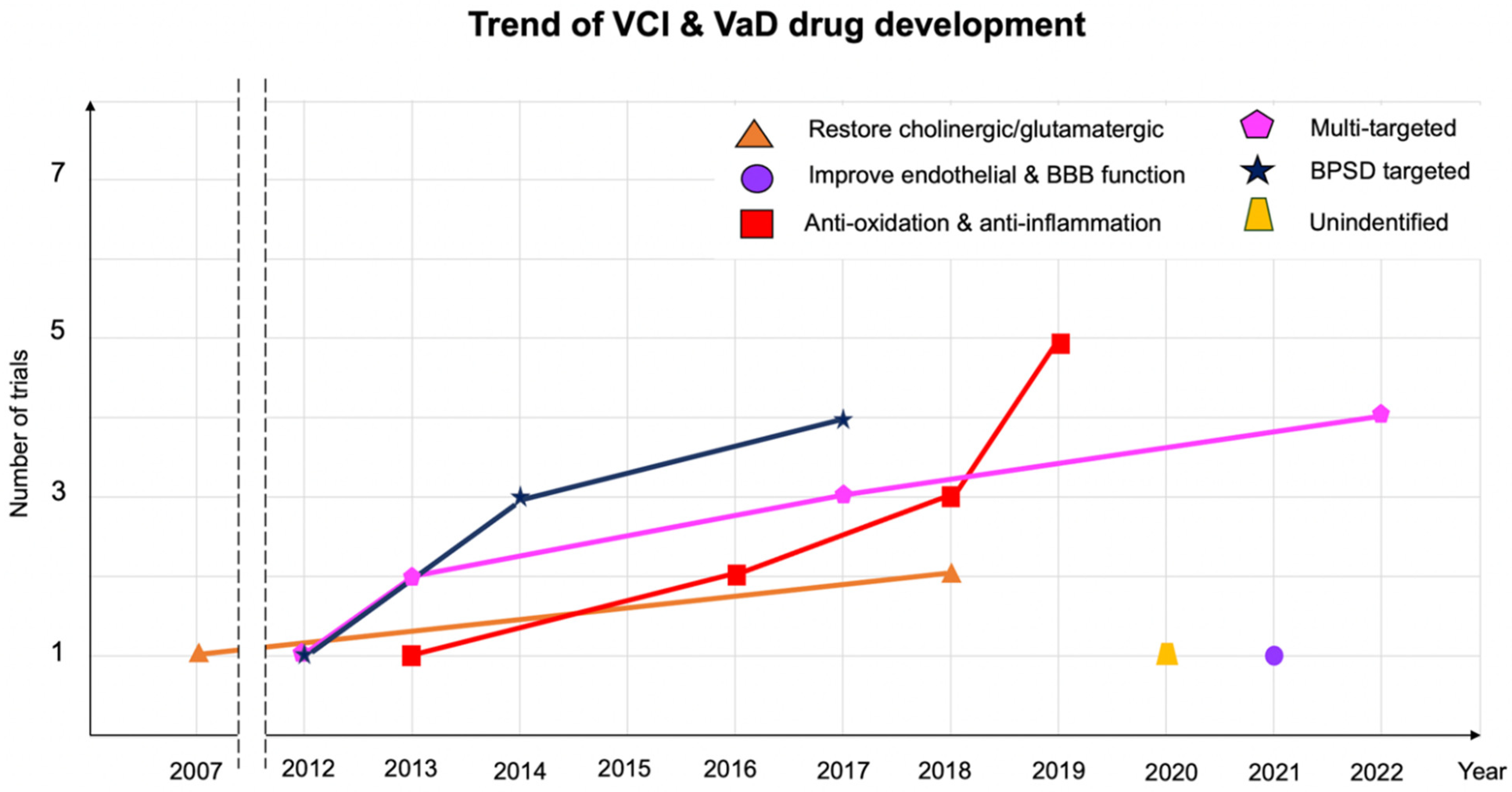
3. Results
3.1. Antioxidant and Anti-Inflammatory Agents
3.2. Agents to Mitigate Endothelial Dysfunction
3.3. Multitarget Agents
3.4. Agents for Restoring the Central Cholinergic or Glutamatergic System
3.5. Agents for Treating Behavioral Psychological Symptoms of Dementia (BPSD)
4. Discussion
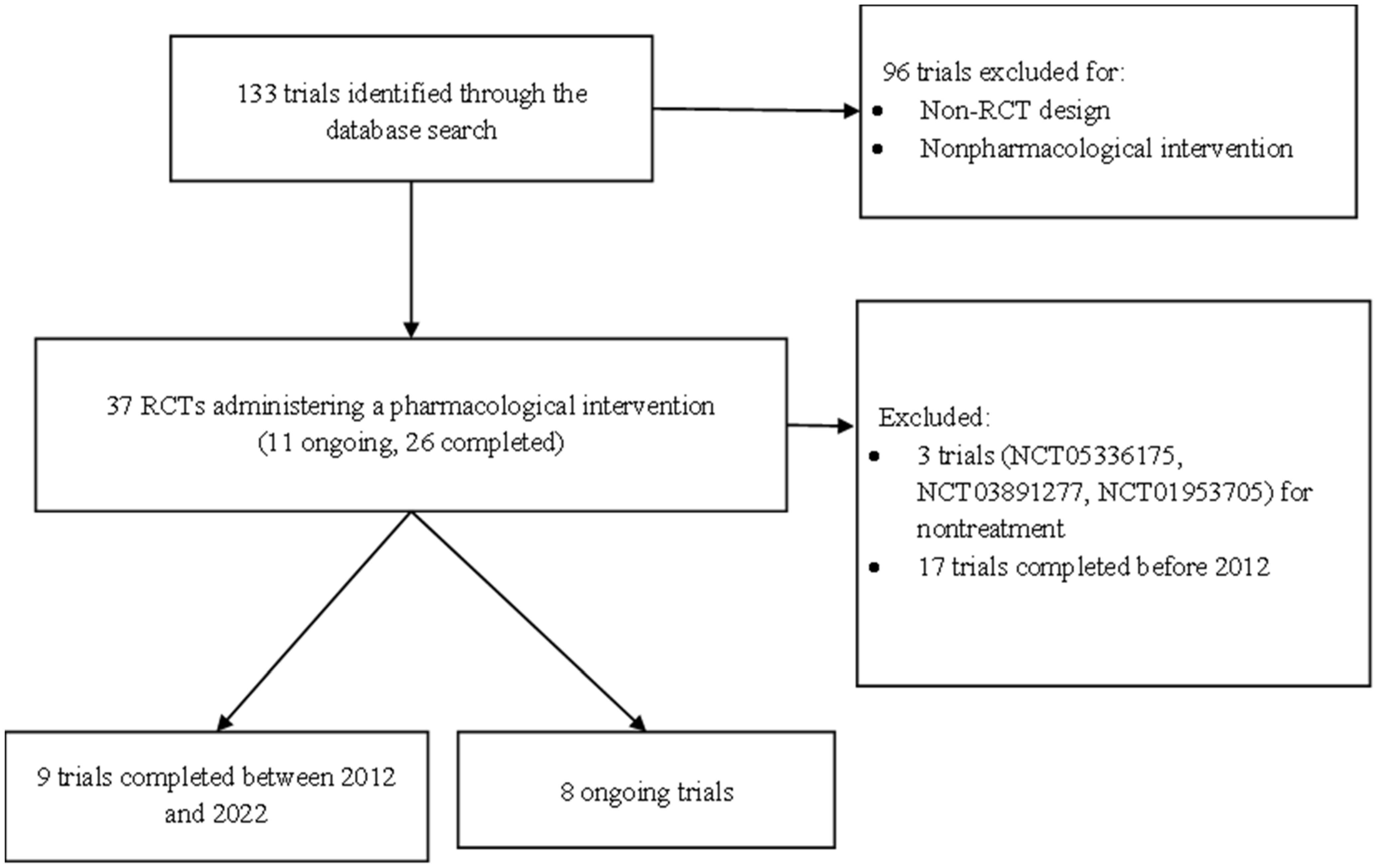
5. Materials and Methods
5.1. Type of Trials
5.2. Type of Participants
5.3. Types of Interventions
5.4. Type of Outcome
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; Decarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef] [PubMed]
- Moorhouse, P.; Rockwood, K. Vascular cognitive impairment: Current concepts and clinical developments. Lancet Neurol. 2008, 7, 246–255. [Google Scholar] [CrossRef]
- Erkinjuntti, T. Subcortical Vascular Dementia. Cerebrovasc. Dis. 2002, 13 (Suppl. S2), 58–60. [Google Scholar] [CrossRef]
- Cao, Q.; Tan, C.-C.; Xu, W.; Hu, H.; Cao, X.-P.; Dong, Q.; Tan, L.; Yu, J.-T. The Prevalence of Dementia: A Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2020, 73, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Douiri, A.; Rudd, A.G.; Wolfe, C.D. Prevalence of poststroke cognitive impairment: South London Stroke Register 1995–2010. Stroke 2013, 44, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, P.S.; Brodaty, H.; Valenzuela, M.J.; Lorentz, L.; Looi, J.C.; Berman, K.; Ross, A.; Wen, W.; Zagami, A.S. Clinical Determinants of Dementia and Mild Cognitive Impairment following Ischaemic Stroke: The Sydney Stroke Study. Dement. Geriatr. Cogn. Disord. 2006, 21, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Pendlebury, S.T.; Rothwell, P.M. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: A systematic review and meta-analysis. Lancet Neurol. 2009, 8, 1006–1018. [Google Scholar] [CrossRef]
- Kokmen, E.; Whisnant, J.P.; O’Fallon, W.M.; Chu, C.P.; Beard, C.M. Dementia after ischemic stroke: A population-based study in Rochester, Minnesota (1960–1984). Neurology 1996, 46, 154–159. [Google Scholar] [CrossRef]
- Pendlebury, S.T.; Rothwell, P.M. Incidence and prevalence of dementia associated with transient ischaemic attack and stroke: Analysis of the population-based Oxford Vascular Study. Lancet Neurol. 2019, 18, 248–258. [Google Scholar] [CrossRef]
- Wu, Y.-T.; Beiser, A.S.; Breteler, M.M.; Fratiglioni, L.; Helmer, C.; Hendrie, H.C.; Honda, H.; Ikram, M.A.; Langa, K.; Lobo, A.; et al. The changing prevalence and incidence of dementia over time—Current evidence. Nat. Rev. Neurol. 2017, 13, 327–339. [Google Scholar] [CrossRef]
- Markus, H.S.; Schmidt, R. Genetics of Vascular Cognitive Impairment. Stroke 2019, 50, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Dichgans, M.; Zietemann, V. Prevention of Vascular Cognitive Impairment. Stroke 2012, 43, 3137–3146. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y.; Chételat, G.; Habeck, C.; Arenaza-Urquijo, E.M.; Vemuri, P.; Estanga, A.; Bartrés-Faz, D.; Cantillon, M.; Clouston, S.A.P.; Elman, J.A.; et al. Mechanisms underlying resilience in ageing. Nat. Rev. Neurosci. 2019, 20, 246. [Google Scholar] [CrossRef] [PubMed]
- Diniz, B.S.; Butters, M.A.; Albert, S.M.; Dew, M.A.; Reynolds, C.F., 3rd. Late-life depression and risk of vascular dementia and Alzheimer’s disease: Systematic review and meta-analysis of community-based cohort studies. Br. J. Psychiatry 2013, 202, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Duering, M.; Hachinski, V.; Joutel, A.; Pendlebury, S.T.; Schneider, J.A.; Dichgans, M. Vascular Cognitive Impairment and Dementia: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019, 73, 3326–3344. [Google Scholar] [CrossRef]
- Silva, N.; Bracko, O.; Nelson, A.R.; de Oliveira, F.F.; Robison, L.S.; Shaaban, C.E.; Hainsworth, A.H.; Price, B.R. Vascular cognitive impairment and dementia: An early career researcher perspective. Alzheimers Dement. 2022, 14, e12310. [Google Scholar] [CrossRef]
- Iadecola, C. The Pathobiology of Vascular Dementia. Neuron 2013, 80, 844–866. [Google Scholar] [CrossRef]
- Wallin, A.; Alafuzoff, I.; Carlsson, A.; Eckernäs, S.-A.; Gottfries, C.-G.; Karlsson, I.; Svennerholm, L.; Winblad, B. Neurotransmitter deficits in a non-multi-infarct category of vascular dementia. Acta Neurol. Scand. 1989, 79, 397–406. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, Z.; Teipel, S.J.; Yang, J.; Xing, Y.; Tang, Y.; Jia, J. White Matter Damage in the Cholinergic System Contributes to Cognitive Impairment in Subcortical Vascular Cognitive Impairment, No Dementia. Front. Aging Neurosci. 2017, 9, 47. [Google Scholar] [CrossRef]
- Sharp, S.I.; Francis, P.T.; Elliott, M.S.J.; Kalaria, R.N.; Bajic, N.; Hortobagyi, T.; Ballard, C.G. Choline Acetyltransferase Activity in Vascular Dementia and Stroke. Dement. Geriatr. Cogn. Disord. 2009, 28, 233–238. [Google Scholar] [CrossRef]
- Lnsis, A. Atherosclenrosis. Nature 2000, 407, 233–241. [Google Scholar]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; De Ferranti, S.; Després, J.-P.; Fullerton, H.J.; Howard, V.J. Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation 2015, 131, e29–e322. [Google Scholar] [CrossRef] [PubMed]
- Hoth, K.F.; Poppas, A.; Moser, D.J.; Paul, R.H.; Cohen, R.A. Cardiac Dysfunction and Cognition in Older Adults with Heart Failure. Cogn. Behav. Neurol. 2008, 21, 65–72. [Google Scholar] [CrossRef]
- Jefferson, A.L.; Beiser, A.S.; Himali, J.J.; Seshadri, S.; O’Donnell, C.J.; Manning, W.J.; Wolf, P.A.; Au, R.; Benjamin, E.J. Low cardiac index is associated with incident dementia and Alzheimer disease: The Framingham Heart Study. Circulation 2015, 131, 1333–1339. [Google Scholar] [CrossRef]
- Zlokovic, B.V.; Gottesman, R.F.; Bernstein, K.E.; Seshadri, S.; McKee, A.; Snyder, H.; Greenberg, S.M.; Yaffe, K.; Schaffer, C.B.; Yuan, C.; et al. Vascular contributions to cognitive impairment and dementia (VCID): A report from the 2018 National Heart, Lung, and Blood Institute and National Institute of Neurological Disorders and Stroke Workshop. Alzheimer’s Dement. 2020, 16, 1714–1733. [Google Scholar] [CrossRef]
- Kalaria, R.N. The pathology and pathophysiology of vascular dementia. Neuropharmacology 2017, 134, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R.; Grinberg, L.T.; Attems, J. Vascular dementia: Different forms of vessel disorders contribute to the development of dementia in the elderly brain. Exp. Gerontol. 2012, 47, 816–824. [Google Scholar] [CrossRef]
- Iadecola, C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurosci. 2004, 5, 347–360. [Google Scholar] [CrossRef]
- Duncombe, J.; Kitamura, A.; Hase, Y.; Ihara, M.; Kalaria, R.N.; Horsburgh, K. Chronic cerebral hypoperfusion: A key mechanism leading to vascular cognitive impairment and dementia. Closing the translational gap between rodent models and human vascular cognitive impairment and dementia. Clin. Sci. 2017, 131, 2451–2468. [Google Scholar] [CrossRef]
- Marshall, R.S. Effects of Altered Cerebral Hemodynamics on Cognitive Function. J. Alzheimer’s Dis. 2012, 32, 633–642. [Google Scholar] [CrossRef]
- Alosco, M.L.; Brickman, A.M.; Spitznagel, M.B.; Garcia, S.L.; Narkhede, A.; Griffith, E.Y.; Raz, N.; Cohen, R.; Sweet, L.H.; Colbert, L.H.; et al. Cerebral perfusion is associated with white matter hyperintensities in older adults with heart failure. Congest. Heart Fail. 2013, 19, E29–E34. [Google Scholar] [CrossRef] [PubMed]
- Justin, B.N.; Turek, M.; Hakim, A.M. Heart disease as a risk factor for dementia. Clin. Epidemiol. 2013, 5, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Tatemichi, T.K.; Desmond, D.; Prohovnik, I.; Eidelberg, D. Dementia associated with bilateral carotid occlusions: Neuropsychological and haemodynamic course after extracranial to intracranial bypass surgery. J. Neurol. Neurosurg. Psychiatry 1995, 58, 633–636. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Back, S.A.; Kroenke, C.D.; Sherman, L.S.; Lawrence, G.; Gong, X.; Taber, E.N.; Sonnen, J.A.; Larson, E.B.; Montine, T.J. White matter lesions defined by diffusion tensor imaging in older adults. Ann. Neurol. 2011, 70, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Fernando, M.S.; Simpson, J.E.; Matthews, F.; Brayne, C.; Lewis, C.E.; Barber, R.; Kalaria, R.N.; Forster, G.; Esteves, F.; Wharton, S.B.; et al. White matter lesions in an unselected cohort of the elderly: Molecular pathology suggests origin from chronic hypoperfusion injury. Stroke 2006, 37, 1391–1398. [Google Scholar] [CrossRef]
- Akiguchi, I.; Tomimoto, H.; Suenaga, T.; Wakita, H.; Budka, H. Alterations in Glia and Axons in the Brains of Binswanger’s Disease Patients. Stroke 1997, 28, 1423–1429. [Google Scholar] [CrossRef]
- Nation, D.A.; Sweeney, M.D.; Montagne, A.; Sagare, A.P.; D’Orazio, L.M.; Pachicano, M.; Sepehrband, F.; Nelson, A.R.; Buennagel, D.P.; Harrington, M.G.; et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 2019, 25, 270–276. [Google Scholar] [CrossRef]
- Siracusa, R.; Impellizzeri, D.; Cordaro, M.; Crupi, R.; Esposito, E.; Petrosino, S.; Cuzzocrea, S. Anti-Inflammatory and Neuroprotective Effects of Co-UltraPEALut in a Mouse Model of Vascular Dementia. Front. Neurol. 2017, 8, 233. [Google Scholar] [CrossRef]
- Ames, B.N. Delaying the mitochondrial decay of aging. Ann. New York Acad. Sci. 2004, 1019, 406–411. [Google Scholar] [CrossRef]
- Perluigi, M.; Swomley, A.M.; Butterfield, D.A. Redox proteomics and the dynamic molecular landscape of the aging brain. Ageing Res. Rev. 2014, 13, 75–89. [Google Scholar] [CrossRef]
- Wang, F.; Cao, Y.; Ma, L.; Pei, H.; Rausch, W.D.; Li, H. Dysfunction of Cerebrovascular Endothelial Cells: Prelude to Vascular Dementia. Front. Aging Neurosci. 2018, 10, 376. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, H.-Y.; Tang, X.-C. Cholinergic deficiency involved in vascular dementia: Possible mechanism and strategy of treatment. Acta Pharmacol. Sin. 2009, 30, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. The pathology of ischemic-vascular dementia: An update. J. Neurol. Sci. 2002, 203–204, 153–157. [Google Scholar] [CrossRef]
- Jellinger, K.A. The Pathology of “Vascular Dementia”: A Critical Update. J. Alzheimer’s Dis. 2008, 14, 107–123. [Google Scholar] [CrossRef]
- Wang, S.; Ma, F.; Huang, L.; Zhang, Y.; Peng, Y.; Xing, C.; Feng, Y.; Wang, X.; Peng, Y. Dl-3-n-Butylphthalide (NBP): A Promising Therapeutic Agent for Ischemic Stroke. CNS Neurol. Disord. Drug Targets 2018, 17, 338–347. [Google Scholar] [CrossRef]
- Chen, X.-Q.; Qiu, K.; Liu, H.; He, Q.; Bai, J.-H.; Lu, W. Application and prospects of butylphthalide for the treatment of neurologic diseases. Chin. Med. J. 2019, 132, 1467–1477. [Google Scholar] [CrossRef]
- Abdoulaye, I.A.; Guo, Y.J. A Review of Recent Advances in Neuroprotective Potential of 3-N-Butylphthalide and Its Derivatives. BioMed Res. Int. 2016, 2016, 5012341. [Google Scholar] [CrossRef]
- Jia, J.; Wei, C.; Liang, J.; Zhou, A.; Zuo, X.; Song, H.; Wu, L.; Chen, X.; Chen, S.; Zhang, J.; et al. The effects of DL-3-n-butylphthalide in patients with vascular cognitive impairment without dementia caused by subcortical ischemic small vessel disease: A multicentre, randomized, double-blind, placebo-controlled trial. Alzheimer’s Dement. 2016, 12, 89–99. [Google Scholar] [CrossRef]
- Fan, X.; Shen, W.; Wang, L.; Zhang, Y. Efficacy and Safety of DL-3-n-Butylphthalide in the Treatment of Poststroke Cognitive Impairment: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2022, 12, 810297. [Google Scholar] [CrossRef]
- Chang, D.; Liu, J.; Bilinski, K.; Xu, L.; Steiner, G.Z.; Seto, S.W.; Bensoussan, A. Herbal Medicine for the Treatment of Vascular Dementia: An Overview of Scientific Evidence. Evidence-Based Complement. Altern. Med. 2016, 2016, 7293626. [Google Scholar] [CrossRef]
- Li, T.; Liu, H.; Lu, Y.; Jia, Z.; Xu, L.; Gao, R.; Liu, J.; Liu, J.; Tang, X. A phase I tolerance and safety study of Sailuotong capsule. Chin. J. New Drugs 2012, 21, 62–67. [Google Scholar]
- Steiner, G.Z.; Yeung, A.; Liu, J.-X.; Camfield, D.A.; de Blasio, F.M.; Pipingas, A.; Scholey, A.B.; Stough, C.; Chang, D.H. The effect of Sailuotong (SLT) on neurocognitive and cardiovascular function in healthy adults: A randomised, double-blind, placebo controlled crossover pilot trial. BMC Complement. Altern. Med. 2016, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chang, D.; Chan, D.; Liu, J.; Bensoussan, A. A randomised placebo-controlled clinical trial of a Chinese herbal medicine for the treatment of vascular dementia. In Proceedings of the 2nd International Congress for Complementary Medicine Research, Munich, Germany, 11–13 May 2007. [Google Scholar]
- Genevieve, S.; Alan, Y.; David, C.; Frances, D.B.; Andrew, P.; Andrew, S.; Con, S.; Dennis, C. The effect of a standardised Chinese herbal medicine formula (Sailuotong) on N1, PN, P2, MMN, P3a, and P3b amplitudes: A pilot study. In Proceedings of the Conference Abstract: Australasian Society for Psychophysiology, Inc., Coffs Harbour, Australia, 26–28 November 2014. [Google Scholar] [CrossRef]
- Shi, J.; Wei, M.; Ni, J.; Sun, F.; Sun, L.; Wang, J.; Yu, T.; Wang, K.; Lv, P.; Zhang, Y.; et al. Tianzhi granule improves cognition and BPSD of vascular dementia: A randomized controlled trial. J. Transl. Med. 2020, 18, 76. [Google Scholar] [CrossRef]
- Li, L.-J.; Li, H.-X.; Wu, X.-T.; Yan, B.; Zhou, N. Effect of geniposide on vascular dementia in rats. Sichuan Da Xue Xue Bao Yi Xue Ban 2009, 40, 604–607. [Google Scholar] [PubMed]
- Farr, S.A.; Poon, H.F.; Dogrukol-Ak, D.; Drake, J.; Banks, W.A.; Eyerman, E.L.; Butterfield, D.A.; Morley, J.E. The antioxidants α-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J. Neurochem. 2003, 84, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Bernardi, J.; Fiuza, T.; Costa, L.; Brandão, R.; Pereira, M.E. N-acetylcysteine protects memory decline induced by streptozotocin in mice. Chem. Interact. 2016, 253, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Remington, R.; Lortie, J.J.; Hoffmann, H.; Page, R.; Morrell, C.; Shea, T.B. A Nutritional Formulation for Cognitive Performance in Mild Cognitive Impairment: A Placebo-Controlled Trial with an Open-Label Extension. J. Alzheimer’s Dis. 2015, 48, 591–595. [Google Scholar] [CrossRef]
- Remington, R.; Bechtel, C.; Larsen, D.; Samar, A.; Doshanjh, L.; Fishman, P.; Luo, Y.; Smyers, K.; Page, R.; Morrell, C.; et al. A Phase II Randomized Clinical Trial of a Nutritional Formulation for Cognition and Mood in Alzheimer’s Disease. J. Alzheimer’s Dis. 2015, 45, 395–405. [Google Scholar] [CrossRef]
- Thein, S.; Konis, G.; Lacey, D.M.; Hudefi, F.; Vandersluis, J.; Papka, M.; Sharma, S.; Anderson, C.G.; Jagadeesan, S.; Fink, E.; et al. A Randomized, Double Blind, Vehicle Controlled, Parallel, Phase Ii Study to Evaluate Efficacy and Safety of Bac in Patients with Alzheimer’s Disease or Vascular Dementia. Res. Sq. 2020. Preprint. [Google Scholar] [CrossRef]
- Stephan, B.C.M.; Harrison, S.L.; Keage, H.A.D.; Babateen, A.; Robinson, L.; Siervo, M. Cardiovascular Disease, the Nitric Oxide Pathway and Risk of Cognitive Impairment and Dementia. Curr. Cardiol. Rep. 2017, 19, 1–8. [Google Scholar] [CrossRef]
- Aissa, M.; Lee, S.; Bennett, B.; Thatcher, G. Targeting NO/cGMP Signaling in the CNS for Neurodegeneration and Alzheimer’s Disease. Curr. Med. Chem. 2016, 23, 2770–2788. [Google Scholar] [CrossRef] [PubMed]
- Garthwaite, J. NO as a multimodal transmitter in the brain: Discovery and current status. Br. J. Pharmacol. 2019, 176, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Correia, S.S.; Iyengar, R.R.; Germano, P.; Tang, K.; Bernier, S.G.; Schwartzkopf, C.D.; Tobin, J.; Lee, T.W.-H.; Liu, G.; Jacobson, S.; et al. The CNS-Penetrant Soluble Guanylate Cyclase Stimulator CY6463 Reveals its Therapeutic Potential in Neurodegenerative Diseases. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, F.; Shi, J.; Yang, D.; Deng, Y.; Gong, Q. Gastrodin ameliorates subacute phase cerebral ischemia-reperfusion injury by inhibiting inflammation and apoptosis in rats. Mol. Med. Rep. 2016, 14, 4144–4152. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Chen, M.; Lu, J.; Chen, C.; Jiao, B.-H. Effect of tanshinone II A on MMP-2 and iNOS expression and free radical release in hippocampus of rat Alzheimer’s disease model. Acad. J. Second Mil. Med Univ. 2010, 30, 380–384. [Google Scholar] [CrossRef]
- Habtemariam, S. Molecular Pharmacology of Rosmarinic and Salvianolic Acids: Potential Seeds for Alzheimer’s and Vascular Dementia Drugs. Int. J. Mol. Sci. 2018, 19, 458. [Google Scholar] [CrossRef]
- Zhang, Q.-L.; Fu, B.M.; Zhang, Z.-J. Borneol, a novel agent that improves central nervous system drug delivery by enhancing blood–brain barrier permeability. Drug Deliv. 2017, 24, 1037–1044. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Qin, X.; Zhang, Y.F.; Lin, J.; Wang, D.Q.; Qin, R.A. Protective effects of Compound Danshen Tablets on rat with vascular dementia caused by ischemia and reperfusion in middle cerebral artery. Pharmacol. Clin. Chin. Materia Med. 2015, 12, 177–180. [Google Scholar]
- Oliveira, W.H.; Nunes, A.K.; França, M.E.R.; Santos, L.A.; Lós, D.B.; Rocha, S.W.; Barbosa, K.P.; Rodrigues, G.B.; Peixoto, C.A. Effects of metformin on inflammation and short-term memory in streptozotocin-induced diabetic mice. Brain Res. 2016, 1644, 149–160. [Google Scholar] [CrossRef]
- Isoda, K.; Young, J.L.; Zirlik, A.; MacFarlane, L.A.; Tsuboi, N.; Gerdes, N.; Schönbeck, U.; Libby, P. Metformin inhibits proinflammatory responses and nuclear factor-κb in human vascular wall cells. Arter. Thromb. Vasc. Biol. 2006, 26, 611–617. [Google Scholar] [CrossRef]
- Bhutada, P.; Mundhada, Y.; Bansod, K.; Tawari, S.; Patil, S.; Dixit, P.; Umathe, S.; Mundhada, D. Protection of cholinergic and antioxidant system contributes to the effect of berberine ameliorating memory dysfunction in rat model of streptozotocin-induced diabetes. Behav. Brain Res. 2011, 220, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Arafa, N.M.; Marie, M.-A.S.; AlAzimi, S.A.M. Effect of canagliflozin and metformin on cortical neurotransmitters in a diabetic rat model. Chem. Interact. 2016, 258, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Luchsinger, J.A.; Perez, T.; Chang, H.; Mehta, P.; Steffener, J.; Pradabhan, G.; Ichise, M.; Manly, J.; Devanand, D.P.; Bagiella, E. Metformin in Amnestic Mild Cognitive Impairment: Results of a Pilot Randomized Placebo Controlled Clinical Trial. J. Alzheimer’s Dis. 2016, 51, 501–514. [Google Scholar] [CrossRef]
- Koenig, A.M.; Mechanic-Hamilton, D.; Xie, S.X.; Combs, M.F.; Cappola, A.R.; Xie, L.; Detre, J.A.; Wolk, D.A.; Arnold, S.E. Effects of the Insulin Sensitizer Metformin in Alzheimer Disease: Pilot Data From a Randomized Placebo-controlled Crossover Study. Alzheimer Dis. Assoc. Disord. 2017, 31, 107–113. [Google Scholar] [CrossRef]
- Jin, B.R.; Liu, H.Y. Comparative efficacy and safety of cognitive enhancers for treating vascular cognitive impairment: Systematic review and Bayesian network meta-analysis. Neural Regen. Res. 2019, 14, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.; Róman, G.; Salloway, S.; Hecker, J.; Boundy, K.; Kumar, D.; Posner, H.; Schindler, R. The long-term efficacy and tolerability of donepezil in patients with vascular dementia. Int. J. Geriatr. Psychiatry 2010, 25, 305–313. [Google Scholar] [CrossRef]
- Ballard, C.; Sauter, M.; Scheltens, P.; He, Y.; Barkhof, F.; Van Straaten, E.C.W.; van der Flier, W.; Hsu, C.; Wu, S.; Lane, R. Efficacy, safety and tolerability of rivastigmine capsules in patients with probable vascular dementia: The VantagE study. Curr. Med Res. Opin. 2008, 24, 2561–2574. [Google Scholar] [CrossRef]
- Birks, J.; McGuinness, B.; Craig, D. Rivastigmine for vascular cognitive impairment. Cochrane Database Syst. Rev. 2013, 5, CD004744. [Google Scholar] [CrossRef]
- Auchus, A.P.; Brashear, H.R.; Salloway, S.; Korczyn, A.; De Deyn, P.P.; Gassmann-Mayer, C. For the GAL-INT-26 Study Group Galantamine treatment of vascular dementia: A randomized trial. Neurology 2007, 69, 448–458. [Google Scholar] [CrossRef]
- Smith, E.E.; Barber, P.; Field, T.S.; Ganesh, A.; Hachinski, V.; Hogan, D.B.; Lanctôt, K.L.; Lindsay, M.P.; Sharma, M.; Swartz, R.H.; et al. Canadian Consensus Conference on Diagnosis and Treatment of Dementia (CCCDTD)5: Guidelines for management of vascular cognitive impairment. Alzheimer’s Dement. 2020, 6, e12056. [Google Scholar] [CrossRef]
- McShane, R.; Westby, M.J.; Roberts, E.; Minakaran, N.; Schneider, L.; Farrimond, L.E.; Maayan, N.; Ware, J.; Debarros, J. Memantine for dementia. Cochrane Database Syst. Rev. 2019, 3, Cd003154. [Google Scholar] [CrossRef] [PubMed]
- Finkel, S.I.; Costa e Silva, J.; Cohen, G.; Miller, S.; Sartorius, N. Behavioral and Psychological Signs and Symptoms of Dementia: A Consensus Statement on Current Knowledge and Implications for Research and Treatment. Int. Psychogeriatr. 1996, 8 (Suppl. S3), 497–500. [Google Scholar] [CrossRef] [PubMed]
- Fuh, J.-L.; Wang, S.-J.; Cummings, J.L. Neuropsychiatric profiles in patients with Alzheimer’s disease and vascular dementia. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1337–1341. [Google Scholar] [CrossRef]
- Schwertner, E.; Pereira, J.B.; Xu, H.; Secnik, J.; Winblad, B.; Eriksdotter, M.; Nägga, K.; Religa, D. Behavioral and Psychological Symptoms of Dementia in Different Dementia Disorders: A Large-Scale Study of 10,000 Individuals. medRxiv 2021. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Chen, P.K.; Wang, S.H.; Lane, H.Y. Effect of Sodium Benzoate on Cognitive Function Among Patients With Behavioral and Psychological Symptoms of Dementia: Secondary Analysis of a Randomized Clinical Trial. JAMA Netw Open 2021, 4, e216156. [Google Scholar] [CrossRef] [PubMed]
- van den Elsen, G.A.; Ahmed, A.I.; Verkes, R.-J.; Kramers, C.; Feuth, T.; Rosenberg, P.B.; Van Der Marck, M.A.; Olde Rikkert, M.G.O. Tetrahydrocannabinol for neuropsychiatric symptoms in dementia: A randomized controlled trial. Neurology 2015, 84, 2338–2346. [Google Scholar] [CrossRef]
- Toledo, J.B.; Arnold, S.E.; Raible, K.; Brettschneider, J.; Xie, S.X.; Grossman, M.; Monsell, S.E.; Kukull, W.A.; Trojanowski, J.Q. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain 2013, 136 Pt 9, 2697–2706. [Google Scholar] [CrossRef]
- Smith, E.E.; Cieslak, A.; Barber, P.; Chen, J.; Chen, Y.W.; Donnini, I.; Edwards, J.D.; Frayne, R.; Field, T.S.; Hegedus, J.; et al. Therapeutic Strategies and Drug Development for Vascular Cognitive Impairment. J. Am. Heart Assoc. 2017, 6, e005568. [Google Scholar] [CrossRef]
| Drug | Target Type | Mechanism of Action | Therapeutic Purpose | NCT Number | Status | Country | Start Year | Estimated Year of Completion |
|---|---|---|---|---|---|---|---|---|
| Akatinol memantine | Enhance the effect of the glutamatergic system | N-methyl-D-aspartate(NMDA) glutamate receptor agonist Neurotransmitter based Antagonizes glutamate toxicity Neuroprotection | Cognitive function enhancer | NCT03986424 | Active, not recruiting | Russia | 2018 | 2022 |
| Butylphthalide soft capsules | Antioxidation and anti-inflammation | DL-3-n-butylphthalide Improves the oxidative stress response of the nervous system Inhibits neuronal apoptosis and autophagy Regulates the function of the central cholinergic system Promotes neuroplasticity | Cognitive function enhancer | NCT03804229 | Recruiting | China | 2019 | 2024 |
| SaiLuoTong capsules (traditional Chinese medicine) | Antioxidant and anti-inflammatory | Ginkgo biloba—anti-inflammation and neurogenesis Ginsenoside Rg1—antioxidation and stress-induced neuronal apoptosis Saffron—scavenging of oxygen free radicals | Cognitive function enhancer | NCT03789760 | Recruiting | China | 2019 | 2023 |
| Prospecta | Unknown | Unidentified | Cognitive function enhancer | NCT04552041 | Active, not recruiting | Russia | 2020 | 2023 |
| Tianzhi granules (traditional Chinese medicine) | Antioxidant and anti-inflammatory | Reduces oxidative stress Mitigates apoptosis and necrosis | Cognitive function enhancer | NCT02453932 | Completed | China | 2013 | 2017 |
| Pimavanserin | Neuropsychiatric | Antipsychotic | Reduces the relapse of psychotic symptoms | NCT03325556 | Completed | Multiple countries | 2017 | 2019 |
| Drug | Target Type | Mechanism of Action | Therapeutic Purpose | NCT Number | Status | Country | Phases | Start Year | Estimated Year of Completion |
|---|---|---|---|---|---|---|---|---|---|
| Donepezil | Restores the function of the central cholinergic system | Cholinesterase inhibitor Inhibits acetylcholinesterase (AChE) and increases acetylcholine release | Cognitive function enhancer | NCT00457769 | Active, not recruiting | United States | Phase I | 2007 | 2021 |
| N-acetylcysteine | Antioxidant and anti-inflammatory | Glutathione precursor Exerts antioxidant and anti-inflammatory effects | Cognitive function enhancer | NCT03306979 | Recruiting | Canada | Phase II | 2018 | 2022 |
| CY6463 | Improves endothelial and BBB dysfunction | Central nervous system–penetrant guanylyl cyclase stimulator Compensates for NO deficiency | Cognitive function enhancer | NCT04798989 | Recruiting | United States | Phase II | 2021 | 2022 |
| Tianmabianchunzhigan (traditional Chinese medicine) | Multitarget | Gastrodia elata Reduces inflammation Mitigates apoptosis Suppresses the formation of beta-amyloid plaques | Cognitive function enhancer | NCT05371639 | Not yet recruiting | Not mentioned | Phase II | 2022 | 2025 |
| NCT03230071 | Completed | China | Phase II | 2017 | 2021 | ||||
| Fufangdanshen tablets (traditional Chinese medicine) | Multitarget | Tanshinone—inhibits inducible NO synthase (iNOS) and matrix metalloproteinase 2 (MMP 2), reduces free radicals and oxidation Salvianolic acid—inhibits oxidation and inflammation Borneol—increases BBB permeability | Cognitive function enhancer | NCT01761227 | Completed | China | Phase II | 2012 | 2015 |
| Delta-9-tetrahydrocannabinol (delta-THC) + acetaminophen | Neuropsychiatric | Analgesic | Treatment of pain-induced behavioral disturbances | NCT01608217 | Completed | Netherland | Phase II | 2012 | 2014 |
| Metformin | Multitarget Atherosclerosis | Hypoglycemic agent Antidiabetic Reduces oxidative stress and inflammation Improves endothelial function Inhibits AChE activity | Cognitive function enhancer | NCT01965756 | Completed | USA | Phase II | 2013 | 2017 |
| D-amino acid oxidase inhibitor | Neuropsychiatric | NMDA receptor enhancer | Treatment of behavioral disturbances Cognitive function enhancer | NCT02103673 | Completed | Taiwan | Phase II | 2014 | 2017 |
| BAC | Antioxidant and neuroinflammatory | Glycine max(L.) Merr Reduce levels of procytokine interleukin 1-β | Cognitive function enhancer | NCT02886494 | Completed | USA | Phase II | 2016 | 2018 |
| Drug | Target Type | Mechanism of Action | Therapeutic Purpose | NCT Number | Status | Country | Phases | Start Year | Estimated Year of Completion |
|---|---|---|---|---|---|---|---|---|---|
| Paracetamol | Neuropsychiatric | Analgesic | Treatment of pain-induced depression | NCT02267057 | Completed | Norway | Phase IV | 2014 | 2016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linh, T.T.D.; Hsieh, Y.-C.; Huang, L.-K.; Hu, C.-J. Clinical Trials of New Drugs for Vascular Cognitive Impairment and Vascular Dementia. Int. J. Mol. Sci. 2022, 23, 11067. https://doi.org/10.3390/ijms231911067
Linh TTD, Hsieh Y-C, Huang L-K, Hu C-J. Clinical Trials of New Drugs for Vascular Cognitive Impairment and Vascular Dementia. International Journal of Molecular Sciences. 2022; 23(19):11067. https://doi.org/10.3390/ijms231911067
Chicago/Turabian StyleLinh, Tran Thanh Duy, Yi-Chen Hsieh, Li-Kai Huang, and Chaur-Jong Hu. 2022. "Clinical Trials of New Drugs for Vascular Cognitive Impairment and Vascular Dementia" International Journal of Molecular Sciences 23, no. 19: 11067. https://doi.org/10.3390/ijms231911067
APA StyleLinh, T. T. D., Hsieh, Y.-C., Huang, L.-K., & Hu, C.-J. (2022). Clinical Trials of New Drugs for Vascular Cognitive Impairment and Vascular Dementia. International Journal of Molecular Sciences, 23(19), 11067. https://doi.org/10.3390/ijms231911067






