Cardiac Toxicity Associated with Immune Checkpoint Inhibitors: A Systematic Review
Abstract
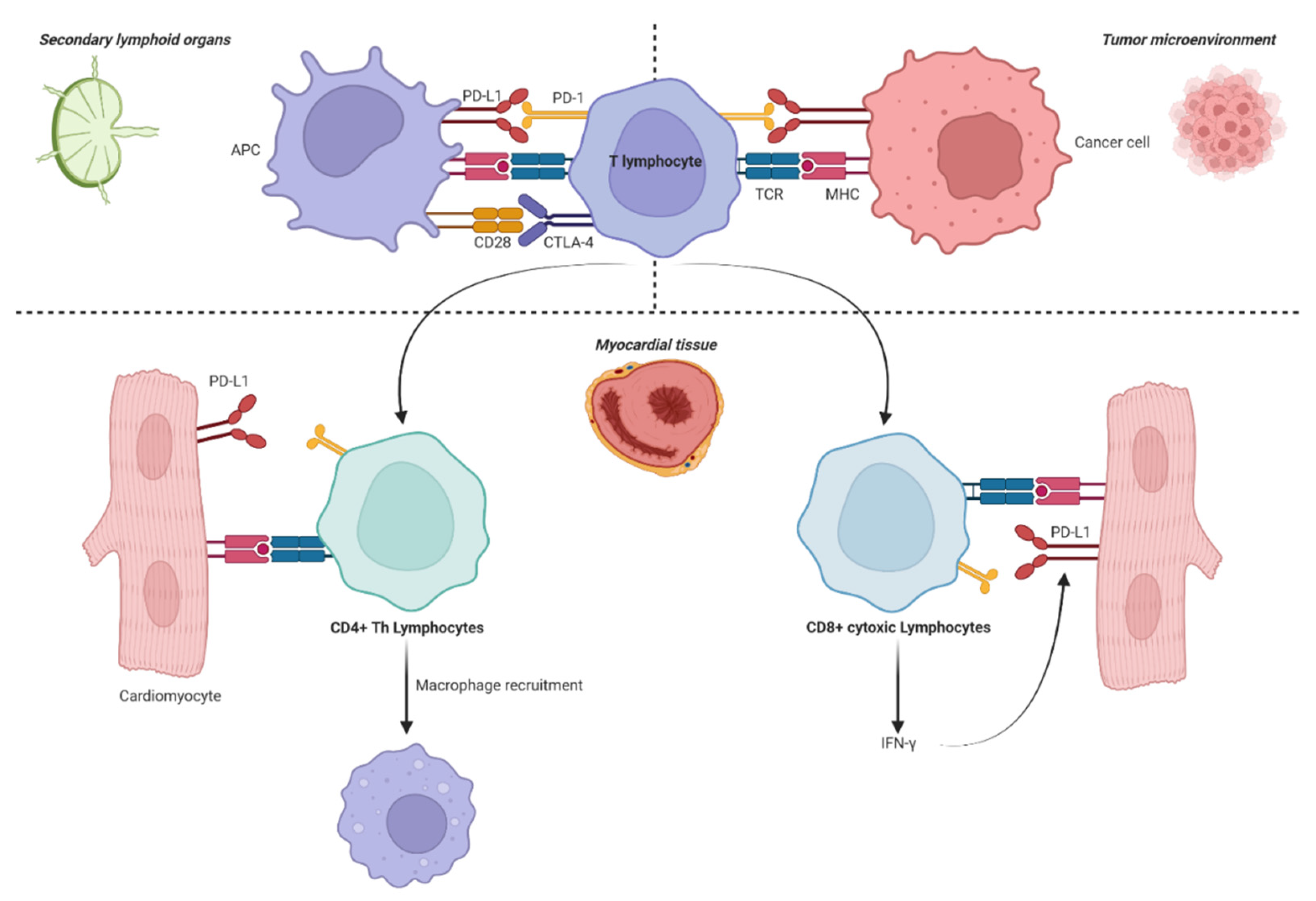
1. Introduction
2. Results
2.1. ICI-Induced Myocarditis
2.1.1. Treatment of ICI-Induced Myocarditis
2.1.2. Evolution of ICI-Induced Myocarditis
2.2. ICI-Induced Pericarditis
2.3. ICI-Induced Arrythmias
2.4. Takotsubo Cardiomyopathy and Acute Heart Failure
2.5. Acute Coronary Syndrome
2.6. IrAEs Reported in Clinical Trials
Limitations of the Study
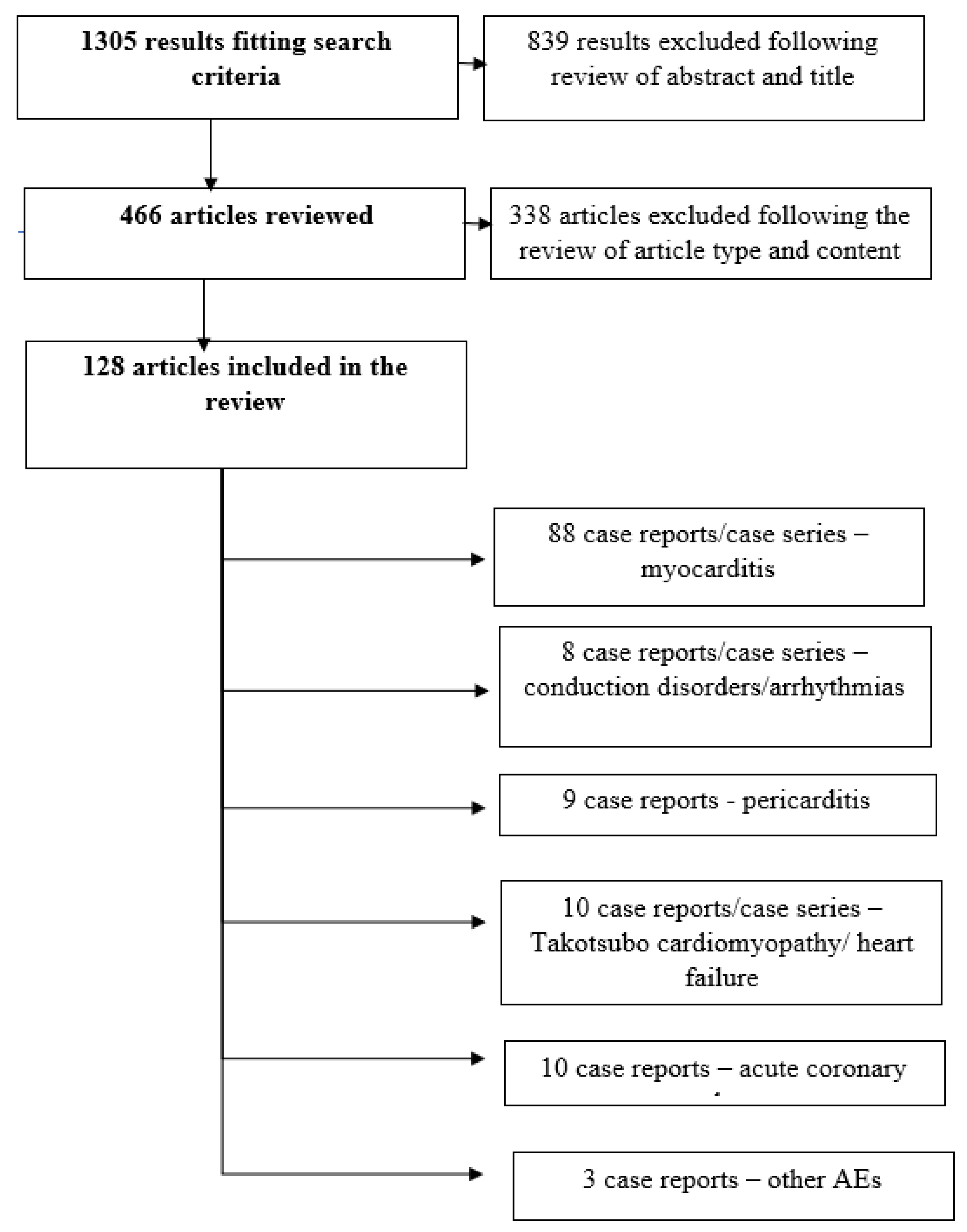
3. Material and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune Checkpoint Blockade in Cancer Therapy. J. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Nardi Agmon, I.; Itzhaki Ben Zadok, O.; Kornowski, R. The Potential Cardiotoxicity of Immune Checkpoint Inhibitors. J. Clin. Med. 2022, 11, 865. [Google Scholar] [CrossRef]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O.; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Hodi, F.; Wolchok, J. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 1270–1271. [Google Scholar] [CrossRef] [PubMed]
- Grabie, N.; Lichtman, A.H.; Padera, R. T cell checkpoint regulators in the heart. Cardiovasc. Res. 2019, 115, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Arangalage, D.; Degrauwe, N.; Michielin, O.; Monney, P.; Özdemir, B.C. Pathophysiology, diagnosis and management of cardiac toxicity induced by immune checkpoint inhibitors and BRAF and MEK inhibitors. Cancer Treat. Rev. 2021, 100, 102282. [Google Scholar] [CrossRef]
- Okazaki, T.; Tanaka, Y.; Nishio, R.; Mitsuiye, T.; Mizoguchi, A.; Wang, J.; Ishida, M.; Hiai, H.; Matsumori, A.; Minato, N.; et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat. Med. 2003, 9, 1477–1483. [Google Scholar] [CrossRef]
- Wang, J.; Okazaki, I.; Yoshida, T.; Chikuma, S.; Kato, Y.; Nakaki, F.; Hiai, H.; Honjo, T.; Okazaki, T. PD-1 deficiency results in the development of fatal myocarditis in MRL mice. Int. Immunol. 2010, 22, 443–452. [Google Scholar] [CrossRef]
- Tarrio, M.L.; Grabie, N.; Bu, D.; Sharpe, A.H.; Lichtman, A.H. PD-1 Protects against Inflammation and Myocyte Damage in T Cell-Mediated Myocarditis. J. Immunol. 2012, 188, 4876–4884. [Google Scholar] [CrossRef]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloom-er, T.L.; et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N. Engl. J. Med. 2016, 375, 1749–1755. [Google Scholar] [CrossRef]
- Balanescu, D.V.; Donisan, T.; Palaskas, N.; Lopez-Mattei, J.; Kim, P.Y.; Buja, L.M.; McNamara, D.M.; Kobashigawa, J.A.; Durand, J.-B.; Iliescu, C.A. Immunomodulatory treatment of immune checkpoint inhibitor-induced myocarditis: Pathway toward precision-based therapy. Cardiovasc. Pathol. 2020, 47, 107211. [Google Scholar] [CrossRef] [PubMed]
- Tajmir-Riahi, A.; Bergmann, T.; Schmid, M.; Agaimy, A.; Schuler, G.; Heinzerling, L. Life-threatening Autoimmune Cardio-myopathy Reproducibly Induced in a Patient by Checkpoint Inhibitor Therapy. J. Immunother. 2018, 41, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, J.J.; Salem, J.-E.; Sosman, J.A.; Lebrun-Vignes, B.; Johnson, D.B. Increased reporting of fatal immune checkpoint in-hibitor-associated myocarditis. Lancet 2018, 391, 933. [Google Scholar] [CrossRef]
- Bu, D.; Tarrio, M.; Maganto-Garcia, E.; Stavrakis, G.; Tajima, G.; Lederer, J.; Jarolim, P.; Freeman, G.J.; Sharpe, A.H.; Licht-man, A.H. Impairment of the Programmed Cell Death-1 Pathway Increases Atherosclerotic Lesion Development and In-flammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Gelsomino, F.; Fiorentino, M.; Zompatori, M.; Poerio, A.; Melotti, B.; Sperandi, F.; Gargiulo, M.; Borghi, C.; Ardizzoni, A. Pro-grammed death-1 inhibition and atherosclerosis: Can nivolumab vanish complicated atheromatous plaques? Ann. Oncol. 2018, 29, 284–286. [Google Scholar] [CrossRef]
- Chocarro, L.; Blanco, E.; Zuazo, M.; Arasanz, H.; Bocanegra, A.; Fernández-Rubio, L.; Morente, P.; Fernández-Hinojal, G.; Echaide, M.; Garnica, M.; et al. Understanding LAG-3 Signaling. Int. J. Mol. Sci. 2021, 22, 5282. [Google Scholar] [CrossRef]
- Xiong, X.; Luo, Z.; Zhou, H.; Duan, Z.; Niu, L.; Zhang, K.; Huang, G.; Li, W. Downregulation of TIGIT Expression in FOXP3+Regulatory T Cells in Acute Coronary Syndrome. J. Inflamm. Res. 2022, 15, 1195–1207. [Google Scholar] [CrossRef]
- Zhang, J.; Zhan, F.; Liu, H. Expression Level and Significance of Tim-3 in CD4+ T Lymphocytes in Peripheral Blood of Pa-tients with Coronary Heart Disease. Braz. J. Cardiovasc. Surg. 2022, 37, 350–355. [Google Scholar] [CrossRef]
- Nguyen, L.S.; Bretagne, M.; Arrondeau, J.; Zahr, N.; Ederhy, S.; Abbar, B.; Pinna, B.; Allenbach, Y.; Mira, J.P.; Moslehi, J.; et al. Reversal of immune-checkpoint inhibitor fulminant myocarditis using personalized-dose-adjusted abatacept and rux-olitinib: Proof of concept. J. Immunother. Cancer 2022, 10, e004699. [Google Scholar] [CrossRef]
- Diamantopoulos, P.T.; Tsatsou, K.; Benopoulou, O.; Bonou, M.; Anastasopoulou, A.; Mastrogianni, E.; Gogas, H. Concomi-tant development of neurologic and cardiac immune-related adverse effects in patients treated with immune checkpoint in-hibitors for melanoma. Melanoma Res. 2020, 30, 484–491. [Google Scholar] [CrossRef]
- Fazal, M.; Prentice, D.A.; Kho, L.K.; Fysh, E. Nivolumab-associated myositis myocarditis and myasthenia and anti-striated muscle antibodies. Intern. Med. J. 2020, 50, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Samara, Y.; Yu, C.L.; Dasanu, C.A. Acute autoimmune myocarditis and hepatitis due to ipilimumab monotherapy for malig-nant melanoma. J. Oncol. Pharm. Pract. 2019, 25, 966–968. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chan, J.; Brinc, D.; Gandhi, S.; Izenberg, A.; Delgado, D.; Abdel-Qadir, H.; Wintersperger, B.J.; Thavendiranathan, P. Immune Checkpoint Inhibitor-Associated Myocarditis With Persistent Troponin Elevation Despite Abatacept and Prolonged Immunosuppression. JACC Cardio Oncol. 2020, 2, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Barham, W.; Guo, R.; Park, S.S.; Herrmann, J.; Dong, H.; Yan, Y. Case Report: Simultaneous Hyperprogression and Fulmi-nant Myocarditis in a Patient with Advanced Melanoma Following Treatment With Immune Checkpoint Inhibitor Therapy. Front. Immunol. 2021, 11, 561083. [Google Scholar] [CrossRef] [PubMed]
- Wintersperger, B.J.; Calvillo-Argüelles, O.; Lheureux, S.; Houbois, C.P.; Spreafico, A.; Bedard, P.L.; Neilan, T.G.; Thavendiranathan, P. Immune checkpoint inhibitor-related myocarditis: An illustrative case series of applying the updated Cardiovascular Magnetic Resonance Lake Louise Criteria. Eur. Heart J.-Case Rep. 2022, 6, ytab478. [Google Scholar] [CrossRef]
- Saishu, Y.; Yoshida, T.; Seino, Y.; Nomura, T. Nivolumab-related myasthenia gravis with myositis requiring prolonged me-chanical ventilation: A case report. J. Med. Case Rep. 2022, 16, 61. [Google Scholar] [CrossRef]
- Saibil, S.D.; Bonilla, L.; Majeed, H.; Sotov, V.; Hogg, D.; Chappell, M.A.; Cybulsky, M.; Butler, M.O. Fatal Myocarditis and Rhabdomyositis in a Patient with Stage Iv Melanoma Treated with Combined Ipilimumab and Nivolumab. Curr. Oncol. 2019, 26, 418–421. [Google Scholar] [CrossRef]
- Norwood, T.G.; Westbrook, B.C.; Johnson, D.B.; Litovsky, S.H.; Terry, N.L.; McKee, S.B.; Gertler, A.S.; Moslehi, J.J.; Conry, R.M. Smoldering myocarditis following immune checkpoint blockade. J. Immunother. Cancer 2017, 5, 91. [Google Scholar] [CrossRef]
- Giblin, G.T.; Dennehy, C.; Featherstone, H.; Clarke, R.; Murphy, L.; Timlin, D.; O’Keane, C.; Mulligan, N.; Kelly, C.M.; Joyce, E. Subclinical Myocarditis After Combination Immune Checkpoint Inhibitor Therapy. Circ. Heart Fail. 2021, 14, e007524. [Google Scholar] [CrossRef] [PubMed]
- Arangalage, D.; Delyon, J.; Lermuzeaux, M.; Ekpe, K.; Ederhy, S.; Pages, C.; Lebbé, C. Survival After Fulminant Myocarditis Induced by Immune-Checkpoint Inhibitors. Ann. Intern. Med. 2017, 167, 683. [Google Scholar] [CrossRef]
- Fazel, M.; Jedlowski, P.M. Severe Myositis, Myocarditis, and Myasthenia Gravis with Elevated Anti-Striated Muscle Antibody following Single Dose of Ipilimumab-Nivolumab Therapy in a Patient with Metastatic Melanoma. Case Rep. Immunol. 2019, 2019, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Ida, M.; Nakamori, S.; Yamamoto, S.; Watanabe, S.; Imanaka-Yoshida, K.; Ishida, M.; Sakuma, H.; Yamanaka, K.; Dohi, K. Subtle-but-smouldering myocardial injury after immune checkpoint inhibitor treatment accompanied by amyloid deposits. ESC Heart Fail. 2022, 9, 2027–2031. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Talamo, L.; Dillon, P.; Gentzler, R.D.; Millard, T.; Salerno, M.; Slingluff, C.L.; Gaughan, E.M. Severe combined car-diac and neuromuscular toxicity from immune checkpoint blockade: An institutional case series. Cardio Oncol. 2020, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- So, H.; Ikeguchi, R.; Kobayashi, M.; Suzuki, M.; Shimizu, Y.; Kitagawa, K. PD-1 inhibitor-associated severe myasthenia gravis with necrotizing myopathy and myocarditis. J. Neurol. Sci. 2019, 399, 97–100. [Google Scholar] [CrossRef]
- Esfahani, K.; Buhlaiga, N.; Thébault, P.; Lapointe, R.; Johnson, N.A.; Miller, W.H. Alemtuzumab for Immune-Related Myo-carditis Due to PD-1 Therapy. N. Engl. J. Med. 2019, 380, 2375–2376. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Morimoto, R.; Okumura, T.; Yamashita, Y.; Haga, T.; Kuwayama, T.; Yokoi, T.; Hiraiwa, H.; Kondo, T.; Sugiura, Y.; et al. Late-Onset Fulminant Myocarditis with Immune Checkpoint Inhibitor Nivolumab. Can. J. Cardiol. 2018, 34, 812.e1–812.e3. [Google Scholar] [CrossRef]
- Ansari-Gilani, K.; Tirumani, S.H.; Smith, D.A.; Nelson, A.; Alahmadi, A.; Hoimes, C.J.; Ramaiya, N.H. Myocarditis associat-ed with immune checkpoint inhibitor therapy: A case report of three patients. Emerg. Radiol. 2020, 27, 455–460. [Google Scholar] [CrossRef]
- Shalata, W.; Peled, N.; Gabizon, I.; Abu Saleh, O.; Kian, W.; Yakobson, A. Associated Myocarditis: A Predictive Factor for Response? Case Rep. Oncol. 2020, 13, 550–557. [Google Scholar] [CrossRef]
- Leaver, P.J.; Jang, H.S.-I.; Vernon, S.T.; Fernando, S.L. Immune checkpoint inhibitor-mediated myasthenia gravis with focal subclinical myocarditis progressing to symptomatic cardiac disease. BMJ Case Rep. 2020, 13, e232920. [Google Scholar] [CrossRef]
- Hardy, T.; Yin, M.; Chavez, J.A.; Ivanov, I.; Chen, W.; Nadasdy, T.; Brodsky, S.V. Acute fatal myocarditis after a single dose of anti-PD-1 immunotherapy, autopsy findings: A case report. Cardiovasc. Pathol. 2020, 46, 107202. [Google Scholar] [CrossRef]
- Sato, T.; Nakamori, S.; Watanabe, S.; Nishikawa, K.; Inoue, T.; Imanaka-Yoshida, K.; Ishida, M.; Sakuma, H.; Ito, M.; Dohi, K. Monitoring of the Evolution of Immune Checkpoint Inhibitor Myocarditis With Cardiovascular Magnetic Resonance. Circ. Cardiovasc. Imaging 2020, 13, e010633. [Google Scholar] [CrossRef] [PubMed]
- Mirabel, M.; Callon, D.; Bruneval, P.; Lebreil, A.-L.; Mousseaux, E.; Oudard, S.; Hulot, J.-S.; Andreoletti, L. Late-Onset Giant Cell Myocarditis Due to Enterovirus During Treatment With Immune Checkpoint Inhibitors. JACC Cardio Oncol. 2020, 2, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Yanase, T.; Moritoki, Y.; Kondo, H.; Ueyama, D.; Akita, H.; Yasui, T. Myocarditis and myasthenia gravis by combined nivolumab and ipilimumab immunotherapy for renal cell carcinoma: A case report of successful management. Urol. Case Rep. 2021, 34, 101508. [Google Scholar] [CrossRef] [PubMed]
- Thibault, C.; Vano, Y.; Soulat, G.; Mirabel, M. Immune checkpoint inhibitors myocarditis: Not all cases are clinically patent. Eur. Heart J. 2018, 39, 3553. [Google Scholar] [CrossRef]
- Rota, E.; Varese, P.; Agosti, S.; Celli, L.; Ghiglione, E.; Pappalardo, I.; Zaccone, G.; Paglia, A.; Morelli, N. Concomitant myas-thenia gravis, myositis, myocarditis and polyneuropathy, induced by immune-checkpoint inhibitors: A life–threatening con-tinuum of neuromuscular and cardiac toxicity. eNeurologicalSci 2019, 14, 4–5. [Google Scholar] [CrossRef]
- Miyauchi, Y.; Naito, H.; Tsunemori, H.; Tani, R.; Hasui, Y.; Miyake, Y.; Minamino, T.; Ishikawa, R.; Kushida, Y.; Haba, R.; et al. Myocarditis as an immune-related adverse event following treatment with ipilimumab and nivolumab combination thera-py for metastatic renal cell carcinoma: A case report. J. Med. Case Rep. 2021, 15, 508. [Google Scholar] [CrossRef]
- Jespersen, M.S.; Fanø, S.; Stenør, C.; Møller, A.K. A case report of immune checkpoint inhibitor-related steroid-refractory my-ocarditis and myasthenia gravis-like myositis treated with abatacept and mycophenolate mofetil. Eur. Heart J.-Case Rep. 2021, 5, ytab342. [Google Scholar] [CrossRef]
- Lorente-Ros, Á.; Rajjoub-Al-Mahdi, E.A.; Monteagudo Ruiz, J.M.; Rivas García, S.; Ortega Pérez, R.; Fernández Golfín, C.; Álvarez-García, J.; Zamorano Gómez, J.L. Checkpoint Immunotherapy-Induced Myocarditis and Encephalitis Complicated With Complete AV Block: Not All Hope Is Lost. JACC Case Rep. 2022, 4, 1032–1036. [Google Scholar] [CrossRef]
- Tanabe, J.; Watanabe, N.; Endo, A.; Nagami, T.; Inagaki, S.; Tanabe, K. Asymptomatic Immune Checkpoint Inhibitor-associated Myocarditis. Intern. Med. 2021, 60, 569–573. [Google Scholar] [CrossRef]
- Xie, X.; Wang, F.; Qin, Y.; Lin, X.; Xie, Z.; Liu, M.; Ouyang, M.; Luo, B.; Gu, Y.; Li, S.; et al. Case Report: Fatal Multiorgan Failure and Heterochronous Pneumonitis Following Pembrolizumab Treatment in a Patient with Large-Cell Neuroendocrine Carcinoma of Lung. Front. Pharmacol. 2021, 11, 569466. [Google Scholar] [CrossRef]
- Tomoaia, R.; Beyer, R.Ș.; Pop, D.; Minciună, I.A.; Dădârlat-Pop, A. Fatal association of fulminant myocarditis and rhabdo-myolysis after immune checkpoint blockade. Eur. J. Cancer 2020, 132, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Yahagi, K.; Hoshino, T.; Yokota, T.; Tanabe, K.; Mori, M.; Ikeda, S. Nivolumab-induced myocardial necrosis in a patient with lung cancer: A case report. Respir. Med. Case Rep. 2019, 27, 100839. [Google Scholar] [CrossRef]
- von Itzstein, M.S.; Khan, S.; Popat, V.; Lu, R.; Khan, S.A.; Fattah, F.J.; Park, J.Y.; Bermas, B.L.; Karp, D.R.; Ahmed, M.; et al. Statin Intolerance, Anti-HMGCR Antibodies, and Immune Checkpoint Inhibitor-Associated Myositis: A “Two-Hit” Autoim-mune Toxicity or Clinical Predisposition? Oncologist 2020, 25, e1242–e1245. [Google Scholar] [CrossRef] [PubMed]
- Salido Iniesta, M.; López López, L.; Carreras Costa, F.; Sionis, A. A different type of acute myocarditis: A case report of acute autoimmune myocarditis mediated by anti-PD-1 T lymphocyte receptor (pembrolizumab). Eur. Heart J.-Case Rep. 2020, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tsuruda, T.; Yoshikawa, N.; Kai, M.; Yamaguchi, M.; Toida, R.; Kodama, T.; Kajihara, K.; Kawabata, T.; Nakamura, T.; Sa-kata, K.; et al. The Cytokine Expression in Patients with Cardiac Complication after Immune Checkpoint Inhibitor Therapy. Intern. Med. 2021, 60, 423–429. [Google Scholar] [CrossRef]
- Doms, J.; Prior, J.O.; Peters, S.; Obeid, M. Tocilizumab for refractory severe immune checkpoint inhibitor-associated myocar-ditis. Ann. Oncol. 2020, 31, 1273–1275. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, Y.; Yang, B.; Jin, C.; Ren, H.; Wu, J.; Wang, Z.; Wei, Y.; Yang, L.; Hu, Y. Immunotherapy-Related Cardiotoxicity Re-Emergence in Non-Small Cell Lung Cancer—A Case Report. Onco. Targets. Ther. 2021, 14, 5309–5314. [Google Scholar] [CrossRef]
- Fukasawa, Y.; Sasaki, K.; Natsume, M.; Nakashima, M.; Ota, S.; Watanabe, K.; Takahashi, Y.; Kondo, F.; Kozuma, K.; Seki, N. Nivolumab-Induced Myocarditis Concomitant with Myasthenia Gravis. Case Rep. Oncol. 2017, 10, 809–812. [Google Scholar] [CrossRef]
- Matson, D.R.; Accola, M.A.; Rehrauer, W.M.; Corliss, R.F. Fatal Myocarditis Following Treatment with the PD-1 Inhibitor Nivolumab. J. Forensic. Sci. 2018, 63, 954–957. [Google Scholar] [CrossRef]
- Okauchi, S.; Sasatani, Y.; Yamada, H.; Satoh, H. Late-onset pulmonary and cardiac toxicities in a patient treted with immune checkpoint inhibitor monotherapy. Klin. Onkol. 2022, 35, 150–154. [Google Scholar] [CrossRef]
- Ederhy, S.; Fenioux, C.; Cholet, C.; Rouvier, P.; Redheuil, A.; Cohen, A.; Salem, J.-E. Immune Checkpoint Inhibitor Myocardi-tis with Normal Cardiac Magnetic Resonance Imaging: Importance of Cardiac Biopsy and Early Diagnosis. Can. J. Cardiol. 2021, 37, 1654–1656. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Li, M.; Chen, T.; She, J. Case Report: Acute Myocarditis Due to PD-L1 Inhibitor Durvalumab Monotherapy in a Patient with Lung Squamous Cell Carcinoma. Front. Med. 2022, 9, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Xing, Q.; Zhang, Z.-W.; Lin, Q.-H.; Shen, L.-H.; Wang, P.-M.; Zhang, S.; Fan, M.; Zhu, B. Myositis-myasthenia gravis overlap syndrome complicated with myasthenia crisis and myocarditis associated with anti-programmed cell death-1 (sintilimab) therapy for lung adenocarcinoma. Ann. Transl. Med. 2020, 8, 250. [Google Scholar] [CrossRef] [PubMed]
- Chatzantonis, G.; Evers, G.; Meier, C.; Bietenbeck, M.; Florian, A.; Klingel, K.; Bleckmann, A.; Yilmaz, A. Immune Check-point Inhibitor-Associated Myocarditis. JACC Case Rep. 2020, 2, 630–635. [Google Scholar] [CrossRef]
- Fuentes-Antrás, J.; Peinado, P.; Guevara-Hoyer, K.; del Arco, C.D.; Sánchez-Ramón, S.; Aguado, C. Fatal autoimmune storm after a single cycle of anti-PD-1 therapy: A case of lethal toxicity but pathological complete response in metastatic lung ade-nocarcinoma. Hematol. Oncol. Stem Cell Ther. 2020, in press. [Google Scholar] [CrossRef]
- Frigeri, M.; Meyer, P.; Banfi, C.; Giraud, R.; Hachulla, A.-L.; Spoerl, D.; Friedlaender, A.; Pugliesi-Rinaldi, A.; Dietrich, P.-Y. Immune Checkpoint Inhibitor-Associated Myocarditis: A New Challenge for Cardiologists. Can. J. Cardiol. 2018, 34, 92.e1–92.e3. [Google Scholar] [CrossRef]
- Nierstedt, R.T.; Yeahia, R.; Barnett, K.M. Unanticipated Myocarditis in a Surgical Patient Treated with Pembrolizumab: A Case Report. AA Pract. 2020, 14, e01177. [Google Scholar] [CrossRef]
- Valenti-Azcarate, R.; Esparragosa Vazquez, I.; Toledano Illan, C.; Idoate Gastearena, M.A.; Gállego Pérez-Larraya, J. Nivolumab and Ipilimumab-induced myositis and myocarditis mimicking a myasthenia gravis presentation. Neuromuscul. Disord. 2020, 30, 67–69. [Google Scholar] [CrossRef]
- Tu, L.; Liu, J.; Li, Z.; Liu, Y.; Luo, F. Early detection and management of immune-related myocarditis: Experience from a case with advanced squamous cell lung carcinoma. Eur. J. Cancer 2020, 131, 5–8. [Google Scholar] [CrossRef]
- Möhn, N.; Beutel, G.; Gutzmer, R.; Ivanyi, P.; Satzger, I.; Skripu, T. Neurological Immune Related Adverse Events Associated with Nivolumab, Ipilimumab, and Pembrolizumab Therapy—Review of the Literature and Future Outlook. J. Clin. Med. 2019, 8, 1777. [Google Scholar] [CrossRef]
- Tajiri, K.; Aonuma, K.; Sekine, I. Immune checkpoint inhibitor-related myocarditis. Jpn. J. Clin. Oncol. 2018, 48, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Bomze, D.; Hasan Ali, O.; Bate, A.; Flatz, L. Association Between Immune-Related Adverse Events During Anti–PD-1 Ther-apy and Tumor Mutational Burden. JAMA Oncol. 2019, 5, 1633. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S. Autoimmune Targets of Heart and Skeletal Muscles in Myasthenia Gravis. Arch. Neurol. 2009, 66, 1334. [Google Scholar] [CrossRef] [PubMed]
- Romi, F.; Skeie, G.O.; Gilhus, N.E.; Aarli, J.A. Striational Antibodies in Myasthenia Gravis. Arch. Neurol. 2005, 62, 442. [Google Scholar] [CrossRef] [PubMed]
- Shivamurthy, P.; Parker, M.W. Cardiac manifestations of myasthenia gravis: A systematic review. IJC Metab. Endocr. 2014, 5, 3–6. [Google Scholar] [CrossRef]
- Hughes, M.; Lilleker, J.B.; Herrick, A.L.; Chinoy, H. Cardiac troponin testing in idiopathic inflammatory myopathies and systemic sclerosis-spectrum disorders: Biomarkers to distinguish between primary cardiac involvement and low-grade skeletal muscle disease activity. Ann. Rheum. Dis. 2015, 74, 795–798. [Google Scholar] [CrossRef]
- Shen, L.; Chen, H.; Wei, Q. Immune-Therapy-Related Toxicity Events and Dramatic Remission After a Single Dose of Pem-brolizumab Treatment in Metastatic Thymoma: A Case Report. Front. Immunol. 2021, 12, e621858. [Google Scholar] [CrossRef]
- Cao, J.; Li, Q.; Zhi, X.; Yang, F.; Zhu, W.; Zhou, T.; Hou, X.; Chen, D. Pembrolizumab-induced autoimmune Stevens-Johnson syndrome/toxic epidermal necrolysis with myositis and myocarditis in a patient with esophagogastric junction carcinoma: A case report. Transl. Cancer Res. 2021, 10, 3870–3876. [Google Scholar] [CrossRef]
- Ai, L.; Gao, J.; Zhao, S.; Li, Q.; Cui, Y.-H.; Liu, Q.; Wu, D.; Wang, Y.; Jin, X.; Ji, Y.; et al. Nivolumab-associated DRESS in a genetic susceptible individual. J. Immunother. Cancer 2021, 9, e002879. [Google Scholar] [CrossRef]
- Luo, Y.-B.; Tang, W.; Zeng, Q.; Duan, W.; Li, S.; Yang, X.; Bi, F. Case Report: The Neuromusclar Triad of Immune Checkpoint Inhibitors: A Case Report of Myositis, Myocarditis, and Myasthenia Gravis Overlap Following Toripalimab Treatment. Front. Cardiovasc. Med. 2021, 8, 714460. [Google Scholar] [CrossRef]
- Zhao, L.-Z.; Liu, G.; Li, Q.-F.; Chen, G.; Jin, G.-W. A case of carrelizumab-associated immune myocarditis. Asian J. Surg. 2022, 45, 496–497. [Google Scholar] [CrossRef] [PubMed]
- Monge, C.; Maeng, H.; Brofferio, A.; Apolo, A.B.; Sathya, B.; Arai, A.E.; Gulley, J.L.; Bilusic, M. Myocarditis in a patient treat-ed with Nivolumab and PROSTVAC: A case report. J. Immunother. Cancer 2018, 6, 150. [Google Scholar] [CrossRef]
- Iwasaki, S.; Hidaka, H.; Uojima, H.; Hashimura, M.; Nabeta, T.; Sanoyama, I.; Wada, N.; Kubota, K.; Nakazawa, T.; Shibuya, A.; et al. A case of immune checkpoint inhibitor-associated myocarditis after initiation of atezolizumab plus bevaci-zumab therapy for advanced hepatocellular carcinoma. Clin. J. Gastroenterol. 2021, 14, 1233–1239. [Google Scholar] [CrossRef]
- Stein-Merlob, A.F.; Hsu, J.J.; Colton, B.; Berg, C.J.; Ferreira, A.; Price, M.M.; Wainberg, Z.; Baas, A.S.; Deng, M.C.; Parikh, R.V.; et al. Keeping immune checkpoint inhibitor myocarditis in check: Advanced circulatory mechanical support as a bridge to recovery. ESC Heart Fail. 2021, 8, 4301–4306. [Google Scholar] [CrossRef] [PubMed]
- Szuchan, C.; Elson, L.; Alley, E.; Leung, K.; Camargo, A.L.; Elimimian, E.; Nahleh, Z.; Sadler, D. Checkpoint inhibitor-induced myocarditis and myasthenia gravis in a recurrent/metastatic thymic carcinoma patient: A case report. Eur. Heart J.-Case Rep. 2020, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jeyakumar, N.; Etchegaray, M.; Henry, J.; Lelenwa, L.; Zhao, B.; Segura, A.; Buja, L.M. The Terrible Triad of Checkpoint In-hibition: A Case Report of Myasthenia Gravis, Myocarditis, and Myositis Induced by Cemiplimab in a Patient with Metastatic Cutaneous Squamous Cell Carcinoma. Case Rep. Immunol. 2020, 2020, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Huang, D.-S.; Zhang, L.-W.; Li, Y.-Q.; Wang, H.-W.; Liu, H. Fatal myocarditis and rhabdomyolysis induced by nivolumab during the treatment of type B3 thymoma. Clin. Toxicol. 2018, 56, 667–671. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, B. Successful therapy for autoimmune myocarditis with pembrolizumab treatment for nasopharyngeal carci-noma. Ann. Transl. Med. 2019, 7, 247. [Google Scholar] [CrossRef]
- Portolés Hernández, A.; Blanco Clemente, M.; Escribano García, D.; Velasco Calvo, R.; Núñez García, B.; Oteo Domínguez, J.F.; Salas Antón, C.; Méndez García, M.; Segovia Cubero, J.; Domínguez, F. Checkpoint inhibitor-induced fulminant myo-carditis, complete atrioventricular block and myasthenia gravis—A case report. Cardiovasc. Diagn. Ther. 2021, 11, 1013–1019. [Google Scholar] [CrossRef]
- Thiene, G.; Bruneval, P.; Veinot, J.; Leone, O. Diagnostic use of the endomyocardial biopsy: A consensus statement. Virchows Arch. 2013, 463, 1–5. [Google Scholar] [CrossRef]
- Love, V.A.; Grabie, N.; Duramad, P.; Stavrakis, G.; Sharpe, A.; Lichtman, A. CTLA-4 Ablation and Interleukin-12–Driven Differentiation Synergistically Augment Cardiac Pathogenicity of Cytotoxic T Lymphocytes. Circ. Res. 2007, 101, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Zou, C.; Chen, H.; Xie, C.; Hou, M. Immune checkpoint inhibitor induces cardiac injury through polarizing macro-phages via modulating microRNA-34a/Kruppel-like factor 4 signaling. Cell Death Dis. 2020, 11, 575. [Google Scholar] [CrossRef] [PubMed]
- Hua, C.-C.; Liu, X.-M.; Liang, L.-R.; Wang, L.-F.; Zhong, J.-C. Targeting the microRNA-34a as a Novel Therapeutic Strategy for Cardiovascular Diseases. Front. Cardiovasc. Med. 2022, 8, 784044. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, L. Tofacitinib for treatment in immune-mediated myocarditis: The first reported cases. J. Oncol. Pharm. Pract. 2021, 27, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Safa, H.; Johnson, D.H.; Trinh, V.A.; Rodgers, T.E.; Lin, H.; Suarez-Almazor, M.E.; Fa’ak, F.; Saberian, C.; Yee, C.; Davies, M.A.; et al. Immune checkpoint inhibitor related myasthenia gravis: Single center experience and systematic review of the lit-erature. J. Immunother. Cancer 2019, 7, 319. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Sabichi, A.L.; Sada, Y.H. Myasthenia Gravis After Nivolumab Therapy for Squamous Cell Carcinoma of the Blad-der. J. Immunother. 2017, 40, 114–116. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
- Dhawan, P.S.; Goodman, B.P.; Harper, C.M.; Bosch, P.E.; Hoffman-Snyder, C.R.; Wellik, K.E.; Wingerchuk, D.M.; Demaerschalk, B.M. IVIG Versus PLEX in the Treatment of Worsening Myasthenia Gravis. Neurologist 2015, 19, 145–148. [Google Scholar] [CrossRef]
- Martinez-Calle, N.; Rodriguez-Otero, P.; Villar, S.; Mejías, L.; Melero, I.; Prosper, F.; Marinello, P.; Paiva, B.; Idoate, M.; San-Miguel, J. Anti-PD1 associated fulminant myocarditis after a single pembrolizumab dose: The role of occult pre-existing auto-immunity. Haematologica 2018, 103, e318–e321. [Google Scholar] [CrossRef]
- Koul, D.; Kanwar, M.; Jefic, D.; Kolluru, A.; Singh, T.; Dhar, S.; Kumar, P.; Cohen, G. Fulminant Giant Cell Myocarditis and Cardiogenic Shock: An Unusual Presentation of Malignant Thymoma. Cardiol. Res. Pract. 2010, 2010, 1–4. [Google Scholar] [CrossRef]
- Furuta, C.; Yano, M.; Numanami, H.; Yamaji, M.; Taguchi, R.; Haniuda, M. A case of thymoma-associated multiorgan auto-immunity including polymyositis and myocarditis. Surg. Case Rep. 2021, 7, 226. [Google Scholar] [CrossRef] [PubMed]
- Mygland, Å.; Vincent, A.; Newsom-Davis, J.; Kaminski, H.; Zorzato, F.; Agius, M.; Gilhus, N.E.; Aarli, J.A. Autoantibodies in Thymoma-Associated Myasthenia Gravis With Myositis or Neuromyotonia. Arch. Neurol. 2000, 57, 527. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, D.V.P.; Gomes, J.R.; Haddad, F.J.; Buzaid, A.C. Immune-mediated Pericarditis With Pericardial Tamponade During Nivolumab Therapy. J. Immunother. 2018, 41, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, S.; Fukata, M.; Tatsumoto, R.; Kono, M. Refractory constrictive pericarditis caused by an immune checkpoint in-hibitor properly managed with infliximab: A case report. Eur. Heart J.-Case Rep. 2021, 5, ytab002. [Google Scholar] [CrossRef] [PubMed]
- Oristrell, G.; Bañeras, J.; Ros, J.; Muñoz, E. Cardiac tamponade and adrenal insufficiency due to pembrolizumab: A case report. Eur. Heart J.-Case Rep. 2018, 2, yty038. [Google Scholar] [CrossRef]
- Dhenin, A.; Samartzi, V.; Lejeune, S.; Seront, E. Cascade of immunologic adverse events related to pembrolizumab treatment. BMJ Case Rep. 2019, 12, e229149. [Google Scholar] [CrossRef]
- Dasanu, C.A.; Jen, T.; Skulski, R. Late-onset pericardial tamponade, bilateral pleural effusions and recurrent immune mono-arthritis induced by ipilimumab use for metastatic melanoma. J. Oncol. Pharm. Pract. 2017, 23, 231–234. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Chinelis, P.; Athanasiadou, A.; Tsiouda, T.; Trakada, G.; Kallianos, A.; Veletza, L.; Hatzibougias, D.; Mi-halopoulou, E.; Goupou, E.; et al. Possible adverse effects of immunotherapy in non-small cell lung cancer; treatment and fol-low-up of three cases. Respir. Med. Case Rep. 2017, 22, 101–105. [Google Scholar] [CrossRef]
- Öztürk, C.; Luetkens, J.A. Cardiac MRI in Immune Checkpoint Inhibitor Associated Pericarditis. Clin. Case Rep. 2021, 9, e04926. [Google Scholar] [CrossRef]
- Khan, A.M.; Munir, A.; Thalody, V.; Munshi, M.K.; Mehdi, S. Cardiac tamponade in a patient with stage IV lung adenocar-cinoma treated with pembrolizumab. Immunotherapy 2019, 11, 1533–1540. [Google Scholar] [CrossRef]
- Jacobs, F.; Carnio, S.; De Stefanis, P.; Luciano, A.; Novello, S. Pericarditis during chemoimmunotherapy for non–small cell lung cancer: An adverse event to prevent and recognise. Eur. J. Cancer 2021, 149, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Joseph, L.; Nickel, A.; Patel, A.; Saba, N.; Leon, A.; El-Chami, M.; Merchant, F. Incidence of Cancer Treatment Induced Ar-rhythmia Associated with Immune Checkpoint Inhibitors. J. Atr. Fibrillation 2021, 13, 2461. [Google Scholar] [PubMed]
- Reddy, N.; Moudgil, R.; Lopez-Mattei, J.C.; Karimzad, K.; Mouhayar, E.N.; Somaiah, N.; Conley, A.P.; Patel, S.; Giza, D.E.; Iliescu, C. Progressive and Reversible Conduction Disease with Checkpoint Inhibitors. Can. J. Cardiol. 2017, 33, 1335.e13–1335.e15. [Google Scholar] [CrossRef]
- Giancaterino, S.; Abushamat, F.; Duran, J.; Lupercio, F.; DeMaria, A.; Hsu, J.C. Complete heart block and subsequent sudden cardiac death from immune checkpoint inhibitor–associated myocarditis. Heart Case Rep. 2020, 6, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Behling, J.; Kaes, J.; Münzel, T.; Grabbe, S.; Loquai, C. New-onset third-degree atrioventricular block because of autoimmune-induced myositis under treatment with anti-programmed cell death-1 (nivolumab) for metastatic melanoma. Melanoma Res. 2017, 27, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Pohl, J.; Mincu, R.-I.; Mrotzek, S.M.; Hinrichs, L.; Michel, L.; Livingstone, E.; Zimmer, L.; Wakili, R.; Schadendorf, D.; Rassaf, T.; et al. ECG Changes in Melanoma Patients Undergoing Cancer Therapy—Data from the ECoR Registry. J. Clin. Med. 2020, 9, 2060. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Riaz, S.; Carhart, R. Pembrolizumab-Induced Mobitz Type 2 Second-Degree Atrioventricular Block. Case Rep. Cardiol. 2020, 2020, 1–5. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Su, Y.-W.; Chen, S.-C. Sick sinus syndrome associated with anti-programmed cell death-1. J. Immunother. Cancer 2018, 6, 72. [Google Scholar] [CrossRef]
- Katsume, Y.; Isawa, T.; Toi, Y.; Fukuda, R.; Kondo, Y.; Sugawara, S.; Ootomo, T. Complete Atrioventricular Block Associated with Pembrolizumab-induced Acute Myocarditis: The Need for Close Cardiac Monitoring. Intern. Med. 2018, 57, 3157–3162. [Google Scholar] [CrossRef]
- Serzan, M.; Rapisuwon, S.; Krishnan, J.; Chang, I.C.; Barac, A. Takotsubo Cardiomyopathy Associated with Checkpoint In-hibitor Therapy. JACC Cardio Oncol. 2021, 3, 330–334. [Google Scholar] [CrossRef]
- Oldfield, K.; Jayasinghe, R.; Niranjan, S.; Chadha, S. Immune checkpoint inhibitor-induced takotsubo syndrome and diabetic ketoacidosis: Rare reactions. BMJ Case Rep. 2021, 14, e237217. [Google Scholar] [CrossRef] [PubMed]
- Ederhy, S.; Cautela, J.; Ancedy, Y.; Escudier, M.; Thuny, F.; Cohen, A. Takotsubo-like Syndrome in Cancer Patients Treated with Immune Checkpoint Inhibitors. JACC Cardiovasc. Imaging 2018, 11, 1187–1190. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.J.; Pacioles, T.; Alsharedi, M. Atypical Takotsubo Cardiomyopathy Secondary to Combination of Chemo-Immunotherapy in a Patient with Non-Small Cell Lung Cancer. Cureus 2020, 12, e9429. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.E.; Muluneh, B.; Jensen, B.C.; Madamanchi, C.; Lee, C.B. Left ventricular dysfunction after treatment with ipili-mumab for metastatic melanoma. Am. J. Ther. 2016, 23, e1925–e1928. [Google Scholar] [CrossRef] [PubMed]
- Al-Obaidi, A.; Parker, N.; Choucair, K.; Alderson, J.; Deutsch, J.M. A Case of Acute Heart Failure Following Immunotherapy for Metastatic Lung Cancer. Cureus 2020, 12, e8093. [Google Scholar] [CrossRef]
- Samejima, Y.; Iuchi, A.; Kanai, T.; Noda, Y.; Nasu, S.; Tanaka, A.; Morishita, N.; Suzuki, H.; Okamoto, N.; Harada, H.; et al. Development of Severe Heart Failure in a Patient with Squamous Non-small-cell Lung Cancer During Nivolumab Treatment. Intern. Med. 2020, 59, 2003–2008. [Google Scholar] [CrossRef]
- Tan, N.Y.L.; Anavekar, N.S.; Wiley, B.M. Concomitant myopericarditis and takotsubo syndrome following immune check-point inhibitor therapy. BMJ Case Rep. 2020, 13, e235265. [Google Scholar] [CrossRef]
- Anderson, R.D.; Brooks, M. Apical takotsubo syndrome in a patient with metastatic breast carcinoma on novel immunother-apy. Int. J. Cardiol. 2016, 222, 760–761. [Google Scholar] [CrossRef]
- Schwab, K.S.; Kristiansen, G.; Isaak, A.; Held, S.E.A.; Heine, A.; Brossart, P. Long Term Remission and Cardiac Toxicity of a Combination of Ipilimumab and Nivolumab in a Patient With Metastatic Head and Neck Carcinoma After Progression Fol-lowing Nivolumab Monotherapy. Front. Oncol. 2019, 9, 00403. [Google Scholar] [CrossRef]
- Cheng, Y.; Nie, L.; Ma, W.; Zheng, B. Early Onset Acute Coronary Artery Occlusion After Pembrolizumab in Advanced Non-Small Cell Lung Cancer: A Case Report. Cardiovasc. Toxicol. 2021, 21, 683–686. [Google Scholar] [CrossRef]
- Tomita, Y.; Sueta, D.; Kakiuchi, Y.; Saeki, S.; Saruwatari, K.; Sakata, S.; Jodai, T.; Migiyama, Y.; Akaike, K.; Hirosako, S.; et al. Acute coronary syndrome as a possible immune-related adverse event in a lung cancer patient achieving a complete response to anti-PD-1 immune checkpoint antibody. Ann. Oncol. 2017, 28, 2893–2895. [Google Scholar] [CrossRef] [PubMed]
- Kwan, J.M.; Cheng, R.; Feldman, L.E. Hepatotoxicity and Recurrent NSTEMI While on Pembrolizumab for Metastatic Giant Cell Bone Tumor. Am. J. Med. Sci. 2019, 357, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Cancela-Díez, B.; Gómez-De Rueda, F.; Antolinos Pérez, M.J.; Jiménez-Morales, A.; López-Hidalgo, J.L. Acute coronary syn-drome and recurrent colitis as immune-related adverse events in a lung cancer patient. J. Oncol. Pharm. Pract. 2020, 26, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Cautela, J.; Rouby, F.; Salem, J.-E.; Alexandre, J.; Scemama, U.; Dolladille, C.; Cohen, A.; Paganelli, F.; Ederhy, S.; Thuny, F. Acute Coronary Syndrome with Immune Checkpoint Inhibitors: A Proof-of-Concept Case and Pharmacovigilance Analysis of a Life-Threatening Adverse Event. Can. J. Cardiol. 2020, 36, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Masson, R.; Manthripragada, G.; Liu, R.; Tavakoli, J.; Mok, K. Possible Precipitation of Acute Coronary Syndrome with Im-mune Checkpoint Blockade: A Case Report. Perm. J. 2020, 24, 1. [Google Scholar] [CrossRef]
- Otsu, K.; Tajiri, K.; Sakai, S.; Ieda, M. Vasospastic angina following immune checkpoint blockade. Eur. Heart J. 2020, 41, 1702. [Google Scholar] [CrossRef]
- Kumamoto, T.; Kawano, H.; Kurobe, M.; Akashi, R.; Yonekura, T.; Ikeda, S.; Maemura, K. Vasospastic Angina: An Immune-related Adverse Event. Intern. Med. 2022, 61, 1983–1986. [Google Scholar] [CrossRef]
- Guo, K.; Chen, M.; Li, J. PD-1 Inhibitor-Induced Thyrotoxicosis Associated with Coronary Artery Spasm and Ventricular Tachycardia. Cardiovasc. Toxicol. 2022. [Google Scholar] [CrossRef]
- Maio, M.; Scherpereel, A.; Calabrò, L.; Aerts, J.; Perez, S.C.; Bearz, A.; Nackaerts, K.; Fennell, D.A.; Kowalski, D.; Tsao, A.S.; et al. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): A multicen-tre, international, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol. 2017, 18, 1261–1273. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Larkin, J.; Oya, M.; Thistlethwaite, F.; Martignoni, M.; Nathan, P.; Powles, T.; McDermott, D.; Robbins, P.B.; Chism, D.D.; et al. Preliminary results for avelumab plus axitinib as first-line therapy in patients with advanced clear-cell re-nal-cell carcinoma (JAVELIN Renal 100): An open-label, dose-finding and dose-expansion, phase 1b trial. Lancet Oncol. 2018, 19, 451–460. [Google Scholar] [CrossRef]
- Juergens, R.A.; Hao, D.; Ellis, P.M.; Tu, D.; Mates, M.; Kollmannsberger, C.; Bradbury, P.A.; Tehfe, M.; Wheatley-Price, P.; Robinson, A.; et al. A phase IB study of durvalumab with or without tremelimumab and platinum-doublet chemotherapy in advanced solid tumours: Canadian Cancer Trials Group Study IND226. Lung Cancer 2020, 143, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xu, J.; Chen, Y.; Zhuang, W.; Zhang, Y.; Chen, Z.; Chen, J.; Zhang, H.; Niu, Z.; Fan, Q.; et al. Camrelizumab ver-sus investigator’s choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell car-cinoma (ESCORT): A multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2020, 21, 832–842. [Google Scholar] [CrossRef]
- Peleg Hasson, S.; Salwen, B.; Sivan, A.; Shamai, S.; Geva, R.; Merimsky, O.; Raphael, A.; Shmilovich, H.; Moshkovits, Y.; Kapusta, L.; et al. Re-introducing immunotherapy in patients surviving immune checkpoint inhibitors-mediated myocarditis. Clin. Res. Cardiol. 2021, 110, 50–60. [Google Scholar] [CrossRef] [PubMed]
| Study | Patient Characteristics | Medical History | Symptoms | Diagnosis | CV Side Effect | ICI | Type of Cancer | Myocarditis Onset | Myocarditis Treatment | Evolution |
|---|---|---|---|---|---|---|---|---|---|---|
| Ida, 2022 | ♀, 81 | HBP dyslipidemia | high-grade fever whole-body rash altered consciousness | ↑CK; ↑CKMB, troponin I, C-reactive protein, ECG, TTE CMR endomyocardial biopsy | Myocarditis | PD-L1i + PD-1i | advanced melanoma, | 1 week | Methylprednisolone followed by prednisolone IGIV | favorable |
| Nguyen, 2022 | ♂, 25 | N/A | chest pain, subtle myalgia | Coronary angiography cardiac MRI endomyocardial biopsy Troponin-T creatine-kinase | Myocarditis myositis | PD-1i | hymoma | 2 weeks | Methylprednisolone mycophenolate-mofetil loading dose of intravenous abatacept oral ruxolitinib | cardiogenic shock VT extracorporeal life support On day 40, the patient fully recovered clinically |
| Okauchi, 2022 | ♂, 60 | Smoking history | Cough, dyspnea | Chest RX BNP TTE | Myocarditis | PD-1i | Squamous cell carcinoma | 130 weeks from initiation | Diuretics, beta-blocker | Favorable |
| Zhou, 2022 | ♂, 67 | N/A | fever, chest pain and dyspnea | Chest computed tomography, TTE, ECG, BNP, troponin T, CK | myocarditis | PD-L1i | lung squamous cell carcinoma stage IV | days after lastcycle | methylprednisolone | favorable |
| Zhao, 2022 | ♂, 60 | N/A | fever, tachycardia, hypotension, fatigue, dyspnea | ECG, TTE | Myocarditis + hypothyroidism | PD-1i | soft tissue sarcoma | 8 weeks | IV mPSL | favorable |
| Lorente-Ros, 2022 | ♂, 70 | nephrectomy | 2 episodes in the previous 12 h of severe dizziness, dyspnea, and profuse sweating. | ECG troponin I,C- reactive protein, TTE ECG- 3rd AVB, coronary angiography, brain CT autoimmunity lab tests, brain, MRI, lumbar puncture, EEG | Myocarditis + Encephalitis | PD-1i + CTLA-4i | renal cell carcinoma | 19 days | Temporary pacemaker high-dose iv corticosteroids intravenous immunoglobulins | delirium deterioration in his level of consciousness, intubation. Extubate -> reintubation discharged |
| Saishu 2022 | ♀, 55 | N/A | quadrantanopia, ocular motility disorder, diplopia, dysphagia, ocular motility disorder, muscle weakness of the extremities, bilateral ptosis muscle weakness. | ↑CK; ECG, TTE; anti-AchR ab. | Myocarditis, myositis/MG | PD-1i | mela- noma | 2 weeks | IGIV, prednisolone Intubation for MV, mPSL plasma exchange Tracheostomy | favorable |
| Yang, 2022 | ♀, 51 | contrast agent allergy | high fever, mild dyspnea, and systemic rash. | Liver function indexes cardiac markers CT examination | myocarditis hepatitis | PD-1i | breast cancer (TNBC) | 3 days | Iv methylprednisolone Antibiotics hepatic protectors | favorable |
| Ederhy, 2021 | ♀, 60–70 approx. | N/A | diplopia | ↑TnI; ECG; CMR, coronary angiography, EMB | Myocarditis (subclinical) | PD-1i | Lung cancer (unmentioned type, metastatic) | 3 infusions + 10 days | steroids, plasmapheresis | favorable |
| Tsuruda, 2021 | ♂, 75 | N/A | asymptomatic | ↑cTnT, CK, CK-MB; ECG, Echocardiogram, CMR, EMB | Myocarditis (subclinical), TTS | PD-1i | NSCLS (squamous, recurrent) | 3 weeks | mPSL | fatal |
| ♂, 47 | N/A | asymptomatic | ↑cTnT, CK, CK-MB; CMR | Myocarditis (subclinical) | PD-1i | Ethmoid sinus cancer | 3 infusions + 16 days | mPSL; IVIG (progressive thrombocytopenia); cyclosporine (hemophagocytic syndrome) | favorable | |
| ♂, 63 | N/A | hypotension (84/42 mmHg), tachycardia (132 bpm), tachypnea (22 rpm); high fever, decreased appetite, | ↑cTnT, CK, AST, ALT, CRP, Cr; ↓WBC, Hb, PLT; ECG, Echocardiogram | cardiac complication of cytokine-releasing syndrome | PD-1i | Hypopharyngeal cancer | 5 infusions + 32 days | cardioversion, extracorporeal hemoperfusion with polymyxin B + continuous hemodiafiltration, catechocardiographylamines, broad-spectrum antibiotics, recombinant thrombomodulin, IVIG, high-dose corticosteroids | favorable | |
| Tanabe, 2021 | ♂, 75 | N/A | posterior neck pain, neck drop | ↑TnI, CK, CK-MB; ↑Eo (834/μL), ↓eRFG; DLST (+); Echocardiogram, coronary angiography, CMR, | Myocarditis (subclinical) | PD-1i + CTLA-4i | RCC (clear cell, metastatic) | 53 days | prednisolone | favorable |
| Barham, 2021 | ♀, 79 | N/A | dizziness, abdominal bloating, hypoxic | ↑LDH; ECG; EMB | Myocarditis (grade 4); hyperprogression | PD-1i + CTLA-4i | Melanoma (vaginal, metastatic) | 23 days | steroids; carboplatin + paclitaxel (salvage therapy), atropine, pacemaker (for AVB III) | fatal |
| Xie, 2021 | ♂, 67 | N/A | exertional dyspnea, ptosis, blurred vision, quadriparesis | ↑TnI, CK, CK-MB, AST, ALT, BNP, Mb; ECG; Echocardiogram; coronary angiography | Myocarditis (fulminant), MG crisis, hepatic dysfunction; delayed ir pneumonitis | PD-1i + pemetrexed + carboplatin | LCNEC (metastatic) | 2 weeks | mPSL; pacemaker (temporary permanent); ganciclovir/cefmetazole | favorable |
| Hu, 2021 | ♂, 63 | N/A | chest tightness, limb weakness, dorsal myasthenia, diplopia, dysphagia | ↑Hs-TnI, CK-MB, NT-proBNP, CK; Echocardiogram, CMR; anti-β1AR ab, CC ab, anti-myosin heavy chain ab, ribonucleoprotein ab | Myocarditis + MG | PD-1i | ureteral urothelial cancer IV | 3 weeks | mPSL IVIG | favorable |
| Wintersperger, 2021 | ♂, 52 | N/A | fatigue dyspnea | ↑hsTnI, CK, BNP; ECG, Chest CT, Echocardiogram, CMR, EMB | Myocarditis | PD-L1i + investigational ICI | melanoma | 3 weeks | mPSL prednisone infliximab IV MMF | favorable |
| ♀, 60 | N/A | general- ized weakness muscle pain fatigue fever | ↑CK, hsTnI; ECG, Coronary angiography, CMR, EMB | Myocarditis | PD-L1i | gynecological cancer | 2 weeks | mPSL prednisone | favorable | |
| ♀, 49 | N/A | fever cough | ↑hsTnI, BNP; chest CT, ECG, CMR | Myocarditis | PD-L1i | triple-negative breast cance | 2 weeks | MMF prednisone | favorable | |
| ♀, 74 | N/A | general pain, progressive muscle weakness diplopia | ↑hsTnI, BNP, ECG, Coronary angiography, CMR | Myocarditis | PD-L1i | gynecological cancer | 2 weeks | mPSL prednisone MMF | favorable | |
| Stein-Merlob, 2021 | ♀, 60 | N/A | palpitations reduced exercise tolerance, cool extremities altered mental status | ↑Tn, BNP; ECG, Echocardiogram, Coronary angiography, CMR | Myocarditis Ocular myasthenia, Colitis hepatitis | PD-1i | Colon cancer | Metoprolol succinate, lisinopril Continued immunosuppression spironolactone, Oral amiodarone, wearable defibrillator.dopamine Nitroprusside milrinone, VA-ECMO | favorable | |
| Shen, 2021 | ♀, 53 | N/A | cough chest congestion, muscle weakness fatigability drooping eyelids, | ↑CK, CK-MB; ECG | Myocarditis, hepatitis, renal dysfunction, hypothyroidism | PD-1i + paclitaxel + platinum | type B3 thymoma | 3 weeks | Magnesium isoglycyrrhizinate reduced glutathione injections, prednisone mPSL euthyrox pyridostigmine | favorable |
| Miyauchi, 2021 | ♂, 71 | hypertension, DM2, hyperuricemia | Asymptomatic, chest tightness, shortness of breath, cardiogenic shock | ↑CK, CM-MB, TnI, NT-proBNP; ECG, catheterization, EMB, CMR | Myocarditis | CTLA-4i + PD-1i | RCC | 8 weeks | dopamine, dobutamine, noradrenaline intra-aortic balloon pump was inserted, adaptive servo ventilation mPSL prednisolone | favorable |
| Luo, 2021 | ♀, 47 | N/A | diplopia, myalgia, limb weakness, dysphagia, dyspnea | ↑TnI, CK, ECG, EMG; RyR-ab, AChR-ab, anti-fibrillarin ab, anti-NOR-90 ab anti-Ro-52 ab | Myocarditis, myositis, MG | PD-1i | thymoma | 3 weeks | neostigmine IVIG mPSL prednisolone pacemaker | favorable |
| Li, 2021 | ♂, 62 | hypertension, coronary heart disease | fever lethargy, cognitive dysfunction tachypnea hypoxia hypotension oliguria | ↑Mb, Tn, CK-MB; ECG | cardiotoxicity kidney toxicity. | PD-1i | lung adenocarcinoma | 48 weeks | mPSL continuous renal replacement therapy | favorable |
| Jespersen, 2021 | ♂, 57 | N/A | headache myalgia, palpitations binocular diplopia, ptosis, muscle weakness | ↑TnI, CK-MB, Mb, CK; ECG, EMG, Echocardiogram, CMR, AChR-ab, | Myocarditis + myositis | CTLA-4i + PD-1i | RCC | 2 weeks | temporary pacemaker. mPSL abatacept MMF implantable cardio-defibrilator | favorable |
| Iwasaki, 2021 | ♀, 70 | hypertension, aortic stenosis, chronic renal failure | shortness of breath fatigue | ↑CK, CK-MB, TnT, NT-proBNP; ECG, Echocardiogram, CMR, Cardiac catheterization, EBMCoronary angiography | Myocarditis + myositis | PD-L1i | HCC | <1 week | cariperitide, furosemide, mPSL prednisolone | favorable |
| Hernández, 2021 | ♀, 48 | N/A | shortness of breath, dyspnea, bilateral ptosis blurred vision | ↑Hs-TnI, NT-proBNP, CRP, CK; ECG, Echocardiogram, Coronary angiography, EMB; AChR-ab | Myocarditis + myositis (MG) | PD-1i | thymoma | <2 weeks | IV isoproterenol drip mPSL Infliximab temporary pacemaker, dual-chamber pacemaker. intravenous amiodarone, noradrenaline dobutamine, intravenous anti-thymocyte globuline, pyridostigmine, ECMO | fatal |
| Giblin, 2021 | ♀, 47 | N/A | dermatitisdiarrhea, palpitations | ↑hS-TnI, BNP, Echocardiogram, CMR, Coronary angiography, EMB | Myocarditis (subclinical) | CTLA-4i + PD-1i | melanoma | 1 week | mPSL prednisolon, IVIG | favorable |
| Cao, 2021 | ♂, 69 | N/A | ptosis, diplopia, shortness of breath, | ↑CK, CK-MB, Mb, hs-TnT, NT-proBNP, LDH, ECG; Echocardiogram, EMG | Myositis, Myocarditis SJS/TEN | PD-1i | esophagogastric junction carcinoma | 2 weeks | mPSL IVIG plasmapheresis | favorable |
| Ai, 2021 | ♂, 72 | N/A | asymptomatic | ECG; CMR | Myocarditis (DRESS) | PD-1i | gastric adenocarcinoma | 3 weeks | SCS (for DRESS) |
| Study | Patient Characteristics | CV Side Effect | Symptoms | Diagnosis | Pre-Existent CVD | ICI | Type of Cancer | Pericarditis Onset | Concomitant Treatments | Pericarditis Treatment | Evolution | Concomitant AID |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Khan, 2019 | ♂, 62 | pericarditis | dyspnea | CT, echocardiogram, ECG, TnI, BNP | not mentioned | PD-1i | tonsillar cancer (squamous) | 15 weeks | not mentioned | pericardiocentesis, prednisone | resolution | not mentioned |
| Arora, 2020 | ♂, 83 | pericarditis | marked fatigue weakness chest pain orthopnea left eye ptosis | TnI, CK, TTE, brain MRI | Hypertension, HyperlipidemiaAtrial fibrillation | PD-1i | melanoma | 1 month after first dose | not mentioned | colchicine and naproxen IV methylprednisolonePlasmapheresis Intubation | inability to reduce ventilatory support–transitioned to comfort measures | Hepatitis MG |
| de Almeida, 2018 | ♂, 69 | pericarditis | dyspnea, tachycardia, low-grade fever | CT, echocardiogram | not mentioned | PD-1i | NSCLC (adenocarcinoma) | 48 weeks | not mentioned | pericardiocentesis, prednisone | favorable | thyroiditis |
| Oristrell, 2018 | ♀, 55 | pericarditis | pericardial chest pain | echocardiogram, ECG, TnI | not mentioned | PD-1i | ductal carcinoma of the left breast | 30 weeks | not mentioned | Pericardiocentesis followed by pericardiectomy steroids | favorable | not mentioned |
| Zarogoulidis, 2017 | ♂, 60 | pericarditis | not mentioned | not mentioned | not mentioned | PD-1i | NSCLC | 17 weeks | not mentioned | pericardiocentesis mPSL | favorable | not mentioned |
| Öztürk, 2021 | ♂, 61 | pericarditis | not mentioned | CT, echocardiogram, MRI | not mentioned | PD-1i | NSCLC | 17 weeks | pemetrexed | not mentioned | not mentioned | not mentioned |
| Moriyama, 2021 | ♂, 58 | pericarditis | fatigue, limb oedema, increased body weight | echocardiogram, cardiac CT, cardiac catheterization, EMB, MRI, ECG, TnT | not mentioned | PD-1i | NSCLC | 77 weeks | not mentioned | prednisolone, furosemide, mPSL, infliximab | favorable | autoimmune hepatitis |
| Jacobs, 2021 | ♂, 54 | pericarditis | chest pain, general malaise, dyspnea | echocardiogram, CT, MRI, ECG, TnI | not mentioned | PD-1i | NSCLC (adenocarcinoma) | 5 weeks | carboplatin + pemetrexed + | mPSL | fatal | not mentioned |
| Dasanu, 2016 | ♀, 65 | pericarditis | progressive dyspnea, chest discomfort | X-ray, CT, echocardiogram, ECG | not mentioned | PD-1i | melanoma (nodular type) | 37 weeks | not mentioned | pericardiocentesis, mPSL | favorable | abnormal thyroid function, hepatitis, rash |
| Dhenin, 2019 | ♀, 79 | pericarditis | intense thoracic pain, increasingwhen leaning, fatigue, general malaise | echocardiogram, ECG | hypertension | PD-1i | NSCLC (adenocarcinoma) | 3 weeks | not mentioned | mPSL | favorable | rash, colitis, MG |
| Study | Patient Characteristics | CV Side Effect | Symptoms | Diagnosis | Pre-Existent CVD | ICI | Type of Cancer | Arrhythmias Onset | Concomitant Treatments | Arrhythmias Treatment | Evolution | Concomitant AID |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Joseph, 2021 | ♂, 78 | AF | not mentioned | ECG | hypertension | PD-1i | metastatic melanoma | 35 weeks | not mentioned | TEE-guided cardioversion | favorable | not mentioned |
| ♂, 68 | AF | not mentioned | ECG | hypertension | PD-1i | metastatic melanoma | 14 weeks | not mentioned | self-limited AF (48 h) | favorable | not mentioned | |
| ♂, 66 | AF | not mentioned | ECG | hypertension | PD-1i | metastatic melanoma | 2 weeks | not mentioned | beta-blockers | favorable | hyperthyroidism | |
| ♂, 74 | sinus bradycardia, AF with RVR | fatigue and dizziness | ECG | 3 ablations for AF | PD-1i | metastatic melanoma | 21 weeks | not mentioned | beta-blockers | favorable | not mentioned | |
| Reddy, 2017 | ♂, 68 | sinus tachycardia, 1st degree AVB, RBBB, LAFB followed bycomplete AVB) | fatigue, generalized malaise, weakness with ambulation NYHA IIB symptoms | ECG, Tn, CK-MB, echocardiogram, cardiac catheterization | not mentioned | PD-1i + CTLA-4i | metastatic sarcoma | 2 weeks | not mentioned | high-dose IV steroids, temporary transvenous pacemaker, MMF | favorable | not mentioned |
| Giancaterino, 2020 | ♂, 88 | ECGs–progression normal SR + with PAC- 3rd AVB (hospital day 5) | generalized weakness | ECG, Tn, CK-MB, TTE | not mentioned | invasive melanoma | first - 22 days prior | prednisone 40 mg daily nivolumab infusions were held IV methylprednisolone Infliximab dual-chamber pacemaker—day 10 | decline clinically- VF- fatal | myositis | ||
| Behling, 2017 | ♂, 63 | complete AVB (44 bpm) | worsening of a pre-existing dyspnea | ECG, echocardiogram, cardiac catheterization, myoglobin, Tn | hypertension | PD-1i | metastatic melanoma | 3 weeks | not mentioned | temporary pacemaker, corticosteroids, oxygen therapy | fatal | not mentioned |
| Katsume, 2018 | ♂, 73 | complete AVB (wide QRS complexes) | fatigue, faintness, syncope, palpitations | ECG, echocardiogram, cardiac catheterization, TnT, CK | not mentioned | PD-1i | metastatic NSCLC | 2 weeks | not mentioned | IV steroids, pacemaker (temporary permanent) | favorable | not mentioned |
| Hsu, 2018 | ♂, 42 | sinus bradycardia (40 bpm) | fatigue, dizziness, anorexia, hypotension | ECG, TnI | not mentioned | PD-1i | metastatic liver cancer | not mentioned | not mentioned | PO steroids | favorable | not mentioned |
| Pohl, 2020 | ♀, 61 | new 1st degree AVB, QTc prolongation | not mentioned | ECG, echocardiogram | not mentioned | PD-1i | metastatic melanoma | 4–12 weeks | not mentioned | not mentioned | not mentioned | not mentioned |
| Khan, 2020 | ♀, 67 | Mobitz type 2 2nd degree AVB (30 bpm) complete AVB (22 bpm) after 3 h | asymptomatic | ECG, echocardiogram, TnT | hypertension, hyperlipidemia | PD-1i | metastatic NSCLC | 3 weeks | not mentioned | dobutamine, pacemaker (temporary permanent) | favorable | not mentioned |
| Study | Patient | CV Side Effect | Symptoms | Diagnosis | Pre-Existent CVD | ICI | Type of Cancer | CVD Onset | CVD Treatment | Evolution |
|---|---|---|---|---|---|---|---|---|---|---|
| Serzan, (2021) | ♀, 66 | Takotsubo cardiomyopathy | Exertional dyspnea, generalized pain | TNI, ECG, TTE, CT angiography, RV catheterization, EMB, CMR | not mentioned | CTLA-4i + PD-1i | Choroidal melanoma | 16 weeks | Metoprolol | Favorable |
| Oldfield, (2021) | ♂, 76 | Takotsubo cardiomyopathy, diabetic ketoacidosis | Chest pain, diaphoresis | ECG, TNI, Coronary angiography, TTE, CMR | T2DM, dyslipidemia, hypertension | CTLA-4i + PD-1i | Melanoma | 4 days | Aspirin, bisoprolol, ramipril | Favorable |
| Schwab, (2018) | ♂, 69 | Takotsubo cardiomyopathy | Chest pain, shortness of breath | Coronary angiography, TTE, CMR | not mentioned | PD-1i and CTLA-4i + PD-1i | Squamous cell cancer of the lower lip | 7 cycles | Heart failure treatment, prednisolone | Favorable |
| Ederhy, (2018) | ♂, 41 | Takotsubo-like syndrome | not mentioned | ECG, TNI, TTE, Coronary angiography, CMR | not mentioned | CTLA-4i + PD-1i | melanoma | 5 days | IV mPSL | Favorable |
| ♂, 77 | Takotsubo-like syndrome | not mentioned | ECG, CMR, TNI, Coronary angiography | not mentioned | CTLA-4i + PD-1i | esophageal melanoma | 2 cycles of ipilimumab + nivolumab and 1 cycle of nivolumab | mPSL, ACEi, beta blockers | Favorable | |
| Tan, (2020) | ♂, 62 | Takotsubo cardiomyopathy | Chest pain, nausea, vomiting | ECG, Coronary angiography, TNI, NT-proBNP, TTE, CMR | not mentioned | PD-1i | HCC | 3 weeks | IV mPSL, Prednisone | Favorable |
| Al-Obaidi, (2020) | ♀, 52 | Acute HF | Dyspnea on exertion, angina-like chest pain, lower extremity oedema | TTE, chest X-ray, CT angiography, ECG | not mentioned | CTLA-4i + PD-1i | NSCLC | 1 year | IV mPSL, oral prednisone | Favorable |
| Khan, (2020) | ♀, 57 | Atypical Takotsubo cardiomyopathy | Chest pain, palpitations, tachypnoea, tachycardia | Chest X-ray, ECG, TNI, TTE, Coronary angiography | not mentioned | PD-L-1i | NSCLC (adenocarcinoma) | 2 weeks following 4th cycle | Guideline-directed HF treatment | Favorable |
| Samejima, (2020) | ♂, 79 | Acute HF | Dyspnea | Chest X-ray, CT, ECG, CRP, CK, CK-MB, TnI, BNP, TTE, Coronary angiography, EMB | not mentioned | PD-1i | NSCLC | 20 days | Furosemide, dopamine, tolvaptan, bisoprolol, spironolactone, enalapril | Favorable |
| Roth, (2016) | ♂,60 | Left Ventricular Dysfunction | heart palpitations | ECG, TTE, pharmacologic stress test | hypertension, anxiety, and Raynaud syndrome | CTLA-4i | BRAFwild-type stage IIIA (T2, N1a) melanoma | 4 cycles + another 4 cycles (liver metastasis)–after 4 months | beta blockers, ACEI | Favorable |
| Andersen, (2016) | ♀, 56 | Apical takotsubo syndrome | chest pain after severe episode of abdominal cramping with diarrhea | ECG, highly sensitive troponin Chest X-ray, TTE, Coronary angiography | no cardiac risk factors. No CVD | PD-1i | breast carcinoma | 3 weeks | ACEi, beta blockers | Favorable |
| Study | Patient | CV Side Effect | Symptoms | Diagnosis | Preexistent CVD | ICI | Type of Cancer | CVD Onset | CVD Treatment | Evolution |
|---|---|---|---|---|---|---|---|---|---|---|
| Arora, 2020 | ♂,69 | ACS (NSTEMI) | diffuse body pain weakness | ECG, CK-MB, cTNI, TTE Coronary angiography | CKD, Hypertension, Hyperlipidemia Type 2 DM CAD | PD-1i | metastatic urothelial carcinoma | cycle day 2 | IV Steroids, MMF | transition to comfort measures |
| Cheng, (2021) | ♀, 87 | ACS (NSTEMI) | Chest pain and dyspnea | ECG, CK-MB, cTNI, CRP, Coronary angiography | Hypertension, 3-vessel CAD | PD-1i | NSCLC (adenocarcinoma) | 2 days | PCI + DES | Favorable |
| Tomita, (2017) | ♂, 61 | ACS (NSTEMI) | not mentioned | CK, CK-MB, cTNI, Coronary angiography, OCT | Dyslipidemia | PD-1i | NSCLC (adenocarcinoma) | 11th cycle | PCI + DES; thrombus aspiration | Favorable |
| Kwan, (2019) | ♀, 71 | ACS (NSTEMI) | Chest pain | cTNI, ECG, Coronary angiography | Hypertension, T2DM, peripheral artery disease | PD-1i | Giant cell tumor of the bone | 2 Years | First ACS: atherectomy of the LAD with 3 x DES, aspirin + clopidogrel, atorvastatin; Second ACS: DES, DAPT | Favorable |
| Cancela-Díez, (2019) | ♂, 79 | ACS (STEMI) | Chest pain, oppression and dyspnea. | TNI, TTE, ECG, Coronary angiography | Infrarenal abdominal aortic aneurysm | PD-1i | NSCLC (epidermoid) | 10 days after the last cycle (10th) | PCI + DES, aspirin + clopidogrel, nitro-glycerine, beta blockers, enalapril | Favorable |
| Masson, (2020) | ♂, 62 | ACS (NSTEMI) | Chest pain | ECG, TNI, BNP, TTE, Coronary angiography | T2DM, multi-vessel CAD, STEMI | PD-1i | Melanoma | 1 week after cycle 4 of Nivolumab therapy | CABG | Favorable |
| Cautela, (2020) | ♀, 52 | ACS (NSTEMI) | Chest pain | ECG, TNT, NT-proBNP, TTE, Coronary angiography, CMR | not mentioned | PD-1i | NSCLC (?) | 5 days | Methylprednisolone prednisolone; aspirin + clopidogrel, statins | Fatal (due to refractory shock) |
| Otsu, (2020) | ♂, 57 | Vasospastic angina | Rest angina | ECG, Coronary angiography | not mentioned | PD-1i | Renal cell carcinoma | 4 weeks | CCB, nitrates | Favorable |
| Kumamato, (2022) | ♀, 54 | Vasospastic angina | chest pain at rest for 2 months | ECG, Chest radiography, TTE, Gadolinium enhanced cardiac MRI cardiac catheterization–coronary vasospasm provoked by ergonovine | not mentioned | PD-1i | Hypopharyngeal cancer | 21 months | Benidipine 8 mg | Favorable |
| Guo, (2022) | ♂, 6o | Coronary Artery Spasm ventricular tachycardia | 1-week history of chest tightness and palpitation | ECG Thyroid function Holter ECG TTE | acute coronary syndrome 8 months ago–complete revascularization with stents | PD-1i | Metastatic liver cancer | Pre-evaluation of 3rd dose | isosorbide mononitrate and diltiazem sedative drug aspirin, clopidogrel, and atorvastatin | 1 week later–discharged |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cozma, A.; Sporis, N.D.; Lazar, A.L.; Buruiana, A.; Ganea, A.M.; Malinescu, T.V.; Berechet, B.M.; Fodor, A.; Sitar-Taut, A.V.; Vlad, V.C.; et al. Cardiac Toxicity Associated with Immune Checkpoint Inhibitors: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 10948. https://doi.org/10.3390/ijms231810948
Cozma A, Sporis ND, Lazar AL, Buruiana A, Ganea AM, Malinescu TV, Berechet BM, Fodor A, Sitar-Taut AV, Vlad VC, et al. Cardiac Toxicity Associated with Immune Checkpoint Inhibitors: A Systematic Review. International Journal of Molecular Sciences. 2022; 23(18):10948. https://doi.org/10.3390/ijms231810948
Chicago/Turabian StyleCozma, Angela, Nicolae Dan Sporis, Andrada Luciana Lazar, Andrei Buruiana, Andreea Maria Ganea, Toma Vlad Malinescu, Bianca Mihaela Berechet, Adriana Fodor, Adela Viviana Sitar-Taut, Vasile Calin Vlad, and et al. 2022. "Cardiac Toxicity Associated with Immune Checkpoint Inhibitors: A Systematic Review" International Journal of Molecular Sciences 23, no. 18: 10948. https://doi.org/10.3390/ijms231810948
APA StyleCozma, A., Sporis, N. D., Lazar, A. L., Buruiana, A., Ganea, A. M., Malinescu, T. V., Berechet, B. M., Fodor, A., Sitar-Taut, A. V., Vlad, V. C., Negrean, V., & Orasan, O. H. (2022). Cardiac Toxicity Associated with Immune Checkpoint Inhibitors: A Systematic Review. International Journal of Molecular Sciences, 23(18), 10948. https://doi.org/10.3390/ijms231810948






