Antioxidant Activity of Quercetin in a H2O2-Induced Oxidative Stress Model in Red Blood Cells: Functional Role of Band 3 Protein
Abstract
1. Introduction
2. Results
2.1. Measurement of Percentage Hemolysis
2.2. Evaluation of Erythrocyte Cell Shape
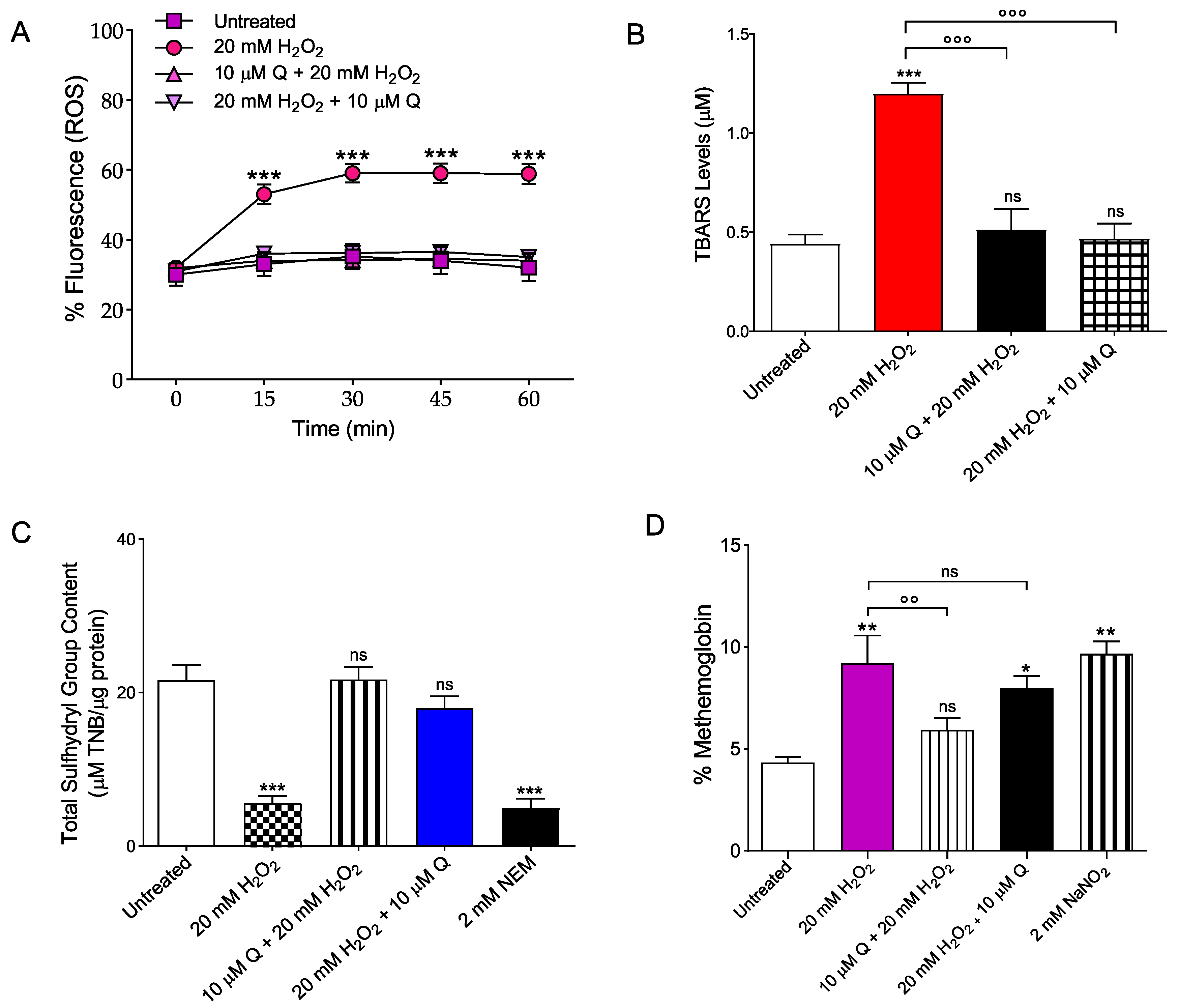
2.3. Oxidative Stress Assessment
2.3.1. Evaluation of Intracellular ROS Levels
2.3.2. Measurement of Thiobarbituric Acid Reactive Substances (TBARS) Levels
2.3.3. Total Sulfhydryl Group Content Measurement
2.3.4. Evaluation of Methemoglobin (MetHb) Levels
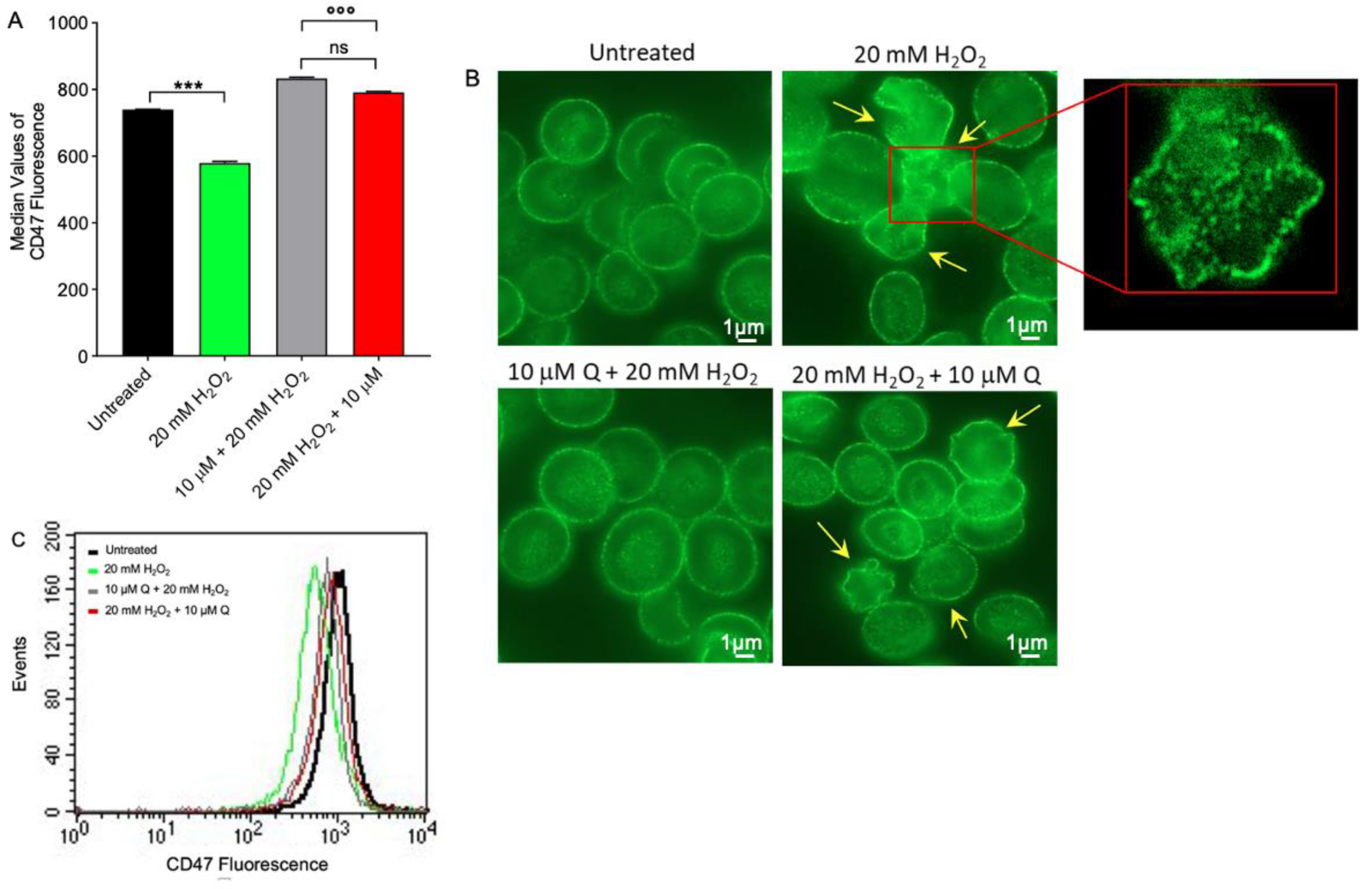
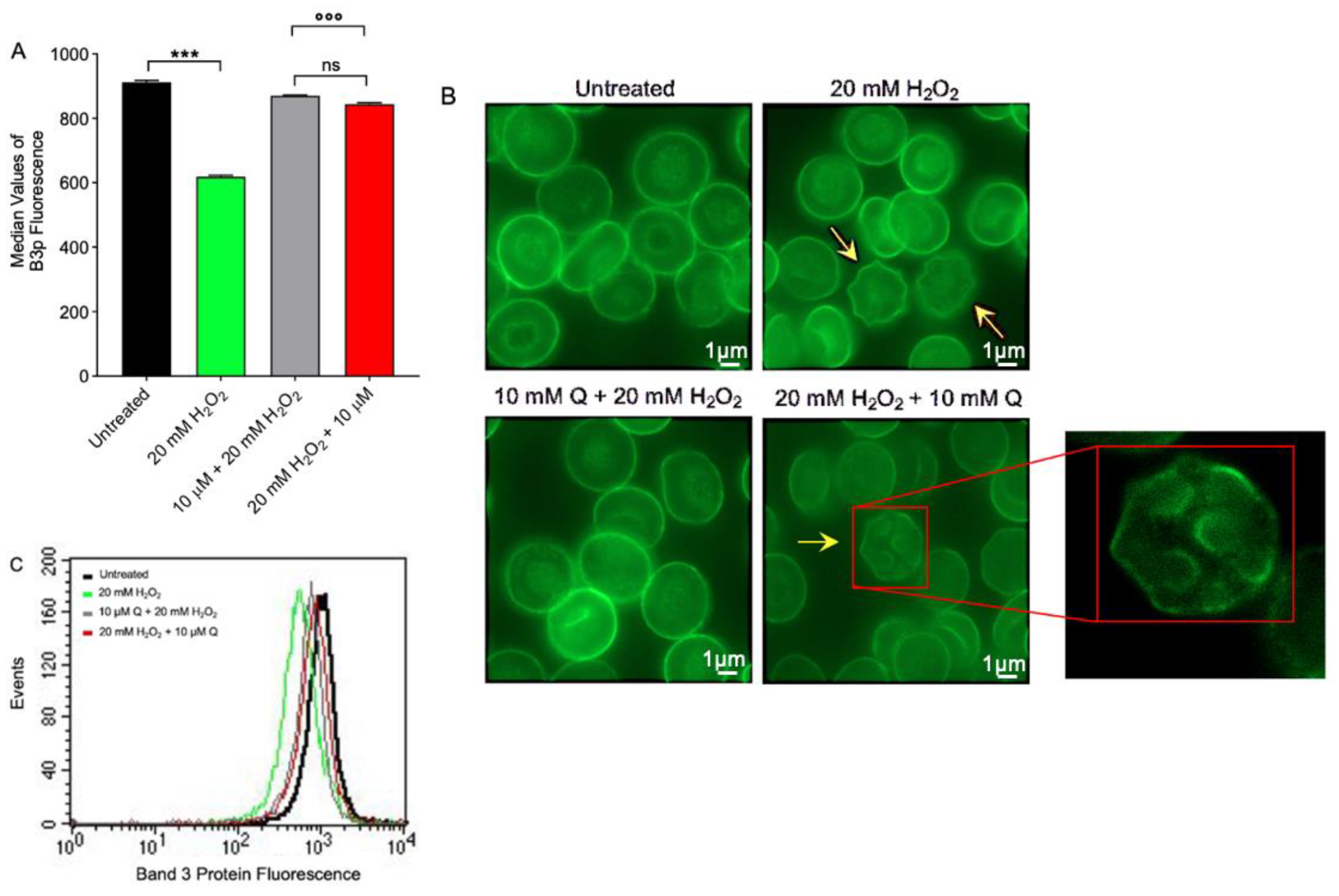
2.4. CD47 Expression Level Determination
2.5. Band 3 Protein Expression Level Determination
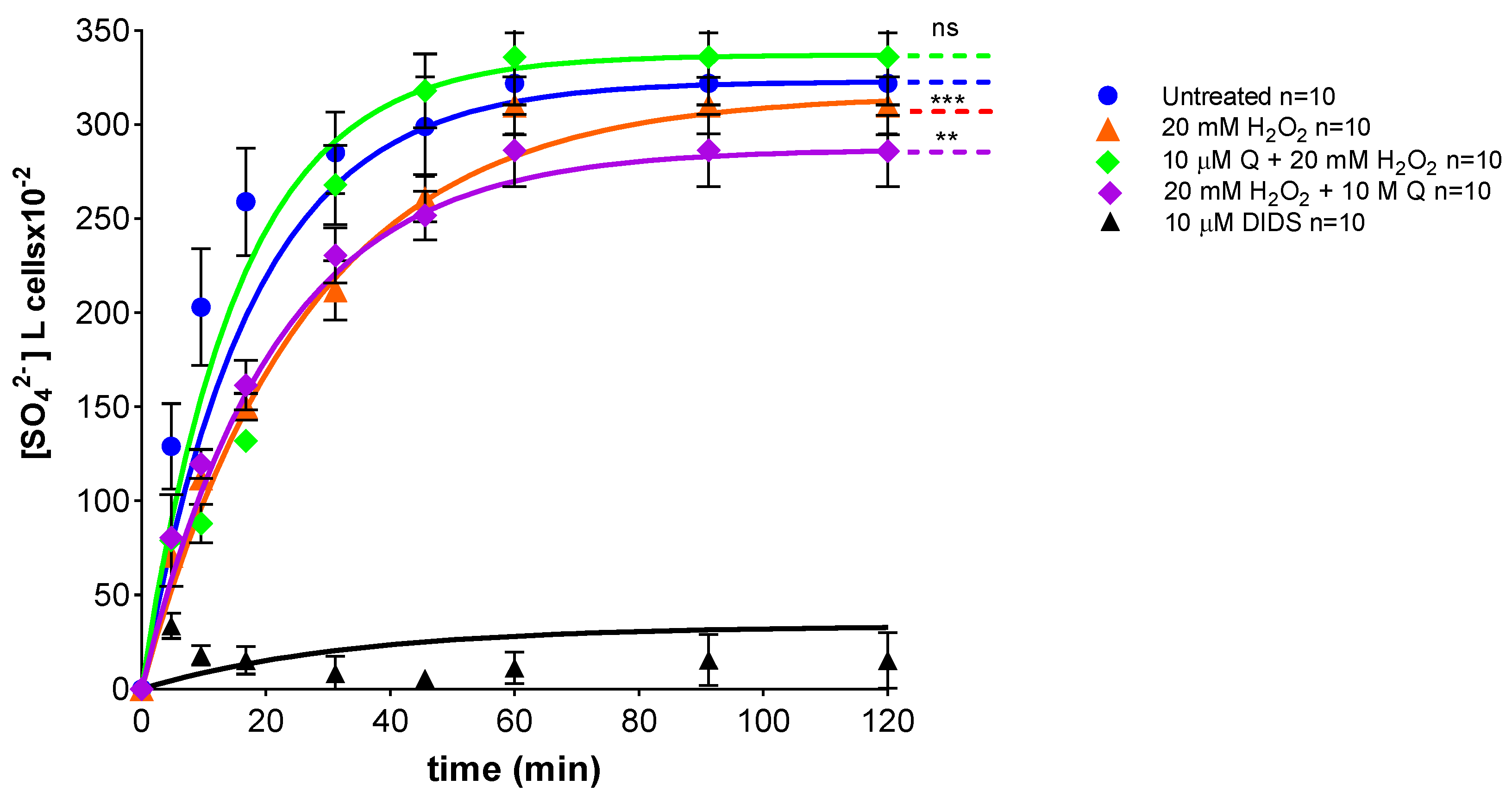
2.6. Measurement of SO42− Uptake via B3p
3. Discussion
4. Materials and Methods
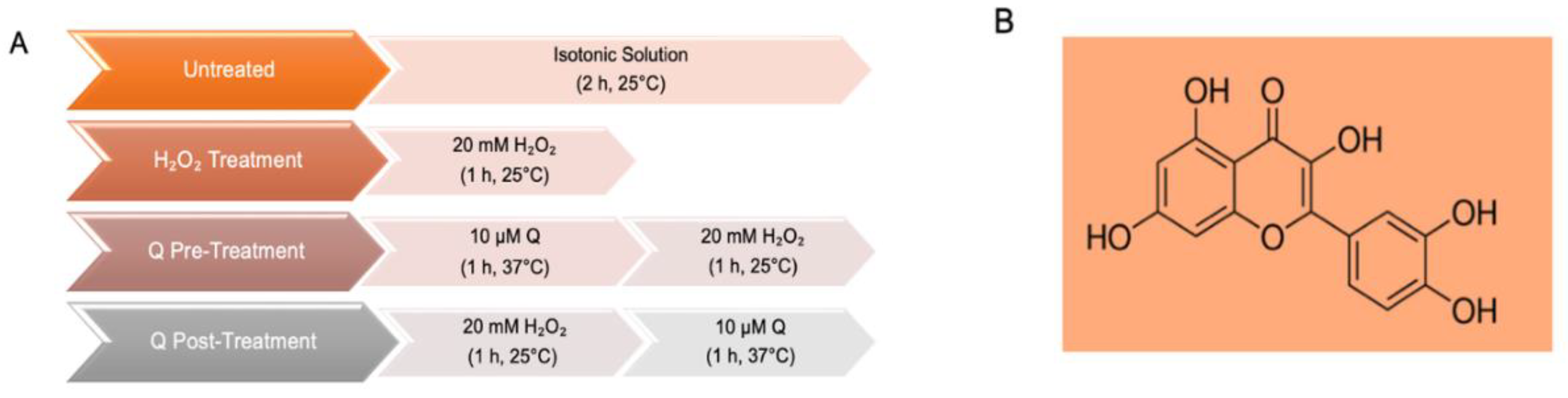
4.1. Solutions and Chemicals
4.2. Erythrocyte Preparation
4.3. Percentage Hemolysis Measurement
4.4. Analysis of Cell Shape by Scanning Electron Microscopy (SEM)
4.5. Measurement of Oxidative Stress Assessment
4.5.1. Detection of Reactive Oxygen Species (ROS)
4.5.2. Thiobarbituric-Acid-Reactive Substances (TBARS) Level Determination
4.5.3. Total Sulfhydryl Group Content Determination
4.5.4. Measurement of Methemoglobin (MetHb) Levels
4.6. Analysis of Analytical Cytology
4.7. SO42− Uptake Measurement
4.7.1. Control Condition
4.7.2. Experimental Conditions
4.8. Experimental Data and Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fujii, J.; Homma, T.; Kobayashi, S.; Warang, P.; Madkaikar, M.; Mukherjee, M.B. Erythrocytes as a preferential target of oxidative stress in blood. Free Radic. Res. 2021, 55, 562–580. [Google Scholar] [CrossRef] [PubMed]
- Remigante, A.; Spinelli, S.; Pusch, M.; Sarikas, A.; Morabito, R.; Marino, A.; Dossena, S. Role of SLC4 and SLC26 solute carriers during oxidative stress. Acta Physiol. 2022, 235, e13796. [Google Scholar] [CrossRef]
- Akki, R.; Siracusa, R.; Cordaro, M.; Remigante, A.; Morabito, R.; Errami, M.; Marino, A. Adaptation to oxidative stress at cellular and tissue level. Arch. Physiol. Biochem. 2019, 128, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Ferrera, L.; Barbieri, R.; Picco, C.; Zuccolini, P.; Remigante, A.; Bertelli, S.; Fumagalli, M.R.; Zifarelli, G.; La Porta, C.A.M.; Gavazzo, P.; et al. TRPM2 Oxidation Activates Two Distinct Potassium Channels in Melanoma Cells through Intracellular Calcium Increase. Int. J. Mol. Sci. 2021, 22, 8359. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Remigante, A.; Civello, D.A.; Bernardinelli, E.; Szabo, Z.; Morabito, R.; Marino, A.; Sarikas, A.; Patsch, W.; Paulmichl, M.; et al. O-GlcNAcylation Suppresses the Ion Current IClswell by Preventing the Binding of the Protein ICln to alpha-Integrin. Front. Cell Dev. Biol. 2020, 8, 607080. [Google Scholar] [CrossRef]
- Remigante, A.; Zuccolini, P.; Barbieri, R.; Ferrera, L.; Morabito, R.; Gavazzo, P.; Pusch, M.; Picco, C. NS-11021 Modulates Cancer-Associated Processes Independently of BK Channels in Melanoma and Pancreatic Duct Adenocarcinoma Cell Lines. Cancers 2021, 13, 6144. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Li, F.; Duan, Y.; Wen, C.; Wang, W.; Zhang, L.; Huang, R.; Yin, Y. Oxidative stress, nutritional antioxidants and beyond. Sci. China Life Sci. 2020, 63, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, S.; Remigante, A.; Zuccolini, P.; Barbieri, R.; Ferrera, L.; Picco, C.; Gavazzo, P.; Pusch, M. Mechanisms of Activation of LRRC8 Volume Regulated Anion Channels. Cell Physiol. Biochem. 2021, 55, 41–56. [Google Scholar] [CrossRef]
- Akki, R.; Siracusa, R.; Morabito, R.; Remigante, A.; Campolo, M.; Errami, M.; La Spada, G.; Cuzzocrea, S.; Marino, A. Neuronal-like differentiated SH-SY5Y cells adaptation to a mild and transient H2O2 -induced oxidative stress. Cell Biochem. Funct. 2018, 36, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Zuccolini, P.; Ferrera, L.; Remigante, A.; Picco, C.; Barbieri, R.; Bertelli, S.; Moran, O.; Gavazzo, P.; Pusch, M. The VRAC blocker DCPIB directly gates the BK channels and increases intracellular Ca2+ in Melanoma and Pancreatic Duct Adenocarcinoma (PDAC) cell lines. Br. J. Pharmacol. 2022, 179, 3452–3469. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef]
- Shiga, T.; Maeda, N.; Kon, K. Erythrocyte rheology. Crit. Rev. Oncol. Hematol. 1990, 10, 9–48. [Google Scholar] [CrossRef]
- Luqman, S.; Rizvi, S.I. Protection of lipid peroxidation and carbonyl formation in proteins by capsaicin in human erythrocytes subjected to oxidative stress. Phytother. Res. 2006, 20, 303–306. [Google Scholar] [CrossRef]
- Gyawali, P.; Richards, R.S.; Bwititi, P.T.; Nwose, E.U. Association of abnormal erythrocyte morphology with oxidative stress and inflammation in metabolic syndrome. Blood Cells Mol. Dis. 2015, 54, 360–363. [Google Scholar] [CrossRef]
- Gyawali, P.; Richards, R.S.; Uba Nwose, E. Erythrocyte morphology in metabolic syndrome. Expert Rev. Hematol. 2012, 5, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Remigante, A.; Morabito, R.; Marino, A. Band 3 protein function and oxidative stress in erythrocytes. J. Cell. Physiol. 2021, 236, 6225–6234. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Y.; Liu, J.; Dao, M.; Du, E. In vitro assay for single-cell characterization of impaired deformability in red blood cells under recurrent episodes of hypoxia. Lab Chip 2021, 21, 3458–3470. [Google Scholar] [CrossRef] [PubMed]
- Pieniazek, A.; Szczepocki, A. Structural component changes of erythrocytes caused by oxidative stress generated by indoxyl sulfate. Toxicol. Vitr. 2021, 70, 105013. [Google Scholar] [CrossRef] [PubMed]
- Gorudko, I.V.; Sokolov, A.V.; Shamova, E.V.; Grigorieva, D.V.; Mironova, E.V.; Kudryavtsev, I.V.; Gusev, S.A.; Gusev, A.A.; Chekanov, A.V.; Vasilyev, V.B.; et al. Binding of human myeloperoxidase to red blood cells: Molecular targets and biophysical consequences at the plasma membrane level. Arch. Biochem. Biophys. 2016, 591, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Olumuyiwa-Akeredolu, O.O.; Soma, P.; Buys, A.V.; Debusho, L.K.; Pretorius, E. Characterizing pathology in erythrocytes using morphological and biophysical membrane properties: Relation to impaired hemorheology and cardiovascular function in rheumatoid arthritis. Biochim. Biophys. Acta Biomembr. 2017, 1859, 2381–2391. [Google Scholar] [CrossRef] [PubMed]
- Himbert, S.; D’Alessandro, A.; Qadri, S.M.; Majcher, M.J.; Hoare, T.; Sheffield, W.P.; Nagao, M.; Nagle, J.F.; Rheinstadter, M.C. The bending rigidity of the red blood cell cytoplasmic membrane. PLoS ONE 2022, 17, e0269619. [Google Scholar] [CrossRef] [PubMed]
- Rinalducci, S.; Ferru, E.; Blasi, B.; Turrini, F.; Zolla, L. Oxidative stress and caspase-mediated fragmentation of cytoplasmic domain of erythrocyte band 3 during blood storage. Blood Transfus. 2012, 10 (Suppl. 2), s55–s62. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, Y.; Xiong, Y.; Zhao, Y.; Tang, F.; Wang, X. Cluster of erythrocyte band 3: A potential molecular target of exhaustive exercise-induced dysfunction of erythrocyte deformability. Can. J. Physiol. Pharmacol. 2013, 91, 1127–1134. [Google Scholar] [CrossRef]
- Remigante, A.; Morabito, R. Cellular and Molecular Mechanisms in Oxidative Stress-Related Diseases. Int. J. Mol. Sci. 2022, 23, 8017. [Google Scholar] [CrossRef]
- Ficarra, S.; Tellone, E.; Giardina, B.; Scatena, R.; Russo, A.; Misiti, F.; Clementi, M.E.; Colucci, D.; Bellocco, E.; Lagana, G.; et al. Derangement of erythrocytic AE1 in beta-thalassemia by caspase 3: Pathogenic mechanisms and implications in red blood cell senescence. J. Membr. Biol. 2009, 228, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Clementi, M.E.; Giardina, B.; Colucci, D.; Galtieri, A.; Misiti, F. Amyloid-beta peptide affects the oxygen dependence of erythrocyte metabolism: A role for caspase 3. Int. J. Biochem. Cell Biol. 2007, 39, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.N. Band 3 protein: Structure, flexibility and function. FEBS Lett. 1994, 346, 26–31. [Google Scholar] [CrossRef]
- Vallese, F.; Kim, K.; Yen, L.Y.; Johnston, J.D.; Noble, A.J.; Cali, T.; Clarke, O.B. Architecture of the human erythrocyte ankyrin-1 complex. Nat. Struct. Mol. Biol. 2022, 29, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Liu, S.; Zhou, Z.H. Structure, dynamics and assembly of the ankyrin complex on human red blood cell membrane. Nat. Struct. Mol. Biol. 2022, 29, 698–705. [Google Scholar] [CrossRef]
- Mohanty, J.G.; Nagababu, E.; Rifkind, J.M. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front. Physiol. 2014, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Remigante, A.; Morabito, R.; Marino, A. Natural Antioxidants Beneficial Effects on Anion Exchange through Band 3 Protein in Human Erythrocytes. Antioxidants 2019, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Giovannetti, A.; Gambardella, L.; Pietraforte, D.; Rosato, E.; Giammarioli, A.M.; Salsano, F.; Malorni, W.; Straface, E. Red blood cell alterations in systemic sclerosis: A pilot study. Cell. Physiol. Biochem. 2012, 30, 418–427. [Google Scholar] [CrossRef]
- Tejero, J.; Shiva, S.; Gladwin, M.T. Sources of Vascular Nitric Oxide and Reactive Oxygen Species and Their Regulation. Physiol. Rev. 2019, 99, 311–379. [Google Scholar] [CrossRef]
- Lopez, J.G. Flavonoids in Health and Disease. Curr. Med. Chem. 2019, 26, 6972–6975. [Google Scholar] [CrossRef] [PubMed]
- Chawla, R.; Arora, R.; Sagar, R.K.; Singh, S.; Puri, S.C.; Kumar, R.; Singh, S.; Sharmaa, A.K.; Prasada, J.; Khan, H.A.; et al. 3-O-beta-D-Galactopyranoside of quercetin as an active principle from high altitude Podophyllum hexandrum and evaluation of its radioprotective properties. Z. Nat. C J. Biosci. 2005, 60, 728–738. [Google Scholar] [CrossRef]
- Duranti, G.; Ceci, R.; Patrizio, F.; Sgro, P.; Di Luigi, L.; Sabatini, S.; Felici, F.; Bazzucchi, I. Chronic consumption of quercetin reduces erythrocytes oxidative damage: Evaluation at resting and after eccentric exercise in humans. Nutr. Res. 2018, 50, 73–81. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Kant, V.; Sharma, M.; Jangir, B.L.; Kumar, V. Acceleration of wound healing by quercetin in diabetic rats requires mitigation of oxidative stress and stimulation of the proliferative phase. Biotech. Histochem. 2022, 97, 461–472. [Google Scholar] [CrossRef]
- Annapurna, A.; Reddy, C.S.; Akondi, R.B.; Rao, S.R. Cardioprotective actions of two bioflavonoids, quercetin and rutin, in experimental myocardial infarction in both normal and streptozotocin-induced type I diabetic rats. J. Pharm. Pharmacol. 2009, 61, 1365–1374. [Google Scholar] [CrossRef]
- Tripathi, A.; Kumar, M.; Kaur, P.; Kumar, B.; Sagi, S.S.K. Efficacy of Quercetin as a potent sensitizer of beta2-AR in combating the impairment of fluid clearance in lungs of rats under hypoxia. Respir. Physiol. Neurobiol. 2020, 273, 103334. [Google Scholar] [CrossRef] [PubMed]
- Mikstacka, R.; Rimando, A.M.; Ignatowicz, E. Antioxidant effect of trans-resveratrol, pterostilbene, quercetin and their combinations in human erythrocytes in vitro. Plant Foods Hum. Nutr. 2010, 65, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Sangai, N.P.; Patel, C.N.; Pandya, H.A. Ameliorative effects of quercetin against bisphenol A-caused oxidative stress in human erythrocytes: An in vitro and in silico study. Toxicol. Res. 2018, 7, 1091–1099. [Google Scholar] [CrossRef]
- Duchnowicz, P.; Broncel, M.; Podsedek, A.; Koter-Michalak, M. Hypolipidemic and antioxidant effects of hydroxycinnamic acids, quercetin, and cyanidin 3-glucoside in hypercholesterolemic erythrocytes (in vitro study). Eur. J. Nutr. 2012, 51, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Jamshidzadeh, A.; Rezaeian Mehrabadi, A. Protective effect of quercetin on oxidative stress in glucose-6-phosphate dehydrogenase-deficient erythrocytes in vitro. Iran. J. Pharm. Res. 2010, 9, 169–175. [Google Scholar]
- Jensen, F.B. The dual roles of red blood cells in tissue oxygen delivery: Oxygen carriers and regulators of local blood flow. J. Exp. Biol. 2009, 212, 3387–3393. [Google Scholar] [CrossRef]
- Forman, H.J.; Bernardo, A.; Davies, K.J. What is the concentration of hydrogen peroxide in blood and plasma? Arch. Biochem. Biophys. 2016, 603, 48–53. [Google Scholar] [CrossRef]
- Morabito, R.; Remigante, A.; Cavallaro, M.; Taormina, A.; La Spada, G.; Marino, A. Anion exchange through band 3 protein in canine leishmaniasis at different stages of disease. Pflug. Arch. 2017, 469, 713–724. [Google Scholar] [CrossRef]
- Morabito, R.; Remigante, A.; Spinelli, S.; Vitale, G.; Trichilo, V.; Loddo, S.; Marino, A. High Glucose Concentrations Affect Band 3 Protein in Human Erythrocytes. Antioxidants 2020, 9, 365. [Google Scholar] [CrossRef]
- Morabito, R.; Remigante, A.; Marino, A. Melatonin Protects Band 3 Protein in Human Erythrocytes against H2O2-Induced Oxidative Stress. Molecules 2019, 24, 2741. [Google Scholar] [CrossRef]
- Morabito, R.; Remigante, A.; Marino, A. Protective Role of Magnesium against Oxidative Stress on SO4= Uptake through Band 3 Protein in Human Erythrocytes. Cell. Physiol. Biochem. 2019, 52, 1292–1308. [Google Scholar] [CrossRef] [PubMed]
- Morabito, R.; Remigante, A.; Di Pietro, M.L.; Giannetto, A.; La Spada, G.; Marino, A. SO4= uptake and catalase role in preconditioning after H2O2-induced oxidative stress in human erythrocytes. Pflug. Arch. 2017, 469, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Morabito, R.; Romano, O.; La Spada, G.; Marino, A. H2O2-Induced Oxidative Stress Affects SO4= Transport in Human Erythrocytes. PLoS ONE 2016, 11, e0146485. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morabito, R.; Remigante, A.; Cordaro, M.; Trichilo, V.; Loddo, S.; Dossena, S.; Marino, A. Impact of acute inflammation on Band 3 protein anion exchange capability in human erythrocytes. Arch. Physiol. Biochem. 2020, 1–7. [Google Scholar] [CrossRef]
- Massaccesi, L.; Galliera, E.; Corsi Romanelli, M.M. Erythrocytes as markers of oxidative stress related pathologies. Mech. Ageing Dev. 2020, 191, 111333. [Google Scholar] [CrossRef]
- Crupi, R.; Morabito, R.; Remigante, A.; Gugliandolo, E.; Britti, D.; Cuzzocrea, S.; Marino, A. Susceptibility of erythrocytes from different sources to xenobiotics-induced lysis. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 221, 68–72. [Google Scholar] [CrossRef]
- Singh, A.K.; Patel, P.K.; Choudhary, K.; Joshi, J.; Yadav, D.; Jin, J.O. Quercetin and Coumarin Inhibit Dipeptidyl Peptidase-IV and Exhibits Antioxidant Properties: In Silico, In Vitro, Ex Vivo. Biomolecules 2020, 10, 207. [Google Scholar] [CrossRef]
- Spinelli, S.; Remigante, A.G.D.; Dossena, S.; Marino, A.; Morabito, R. Hemoglobin Glycation and Oxidative Stress Induce Functional Modification of Band 3 Protein in D-Galactose-Treated Erythrocytes: Double Effect of Quercetin. FASEB J. 2022, 36. [Google Scholar] [CrossRef]
- Grewal, A.K.; Singh, T.G.; Sharma, D.; Sharma, V.; Singh, M.; Rahman, M.H.; Najda, A.; Walasek-Janusz, M.; Kamel, M.; Albadrani, G.M.; et al. Mechanistic insights and perspectives involved in neuroprotective action of quercetin. Biomed. Pharmacother. 2021, 140, 111729. [Google Scholar] [CrossRef]
- Zbikowska, H.M.; Antosik, A.; Szejk, M.; Bijak, M.; Olejnik, A.K.; Saluk, J.; Nowak, P. Does quercetin protect human red blood cell membranes against gamma-irradiation? Redox Rep. 2014, 19, 65–71. [Google Scholar] [CrossRef]
- Yetuk, G.; Pandir, D.; Bas, H. Protective role of catechin and quercetin in sodium benzoate-induced lipid peroxidation and the antioxidant system in human erythrocytes in vitro. Sci. World J. 2014, 2014, 874824. [Google Scholar] [CrossRef] [PubMed]
- Bosman, G.J.; Cluitmans, J.C.; Groenen, Y.A.; Werre, J.M.; Willekens, F.L.; Novotny, V.M. Susceptibility to hyperosmotic stress-induced phosphatidylserine exposure increases during red blood cell storage. Transfusion 2011, 51, 1072–1078. [Google Scholar] [CrossRef]
- Yasin, Z.; Witting, S.; Palascak, M.B.; Joiner, C.H.; Rucknagel, D.L.; Franco, R.S. Phosphatidylserine externalization in sickle red blood cells: Associations with cell age, density, and hemoglobin F. Blood 2003, 102, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Remigante, A.; Spinelli, S.; Straface, E.; Gambardella, L.; Caruso, D.; Falliti, G.; Dossena, S.; Marino, A.; Morabito, R. Acai (Euterpe oleracea) Extract Protects Human Erythrocytes from Age-Related Oxidative Stress. Cells 2022, 11, 2391. [Google Scholar] [CrossRef] [PubMed]
- Anong, W.A.; Franco, T.; Chu, H.; Weis, T.L.; Devlin, E.E.; Bodine, D.M.; An, X.; Mohandas, N.; Low, P.S. Adducin forms a bridge between the erythrocyte membrane and its cytoskeleton and regulates membrane cohesion. Blood 2009, 114, 1904–1912. [Google Scholar] [CrossRef]
- Wu, F.; Satchwell, T.J.; Toye, A.M. Anion exchanger 1 in red blood cells and kidney: Band 3’s in a pod. Biochem. Cell Biol. 2011, 89, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Issaian, A.; Hay, A.; Dzieciatkowska, M.; Roberti, D.; Perrotta, S.; Darula, Z.; Redzic, J.; Busch, M.P.; Page, G.P.; Rogers, S.C.; et al. The interactome of the N-terminus of band 3 regulates red blood cell metabolism and storage quality. Haematologica 2021, 106, 2971–2985. [Google Scholar] [CrossRef]
- Shimo, H.; Arjunan, S.N.; Machiyama, H.; Nishino, T.; Suematsu, M.; Fujita, H.; Tomita, M.; Takahashi, K. Particle Simulation of Oxidation Induced Band 3 Clustering in Human Erythrocytes. PLoS Comput. Biol. 2015, 11, e1004210. [Google Scholar] [CrossRef]
- Remigante, A.S.S.; Trichilo, V.; Loddo, S.; Sarikas, A.; Pusch, M.; Dossena, S.; Marino, A.; Morabito, R. D-Galactose induced early aging in human erythrocytes: Role of Band 3 protein. J. Cell. Physiol. 2021, 237, 1586–1596. [Google Scholar] [CrossRef]
- Remigante, A.; Morabito, R.; Spinelli, S.; Trichilo, V.; Loddo, S.; Sarikas, A.; Dossena, S.; Marino, A. d-Galactose Decreases Anion Exchange Capability through Band 3 Protein in Human Erythrocytes. Antioxidants 2020, 9, 689. [Google Scholar] [CrossRef]
- Oldenborg, P.A. Role of CD47 in erythroid cells and in autoimmunity. Leuk. Lymphoma 2004, 45, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Buttari, B.; Profumo, E.; Rigano, R. Crosstalk between red blood cells and the immune system and its impact on atherosclerosis. Biomed. Res. Int. 2015, 2015, 616834. [Google Scholar] [CrossRef] [PubMed]
- Thangaraju, K.; Neerukonda, S.N.; Katneni, U.; Buehler, P.W. Extracellular Vesicles from Red Blood Cells and Their Evolving Roles in Health, Coagulopathy and Therapy. Int. J. Mol. Sci. 2020, 22, 153. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.P.; Tigges, J.C.; Toxavidis, V.; Ghiran, I. Red Blood Cells: A Source of Extracellular Vesicles. Methods Mol. Biol. 2017, 1660, 15–22. [Google Scholar] [CrossRef]
- Goodhead, L.K.; MacMillan, F.M. Measuring osmosis and hemolysis of red blood cells. Adv. Physiol. Educ. 2017, 41, 298–305. [Google Scholar] [CrossRef]
- Straface, E.; Rivabene, R.; Masella, R.; Santulli, M.; Paganelli, R.; Malorni, W. Structural changes of the erythrocyte as a marker of non-insulin-dependent diabetes: Protective effects of N-acetylcysteine. Biochem. Biophys. Res. Commun. 2002, 290, 1393–1398. [Google Scholar] [CrossRef]
- Lucantoni, G.; Pietraforte, D.; Matarrese, P.; Gambardella, L.; Metere, A.; Paone, G.; Bianchi, E.L.; Straface, E. The red blood cell as a biosensor for monitoring oxidative imbalance in chronic obstructive pulmonary disease: An ex vivo and in vitro study. Antioxid. Redox Signal. 2006, 8, 1171–1182. [Google Scholar] [CrossRef]
- Mendanha, S.A.; Anjos, J.L.; Silva, A.H.; Alonso, A. Electron paramagnetic resonance study of lipid and protein membrane components of erythrocytes oxidized with hydrogen peroxide. Braz. J. Med. Biol. Res. 2012, 45, 473–481. [Google Scholar] [CrossRef]
- Aksenov, M.Y.; Markesbery, W.R. Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci. Lett. 2001, 302, 141–145. [Google Scholar] [CrossRef]
- Morabito, R.; Falliti, G.; Geraci, A.; Spada, G.L.; Marino, A. Curcumin Protects -SH Groups and Sulphate Transport after Oxidative Damage in Human Erythrocytes. Cell. Physiol. Biochem. 2015, 36, 345–357. [Google Scholar] [CrossRef]
- Zavodnik, I.B.; Lapshina, E.A.; Rekawiecka, K.; Zavodnik, L.B.; Bartosz, G.; Bryszewska, M. Membrane effects of nitrite-induced oxidation of human red blood cells. Biochim. Biophys. Acta 1999, 1421, 306–316. [Google Scholar] [CrossRef]
- Romano, L.; Peritore, D.; Simone, E.; Sidoti, A.; Trischitta, F.; Romano, P. Chloride-sulphate exchange chemically measured in human erythrocyte ghosts. Cell. Mol. Biol. 1998, 44, 351–355. [Google Scholar] [PubMed]
- Romano, L.; Passow, H. Characterization of anion transport system in trout red blood cell. Am. J. Physiol. 1984, 246, C330–C338. [Google Scholar] [CrossRef] [PubMed]
- Morabito, R.R.A.; Arcuri, B.; Marino, A.; Giammanco, M.; La Spada, G.M.A. Effect of cadmium on anion exchange capability through Band 3 protein in human erythrocytes. J. Biol. Res. 2018, 91, 7203. [Google Scholar] [CrossRef]
- Jessen, F.; Sjoholm, C.; Hoffmann, E.K. Identification of the anion exchange protein of Ehrlich cells: A kinetic analysis of the inhibitory effects of 4,4′-diisothiocyano-2,2′-stilbene-disulfonic acid (DIDS) and labeling of membrane proteins with 3H-DIDS. J. Membr. Biol. 1986, 92, 195–205. [Google Scholar] [CrossRef]
- Knauf, P.A.; Law, F.Y.; Hahn, K. An oxonol dye is the most potent known inhibitor of band 3-mediated anion exchange. Am. J. Physiol. 1995, 269, C1073–C1077. [Google Scholar] [CrossRef]

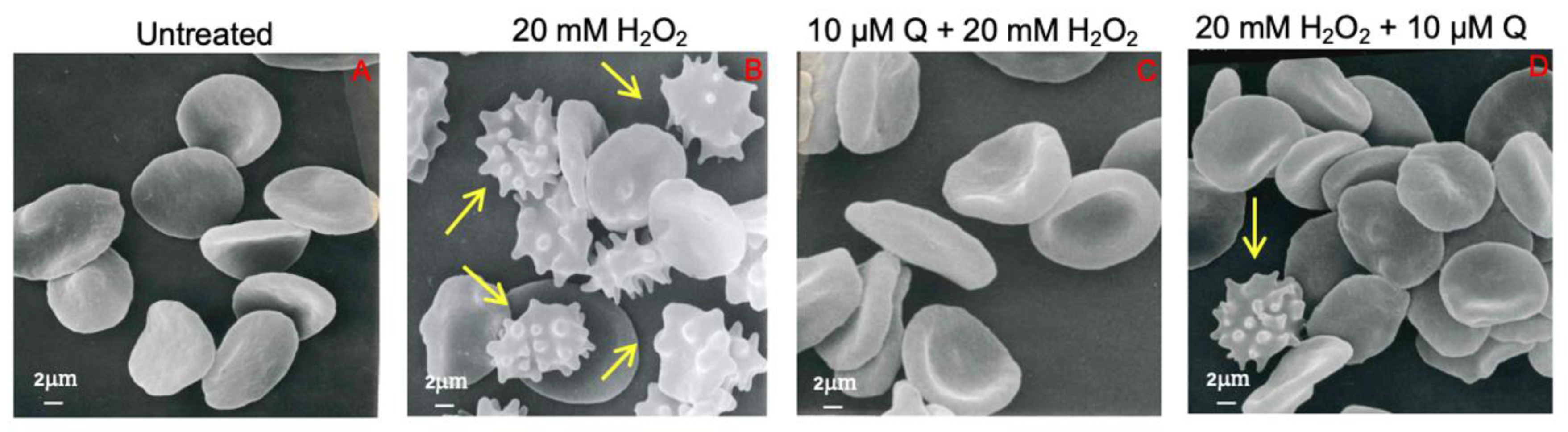
| Biconcave Shape | Acanthocytes | |
|---|---|---|
| Control | 93.8% ± 0.016 | 6.2% ± 0.011 |
| 20 mM H2O2 | 59.9% ± 0.010 *** | 40.1% ± 0.010 *** |
| 10 µM Q + 20 mM H2O2 | 90.4% ± 0.010 ns | 9.6% ± 0.010 ns, ^^^ |
| 20 mM H2O2 + 10 µM Q | 81.6% ± 0.009 ns | 18.4% ± 0.009 **, °° |
| Experimental Conditions | Rate Constant (min−1) | Time (min) | n | SO42− Amount Trapped after 45 min of Incubation in SO42− Medium [SO42−] L Cells × 10−2 |
|---|---|---|---|---|
| Control | 0.056 ± 0.001 | 17.58 | 10 | 299 ± 18.63 |
| 10 µM Q | 0.054 ± 0.001 ns | 17.55 | 10 | 298 ± 17.99 ns |
| 20 mM H2O2 | 0.042 ± 0.001 *** | 26.33 | 10 | 266 ± 16.50 *** |
| 10 µM Q + 20 mM H2O2 | 0.062 ± 0.001 ns | 15.99 | 10 | 286 ± 15.49 ns |
| 20 mM H2O2 + 10 µM Q | 0.047 ± 0.001 **,°° | 21.11 | 10 | 250 ± 19.80 **,ns |
| 10 µM DIDS | 0.017 ± 0.001 *** | 61.50 | 10 | 5.49 ± 3.50 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Remigante, A.; Spinelli, S.; Straface, E.; Gambardella, L.; Caruso, D.; Falliti, G.; Dossena, S.; Marino, A.; Morabito, R. Antioxidant Activity of Quercetin in a H2O2-Induced Oxidative Stress Model in Red Blood Cells: Functional Role of Band 3 Protein. Int. J. Mol. Sci. 2022, 23, 10991. https://doi.org/10.3390/ijms231910991
Remigante A, Spinelli S, Straface E, Gambardella L, Caruso D, Falliti G, Dossena S, Marino A, Morabito R. Antioxidant Activity of Quercetin in a H2O2-Induced Oxidative Stress Model in Red Blood Cells: Functional Role of Band 3 Protein. International Journal of Molecular Sciences. 2022; 23(19):10991. https://doi.org/10.3390/ijms231910991
Chicago/Turabian StyleRemigante, Alessia, Sara Spinelli, Elisabetta Straface, Lucrezia Gambardella, Daniele Caruso, Giuseppe Falliti, Silvia Dossena, Angela Marino, and Rossana Morabito. 2022. "Antioxidant Activity of Quercetin in a H2O2-Induced Oxidative Stress Model in Red Blood Cells: Functional Role of Band 3 Protein" International Journal of Molecular Sciences 23, no. 19: 10991. https://doi.org/10.3390/ijms231910991
APA StyleRemigante, A., Spinelli, S., Straface, E., Gambardella, L., Caruso, D., Falliti, G., Dossena, S., Marino, A., & Morabito, R. (2022). Antioxidant Activity of Quercetin in a H2O2-Induced Oxidative Stress Model in Red Blood Cells: Functional Role of Band 3 Protein. International Journal of Molecular Sciences, 23(19), 10991. https://doi.org/10.3390/ijms231910991











