Human Milk Oligosaccharide 2′-Fucosyllactose Modulates Local Viral Immune Defense by Supporting the Regulatory Functions of Intestinal Epithelial and Immune Cells
Abstract
1. Introduction
2. Results
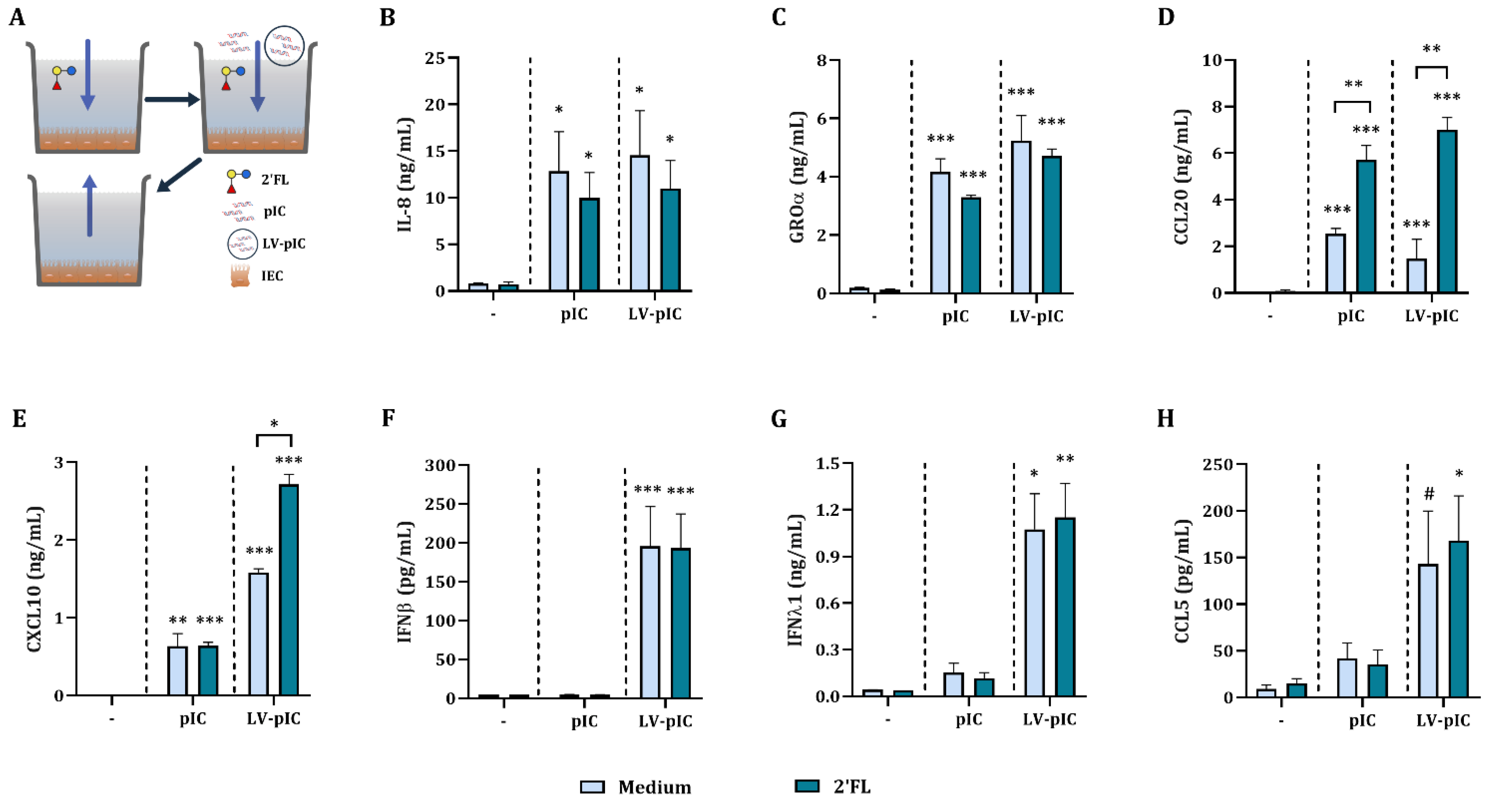
2.1. Poly I:C Stimulated IEC Secrete Viral Defense Related Cytokines
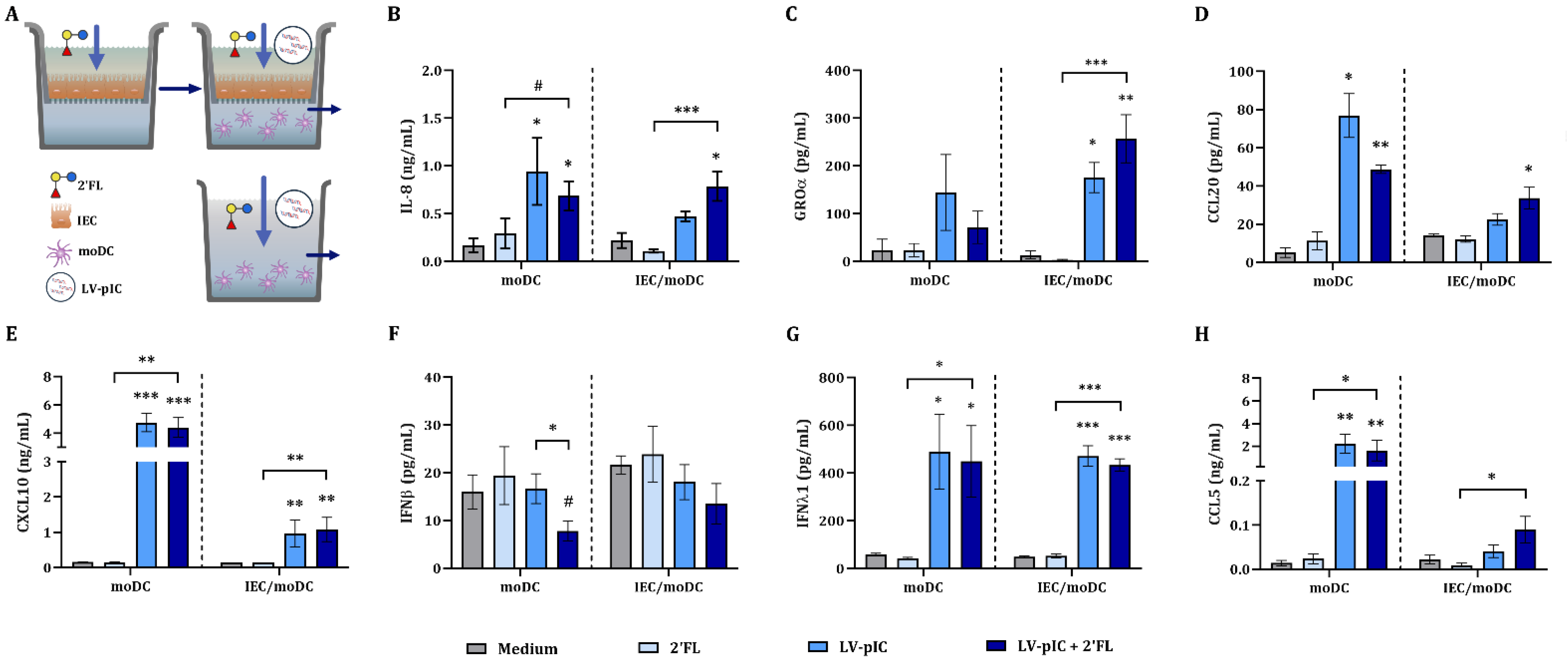
2.2. IEC Regulate the Cytokine Secretion from IEC/moDC Cultures Exposed to pIC
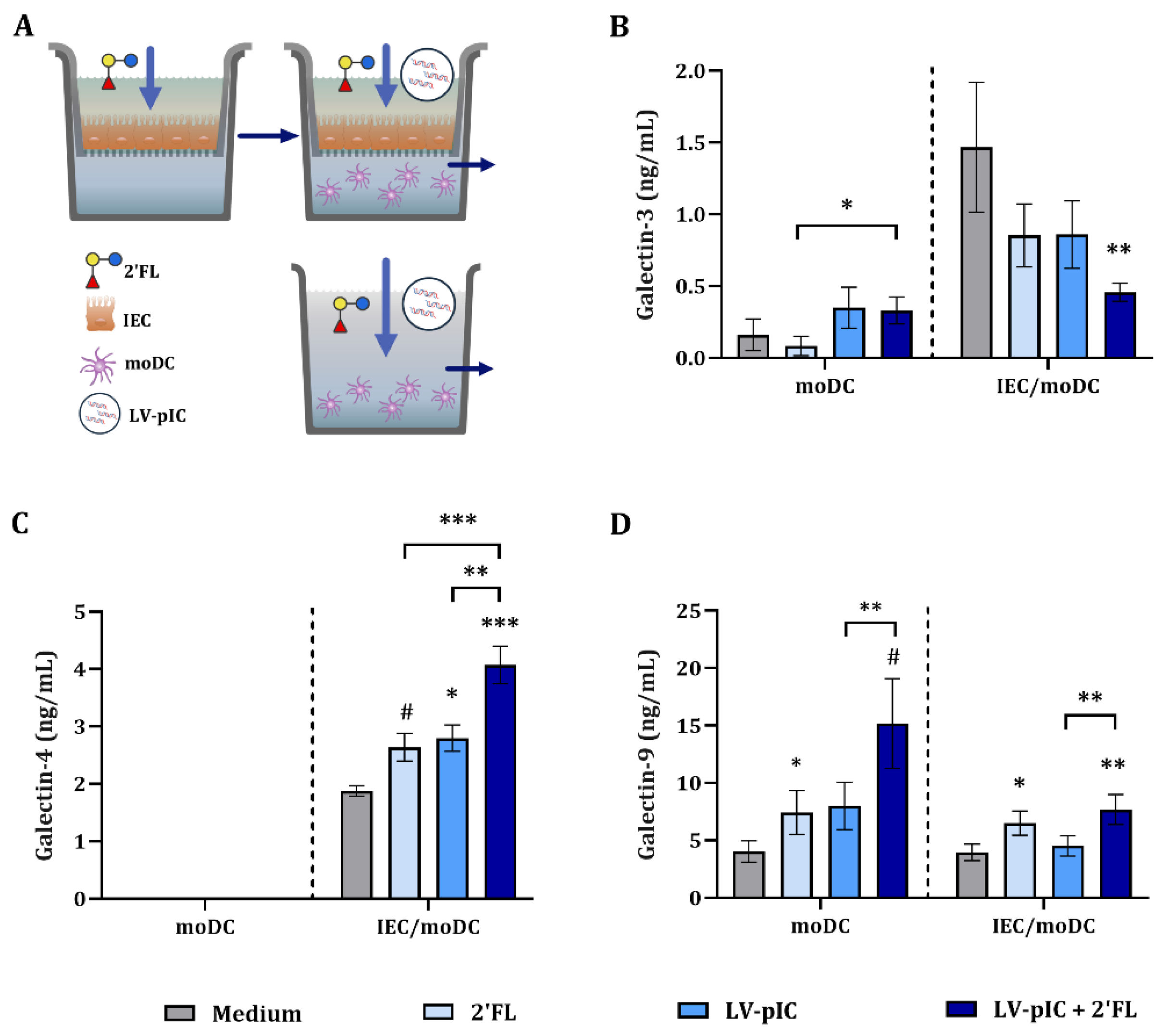
2.3. Modulation of Galectin Secretion by LV-pIC and 2′FL
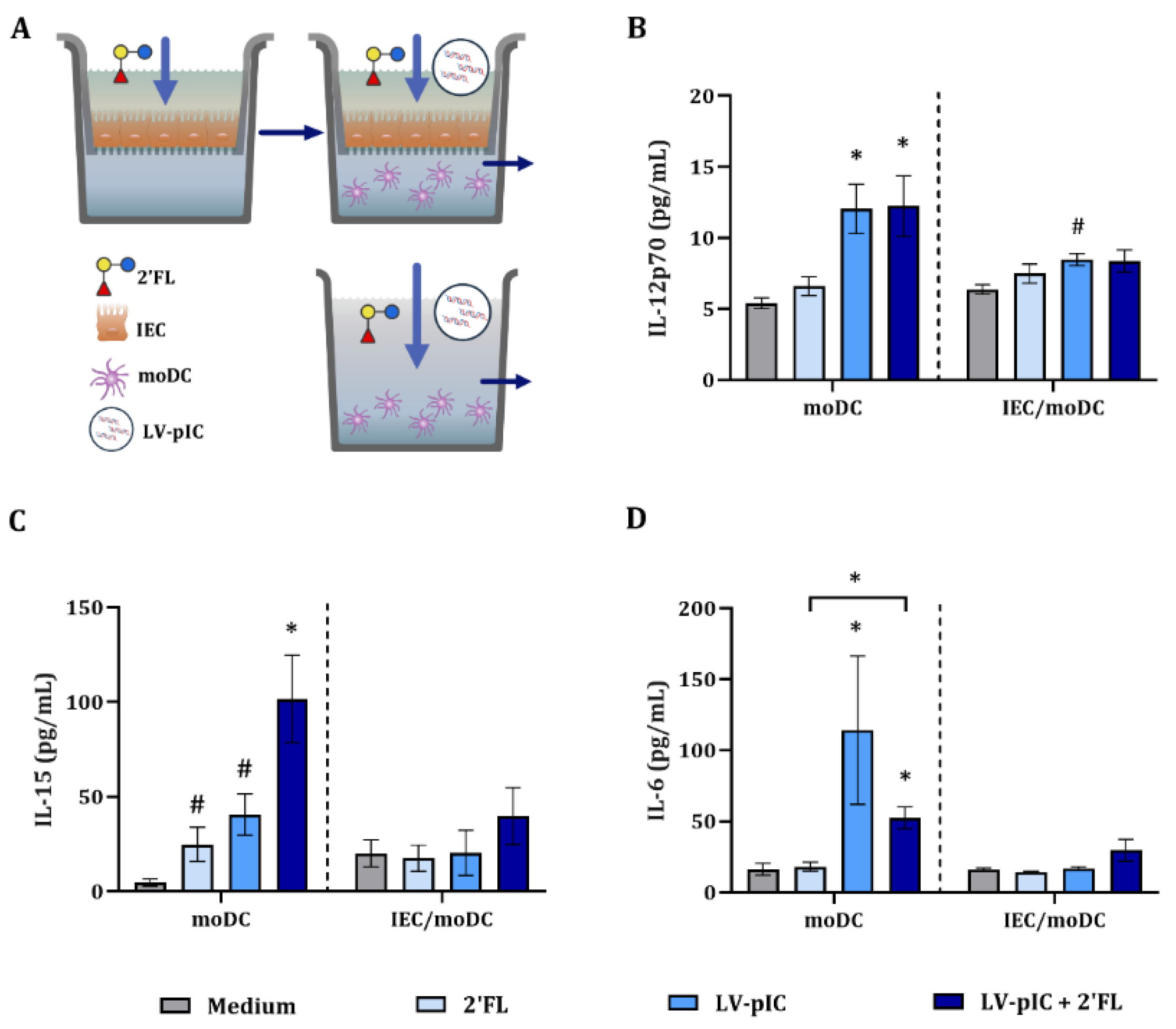
2.4. Dendritic Cell-Related Cytokine Secretion in IEC/moDC Model by LV-pIC
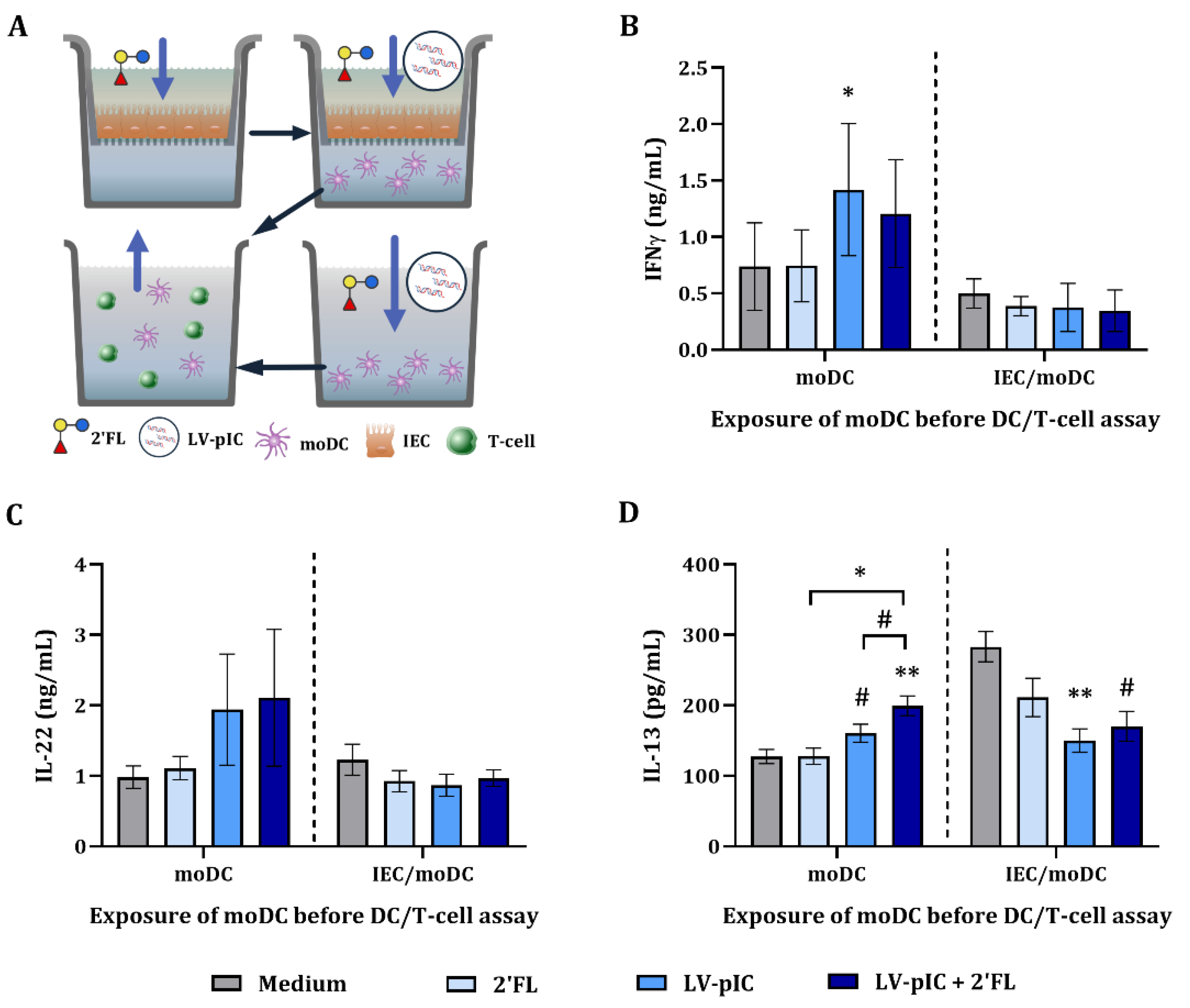
2.5. Cytokine Secretion in DC/T-Cell Assay
3. Discussion
4. Materials and Methods
4.1. Intestinal Epithelial Cell Culture
4.2. IEC Model
4.3. Peripheral Blood Mononuclear Cell Isolation
4.4. Monocyte Isolation and Culture
4.5. IEC/moDC Co-Culture Model
4.6. DC/T-Cell Assay
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Flow Cytometry
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kirk, M.D.; Pires, S.M.; Black, R.E.; Caipo, M.; Crump, J.A.; Devleesschauwer, B.; Döpfer, D.; Fazil, A.; Fischer-Walker, C.L.; Hald, T.; et al. World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLoS Med. 2015, 12, e1001921. [Google Scholar] [CrossRef]
- Troeger, C.; Blacker, B.F.; Khalil, I.A.; Rao, P.C.; Cao, S.; Zimsen, S.R.; Albertson, S.B.; Stanaway, J.D.; Deshpande, A.; Abebe, Z.; et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1211–1228. [Google Scholar] [CrossRef]
- Crawford, S.E.; Ramani, S.; Tate, J.E.; Parashar, U.D.; Svensson, L.; Hagbom, M.; Franco, M.A.; Greenberg, H.B.; O’Ryan, M.; Kang, G.; et al. Rotavirus infection. Nat. Rev. Dis. Prim. 2017, 3, 17083. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Mowat, A.M.; Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef]
- Cieza, R.J.; Cao, A.T.; Cong, Y.; Torres, A.G. Immunomodulation for gastrointestinal infections. Expert Rev. Anti Infect. Ther. 2013, 10, 391–400. [Google Scholar] [CrossRef]
- Burgueño, J.F.; Abreu, M.T. Epithelial Toll-like receptors and their role in gut homeostasis and disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 263–278. [Google Scholar] [CrossRef]
- Villena, J.; Vizoso-Pinto, M.G.; Kitazawa, H. Intestinal innate antiviral immunity and immunobiotics: Beneficial effects against rotavirus infection. Front. Immunol. 2016, 7, 563. [Google Scholar] [CrossRef]
- Holloway, G.; Coulson, B.S. Innate cellular responses to rotavirus infection. J. Gen. Virol. 2013, 94, 1151–1160. [Google Scholar] [CrossRef]
- Medzhitov, R. Recognition of microorganisms and activation of the immune response. Nature 2007, 449, 819–826. [Google Scholar] [CrossRef]
- Fitch, P.M.; Henderson, P.; Schwarze, J. Respiratory and gastrointestinal epithelial modulation of the immune response during viral infection. Innate Immun. 2011, 18, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Innate immune recognition of viral infection. Nat. Immunol. 2006, 7, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Lazear, H.M.; Schoggins, J.W.; Diamond, M.S. 25 Review Shared and Distinct Functions of Type I and Type III Interferons. Immunity 2019, 50, 907–923. [Google Scholar] [CrossRef] [PubMed]
- Soto, J.A.; Gálvez, N.M.S.; Andrade, C.A.; Pacheco, G.A. The Role of Dendritic Cells During Infections Caused by Highly Prevalent Viruses. Front. Immunol. 2020, 11, 1513. [Google Scholar] [CrossRef]
- Marongiu, L.; Valache, M.; Facchini, F.A.; Granucci, F. How dendritic cells sense and respond to viral infections. Clin. Sci. 2021, 135, 2217–2242. [Google Scholar] [CrossRef]
- Worbs, T.; Hammerschmidt, S.I.; Förster, R. Dendritic cell migration in health and disease. Nat. Rev. Immunol. 2017, 17, 30–48. [Google Scholar] [CrossRef]
- Jabri, B.; Abadie, V. IL-15 functions as a danger signal to regulate tissue-resident T cells and tissue destruction. Nat. Rev. Immunol. 2015, 15, 771–783. [Google Scholar] [CrossRef]
- Swain, S.L.; Mckinstry, K.K.; Strutt, T.M. Expanding roles for CD4 + T cells in immunity to viruses. Nat. Rev. Immunol. 2012, 12, 136–148. [Google Scholar] [CrossRef]
- Ma, H.; Tao, W.; Zhu, S. T lymphocytes in the intestinal mucosa: Defense and tolerance. Cell. Mol. Immunol. 2019, 16, 216–224. [Google Scholar] [CrossRef]
- Duijts, L.; Jaddoe, V.W.V.; Hofman, A.; Moll, H.A. Prolonged and exclusive breastfeeding reduces the risk of infectious diseases in infancy. Pediatrics 2010, 126, e18–e25. [Google Scholar] [CrossRef]
- Christensen, N.; Bruun, S.; Søndergaard, J.; Christesen, H.T.; Fisker, N.; Zachariassen, G.; Sangild, P.T.; Husby, S. Breastfeeding and Infections in Early Childhood: A Cohort Study. Pediatrics 2020, 146. [Google Scholar] [CrossRef]
- Plenge-Bönig, A.; Soto-Ramírez, N.; Karmaus, W.; Petersen, G.; Davis, S.; Forster, J. Breastfeeding protects against acute gastroenteritis due to rotavirus in infants. Eur. J. Pediatr. 2010, 169, 1471–1476. [Google Scholar] [CrossRef] [PubMed]
- Hogan, R.; Martinez, J. Breastfeeding as an intervention within diarrhea1 diseases control programs: WHO/CDD activities. Int. J. Gynecol. Obs. 1990, 31, 115–119. [Google Scholar] [CrossRef]
- Mccormick, B.J.J.; Richard, S.A.; Murray-Kolb, L.E.; Kang, G.; Lima, A.A.M.; Mduma, E.; Kosek, M.N.; Rowasky Mcquade, E.T.; Houpt, E.R.; Bessong, P.; et al. Full breastfeeding protection against common enteric bacteria and viruses: Results from the MAL-ED cohort study. Am. Soc. Nutr. 2022, 115, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Carr, L.E.; Virmani, M.D.; Rosa, F.; Munblit, D.; Matazel, K.S.; Elolimy, A.A.; Yeruva, L. Role of Human Milk Bioactives on Infants’ Gut and Immune Health. Front. Immunol. 2021, 12, 604080. [Google Scholar] [CrossRef] [PubMed]
- Ayechu-Muruzabal, V.; van Stigt, A.H.; Mank, M.; Willemsen, L.E.M.; Stahl, B.; Garssen, J.; van’t Land, B. Diversity of human milk oligosaccharides and effects on early life immune development. Front. Pediatr. 2018, 6, 239. [Google Scholar] [CrossRef]
- Xiao, L.; van de Worp, W.R.; Stassen, R.; van Maastrigt, C.; Kettelarij, N.; Stahl, B.; Blijenberg, B.; Overbeek, S.A.; Folkerts, G.; Garssen, J.; et al. Human milk oligosaccharides promote immune tolerance via direct interactions with human dendritic cells. Eur. J. Immunol. 2019, 49, 1001–1014. [Google Scholar] [CrossRef] [PubMed]
- Azagra-Boronat, I.; Massot-Cladera, M.; Knipping, K.; van’t Land, B.; Stahl, B.; Garssen, J.; Rodríguez-Lagunas, M.J.; Franch, À.; Castell, M.; Pérez-Cano, F.J. Supplementation With 2′-FL and scGOS/lcFOS Ameliorates Rotavirus-Induced Diarrhea in Suckling Rats. Front. Cell. Infect. Microbiol. 2018, 8, 372. [Google Scholar] [CrossRef]
- Azagra-Boronat, I.; Massot-Cladera, M.; Knipping, K.; Van’t Land, B.; Tims, S.; Stahl, B.; Knol, J.; Garssen, J.; Franch, À.; Castell, M.; et al. Oligosaccharides Modulate Rotavirus-Associated Dysbiosis and TLR Gene Expression in Neonatal Rats. Cells 2019, 8, 876. [Google Scholar] [CrossRef]
- Li, M.; Monaco, M.H.; Wang, M.; Comstock, S.S.; Kuhlenschmidt, T.B.; Fahey, G.C.; Miller, M.J.; Kuhlenschmidt, M.S.; Donovan, S.M. Human milk oligosaccharides shorten rotavirus-induced diarrhea and modulate piglet mucosal immunity and colonic microbiota. ISME J. 2014, 8, 1609–1620. [Google Scholar] [CrossRef]
- Comstock, S.S.; Li, M.; Wang, M.; Monaco, M.H.; Kuhlenschmidt, T.B.; Kuhlenschmidt, M.S.; Donovan, S.M. Dietary human milk oligosaccharides but not prebiotic oligosaccharides increase circulating natural killer cell and mesenteric lymph node memory T cell populations in noninfected and rotavirus-infected neonatal piglets. J. Nutr. 2017, 147, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Ramani, S.; Stewart, C.J.; Laucirica, D.R.; Ajami, N.J.; Robertson, B.; Autran, C.A.; Shinge, D.; Rani, S.; Anandan, S.; Hu, L.; et al. Human milk oligosaccharides, milk microbiome and infant gut microbiome modulate neonatal rotavirus infection. Nat. Commun. 2018, 9, 5010. [Google Scholar] [CrossRef] [PubMed]
- Laucirica, D.R.; Triantis, V.; Schoemaker, R.; Estes, M.K.; Ramani, S. Milk oligosaccharides inhibit human rotavirus infectivity in MA104 cells. J. Nutr. 2017, 147, 1709–1714. [Google Scholar] [CrossRef] [PubMed]
- He, Y.Y.; Liu, S.B.; Kling, D.E.; Leone, S.; Lawlor, N.T.; Huang, Y.; Feinberg, S.B.; Hill, D.R.; Newburg, D.S. The human milk oligosaccharide 2′-fucosyllactose modulates CD14 expression in human enterocytes, thereby attenuating LPS-induced inflammation. Gut 2016, 65, 33–46. [Google Scholar] [CrossRef]
- Ayechu-Muruzabal, V.; Overbeek, S.A.; Kostadinova, A.I.; Stahl, B.; Garssen, J.; Van’t Land, B.; Willemsen, L.E.M. Exposure of intestinal epithelial cells to 2′-fucosyllactose and CpG enhances galectin release and instructs dendritic cells to drive Th1 and regulatory-type immune development. Biomolecules 2020, 10, 784. [Google Scholar] [CrossRef]
- Xiao, L.; Van’t Land, B.; Engen, P.A.; Naqib, A.; Green, S.J.; Nato, A.; Leusink-Muis, T.; Garssen, J.; Keshavarzian, A.; Stahl, B.; et al. Human milk oligosaccharides protect against the development of autoimmune diabetes in NOD-mice. Sci. Rep. 2018, 8, 3829. [Google Scholar] [CrossRef]
- Bugge, M.; Bergstrom, B.; Eide, O.K.; Solli, H.; Kjønstad, I.F.; Stenvik, J.; Espevik, T.; Nilsen, N.J. Surface toll-like receptor 3 expression in metastatic intestinal epithelial cells induces inflammatory cytokine production and promotes invasiveness. J. Biol. Chem. 2017, 292, 15408–15425. [Google Scholar] [CrossRef]
- Østvik, A.E.; Granlund, A.V.B.; Bugge, M.; Nilsen, N.J.; Torp, S.H.; Waldum, H.L.; Damås, J.K.; Espevik, T.; Sandvik, A.K. Enhanced expression of CXCL10 in inflammatory bowel disease: Potential role of mucosal toll-like receptor 3 stimulation. Inflamm. Bowel Dis. 2013, 19, 265–274. [Google Scholar] [CrossRef]
- Skovdahl, H.K.; Van Beelen Granlund, A.; Østvik, A.E.; Bruland, T.; Bakke, I.; Torp, S.H.; Damas, J.K.; Sandvik, A.K. Expression of CCL20 and its corresponding receptor CCR6 is enhanced in active inflammatory bowel disease, and TLR3 mediates CCL20 expression in colonic epithelial cells. PLoS ONE 2015, 10, e0141710. [Google Scholar] [CrossRef]
- Furrie, E.; Macfarlane, S.; Thomson, G.; Macfarlane, G.T. Toll-like receptors-2, -3 and -4 expression patterns on human colon and their regulation by mucosal-associated bacteria. Immunology 2005, 115, 565–574. [Google Scholar] [CrossRef]
- Hirata, Y.; Broquet, A.H.; Menchén, L.; Kagnoff, M.F. Activation of Innate Immune Defense Mechanisms by Signaling through RIG-I/IPS-1 in Intestinal Epithelial Cells. J. Immunol. 2007, 179, 5425–5432. [Google Scholar] [CrossRef] [PubMed]
- Broquet, A.H.; Hirata, Y.; McAllister, C.S.; Kagnoff, M.F. RIG-I/MDA5/MAVS Are Required to Signal a Protective IFN Response in Rotavirus-Infected Intestinal Epithelium. J. Immunol. 2011, 186, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Eberwine, J.H. Mammalian cell transfection: The present and the future. Anal. Bioanal. Chem. 2010, 397, 3173–3178. [Google Scholar] [CrossRef] [PubMed]
- Casola, A.; Estes, M.K.; Crawford, S.U.E.E.; Ogra, P.L.; Ernst, P.B.; Garofalo, R.P.; Crowe, S.E. Rotavirus Infection of Cultured Intestinal Epithelial Cells Induces Secretion of CXC and CC Chemokines. Gastroenterology 1998, 114, 947–955. [Google Scholar] [CrossRef]
- Sen, A.; Pruijssers, A.J.; Dermody, T.S.; Garcia-Sastre, A.; Greenberg, H.B. The Early Interferon Response to Rotavirus Is Regulated by PKR and Depends on MAVS/IPS-1, RIG-I, MDA-5, and IRF3. J. Virol. 2011, 85, 3717–3732. [Google Scholar] [CrossRef]
- Kato, H.; Takeuchi, O.; Sato, S.; Yoneyama, M.; Yamamoto, M.; Matsui, K.; Uematsu, S.; Jung, A.; Kawai, T.; Ishii, K.J.; et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 2006, 441, 101–105. [Google Scholar] [CrossRef]
- Marineau, A.; Khan, K.A.; Servant, M.J. Roles of GSK-3 and β -Catenin in Antiviral Innate Immune Sensing of Nucleic Acids. Cells 2020, 9, 897. [Google Scholar] [CrossRef]
- Ye, L.; Schnepf, D.; Staeheli, P. Interferon-λ orchestrates innate and adaptive mucosal immune responses. Nat. Rev. Immunol. 2019, 19, 614–625. [Google Scholar] [CrossRef]
- Mcnab, F.; Mayer-barber, K.; Sher, A.; Wack, A.; Garra, A.O. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef]
- Brand, S.; Sakaguchi, T.; Gu, X.; Colgan, S.P.; Reinecker, H.C. Fractalkine-Mediated Signals Regulate Cell-Survival and Immune-Modulatory Responses in Intestinal Epithelial Cells. Gastroenterology 2002, 122, 166–177. [Google Scholar] [CrossRef]
- Ishizuka, T.; Kanmani, P.; Kobayashi, H.; Miyazaki, A. Immunobiotic Bifidobacteria Strains Modulate Rotavirus Immune Response in Porcine Intestinal Epitheliocytes via Pattern Recognition Receptor Signaling. PLoS ONE 2016, 11, e0152416. [Google Scholar] [CrossRef]
- Paludan, S.R.; Pradeu, T.; Masters, S.L.; Mogensen, T.H. Constitutive immune mechanisms: Mediators of host defence and immune regulation. Nat. Rev. Immunol. 2021, 21, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Rancez, M.; Couëdel-Courteille, A.; Cheynier, R. Chemokines at mucosal barriers and their impact on HIV infection. Cytokine Growth Factor Rev. 2012, 23, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Dwinell, M.B.; Johanesen, P.A.; Smith, J.M. Immunobiology of epithelial chemokines in the intestinal mucosa. Surgery 2003, 133, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Schall, T.J.; Bacont, K.; Toy, K.J.; Goeddel, D. V Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature 1990, 347, 669–671. [Google Scholar] [CrossRef]
- Stadnyk, A.W. Intestinal epithelial cells as a source of inflammatory cytokines and chemokines. Can. J. Gastroenterol. 2002, 16, 241–246. [Google Scholar] [CrossRef]
- Wang, W.H.; Lin, C.Y.; Chang, M.R.; Urbina, A.N.; Assavalapsakul, W.; Thitithanyanont, A.; Chen, Y.H.; Liu, F.T.; Wang, S.F. The role of galectins in virus infection—A systemic literature review. J. Microbiol. Immunol. Infect. 2019, 53, 925–935. [Google Scholar] [CrossRef]
- Machala, E.A.; McSharry, B.P.; Rouse, B.T.; Abendroth, A.; Slobedman, B. Gal power: The diverse roles of galectins in regulating viral infections. J. Gen. Virol. 2019, 100, 333–349. [Google Scholar] [CrossRef]
- Vasta, G.R. Current Topics in Innate Immunity II; Lambris, J.D., Hajishengallis, G., Eds.; Springer: New York, NY, USA, 2012; ISBN 9781461401056. [Google Scholar]
- Merani, S.; Chen, W.; Elahi, S. The bitter side of sweet: The role of Galectin-9 in immunopathogenesis of viral infections. Rev. Med. Virol. 2015, 25, 175–186. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.; Chen, Y.; Lin, C.; Huang, C. Serum Galectin-9 and Galectin-3-Binding Protein in Acute Dengue Virus Infection. Int. J. Mol. Sci. 2016, 17, 832. [Google Scholar] [CrossRef]
- Katoh, S.; Ikeda, M.; Shimizu, H.; Mouri, K.; Obase, Y.; Kobashi, Y.; Fukushima, K.; Hirashima, M.; Oka, M. Increased levels of plasma galectin-9 in patients with influenza virus infection. Tohoku J. Exp. Med. 2014, 232, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Tandon, R.; Chew, G.M.; Byron, M.M.; Borrow, P.; Niki, T.; Hirashima, M.; Barbour, J.D.; Norris, P.J.; Lanteri, M.C.; Martin, J.N.; et al. Galectin-9 Is Rapidly Released During Acute HIV-1 Infection and Remains Sustained at High Levels Despite Viral Suppression Even in Elite Controllers. AIDS Res. Hum. Retroviruses 2014, 30, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Tawab, M.S.; Fouad, H.H.; Omran, D.A.; Abdou, A.E.; Zaied, S.M.; Mohamed, A.A. Evaluation of Serum and Gene Expression of Galectin-4, Interleukin-27, and Complement-7 in Hepatitis C Virus-Infected Egyptian Patients. BioMed Res. Int. 2020, 2020, 8879758. [Google Scholar] [CrossRef]
- Cai, Z.; Zeng, Y.; Xu, B.; Gao, Y.; Wang, S.; Zeng, J.; Chen, L.; Huang, A.; Liu, X.; Liu, J. Galectin-4 serves as a prognostic biomarker for the early recurrence⁄metastasis of hepatocellular carcinoma. Cancer Sci. 2014, 105, 1510–1517. [Google Scholar] [CrossRef]
- Giron, L.B.; Dweep, H.; Yin, X.; Wang, H.; Damra, M.; Goldman, A.R.; Gorman, N.; Palmer, C.S.; Tang, H.Y.; Shaikh, M.W.; et al. Plasma Markers of Disrupted Gut Permeability in Severe COVID-19 Patients. Front. Immunol. 2021, 12, 686240. [Google Scholar] [CrossRef]
- Cervantes-Alvarez, E.; Limon-de la Rosa, N.; Salgado-de la Mora, M.; Valdez-Sandoval, P.; Palacios-Jimenez, M.; Rodriguez-Alvarez, F.; Vera-Maldonado, B.I.; Aguirre-Aguilar, E.; Escobar-Valderrama, J.M.; Alanis-Mendizabal, J.; et al. Galectin-3 as a potential prognostic biomarker of severe COVID-19 in SARS-CoV-2 infected patients. Sci. Rep. 2022, 12, 1–9. [Google Scholar] [CrossRef]
- Shete, A.; Dhayarkar, S.; Dhamanage, A.; Kulkarni, S.; Ghate, M.; Sangle, S.; Medhe, U.; Verma, V.; Rajan, S.; Hattori, T.; et al. Possible role of plasma Galectin-9 levels as a surrogate marker of viremia in HIV infected patients on antiretroviral therapy in resource-limited settings. AIDS Res. Ther. 2020, 17, 43. [Google Scholar] [CrossRef]
- Bozorgmehr, N.; Mashhouri, S.; Rosero, P.; Xu, L.; Shahbaz, S.; Sligl, W.; Osman, M. Galectin-9, a Player in Cytokine Release Syndrome and a Surrogate Diagnostic Biomarker in SARS-CoV-2 Infection. mBio 2021, 12, e00384-21. [Google Scholar] [CrossRef]
- Dapat, I.C.; Pascapurnama, D.N.; Iwasaki, H.; Labayo, H.K.; Chagan-Yasutan, H.; Egawa, S.; Hattori, T. Secretion of galectin-9 as a DAMP during dengue virus infection in THP-1 cells. Int. J. Mol. Sci. 2017, 18, 1644. [Google Scholar] [CrossRef]
- Chagan-Yasutan, H.; Ndhlovu, L.C.; Lacuesta, T.L.; Kubo, T.; Leano, P.S.A.; Niki, T.; Oguma, S.; Morita, K.; Chew, G.M.; Barbour, J.D.; et al. Galectin-9 plasma levels reflect adverse hematological and immunological features in acute dengue virus infection. J. Clin. Virol. 2013, 58, 635–640. [Google Scholar] [CrossRef]
- Ji, X.J.; Ma, C.J.; Wang, J.M.; Wu, X.Y.; Niki, T. HCV-infected hepatocytes drive CD4+CD25+Foxp3+ regulatory T-cell development through the Tim-3/Gal-9 pathway. Eur. J. Immunol. 2013, 43, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Rouse, B.T.; Sehrawat, S. Immunity and immunopathology to viruses: What decides the outcome ? Nat. Rev. Immunol. 2010, 10, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Anderson, A.C.; Schubart, A.; Xiong, H.; Imitola, J.; Khoury, S.J.; Zheng, X.X.; Strom, T.B.; Kuchroo, V.K. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005, 6, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Sehrawat, S.; Suryawanshi, A.; Hirashima, M.; Rouse, B.T. Role of Tim-3/Galectin-9 Inhibitory Interaction in Viral Induced Immunopathology: Shifting the Balance Towards Regulators. J. Immunol. 2009, 182, 3191–3201. [Google Scholar] [CrossRef]
- Dai, S.-Y.; Nakagawa, R.; Itoh, A.; Murakami, H.; Kashio, Y.; Abe, H.; Katoh, S.; Kontani, K.; Kihara, M.; Zhang, S.; et al. Galectin-9 Induces Maturation of Human Monocyte-Derived Dendritic Cells. J. Immunol. 2005, 175, 2974–2981. [Google Scholar] [CrossRef] [PubMed]
- Ayechu-Muruzabal, V.; Xiao, L.; Wehkamp, T.; van Ark, I.; Hoogendoorn, E.J.; Leusink-Muis, T.; Folkerts, G.; Garssen, J.; Willemsen, L.E.M.; van’t Land, B. A fermented milk matrix containing postbiotics supports th1-and th17-type immunity in vitro and modulates the influenza-specific vaccination response in vivo in association with altered serum galectin ratios. Vaccines 2021, 9, 254. [Google Scholar] [CrossRef]
- Xu, Y.; Cheng, M.; Shang, P.; Yang, Y. Role of IL-6 in dendritic cell functions. J. Leukoc. Biol. 2022, 111, 695–709. [Google Scholar] [CrossRef]
- Boullart, A.C.I.; Aarntzen, E.H.J.G.; Verdijk, P.; Jacobs, J.F.M.; Schuurhuis, D.H.; Gerty, D.B.; Mandy, S.; Nicole, W.M.M.V.D.R.; De Boer, A.; Kramer, M.; et al. Maturation of monocyte-derived dendritic cells with Toll-like receptor 3 and 7/8 ligands combined with prostaglandin E 2 results in high interleukin-12 production and cell migration. Cancer Immunol. Immunother. 2008, 57, 1589–1597. [Google Scholar] [CrossRef]
- Santini, B.S.M.; Lapenta, C.; Logozzi, M.; Parlato, S.; Spada, M.; Pucchio, T.D.; Belardelli, F. Type I Interferon as a Powerful Adjuvant for Monocyte-derived Dendritic Cell Development and Activity In Vitro and in Hu-PBL-SCID Mice. J. Exp. Med. 2000, 191, 1777–1788. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6. [Google Scholar] [CrossRef]
- Rückeret, R.; Brandt, K.; Bulanova, E.; Mirghomizadeh, F.; Paus, R.; Bulfone-paus, S. Dendritic cell-derived IL-15 controls the induction of CD8 T cell immune responses Ren e. Eur. J. Immunol. 2003, 33, 3493–3503. [Google Scholar] [CrossRef] [PubMed]
- Stonier, S.W.; Ma, L.J.; Castillo, E.F.; Schluns, K.S. Dendritic cells drive memory CD8 T-cell homeostasis via IL-15 transpresentation. Immunobiology 2008, 112, 4546–4554. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayechu-Muruzabal, V.; Poelmann, B.; Berends, A.J.; Kettelarij, N.; Garssen, J.; van’t Land, B.; Willemsen, L.E.M. Human Milk Oligosaccharide 2′-Fucosyllactose Modulates Local Viral Immune Defense by Supporting the Regulatory Functions of Intestinal Epithelial and Immune Cells. Int. J. Mol. Sci. 2022, 23, 10958. https://doi.org/10.3390/ijms231810958
Ayechu-Muruzabal V, Poelmann B, Berends AJ, Kettelarij N, Garssen J, van’t Land B, Willemsen LEM. Human Milk Oligosaccharide 2′-Fucosyllactose Modulates Local Viral Immune Defense by Supporting the Regulatory Functions of Intestinal Epithelial and Immune Cells. International Journal of Molecular Sciences. 2022; 23(18):10958. https://doi.org/10.3390/ijms231810958
Chicago/Turabian StyleAyechu-Muruzabal, Veronica, Bente Poelmann, Alinda J. Berends, Nienke Kettelarij, Johan Garssen, Belinda van’t Land, and Linette E. M. Willemsen. 2022. "Human Milk Oligosaccharide 2′-Fucosyllactose Modulates Local Viral Immune Defense by Supporting the Regulatory Functions of Intestinal Epithelial and Immune Cells" International Journal of Molecular Sciences 23, no. 18: 10958. https://doi.org/10.3390/ijms231810958
APA StyleAyechu-Muruzabal, V., Poelmann, B., Berends, A. J., Kettelarij, N., Garssen, J., van’t Land, B., & Willemsen, L. E. M. (2022). Human Milk Oligosaccharide 2′-Fucosyllactose Modulates Local Viral Immune Defense by Supporting the Regulatory Functions of Intestinal Epithelial and Immune Cells. International Journal of Molecular Sciences, 23(18), 10958. https://doi.org/10.3390/ijms231810958




