Non-Isocyanate Aliphatic–Aromatic Poly(carbonate-urethane)s—An Insight into Transurethanization Reactions and Structure–Property Relationships
Abstract
:1. Introduction
2. Results and Discussion
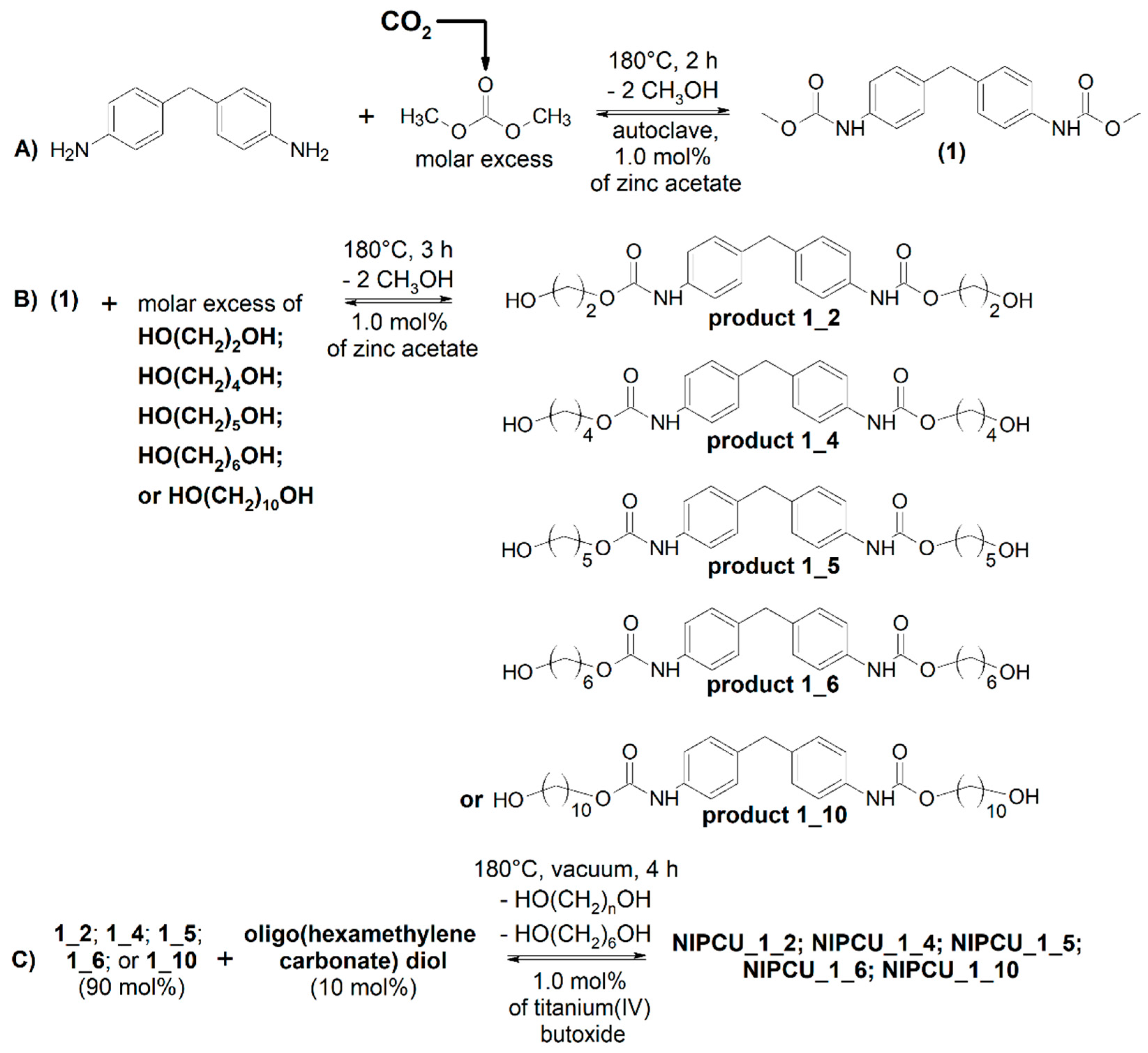
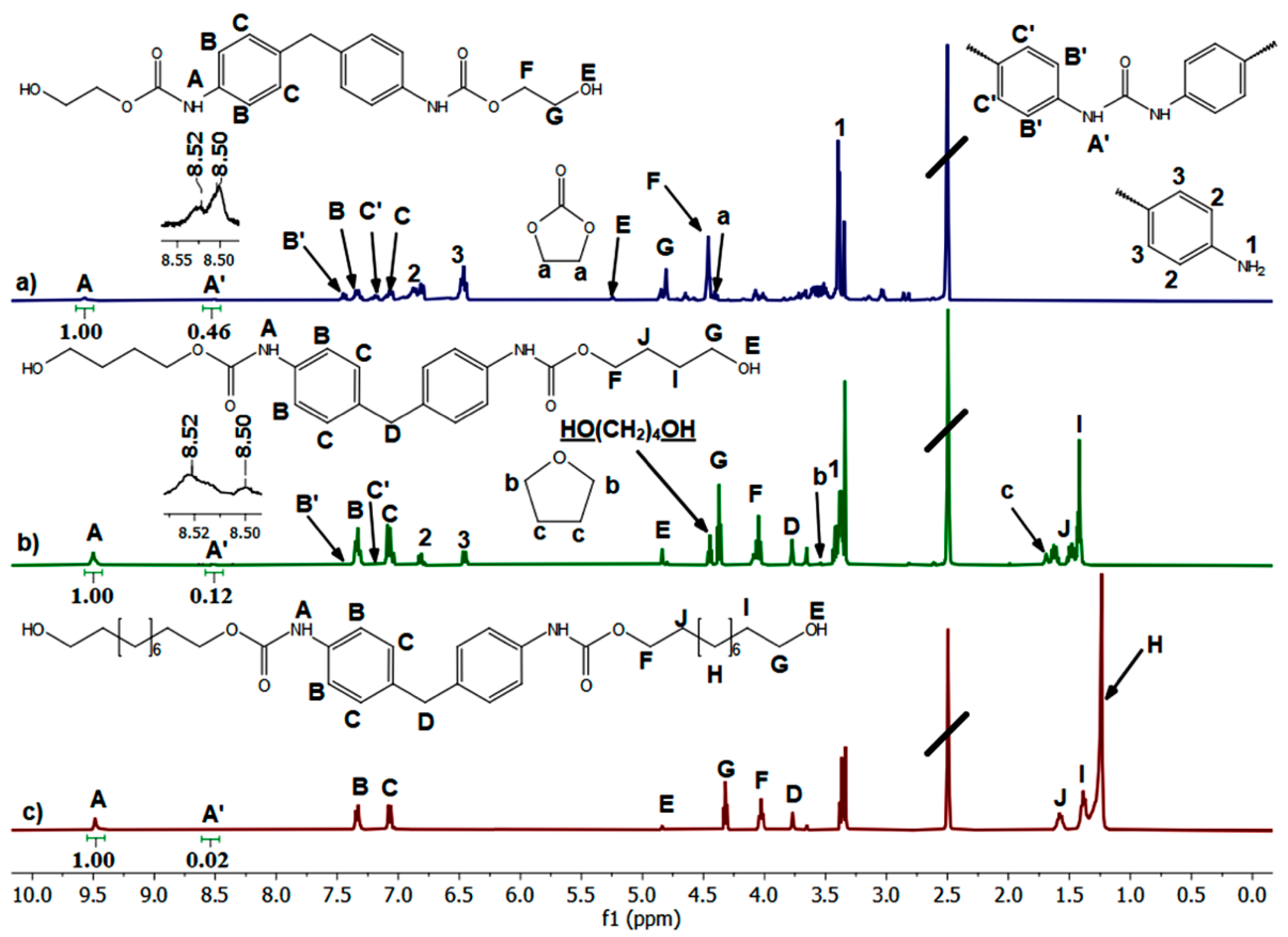
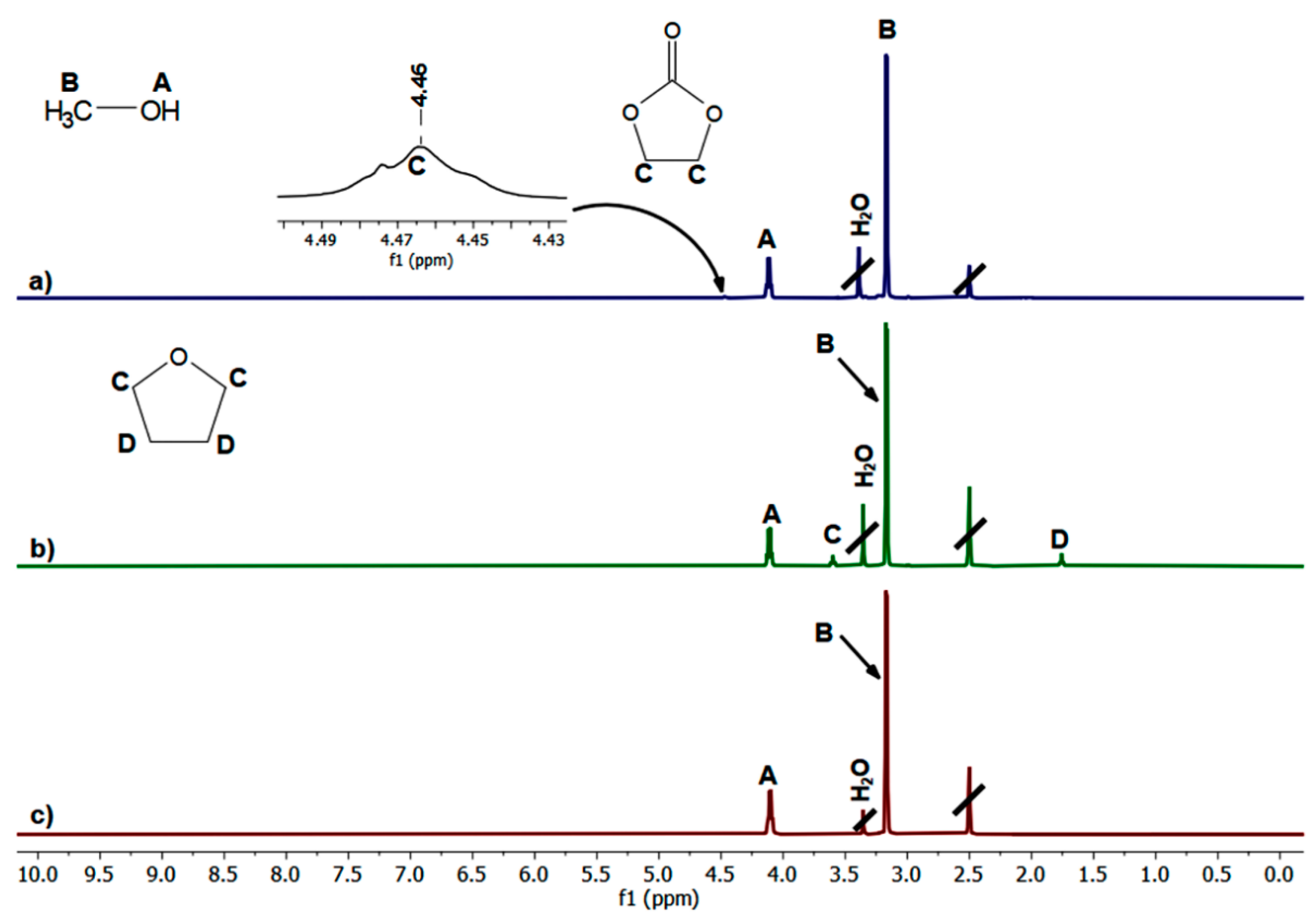
2.1. Transurethanization of Arylene BMC with α,ω-diol (C2, C4, C5, C6, or C10)
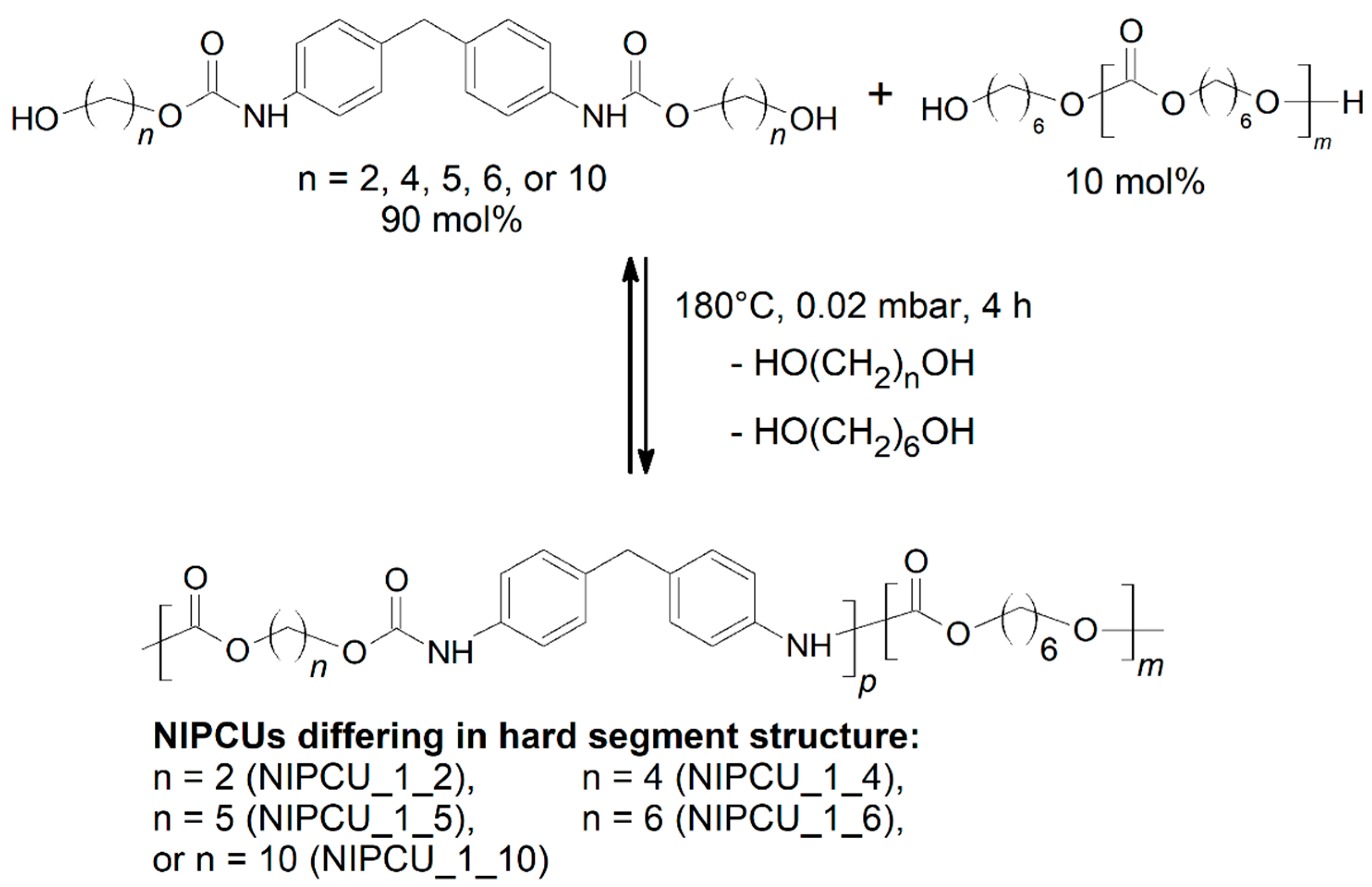
2.2. Transurethane Polycondensation toward the Aliphatic–Aromatic NIPCUs
2.3. Thermal Properties of the Aliphatic–Aromatic NIPCUs
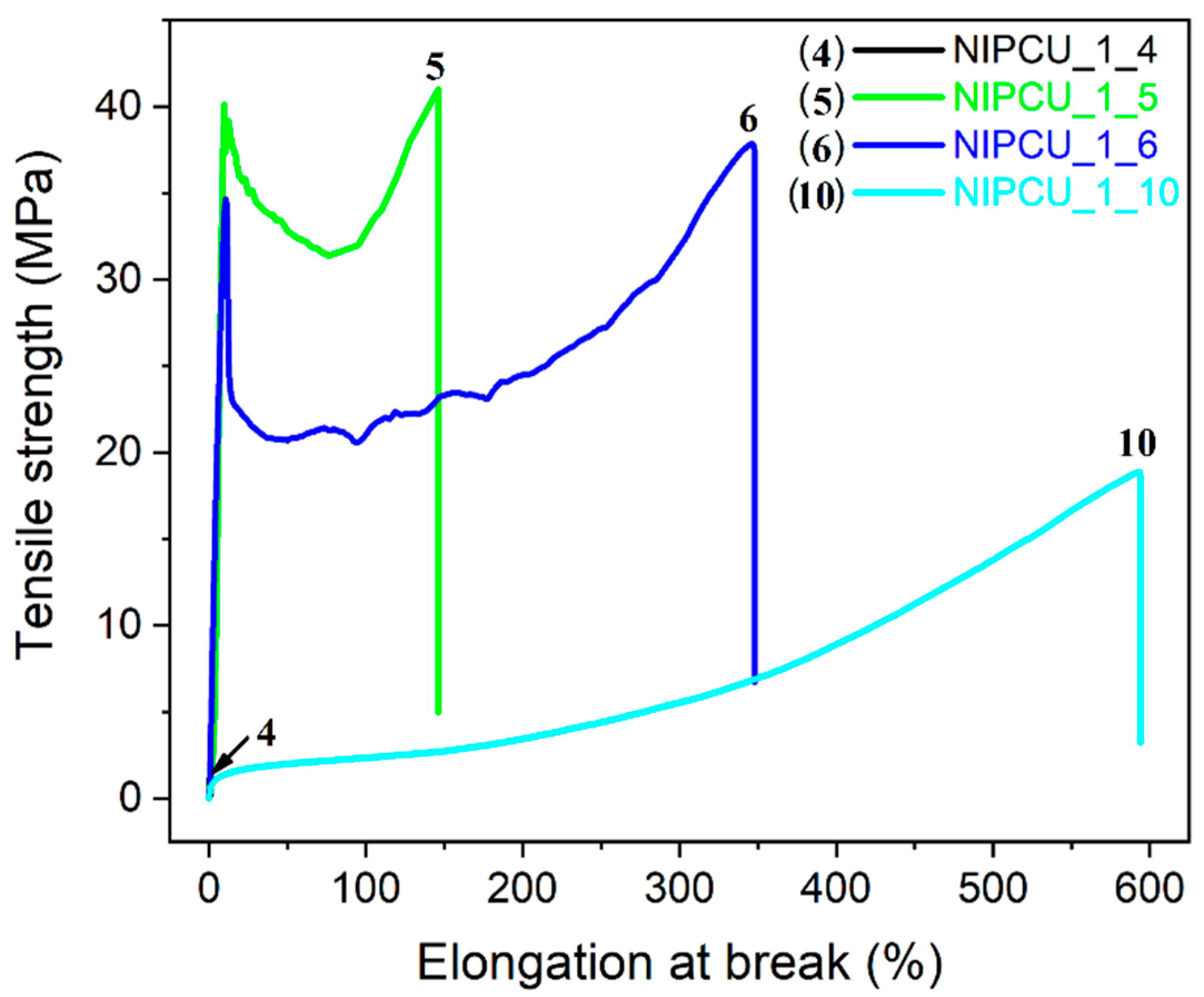
2.4. Mechanical Properties of the Aliphatic–Aromatic NIPCUs
3. Materials and Methods
3.1. Materials
3.2. Characterization Techniques
3.3. Syntheses
3.3.1. Synthesis of Arylene BMC
3.3.2. Transurethanization of the Arylene BMC with α,ω-diol (C2, C4, C5, C6, or C10)
3.3.3. Synthesis of the Aliphatic–Aromatic NIPCUs
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maisonneuve, L.; Lamarzelle, O.; Rix, E.; Grau, E.; Cramail, H. Isocyanate-Free Routes to Polyurethanes and Poly(Hydroxy Urethane)s. Chem. Rev. 2015, 115, 12407–12439. [Google Scholar] [CrossRef]
- Aristri, M.A.; Lubis, M.A.; Yadav, S.M.; Antov, P.; Papadopoulos, A.N.; Pizzi, A.; Fatriasari, W.; Ismayati, M.; Iswanto, A.H. Recent Developments in Lignin- and Tannin-Based Non-isocyanate Polyurethane Resins for Wood Adhesives—A Review. Appl. Sci. 2021, 11, 4242. [Google Scholar] [CrossRef]
- Gomez-Lopez, A.; Elizalde, F.; Calvo, I.; Sardon, H. Trends in Non-isocyanate Polyurethane (NIPU) Development. Chem. Commun. 2021, 57, 12254–12265. [Google Scholar] [CrossRef]
- Oh, J.; Kim, Y.K.; Hwang, S.-H.; Kim, H.-C.; Jung, J.-H.; Jeon, C.-H.; Kim, J.; Lim, S.K. Synthesis of Thermoplastic Polyurethanes Containing Bio-Based Polyester Polyol and Their Fiber Property. Polymers 2022, 14, 2033. [Google Scholar] [CrossRef]
- Parzuchowski, P.G.; Mazurek, M.; Świderska, A.; Roguszewska, M.; Rolińska, K.; Wołosz, D. Preparation and long term stability studies of carbon dioxide adsorbents based on hyperbranched polymers. Polimery 2020, 65, 174–183. [Google Scholar] [CrossRef]
- Wołosz, D.; Parzuchowski, P.G.; Rolińska, K. Environmentally friendly synthesis of urea-free poly(carbonate-urethane) elastomers. Macromolecules 2022, 55, 4995–5008. [Google Scholar] [CrossRef]
- Lapprand, A.; Boisson, F.; Delolme, F.; Méchin, F.; Pascault, J.P. Reactivity of Isocyanates with Urethanes: Conditions for Allophanate Formation. Polym. Degrad. Stab. 2005, 90, 363–373. [Google Scholar] [CrossRef]
- Rokicki, G.; Parzuchowski, P.G.; Mazurek, M. Non-Isocyanate Polyurethanes: Synthesis, Properties, and Applications. Polym. Adv. Technol. 2015, 26, 707–761. [Google Scholar] [CrossRef]
- Monie, F.; Grignard, B.; Detrembleur, C. Divergent Aminolysis Approach for Constructing Recyclable Self-Blown Nonisocyanate Polyurethane Foams. ACS Macro Lett. 2022, 11, 236–242. [Google Scholar] [CrossRef]
- Chen, X.; Pizzi, A.; Fredon, E.; Gerardin, C.; Zhou, X.; Zhang, B.; Du, G. Low Curing Temperature Tannin-Based Non-isocyanate Polyurethane (NIPU) Wood Adhesives: Preparation and properties Evaluation. Int. J. Adhes. Adhes. 2022, 112, 103001. [Google Scholar] [CrossRef]
- Liu, C.; Wu, J.; Zhou, X.; Zhou, X.; Wu, Z.; Qu, J. Synthesis and Properties of Poly(dimethylsiloxane)-Based Non-isocyanate Polyurethanes Coatings with Good Anti-Smudge Properties. Prog. Org. Coat. 2022, 163, 106690. [Google Scholar] [CrossRef]
- Chen, X.; Pizzi, A.; Essawy, H.; Fredon, E.; Gerardin, C.; Guigo, N.; Sbirrazzuoli, N. Non-Furanic Humins-Based Non-isocyanate Polyurethane (NIPU) Thermoset Wood Adhesives. Polymers 2021, 13, 372. [Google Scholar] [CrossRef]
- Xi, X.; Pizzi, A.; Gerardin, C.; Lei, H.; Chen, X.; Amirou, S. Preparation and Evaluation of Glucose Based Non-isocyanate Polyurethane Self-Blowing Rigid Foams. Polymers 2019, 11, 1802. [Google Scholar] [CrossRef]
- Xi, X.; Pizzi, A.; Delmotte, L. Isocyanate-Free Polyurethane Coatings and Adhesives from Mono- and Di-Saccharides. Polymers 2018, 10, 402. [Google Scholar] [CrossRef]
- Cornille, A.; Auvergne, R.; Figovsky, O.; Boutevin, B.; Caillol, S. A Perspective Approach to Sustainable Routes for Non-isocyanate Polyurethanes. Eur. Polym. J. 2017, 87, 535–552. [Google Scholar] [CrossRef]
- Iuliano, A.; Dębowski, M.; Plichta, A.; Kowalczyk, S.; Florjańczyk, Z.; Rokicki, G.; Parzuchowski, P.; Mazurek-Budzyńska, M.; Wołosz, D.; Pilch-Pitera, B. Polycarbonate-Based Polyurethane-Attractive Materials for Adhesives, Binders and Sealants Production. Polimery/Polymers 2020, 65, 497–509. [Google Scholar] [CrossRef]
- Niemczyk, A.; Piegat, A.; Sonseca Olalla, Á.; El Fray, M. New Approach to Evaluate Microphase Separation in Segmented Polyurethanes Containing Carbonate Macrodiol. Eur. Polym. J. 2017, 93, 182–191. [Google Scholar] [CrossRef]
- Chen, L.; Qin, Y.; Wang, X.; Zhao, X.; Wang, F. Plasticizing While Toughening and Reinforcing Poly(Propylene Carbonate) Using Low Molecular Weight Urethane: Role of Hydrogen-Bonding Interaction. Polymers 2011, 52, 4873–4880. [Google Scholar] [CrossRef]
- Wołosz, D.; Parzuchowski, P.G.; Świderska, A. Synthesis and Characterization of the Non-isocyanate poly(Carbonate-Urethane)s Obtained via Polycondensation Route. Eur. Polym. J. 2021, 155, 110574. [Google Scholar] [CrossRef]
- Boisaubert, P.; Kébir, N.; Schuller, A.-S.; Burel, F. Polyurethane Coatings from Formulations with Low Isocyanate Content Using a Transurethane Polycondensation Route. Polymers 2022, 240, 124522. [Google Scholar] [CrossRef]
- Ochiai, B.; Utsuno, T. Non-isocyanate Synthesis and Application of Telechelic Polyurethanes via Polycondensation of Diurethanes Obtained from Ethylene Carbonate and Diamines. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 525–533. [Google Scholar] [CrossRef]
- Li, S.; Zhao, J.; Zhang, Z.; Zhang, J.; Yang, W. Synthesis and Characterization of Aliphatic Thermoplastic Poly(Ether Urethane) Elastomers through a Non-isocyanate Route. Polymers 2015, 57, 164–172. [Google Scholar] [CrossRef]
- Deng, Y.; Li, S.; Zhao, J.; Zhang, Z.; Zhang, J.; Yang, W. Crystallizable and Tough Aliphatic Thermoplastic Poly(Ether Urethane)s Synthesized through a Non-isocyanate Route. RSC Adv. 2014, 4, 43406–43414. [Google Scholar] [CrossRef]
- Han, L.; Dai, J.; Zhang, L.; Ma, S.; Deng, J.; Zhang, R. Diisocyanate Free and Melt Polycondensation Preparation of Bio-Based Unsaturated Poly(Ester-Urethane)s and Their Properties as UV Curable Coating Materials. RSC Adv. 2014, 4, 49471–49477. [Google Scholar] [CrossRef]
- Qin, J.; Jiang, J.; Ye, S.; Wang, S.; Xiao, M.; Tao, Y.; Jie, G.; Meng, Y. High Performance Poly(Urethane-Co-amide) From CO2-Based Dicarbamate: An Alternative to Long Chain Polyamide. RSC Adv. 2019, 9, 26080–26090. [Google Scholar] [CrossRef]
- Yang, X.; Wang, S.; Liu, X.; Huang, Z.; Huang, X.; Xu, X.; Liu, H.; Wang, D.; Shang, S. Preparation of Non-isocyanate Polyurethanes from Epoxy Soybean Oil: Dual Dynamic Networks to Realize Self-Healing and Reprocessing Under Mild Conditions. Green Chem. 2021, 23, 6349–6355. [Google Scholar] [CrossRef]
- Shen, Z.; Zheng, L.; Li, C.; Liu, G.; Xiao, Y.; Wu, S.; Liu, J.; Zhang, B. A Comparison of Non-isocyanate and HDI-Based Poly(Ether Urethane): Structure and Properties. Polymer 2019, 175, 186–194. [Google Scholar] [CrossRef]
- Unverferth, M.; Kreye, O.; Prohammer, A.; Meier, M.A.R. Renewable Non-isocyanate Based Thermoplastic Polyurethanes via Polycondensation of Dimethyl Carbamate Monomers with Diols. Macromol. Rapid Commun. 2013, 34, 1569–1574. [Google Scholar] [CrossRef]
- Sun, D.-L.; Xie, S.-J.; Deng, J.-R.; Huang, C.-J.; Ruckenstein, E.; Chao, Z.-S. CH3COONa as an Effective Catalyst for Methoxycarbonylation of 1,6-Hexanediamine by Dimethyl Carbonate to Dimethylhex-Ane-1,6-Dicarbamate. Green Chem. 2010, 12, 483–490. [Google Scholar] [CrossRef]
- Reixach, E.; Bonet, N.; Rius-Ruiz, F.X.; Wershofen, S.; Vidal-Ferran, A. Zinc Acetates as Efficient Catalysts for the Synthesis of Bis-isocyanate Precursors. Ind. Eng. Chem. Res. 2010, 49, 6362–6366. [Google Scholar] [CrossRef]
- Rokicki, G.; Piotrowska, A. A New Route to Polyurethanes from Ethylene Carbonate, Diamines and Diols. Polymers 2002, 43, 2927–2935. [Google Scholar] [CrossRef]
- Wołosz, D.; Parzuchowski, P.G. Biobased Non-isocyanate Poly(Carbonate-Urethane)s of Exceptional Strength and Flexibility. Polymers 2022, 254, 125026. [Google Scholar] [CrossRef]
- Jagtap, S.R.; Patil, Y.P.; Fujita, S.-I.; Arai, M.; Bhanage, B.M. Heterogeneous Base Catalyzed Synthesis of 2-oxazolidinones/2-Imidiazolidinones Via Transesterification of Ethylene Carbonate with β-aminoalcohols/1,2-Diamines. Appl. Catal. A Gen. 2008, 341, 133–138. [Google Scholar] [CrossRef]
- Kucharski, M.; Kijowska, D. Synthesis of Polyetherols from Melamine and Ethylene Carbonate. J. Appl. Polym. Sci. 2001, 80, 1776–1784. [Google Scholar] [CrossRef]
- Wang, B.; Yang, S.; Min, L.; Gu, Y.; Zhang, Y.; Wu, X.; Zhang, L.; Elageed, E.H.M.; Wu, S.; Gao, G. Eco-Efficient Synthesis of Cyclic Carbamates/Dithiocarbonimidates from Cyclic Carbonates/Trithiocarbonate and Aromatic Amines Catalyzed by Ionic Liquid BmimOAc. Adv. Synth. 2014, 356, 3125–3134. [Google Scholar] [CrossRef]
- Dongdong, P.; Hengshui, T. Polycarbonate Polyurethane Elastomers Synthesized Via a Solvent-Free and Nonisocyanate Melt transesterification Process. J. Appl. Polym. Sci. 2015, 132, 41377. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, J.; Zhu, W.; Zheng, L.; Li, C.; Xiao, Y.; Liu, J.; Wu, S.; Zhang, B. A Solvent-free Route to Non-isocyanate Poly(carbonate urethane) with High Molecular Weight and Competitive Mechanical Properties. Eur. Polym. J. 2018, 107, 258–266. [Google Scholar] [CrossRef]
- Shen, Z.; Zheng, L.; Song, D.; Liu, Y.; Li, C.; Liu, J.; Xiao, Y.; Wu, S.; Zhou, T.; Zhang, B.; et al. A Non-isocyanate Route to Poly(Ether Urethane): Synthesis and Effect of Chemical Structures of Hard Segment. Polymers 2022, 14, 2039. [Google Scholar] [CrossRef]
- Feng, P.; Sun, X.; Su, Y.; Li, X.; Zhang, L.H.; Shi, X.; Jiao, N. Ceric Ammonium Nitrate (CAN) Catalyzed Modification of Ketones via Two C–C Bond Cleavages with the Retention of the Oxo-Group. Org. Lett. 2014, 16, 3388–3391. [Google Scholar] [CrossRef]
- Zhou, L.; Yu, L.; Ding, M.; Li, J.; Tan, H.; Wang, Z.; Fu, Q. Synthesis and Characterization of pH-Sensitive Biodegradable Polyurethane for Potential Drug Delivery Applications. Macromolecules 2011, 44, 857–864. [Google Scholar] [CrossRef]
- Prabhakar, A.; Chattopadhyay, D.K.; Jagadeesh, B.; Raju, K.V.S.N. Structural Investigations of Polypropylene Glycol (PPG) and Isophorone Diisocyanate (IPDI)-Based Polyurethane Prepolymer by 1D and 2D NMR Spectroscopy. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 1196–1209. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. Overview of Recent Developments in the Flame Retardancy of Polycarbonates. Polym. Int. 2005, 54, 981–998. [Google Scholar] [CrossRef]

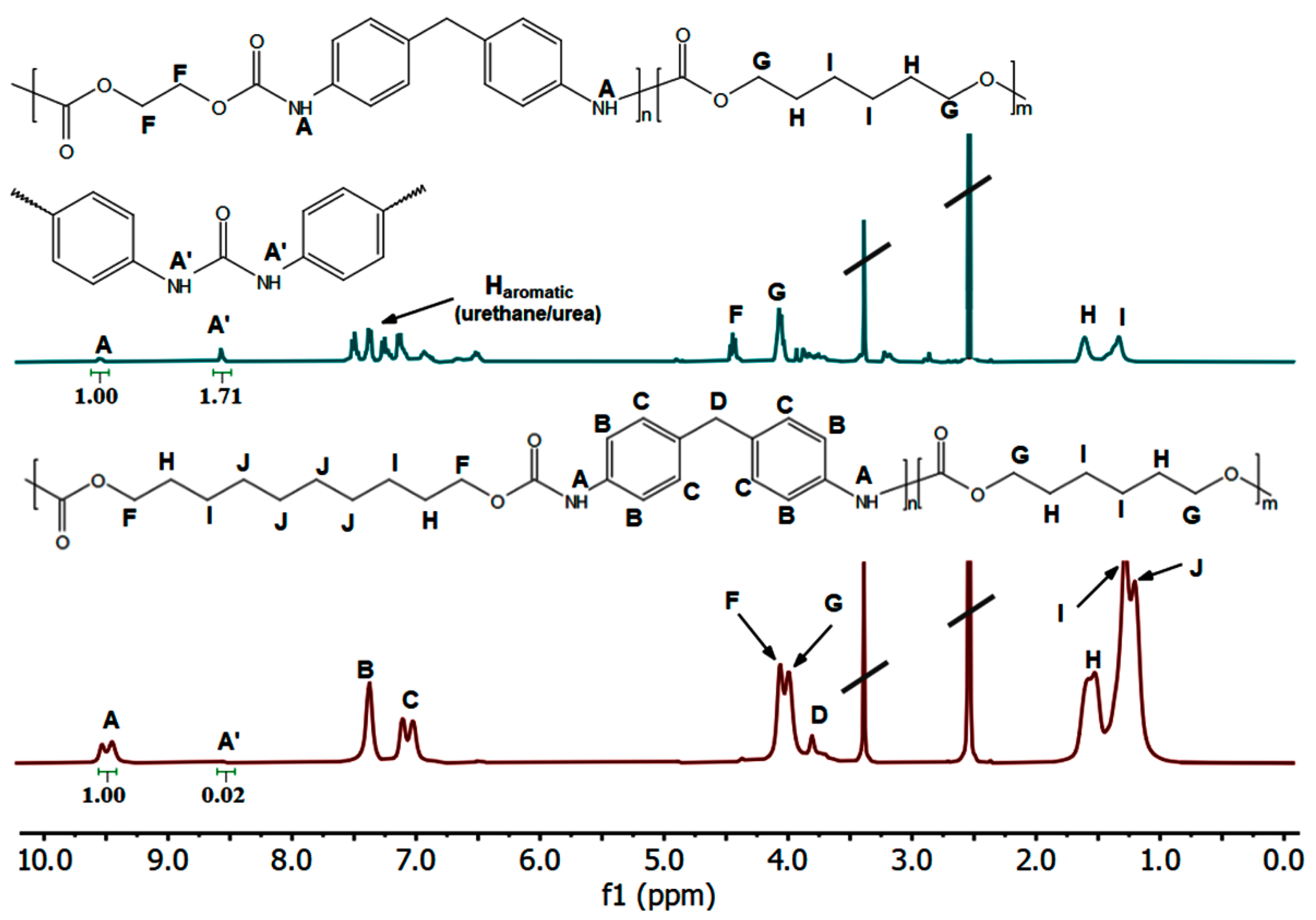


| Urethane Groups | Carbonate Groups | ||||
|---|---|---|---|---|---|
| Theor. | Calc. | Theor. | Calc. | Calc. | |
| /mol% | /mol% | /mol% | /mol% | g·mol−1 | |
| NIPCU_1_2 | 57.93 | 26.51 | 42.07 | 73.49 | 6000 |
| NIPCU_1_4 | 57.93 | 47.38 | 42.07 | 52.62 | 15,000 |
| NIPCU_1_5 | 57.93 | 54.47 | 42.07 | 45.53 | 20,000 |
| NIPCU_1_6 | 57.93 | 55.06 | 42.07 | 44.94 | 28,000 |
| NIPCU_1_10 | 57.93 | 55.49 | 42.07 | 44.51 | 24,000 |
| Sample | Tg /°C | Tc /°C | ΔHc /J∙g−1 | Tm /°C | ΔHm /J∙g−1 |
|---|---|---|---|---|---|
| NIPCU_1_2 | 30 | - | - | - | - |
| NIPCU_1_4 | 30 | - | - | - | - |
| NIPCU_1_5 | 47 | - | - | - | - |
| NIPCU_1_6 | 44 | - | - | - | - |
| NIPCU_1_10 | 18 | 74 | 9 | 107 | 11 |
| NIPCU | Tensile Strength /MPa | Elongation at Break /% | Hardness (Shore D) |
|---|---|---|---|
| NIPCU_1_2 | - a | - a | 44 ± 4 |
| NIPCU_1_4 | 3.1 ± 0.5 | 0.8 ± 0.3 | 57 ± 2 |
| NIPCU_1_5 | 40.2 ± 1.3 | 130 ± 60 | 75 ± 2 |
| NIPCU_1_6 | 37.4 ± 1.1 | 300 ± 40 | 72 ± 3 |
| NIPCU_1_10 | 18.1 ± 0.7 | 550 ± 30 | 54 ± 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wołosz, D. Non-Isocyanate Aliphatic–Aromatic Poly(carbonate-urethane)s—An Insight into Transurethanization Reactions and Structure–Property Relationships. Int. J. Mol. Sci. 2022, 23, 10999. https://doi.org/10.3390/ijms231910999
Wołosz D. Non-Isocyanate Aliphatic–Aromatic Poly(carbonate-urethane)s—An Insight into Transurethanization Reactions and Structure–Property Relationships. International Journal of Molecular Sciences. 2022; 23(19):10999. https://doi.org/10.3390/ijms231910999
Chicago/Turabian StyleWołosz, Dominik. 2022. "Non-Isocyanate Aliphatic–Aromatic Poly(carbonate-urethane)s—An Insight into Transurethanization Reactions and Structure–Property Relationships" International Journal of Molecular Sciences 23, no. 19: 10999. https://doi.org/10.3390/ijms231910999
APA StyleWołosz, D. (2022). Non-Isocyanate Aliphatic–Aromatic Poly(carbonate-urethane)s—An Insight into Transurethanization Reactions and Structure–Property Relationships. International Journal of Molecular Sciences, 23(19), 10999. https://doi.org/10.3390/ijms231910999








