Molecular Pathways of WRKY Genes in Regulating Plant Salinity Tolerance
Abstract
1. Introduction
2. Mechanisms of Plant Tolerance to Salinity
3. WRKY Genes
3.1. WRKY Genes in Biotic and Abiotic Response
3.2. WRKY Genes Involved in Salinity Response
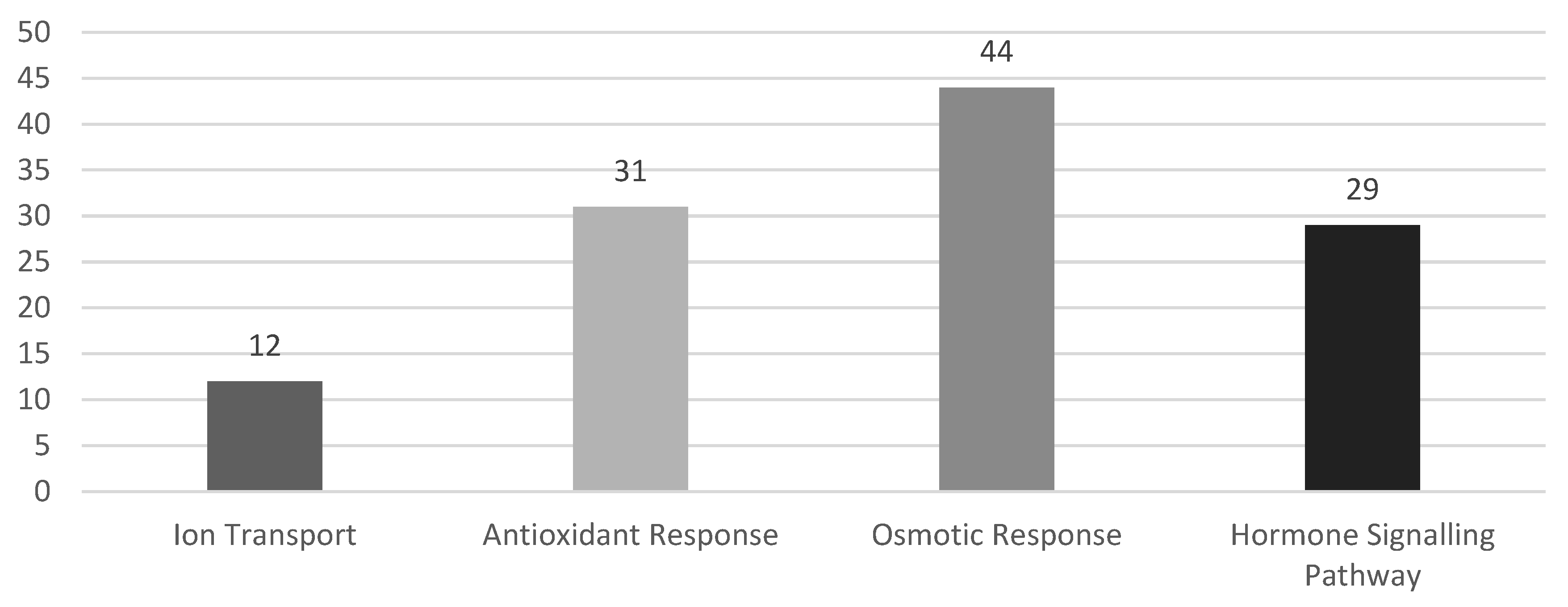
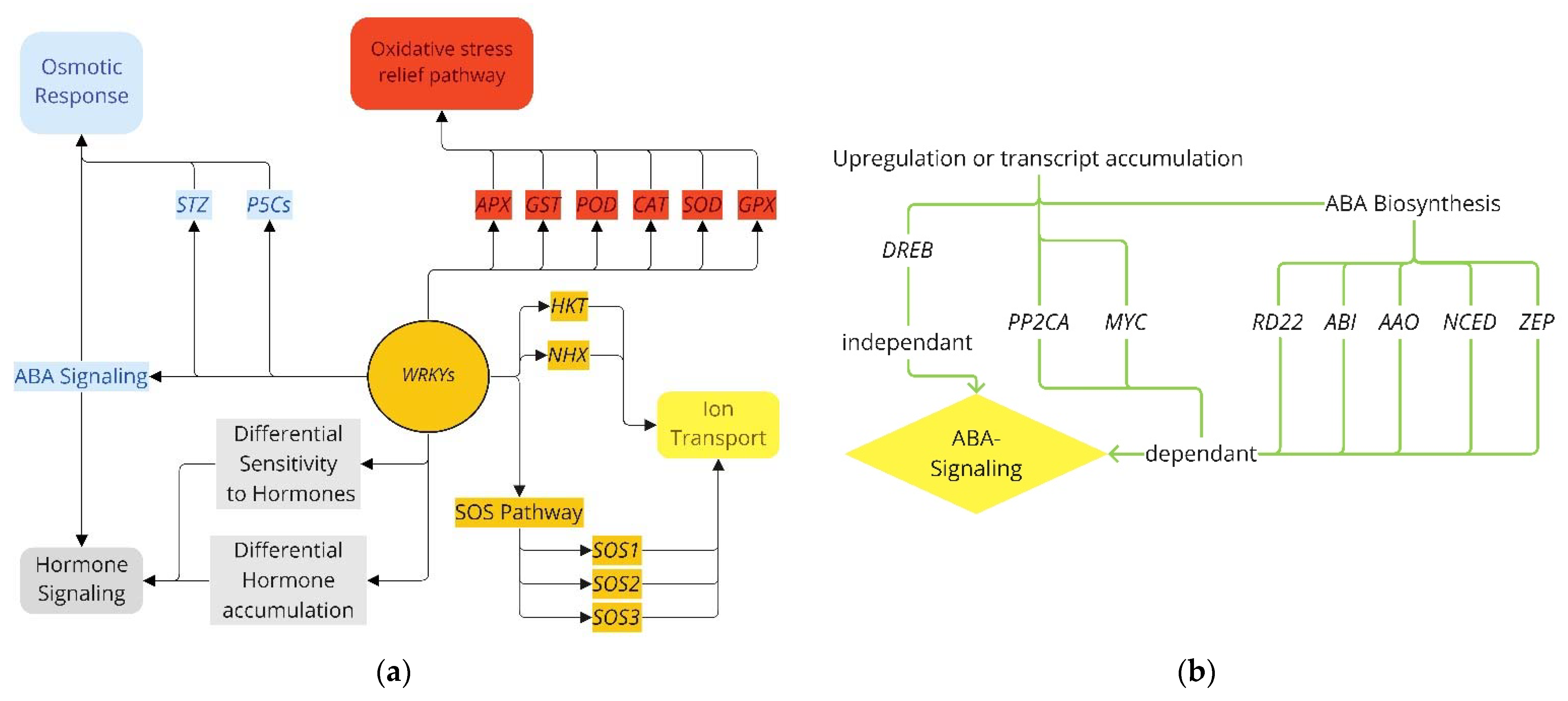
3.3. Pathways for WRKY Mediating Salinity Response
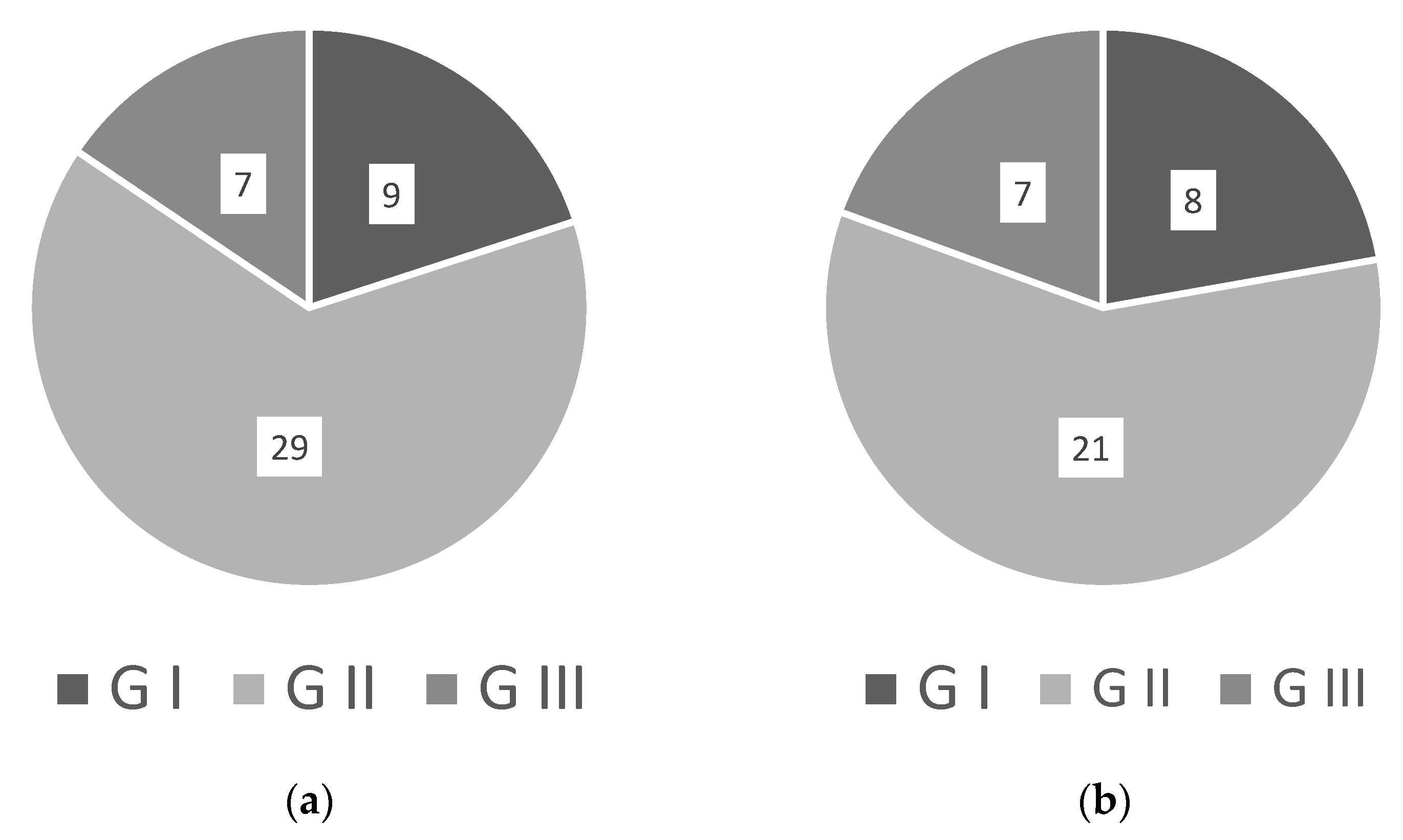
4. Standardizing WRKY Naming
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hossain, M.S. Present scenario of global salt affected soils, its management and importance of salinity research. Int. Res. J. Biol. Sci. 2019, 1, 1–3. [Google Scholar]
- Gaigbe, T.V.; Bassarsk, L.; Gu, D.; Spoorenberg, T.; Zeifman, L. World Population Prospects 2022: Summary of Results; Department of Economic and Social Affairs, United Nations: New York, NY, USA, 2022. [Google Scholar]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Singh, A. An overview of drainage and salinization problems of irrigated lands. Irrig. Drain. 2019, 68, 551–558. [Google Scholar] [CrossRef]
- Corwin, D.L. Climate change impacts on soil salinity in agricultural areas. Eur. J. Soil Sci. 2021, 72, 842–862. [Google Scholar] [CrossRef]
- Zhang, H.; Mittal, N.; Leamy, L.J.; Barazani, O.; Song, B.-H. Back into the wild-Apply untapped genetic diversity of wild relatives for crop improvement. Evol. Appl. 2016, 10, 5–24. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2014, 22, 4056–4075. [Google Scholar] [CrossRef]
- Golldack, D.; Lüking, I.; Yang, O. Plant tolerance to drought and salinity: Stress regulating transcription factors and their functional significance in the cellular transcriptional network. Plant Cell Rep. 2011, 30, 1383–1391. [Google Scholar] [CrossRef]
- Latchman, D.S. Transcription factors: An overview. Int. J. Biochem. Cell Biol. 1997, 29, 1305–1312. [Google Scholar] [CrossRef]
- Maathuis, F.J.M.; Amtmann, A. K+ nutrition and Na+ toxicity: The basis of cellular K+/Na+ ratios. Ann. Bot. 1999, 84, 123–133. [Google Scholar] [CrossRef]
- Jamil, M.; Rehman, S.U.; Lee, K.J.; Kim, J.M.; Kim, H.-S.; Rha, E.S. Salinity reduced growth PS2 photochemistry and chlorophyll content in radish. Sci. Agricola 2007, 64, 111–118. [Google Scholar] [CrossRef]
- Khan, M.A.; Shirazi, M.U.; Khan, M.A.; Mujtaba, S.M.; Islam, E.; Mumtaz, S.; Shereen, A.; Ansari, R.U.; Ashraf, M.Y. Role of proline, K/Na ratio and chlorophyll content in salt tolerance of wheat (Triticum aestivum L.). Pak. J. Bot. 2009, 41, 633–638. [Google Scholar]
- Molazem, D.; Qurbanov, E.M.; Dunyamaliyev, S.A. Role of proline, Na and chlorophyll content in salt tolerance of corn (Zea mays L.). Am.-Eurasian J. Agric. Environ. Sci. 2010, 9, 319–324. [Google Scholar]
- Khatri, K.; Rathore, M. Photosystem photochemistry, prompt and delayed fluorescence, photosynthetic responses and electron flow in tobacco under drought and salt stress. Photosynthetica 2019, 57, 61–74. [Google Scholar] [CrossRef]
- Yeo, A.R.; Lee, Λ.-S.; Izard, P.; Boursier, P.J.; Flowers, T.J. Short- and Long-Term Effects of Salinity on Leaf Growth in Rice (Oryza sativa L.). J. Exp. Bot. 1991, 42, 881–889. [Google Scholar] [CrossRef]
- Amin, I.; Rasool, S.; Mir, M.A.; Wani, W.; Masoodi, K.Z.; Ahmad, P. Ion homeostasis for salinity tolerance in plants: A molecular approach. Physiol. Plant. 2020, 171, 578–594. [Google Scholar] [CrossRef]
- Baillo, E.H.; Kimotho, R.N.; Zhang, Z.; Xu, P. Transcription Factors Associated with Abiotic and Biotic Stress Tolerance and Their Potential for Crops Improvement. Genes 2019, 10, 771. [Google Scholar] [CrossRef]
- Yazdani, B.; Sanjari, S.; Asghari-Zakaria, R.; GhaneGolmohammadi, F.; Pourabed, E.; Shahbazi, M.; Shobbar, Z.-S. Revision of the barley WRKY gene family phylogeny and expression analysis of the candidate genes in response to drought. Biol. Plant. 2020, 64, 9–19. [Google Scholar] [CrossRef]
- Schachtman, D.P.; Schroeder, J.I. Structure and transport mechanism of a high-affinity potassium uptake transporter from higher plants. Nature 1994, 370, 655–658. [Google Scholar] [CrossRef]
- Mäser, P.; Hosoo, Y.; Goshima, S.; Horie, T.; Eckelman, B.; Yamada, K.; Yoshida, K.; Bakker, E.P.; Shinmyo, A.; Oiki, S.; et al. Glycine residues in potassium channel-like selectivity filters determine potassium selectivity in four-loop-per-subunit HKT transporters from plants. Proc. Natl. Acad. Sci. USA 2002, 99, 6428–6433. [Google Scholar] [CrossRef]
- Han, Y.; Yin, S.; Huang, L. Towards plant salinity tolerance-implications from ion transporters and biochemical regulation. Plant Growth Regul. 2014, 76, 13–23. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, Z.-Z.; Zhou, X.-F.; Yin, H.-B.; Li, X.; Xin, X.-F.; Hong, X.-H.; Zhu, J.-K.; Gong, Z. Overexpression of SOS (Salt Overly Sensitive) Genes Increases Salt Tolerance in Transgenic Arabidopsis. Mol. Plant 2009, 2, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.S.; Guo, Y.; Dietrich, M.A.; Schumaker, K.S.; Zhu, J.K. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc. Natl. Acad. Sci. USA 2002, 99, 8436–8441. [Google Scholar] [CrossRef] [PubMed]
- Bassil, E.; Coku, A.; Blumwald, E. Cellular ion homeostasis: Emerging roles of intracellular NHX Na+/H+ antiporters in plant growth and development. J. Exp. Bot. 2012, 63, 5727–5740. [Google Scholar] [CrossRef] [PubMed]
- El Moukhtari, A.; Cabassa-Hourton, C.; Farissi, M.; Savouré, A. How does proline treatment promote salt stress tolerance during crop plant development? Front. Plant Sci. 2020, 11, 1127. [Google Scholar] [CrossRef]
- Zeevaart, J.A.; Creelman, R.A. Metabolism and physiology of abscisic acid. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 439–473. [Google Scholar] [CrossRef]
- Sakamoto, H.; Maruyama, K.; Sakuma, Y.; Meshi, T.; Iwabuchi, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis Cys2/His2-type zinc-finger proteins function as transcription repressors under drought, cold, and high-salinity stress conditions. Plant Physiol. 2004, 136, 2734–2746. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef]
- Liang, Q.-Y.; Wu, Y.-H.; Wang, K.; Bai, Z.-Y.; Liu, Q.-L.; Pan, Y.-Z.; Zhang, L.; Jiang, B.-B. Chrysanthemum WRKY gene DgWRKY5 enhances tolerance to salt stress in transgenic chrysanthemum. Sci. Rep. 2017, 7, 4799. [Google Scholar] [CrossRef]
- Dai, W.; Wang, M.; Gong, X.; Liu, J.H. The transcription factor FcWRKY40 of Fortunella crassifolia functions positively in salt tolerance through modulation of ion homeostasis and proline biosynthesis by directly regulating SOS2 and P5CS1 homologs. New Phytol. 2018, 219, 972–989. [Google Scholar] [CrossRef]
- Zhu, H.; Zhou, Y.; Zhai, H.; He, S.; Zhao, N.; Liu, Q. A Novel Sweetpotato WRKY Transcription Factor, IbWRKY2, Positively Regulates Drought and Salt Tolerance in Transgenic Arabidopsis. Biomolecules 2020, 10, 506. [Google Scholar] [CrossRef]
- Wu, M.; Liu, H.; Han, G.; Cai, R.; Pan, F.; Xiang, Y. A moso bamboo WRKY gene PeWRKY83 confers salinity tolerance in transgenic Arabidopsis plants. Sci. Rep. 2017, 7, 11721. [Google Scholar] [CrossRef] [PubMed]
- Tounekti, T.; Vadel, A.M.; Oñate, M.; Khemira, H.; Munné-Bosch, S. Salt-induced oxidative stress in rosemary plants: Damage or protection? Environ. Exp. Bot. 2011, 71, 298–305. [Google Scholar] [CrossRef]
- Sudhir, P.-R.; Pogoryelov, D.; Kovacs, L.; Garab, G.; Murthy, S.D. The Effects of Salt Stress on Photosynthetic Electron Transport and Thylakoid Membrane Proteins in the Cyanobacterium Spirulina platensis. J. Biochem. Mol. Biol. 2005, 38, 481–485. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Barceló, A.R.; Laura, V. Reactive Oxygen Species in Plant Cell Walls. In Reactive Oxygen Species in Plant Signaling; Springer: Berlin/Heidelberg, Germany, 2009; pp. 73–93. [Google Scholar]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Rajput, V.; Harish; Singh, R.; Verma, K.; Sharma, L.; Quiroz-Figueroa, F.; Meena, M.; Gour, V.; Minkina, T.; Sushkova, S.; et al. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Sappl, P.G.; Carroll, A.J.; Clifton, R.; Lister, R.; Whelan, J.; Millar, A.H.; Singh, K.B. The Arabidopsis glutathione transferase gene family displays complex stress regulation and co-silencing multiple genes results in altered metabolic sensitivity to oxidative stress. Plant J. 2009, 58, 53–68. [Google Scholar] [CrossRef]
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Ding, Z.; Xia, G. How Plant Hormones Mediate Salt Stress Responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef]
- McAdam, S.A.M.; Brodribb, T.J. Stomatal innovation and the rise of seed plants. Ecol. Lett. 2011, 15, 1–8. [Google Scholar] [CrossRef]
- Duan, L.; Dietrich, D.; Ng, C.H.; Chan, P.M.Y.; Bhalerao, R.; Bennett, M.J.; Dinneny, J.R. Endodermal ABA Signaling Promotes Lateral Root Quiescence during Salt Stress in Arabidopsis Seedlings. Plant Cell 2013, 25, 324–341. [Google Scholar] [CrossRef]
- Xu, N.; Chu, Y.; Chen, H.; Li, X.; Wu, Q.; Jin, L.; Wang, G.; Huang, J. Rice transcription factor OsMADS25 modulates root growth and confers salinity tolerance via the ABA–mediated regulatory pathway and ROS scavenging. PLoS Genet. 2018, 14, e1007662. [Google Scholar] [CrossRef] [PubMed]
- Shahba, Z.; Baghizadeh, A.; Vakili, S.M.A.; Yazdanpanah, A.; Yosefi, M. The salicylic acid effect on the tomato (Lycopersicum esculentum Mill.) sugar, protein and proline contents under salinity stress (NaCl). J. Biophys. Struct. Biol. 2011, 2, 35–41. [Google Scholar]
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY transcription factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, S.; Nakamura, K. Characterization of a cDNA encoding a novel DNA-binding protein, SPF1, that recognizes SP8 sequences in the 5′ upstream regions of genes coding for sporamin and β-amylase from sweet potato. Mol. Gen. Genet. MGG 1994, 244, 563–571. [Google Scholar] [CrossRef]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef]
- Rushton, P.J.; Torres, J.T.; Parniske, M.; Wernert, P.; Hahlbrock, K.; Somssich, I. Interaction of elicitor-induced DNA-binding proteins with elicitor response elements in the promoters of parsley PR1 genes. EMBO J. 1996, 15, 5690–5700. [Google Scholar] [CrossRef] [PubMed]
- Ciolkowski, I.; Wanke, D.; Birkenbihl, R.P.; Somssich, I.E. Studies on DNA-binding selectivity of WRKY transcription factors lend structural clues into WRKY-domain function. Plant Mol. Biol. 2008, 68, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Mare, C.; Mazzucotelli, E.; Crosatti, C.; Francia, E.; Stanca, A.; Cattivelli, L. Hv-WRKY38: A new transcription factor involved in cold- and drought-response in barley. Plant Mol. Biol. 2004, 55, 399–416. [Google Scholar] [CrossRef]
- Van Verk, M.C.; Pappaioannou, D.; Neeleman, L.; Bol, J.F.; Linthorst, H.J. A novel WRKY transcription factor is required for induction of PR-1a gene expression by salicylic acid and bacterial elicitors. Plant Physiol. 2008, 146, 1983–1995. [Google Scholar] [CrossRef]
- AgarwalM, P.; Reddy, M.P.; Chikara, J. WRKY: Its structure, evolutionary relationship, DNA-binding selectivity, role in stress tolerance and development of plants. Mol. Biol. Rep. 2010, 38, 3883–3896. [Google Scholar] [CrossRef]
- Maeo, K.; Hayashi, S.; Kojima-Suzuki, H.; Morikami, A.; Nakamura, K. Role of Conserved Residues of the WRKY Domain in the DNA-binding of Tobacco WRKY Family Proteins. Biosci. Biotechnol. Biochem. 2001, 65, 2428–2436. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L. The WRKY transcription factor superfamily: Its origin in eukaryotes and expansion in plants. BMC Evol. Biol. 2005, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Jimmy, J.L.; Babu, S. Variations in the Structure and Evolution of Rice WRKY Genes in Indica and Japonica Genotypes and their Co-expression Network in Mediating Disease Resistance. Evol. Bioinform. 2019, 15, 1176934319857720. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Zhang, Z.-L.; Zou, X.; Huang, J.; Ruas, P.; Thompson, D.; Shen, Q.J. Annotations and Functional Analyses of the Rice WRKY Gene Superfamily Reveal Positive and Negative Regulators of Abscisic Acid Signaling in Aleurone Cells. Plant Physiol. 2005, 137, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Leib, K.; Zhao, P.; Kogel, K.-H.; Langen, G. Phylogenetic analysis of barley WRKY proteins and characterization of HvWRKY1 and -2 as repressors of the pathogen-inducible gene HvGER4c. Mol. Genet. Genom. 2014, 289, 1331–1345. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Kumar, S.R.; Dwivedi, V.; Rai, A.; Pal, S.; Shasany, A.K.; Nagegowda, D.A. A WRKY transcription factor from Withania somnifera regulates triterpenoid withanolide accumulation and biotic stress tolerance through modulation of phytosterol and defense pathways. New Phytol. 2017, 215, 1115–1131. [Google Scholar] [CrossRef]
- Peng, Y.; Bartley, L.; Chen, X.; Dardick, C.; Chern, M.; Ruan, R.; Canlas, P.E.; Ronald, P.C. OsWRKY62 is a Negative Regulator of Basal and Xa21-Mediated Defense against Xanthomonas oryzae pv. oryzae in Rice. Mol. Plant 2008, 1, 446–458. [Google Scholar] [CrossRef]
- Wang, C.-T.; Ru, J.-N.; Liu, Y.-W.; Li, M.; Zhao, D.; Yang, J.-F.; Fu, J.-D.; Xu, Z.-S. Maize WRKY Transcription Factor ZmWRKY106 Confers Drought and Heat Tolerance in Transgenic Plants. Int. J. Mol. Sci. 2018, 19, 3046. [Google Scholar] [CrossRef]
- Shen, H.; Liu, C.; Zhang, Y.; Meng, X.; Zhou, X.; Chu, C.; Wang, X. OsWRKY30 is activated by MAP kinases to confer drought tolerance in rice. Plant Mol. Biol. 2012, 80, 241–253. [Google Scholar] [CrossRef]
- Wang, D.; Wang, L.; Su, W.; Ren, Y.; You, C.; Zhang, C.; Que, Y.; Su, Y. A class III WRKY transcription factor in sugarcane was involved in biotic and abiotic stress responses. Sci. Rep. 2020, 10, 20964. [Google Scholar] [CrossRef]
- Huang, S.; Gao, Y.; Liu, J.; Peng, X.; Niu, X.; Fei, Z.; Cao, S.; Liu, Y. Genome-wide analysis of WRKY transcription factors in Solanum lycopersicum. Mol. Genet. Genom. 2012, 287, 495–513. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ai, C.-R.; Jing, S.-J.; Yu, D.-Q. Research Progress on Functional Analysis of Rice WRKY Genes. Rice Sci. 2010, 17, 60–72. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, F.; Zhu, C.; Li, X.; Dai, X.; Yang, B.; Zou, X.; Ma, Y. Identification, expression, alternative splicing and functional analysis of pepper WRKY gene family in response to biotic and abiotic stresses. PLoS ONE 2019, 14, e0219775. [Google Scholar] [CrossRef]
- Lin, J.; Dang, F.; Chen, Y.; Guan, D.; He, S. CaWRKY27 negatively regulates salt and osmotic stress responses in pepper. Plant Physiol. Biochem. 2019, 145, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Amee, M.; Chen, L. Bermudagrass CdWRKY50 gene negatively regulates plants’ response to salt stress. Environ. Exp. Bot. 2021, 188, 104513. [Google Scholar] [CrossRef]
- Li, P.; Song, A.; Gao, C.; Wang, L.; Wang, Y.; Sun, J.; Jiang, J.; Chen, F.; Chen, S. Chrysanthemum WRKY gene CmWRKY17 negatively regulates salt stress tolerance in transgenic chrysanthemum and Arabidopsis plants. Plant Cell Rep. 2015, 34, 1365–1378. [Google Scholar] [CrossRef]
- Luo, X.; Li, C.; He, X.; Zhang, X.; Zhu, L. ABA signaling is negatively regulated by GbWRKY1 through JAZ1 and ABI1 to affect salt and drought tolerance. Plant Cell Rep. 2019, 39, 181–194. [Google Scholar] [CrossRef]
- Yan, H.; Jia, H.; Chen, X.; Hao, L.; An, H.; Guo, X. The Cotton WRKY Transcription Factor GhWRKY17 Functions in Drought and Salt Stress in Transgenic Nicotiana benthamiana through ABA Signaling and the Modulation of Reactive Oxygen Species Production. Plant Cell Physiol. 2014, 55, 2060–2076. [Google Scholar] [CrossRef]
- Li, Z.; Li, L.; Zhou, K.; Zhang, Y.; Han, X.; Din, Y.; Ge, X.; Qin, W.; Wang, P.; Li, F.; et al. GhWRKY6 Acts as a Negative Regulator in Both Transgenic Arabidopsis and Cotton During Drought and Salt Stress. Front. Genet. 2019, 10, 392. [Google Scholar] [CrossRef]
- Song, Y.; Chen, L.; Zhang, L.; Yu, D. Overexpression of OsWRKY72 gene interferes in the abscisic acid signal and auxin transport pathway of Arabidopsis. J. Biosci. 2010, 35, 459–471. [Google Scholar] [CrossRef]
- Ashwini, N.; Sajeevan, R.S.; Udayakumar, M.; Nataraja, K.N. Identification and Characterization of OsWRKY72 Variant in Indica Genotypes. Rice Sci. 2016, 23, 297–305. [Google Scholar] [CrossRef]
- Jiang, Y.; Tong, S.; Chen, N.; Liu, B.; Bai, Q.; Chen, Y.; Bi, H.; Zhang, Z.; Lou, S.; Tang, H.; et al. The PalWRKY77 transcription factor negatively regulates salt tolerance and abscisic acid signaling in Populus. Plant J. 2020, 105, 1258–1273. [Google Scholar] [CrossRef]
- Bao, W.; Wang, X.; Chen, M.; Chai, T.; Wang, H. A WRKY transcription factor, PcWRKY33, from Polygonum cuspidatum reduces salt tolerance in transgenic Arabidopsis thaliana. Plant Cell Rep. 2018, 37, 1033–1048. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, J.; Sui, Y.; Han, G.; Zhang, Y.; Guo, S.; Sui, N. The sweet sorghum SbWRKY50 is negatively involved in salt response by regulating ion homeostasis. Plant Mol. Biol. 2020, 102, 603–614. [Google Scholar] [CrossRef]
- Yongmei, C.; Zhen, Z.; Dan, Z.; Jie, H.; Lixia, H.; Xin, L. VvWRKY13 from Vitis vinifera negatively modulates salinity tolerance. Plant Cell, Tissue Organ Cult. (PCTOC) 2019, 139, 455–465. [Google Scholar] [CrossRef]
- Cai, R.; Dai, W.; Zhang, C.; Wang, Y.; Wu, M.; Zhao, Y.; Ma, Q.; Xiang, Y.; Cheng, B. The maize WRKY transcription factor ZmWRKY17 negatively regulates salt stress tolerance in transgenic Arabidopsis plants. Planta 2017, 246, 1215–1231. [Google Scholar] [CrossRef]
- Zhu, H.; Jiang, Y.; Guo, Y.; Huang, J.; Zhou, M.; Tang, Y.; Sui, J.; Wang, J.; Qiao, L. A novel salt inducible WRKY transcription factor gene, AhWRKY75, confers salt tolerance in transgenic peanut. Plant Physiol. Biochem. 2021, 160, 175–183. [Google Scholar] [CrossRef]
- Jiang, Y.; Deyholos, M.K. Functional characterization of Arabidopsis NaCl-inducible WRKY25 and WRKY33 transcription factors in abiotic stresses. Plant Mol. Biol. 2009, 69, 91–105. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, L.; Wang, H.; Zhang, L.; Wang, F.; Yu, D. Arabidopsis transcription factor WRKY8 functions antagonistically with its interacting partner VQ9 to modulate salinity stress tolerance. Plant J. 2013, 74, 730–745. [Google Scholar] [CrossRef]
- Liu, Q.-L.; Xu, K.-D.; Pan, Y.-Z.; Jiang, B.-B.; Liu, G.-L.; Jia, Y.; Zhang, H.-Q. Functional Analysis of a Novel Chrysanthemum WRKY Transcription Factor Gene Involved in Salt Tolerance. Plant Mol. Biol. Rep. 2013, 32, 282–289. [Google Scholar] [CrossRef]
- Liu, Q.-L.; Zhong, M.; Li, S.; Pan, Y.-Z.; Jiang, B.-B.; Jia, Y.; Zhang, H.-Q. Overexpression of a chrysanthemum transcription factor gene, DgWRKY3, in tobacco enhances tolerance to salt stress. Plant Physiol. Biochem. 2013, 69, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wu, Y.-H.; Tian, X.-Q.; Bai, Z.-Y.; Liang, Q.-Y.; Liu, Q.-L.; Pan, Y.-Z.; Zhang, L.; Jiang, B.-B. Overexpression of DgWRKY4 Enhances Salt Tolerance in Chrysanthemum Seedlings. Front. Plant Sci. 2017, 8, 1592. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Wu, Q.; Wang, A.; Li, Q.; Dong, Q.; Yang, J.; Zhao, H.; Wang, X.; Chen, H.; Li, C. A WRKY transcription factor, FtWRKY46, from Tartary buckwheat improves salt tolerance in transgenic Arabidopsis thaliana. Plant Physiol. Biochem. 2019, 147, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, N.-N.; Gong, S.-Y.; Lu, R.; Li, Y.; Li, X.-B. Overexpression of a cotton (Gossypium hirsutum) WRKY gene, GhWRKY34, in Arabidopsis enhances salt-tolerance of the transgenic plants. Plant Physiol. Biochem. 2015, 96, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Hao, L.; Li, J.; Liu, D.; Guo, X.; Li, H. The Gossypium hirsutum WRKY gene GhWRKY39-1 promotes pathogen infection defense responses and mediates salt stress tolerance in transgenic Nicotiana benthamiana. Plant Cell Rep. 2013, 33, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, H.; Li, S.; Yang, C.; Ding, Q.; Song, C.-P.; Wang, D. GhWRKY46 from upland cotton positively regulates the drought and salt stress responses in plant. Environ. Exp. Bot. 2021, 186, 104438. [Google Scholar] [CrossRef]
- Ullah, A.; Sun, H.; Hakim; Yang, X.; Zhang, X. A novel cotton WRKY gene, GhWRKY6 -like, improves salt tolerance by activating the ABA signaling pathway and scavenging of reactive oxygen species. Physiol. Plant. 2017, 162, 439–454. [Google Scholar] [CrossRef]
- Kang, G.; Yan, D.; Chen, X.; Yang, L.; Zeng, R. HbWRKY82, a novel IIc WRKY transcription factor from Hevea brasiliensis associated with abiotic stress tolerance and leaf senescence in Arabidopsis. Physiol. Plant. 2020, 171, 151–160. [Google Scholar] [CrossRef]
- Agarwal, P.; Dabi, M.; Sapara, K.K.; Joshi, P.S.; Agarwal, P.K. Ectopic expression of JcWRKY transcription factor confers salinity tolerance via salicylic acid signaling. Front. Plant Sci. 2016, 7, 1541. [Google Scholar] [CrossRef]
- More, P.; Agarwal, P.; Joshi, P.S.; Agarwal, P.K. The JcWRKY tobacco transgenics showed improved photosynthetic efficiency and wax accumulation during salinity. Sci. Rep. 2019, 9, 19617. [Google Scholar] [CrossRef]
- Han, D.; Hou, Y.; Ding, H.; Zhou, Z.; Li, H.; Yang, G. Isolation and preliminary functional analysis of MbWRKY4 gene involved in salt tolerance in transgenic tobacco. Int. J. Agric. Biol. 2018, 20, 2045–2052. [Google Scholar]
- Han, D.; Hou, Y.; Wang, Y.; Ni, B.; Li, Z.; Yang, G. Overexpression of a Malus baccata WRKY transcription factor gene (MbWRKY5) increases drought and salt tolerance in transgenic tobacco. Can. J. Plant Sci. 2019, 99, 173–183. [Google Scholar] [CrossRef]
- Dong, Q.; Zheng, W.; Duan, D.; Huang, D.; Wang, Q.; Liu, C.; Li, C.; Gong, X.; Li, C.; Mao, K.; et al. MdWRKY30, a group IIa WRKY gene from apple, confers tolerance to salinity and osmotic stresses in transgenic apple callus and Arabidopsis seedlings. Plant Sci. 2020, 299, 110611. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.-Y.; Chen, J.; Xu, W.-X.; Qiu, J.-R.; Song, L.; Wang, J.-T.; Tang, R.; Chen, D.; Jiang, C.-Z.; Huang, Z. Dehydration-Induced WRKY Transcriptional Factor MfWRKY70 of Myrothamnus flabellifolia Enhanced Drought and Salinity Tolerance in Arabidopsis. Biomolecules 2021, 11, 327. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Xu, T.; Han, J.; Liu, W.; Wang, Y.; Li, X.; Sun, X.; Wang, X.; Li, T.; Yang, G. Overexpression of MxWRKY53 increased iron and high salinity stress tolerance in Arabidopsis thaliana. Vitr. Cell. Dev. Biol.-Plant 2021, 58, 266–278. [Google Scholar] [CrossRef]
- Han, D.; Zhou, Z.; Du, M.; Li, T.; Wu, X.; Yu, J.; Zhang, P.; Yang, G. Overexpression of a Malus xiaojinensis WRKY transcription factor gene (MxWRKY55) increased iron and high salinity stress tolerance in Arabidopsis thaliana. Vitr. Cell. Dev. Biol.-Plant 2020, 56, 600–609. [Google Scholar] [CrossRef]
- Yan, L.; Baoxiang, W.; Jingfang, L.; Zhiguang, S.; Ming, C.; Yungao, X.; Bo, X.; Bo, Y.; Jian, L.; Jinbo, L.; et al. A novel SAPK10-WRKY87-ABF1 biological pathway synergistically enhance abiotic stress tolerance in transgenic rice (Oryza sativa). Plant Physiol. Biochem. 2021, 168, 252–262. [Google Scholar] [CrossRef]
- Lin, L.; Yuan, K.; Huang, Y.; Dong, H.; Qiao, Q.; Xing, C.; Huang, X.; Zhang, S. A WRKY transcription factor PbWRKY40 from Pyrus betulaefolia functions positively in salt tolerance and modulating organic acid accumulation by regulating PbVHA-B1 expression. Environ. Exp. Bot. 2022, 196, 104782. [Google Scholar] [CrossRef]
- Wang, G.; Wang, X.; Ma, H.; Fan, H.; Lin, F.; Chen, J.; Chai, T.; Wang, H. PcWRKY11, an II-d WRKY Transcription Factor from Polygonum cuspidatum, Enhances Salt Tolerance in Transgenic Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 4357. [Google Scholar] [CrossRef]
- Hichri, I.; Muhovski, Y.; Žižková, E.; Dobrev, P.I.; Gharbi, E.; Franco-Zorrilla, J.M.; Lopez-Vidriero, I.; Solano, R.; Clippe, A.; Errachid, A.; et al. The Solanum lycopersicum WRKY3 Transcription Factor SlWRKY3 Is Involved in Salt Stress Tolerance in Tomato. Front. Plant Sci. 2017, 8, 1343. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, J.; Yang, F.; Zhang, G.; Wang, D.; Zhang, L.; Ou, Y.; Yao, Y. The WRKY transcription factor WRKY8 promotes resistance to pathogen infection and mediates drought and salt stress tolerance in Solanum lycopersicum. Physiol. Plant. 2019, 168, 98–117. [Google Scholar] [CrossRef]
- Wang, C.; Deng, P.; Chen, L.; Wang, X.; Ma, H.; Hu, W.; Yao, N.; Feng, Y.; Chai, R.; Yang, G.; et al. A Wheat WRKY Transcription Factor TaWRKY10 Confers Tolerance to Multiple Abiotic Stresses in Transgenic Tobacco. PLoS ONE 2013, 8, e65120. [Google Scholar]
- Zhou, S.; Zheng, W.-J.; Liu, B.-H.; Zheng, J.-C.; Dong, F.-S.; Liu, Z.-F.; Wen, Z.-Y.; Yang, F.; Wang, H.-B.; Xu, Z.-S.; et al. Characterizing the Role of TaWRKY13 in Salt Tolerance. Int. J. Mol. Sci. 2019, 20, 5712. [Google Scholar] [CrossRef]
- Niu, C.-F.; Wei, W.; Zhou, Q.-Y.; Tian, A.; Hao, Y.-J.; Zhang, W.-K.; Ma, B.; Lin, Q.; Zhang, Z.-B.; Zhang, J.-S.; et al. Wheat WRKY genes TaWRKY2 and TaWRKY19 regulate abiotic stress tolerance in transgenic Arabidopsis plants. Plant Cell Environ. 2012, 35, 1156–1170. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wang, Y.; Xu, P.; Zhang, Z. Overexpression of a WRKY Transcription Factor TaWRKY2 Enhances Drought Stress Tolerance in Transgenic Wheat. Front. Plant Sci. 2018, 9, 997. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, J.; Li, Y.; Rong, X.; Sun, J.; Sun, T.; Li, M.; Wang, L.; Feng, Y.; Chai, R.; et al. Expression of TaWRKY44, a wheat WRKY gene, in transgenic tobacco confers multiple abiotic stress tolerances. Front. Plant Sci. 2015, 6, 615. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Qiao, L.; Guo, H.; Guo, L.; Ren, F.; Bai, J.; Wang, Y. Genome-Wide Identification of Wheat WRKY Gene Family Reveals That TaWRKY75-A Is Referred to Drought and Salt Resistances. Front. Plant Sci. 2021, 12, 663118. [Google Scholar] [CrossRef]
- Qin, Y.; Tian, Y.; Han, L.; Yang, X. Constitutive expression of a salinity-induced wheat WRKY transcription factor enhances salinity and ionic stress tolerance in transgenic Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2013, 441, 476–481. [Google Scholar] [CrossRef]
- Qin, Y.; Tian, Y.; Liu, X. A wheat salinity-induced WRKY transcription factor TaWRKY93 confers multiple abiotic stress tolerance in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2015, 464, 428–433. [Google Scholar] [CrossRef]
- Dai, Z.; Wei, M.; Zhang, B.; Yuan, Y.; Zhang, B. VuWRKY, a group I WRKY gene from Vaccinium uliginosum, confers tolerance to cold and salt stresses in plant. Plant Cell Tissue Organ Cult. (PCTOC) 2021, 147, 157–168. [Google Scholar] [CrossRef]
- Zhu, D.; Hou, L.; Xiao, P.; Guo, Y.; Deyholos, M.K.; Liu, X. VvWRKY30, a grape WRKY transcription factor, plays a positive regulatory role under salinity stress. Plant Sci. 2018, 280, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Li, J.; Zhang, H.; Liu, Y.; Zhang, A. ZmWRKY104 positively regulates salt tolerance by modulating ZmSOD4 expression in maize. Crop J. 2021, 10, 555–564. [Google Scholar] [CrossRef]
- Xu, Z.; Raza, Q.; Xu, L.; He, X.; Huang, Y.; Yi, J.; Zhang, D.; Shao, H.-B.; Ma, H.; Ali, Z. GmWRKY49, a Salt-Responsive Nuclear Protein, Improved Root Length and Governed Better Salinity Tolerance in Transgenic Arabidopsis. Front. Plant Sci. 2018, 9, 809. [Google Scholar] [CrossRef]
- Li, M.; Ding, B.; Wang, J.; Yang, W.; Wang, R.; Bao, S.; Zhong, S.; Xie, X. Wheat TaWRKY10-1 is involved in biological responses to the salinity and osmostresses in transgenic Arabidopsis plants. South. Cross J. 2013, 7, 723–729. [Google Scholar]
- Lee, F.C.; Yeap, W.C.; Appleton, D.R.; Ho, C.-L.; Kulaveerasingam, H. Identification of drought responsive Elaeis guineensis WRKY transcription factors with sensitivity to other abiotic stresses and hormone treatments. BMC Genom. 2022, 23, 164. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xue, H.; Zhang, F.; Jiang, Q.; Yang, S.; Yue, P.; Wang, F.; Zhang, Y.; Li, L.; He, P.; et al. The miR156/SPL module regulates apple salt stress tolerance by activating MdWRKY100 expression. Plant Biotechnol. J. 2021, 19, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Liu, Q.; Yuan, H.; Zhang, Y.; Wang, W.; Huang, S. Molecular cloning and characterization of a novel salt-specific responsive WRKY transcription factor gene IlWRKY2 from the halophyte Iris lactea var. chinensis. Genes Genom. 2018, 40, 893–903. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, W.; Chen, H.; Chen, J.; Liu, X.; Chen, X.; Yang, S. Transcriptomic analysis of salt tolerance-associated genes and diversity analysis using indel markers in yardlong bean (Vigna unguiculata ssp. sesquipedialis). BMC Genom. Data 2021, 22, 34. [Google Scholar] [CrossRef]
- Liu, X.; Song, Y.; Xing, F.; Wang, N.; Wen, F.; Zhu, C. GhWRKY25, a group I WRKY gene from cotton, confers differential tolerance to abiotic and biotic stresses in transgenic Nicotiana benthamiana. Protoplasma 2015, 253, 1265–1281. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, Z.; Tong, T.; Fang, Y.; Zhang, X.; Niu, C.; Li, J.; Wu, Y.; Xue, D.; Zhang, X. Genome-Wide Identification of WRKY Gene Family and Expression Analysis under Abiotic Stress in Barley. Agronomy 2021, 11, 521. [Google Scholar] [CrossRef]
- Aras, S.; Eşitken, A.; Karakurt, Y. Morphological and physiological responses and some WRKY genes expression in cherry rootstocks under salt stress. Span. J. Agric. Res. 2020, 17, e0806. [Google Scholar] [CrossRef]
- He, G.-H.; Xu, J.-Y.; Wang, Y.-X.; Liu, J.-M.; Li, P.-S.; Chen, M.; Ma, Y.-Z.; Xu, Z.-S. Drought-responsive WRKY transcription factor genes TaWRKY1 and TaWRKY33 from wheat confer drought and/or heat resistance in Arabidopsis. BMC Plant Biol. 2016, 16, 116. [Google Scholar] [CrossRef]
- Shi, W.-Y.; Du, Y.-T.; Ma, J.; Min, D.-H.; Jin, L.-G.; Chen, J.; Chen, M.; Zhou, Y.-B.; Ma, Y.-Z.; Xu, Z.-S.; et al. The WRKY Transcription Factor GmWRKY12 Confers Drought and Salt Tolerance in Soybean. Int. J. Mol. Sci. 2018, 19, 4087. [Google Scholar] [CrossRef] [PubMed]
- Rushton, D.L.; Tripathi, P.; Rabara, R.C.; Lin, J.; Ringler, P.; Boken, A.K.; Langum, T.J.; Smidt, L.; Boomsma, D.D.; Emme, N.J.; et al. WRKY transcription factors: Key components in abscisic acid signalling. Plant Biotechnol. J. 2011, 10, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Sato, H.; Takasaki, H.; Takahashi, F.; Suzuki, T.; Iuchi, S.; Mitsuda, N.; Ohme-Takagi, M.; Ikeda, M.; Seo, M.; Yamaguchi-Shinozaki, K.; et al. Arabidopsis thaliana NGATHA1 transcription factor induces ABA biosynthesis by activating NCED3 gene during dehydration stress. Proc. Natl. Acad. Sci. USA 2018, 115, E11178–E11187. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.K.; Jha, B. Transcription factors in plants and ABA dependent and independent abiotic stress signalling. Biol. Plant. 2010, 54, 201–212. [Google Scholar] [CrossRef]
- Narusaka, Y.; Nakashima, K.; Shinwari, Z.K.; Sakuma, Y.; Furihata, T.; Abe, H.; Narusaka, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Interaction between two cis-acting elements, ABRE and DRE, in ABA-dependent expression of Arabidopsis rd29A gene in response to dehydration and high-salinity stresses. Plant J. 2003, 34, 137–148. [Google Scholar] [CrossRef]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Iology 2006, 57, 781–803. [Google Scholar] [CrossRef]
- Yoshida, T.; Mogami, J.; Yamaguchi-Shinozaki, K. ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Curr. Opin. Plant Biol. 2014, 21, 133–139. [Google Scholar] [CrossRef]
- Tan, B.-C.; Joseph, L.M.; Deng, W.-T.; Liu, L.; Li, Q.-B.; Cline, K.; McCarty, D.R. Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J. 2003, 35, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-Y.; Seok, H.-Y.; Park, B.-K.; Kim, S.-H.; Goh, C.-H.; Lee, B.-H.; Lee, C.-H.; Moon, Y.-H. Overexpression of Arabidopsis ZEP enhances tolerance to osmotic stress. Biochem. Biophys. Res. Commun. 2008, 375, 80–85. [Google Scholar] [CrossRef]
- Seo, M.; Aoki, H.; Koiwai, H.; Kamiya, Y.; Nambara, E.; Koshiba, T. Comparative Studies on the Arabidopsis Aldehyde Oxidase (AAO) Gene Family Revealed a Major Role of AAO3 in ABA Biosynthesis in Seeds. Plant Cell Physiol. 2004, 45, 1694–1703. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.; Peeters, A.J.M.; Koiwai, H.; Oritani, T.; Marion-Poll, A.; Zeevaart, J.A.D.; Koornneef, M.; Kamiya, Y.; Koshiba, T. The Arabidopsis aldehyde oxidase 3 (AAO3) gene product catalyzes the final step in abscisic acid biosynthesis in leaves. Proc. Natl. Acad. Sci. USA 2000, 97, 12908–12913. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Urao, T.; Ito, T.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) Function as Transcriptional Activators in Abscisic Acid Signaling. Plant Cell 2002, 15, 63–78. [Google Scholar] [CrossRef]
- Yoshida, T.; Nishimura, N.; Kitahata, N.; Kuromori, T.; Ito, T.; Asami, T.; Shinozaki, K.; Hirayama, T. ABA-Hypersensitive Germination3 Encodes a Protein Phosphatase 2C (AtPP2CA) That Strongly Regulates Abscisic Acid Signaling during Germination among Arabidopsis Protein Phosphatase 2Cs. Plant Physiol. 2005, 140, 115–126. [Google Scholar] [CrossRef]
- Kuhn, J.M.; Boisson-Dernier, A.; Dizon, M.B.; Maktabi, M.H.; Schroeder, J.I. The protein phosphatase AtPP2CA negatively regulates abscisic acid signal transduction in Arabidopsis, and effects of abh1 on AtPP2CA mRNA. Plant Physiol. 2005, 140, 127–139. [Google Scholar] [CrossRef]
- Tak, H.; Negi, S.; Ganapathi, T.R. The 5′-upstream region of WRKY18 transcription factor from banana is a stress-inducible promoter with strong expression in guard cells. Physiol. Plant. 2021, 173, 1335–1350. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Han, Y.; Broughton, S.; Liu, L.; Zhang, X.-Q.; Zeng, J.; He, X.; Li, C. Highly efficient and genotype-independent barley gene editing based on anther culture. Plant Commun. 2021, 2, 100082. [Google Scholar] [CrossRef]
- He, Y.; Zhao, Y. Technological breakthroughs in generating transgene-free and genetically stable CRISPR-edited plants. aBIOTECH 2019, 1, 88–96. [Google Scholar] [CrossRef]
- Jaganathan, D.; Ramasamy, K.; Sellamuthu, G.; Jayabalan, S.; Venkataraman, G. CRISPR for Crop Improvement: An Update Review. Front. Plant Sci. 2018, 9, 985. [Google Scholar] [CrossRef] [PubMed]
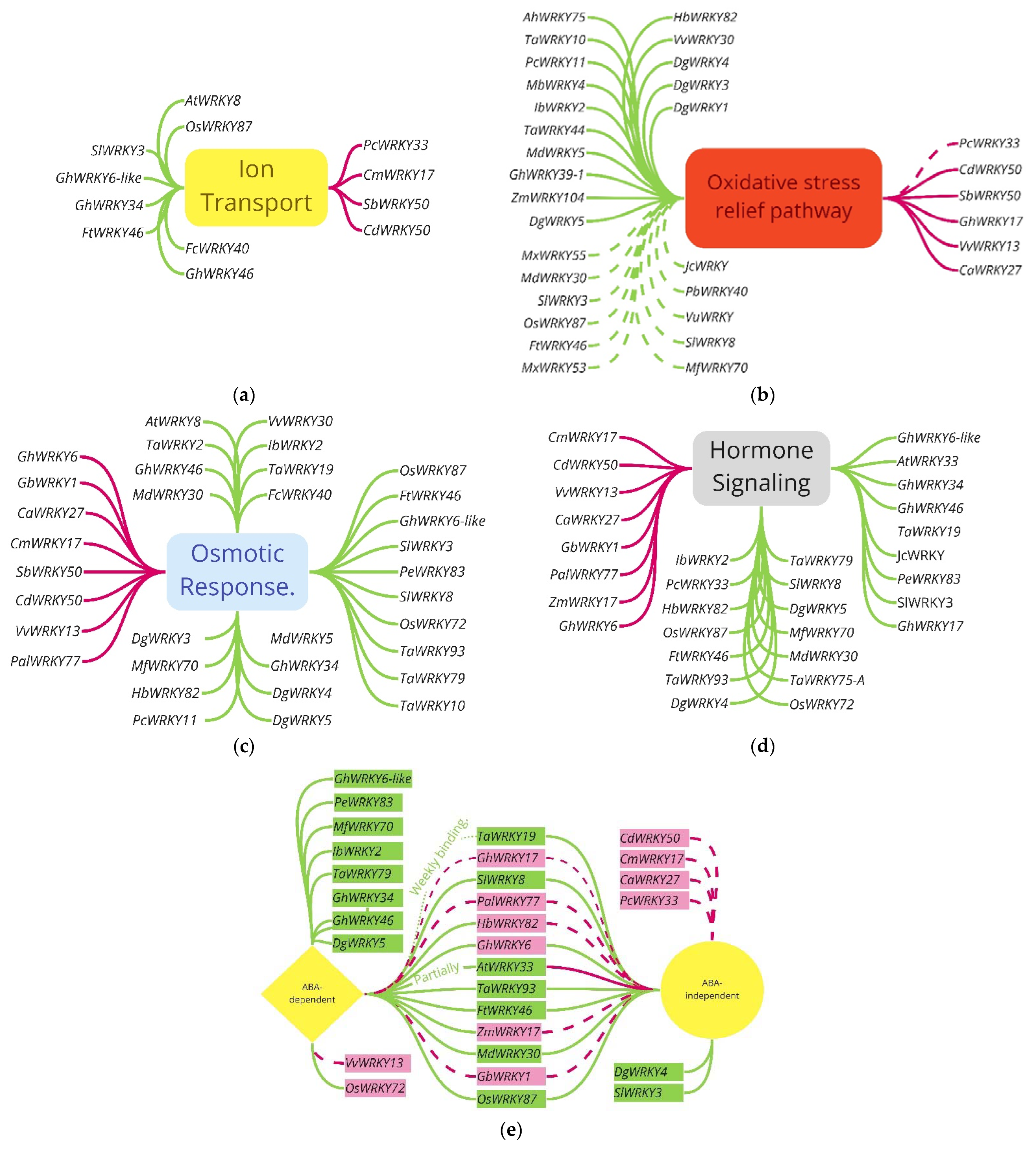

| Plant (Species) Originate from | Expression Tested in | Gene ID | Protein ID | Function | References |
|---|---|---|---|---|---|
| Pepper (Capsicum annuum) | Arabidopsis & Tobacco | CaWRKY27 | n.a. | Insertion reduced ROS-detoxification, hormone signalling, and osmotic response pathways | [66] |
| Bermudagrass (Cynodon dactylon (L). Pers.) | Arabidopsis | CdWRKY50 | n.a. | Overexpression (OE) reduced hormone signalling, ion transport, ROS scavenging, and osmotic regulation pathways | [67] |
| Chrysanthemum (Chrysanthemum morifolium) | Arabidopsis | CmWRKY17 | AJF11725 * | OE reduces hormone signalling and osmotic response pathways | [68] |
| Cotton (Gossypium barbadense) | Arabidopsis | GbWRKY1 | n.a. | OE negatively regulated osmotic response and hormone signalling pathways | [69] |
| Cotton (Gossypium hirsutum) | Tobacco | GhWRKY17 | ADW82098.1 * | OE enhanced sensitivity to saline conditions by reducing ROS regulation and hormone signalling pathways | [70] |
| Cotton (Gossypium hirsutum) | Arabidopsis | GhWRKY6 | n.a. | OE reduced osmotic response and hormone signalling pathways | [71] |
| Rice (Oryza sativa) | Arabidopsis & rice | OsWRKY72 | ALB35168.1 * | OE inhibited osmotic response and interfered with hormone signalling in Arabidopsis, but native expression enhanced rice salinity tolerance | [72,73] |
| Poplar (Populus alba var. pyramidalis) | Populus alba var. pyramidalis | PalWRKY77 | Potri.003G182200.1 *, ⬢ | Negative regulator reduced osmotic and hormone signal responses | [74] |
| Potri.003G182200.2 *, ⬢ | |||||
| Japanese knotweed (Polygonum cuspidatum) | Arabidopsis & native | PcWRKY33 | AYN74370.1 * | OE reduced oxidative stress, osmotic response, and ion transport response pathways in Arabidopsis, but native expression enhanced salinity tolerance | [75] |
| Sorghum (Sorghum bicolor (L.) Moench) | Arabidopsis | SbWRKY50 | Sb09g005700 ** | OE reduced osmotic response, ROS scavenging, and ion transport pathways | [76] |
| Grape (Vitis vinifera) | Arabidopsis | VvWRKY13 | n.a. | OE reduced ROS scavenging, osmotic response, and hormone signalling pathways | [77] |
| Maize (Zea mays) | Arabidopsis | ZmWRKY17 | ACG39023.1 * | OE resulted in salt hypersensitivity and insensitivity to the ABA pathway | [78] |
| Plant | Expressed in | Gene ID | Protein ID | Function | References |
|---|---|---|---|---|---|
| Peanut (Arachis hypogaea) | Peanut | AhWRKY75 | n.a. | OE enhanced fitness and ROS scavenging | [79] |
| Arabidopsis thaliana | Arabidopsis | AtWRKY33 | NP_181381.2 * | OE enhanced osmotic and hormone signaling pathways | [80] |
| Arabidopsis thaliana | Arabidopsis | AtWRKY8 | NP_193551.1 * | OE enhanced osmotic response and ion transport pathways | [81] |
| Chrysanthemum (Dendranthema grandiflorum) | Tobacco | DgWRKY1 | AGI96744.1 * | OE enhanced antioxidant response | [82] |
| Chrysanthemum (Dendranthema grandiflorum) | Tobacco | DgWRKY3 | AGN95658.1 * | Responsive to salt conditions, enhanced oxidative stress relief and osmotic response pathways | [83] |
| Chrysanthemum (Dendranthema grandiflorum) | Chrysanthemum | DgWRKY4 | n.a. | OE enhanced ABA-independent pathways and ROS species | [84] |
| Chrysanthemum (Dendronthema grandiform) | Chrysanthemum | DgWRKY5 | n.a. | OE involved in ABA signaling and pathway, ROS scavenging, osmotic regulator, and adjustment to infer salt stress tolerance | [29] |
| Fortunella crassifolia | Tobacco & Lemon | FcWRKY40 | n.a. | OE enhanced osmotic response and ion transport pathways | [30] |
| Tartary buckwheat (Fagopyrum tataricum) | Arabidopsis | FtWRKY46 | QGT76435.1 * | OE enhanced ROS scavenging and osmotic response and reduced hormone signaling | [85] |
| Cotton (Gossypium hirsutum) | Arabidopsis | GhWRKY34 | AJT43314.1 * | OE enhanced hormone signaling, osmotic response, and ion transport pathways | [86] |
| Cotton (Gossypium hirsutum) | Tobacco | GhWRKY39-1 | AGX27509.1 * | OE enhanced ROS detoxication pathway and enhanced fitness | [87] |
| Cotton (Gossypium hirsutum) | Arabidopsis | GhWRKY46 | n.a. | Enhanced insensitivity to salinity through enhanced osmotic and ion transport response | [88] |
| Cotton (Gossypium hirsutum) | Arabidopsis | GhWRKY6-like | n.a. | OE enhanced ROS scavenging, osmotic response, and hormone signaling pathways | [89] |
| Rubber tree (Hevea brasiliensis) | Arabidopsis | HbWRKY82 | n.a. | OE enhanced ROS scavenging, osmotic response, and hormone signaling pathways | [90] |
| Sweet potato (Ipomoea batatas (L.) Lam.) | Arabidopsis | IbWRKY2 | n.a. | OE enhanced ROS scavenging, osmotic response, and hormone signaling pathways | [31] |
| Jatropha curcas | Tobacco | JcWRKY | AGE81984.1 * | OE enhanced ROS scavenging, osmotic response, and hormone signaling pathways | [91,92] |
| Apple (Malus baccata) | Tobacco | MbWRKY4 | n.a. | OE enhanced antioxidant response and osmotic adjustment | [93] |
| Siberian crab apple (Malus baccata) | Tobacco | MbWRKY5 | MDP0000514115 ** | OE enhanced membrane stability, osmotic response, and AO capabilities | [94] |
| Apple (Malus × domestica borkh) | Arabidopsis & Apple | MdWRKY30 | QDL95022.1 *, ☐ | OE enhanced ROS scavenging, hormone signaling, and osmotic response pathways | [95] |
| Resurrection plant (Myrothamnus flabellifolia) | Arabidopsis | MfWRKY70 | n.a. | OE enhanced hormone signaling, ROS scavenging, and osmotic adjustment pathways | [96] |
| Malus xiaojinensis | Arabidopsis | MxWRKY53 | n.a. | OE enhanced fitness, proline, and ROS scavenging activity | [97] |
| Apple rootstock (Malus xiaojinensis) | Arabidopsis | MxWRKY55 | n.a. | OE enhanced ROS scavenging and osmotic response pathways | [98] |
| Rice (Oryza sativa) | Rice | OsWRKY87 | n.a. | OE enhanced ion transport, osmotic response, and hormone signaling pathways and ROS-scavenging protein activity | [99] |
| Southworth dance (Pyrus betulaefolia) | Arabidopsis | PbWRKY40 | Pbr004885.1 ** | OE enhanced ROS scavenging and Na+ regulation via transporters | [100] |
| Japanese knotweed (Polygonum cuspidatum) | Arabidopsis | PcWRKY11 | MZ734625 **** | OE reduced oxidizing elements and increased proline accumulation | [101] |
| Moso bamboo (Phyllostachys edulis; Bambusoideae) | Arabidopsis | PeWRKY83 | PH01004514G0080 * | OE enhanced hormone signaling and osmotic response pathways | [32] |
| Tomato (Solanum lycopersicum) | Arabidopsis | SlWRKY3 | ADZ15316 * | OE enhanced hormone signaling, osmotic response, ROS scavenging, and ion transport pathways | [102] |
| Tomato (Solanum lycopersicum) | Solanum lycopersicum | SlWRKY8 | Solyc02g093050.2.1 * | OE enhanced osmotic response, ROS scavenging, and hormone signaling pathways | [103] |
| Wheat (Triticum aestivum) | Tobacco | TaWRKY10 | ADY80578.1 * | OE enhanced osmotic response and ROS scavenging pathways | [104] |
| Wheat (Triticum aestivum) | Rice | TaWRKY13 | Traes_2AS_ 6269D889E.1 ** | Reduced ROS activity and enhanced proline accumulation in OE lines | [105] |
| Wheat (Triticum aestivum) | Arabidopsis | TaWRKY19 | ACD80362.1 * | OE enhanced osmotic response pathway | [106] |
| Wheat (Triticum aestivum) | Arabidopsis | TaWRKY2 | ACD80357.1 * | OE enhanced osmotic response pathway | [106,107] |
| Wheat (Triticum aestivum) | Tobacco | TaWRKY44 | ALC04265.1 * | OE enhanced ROS tolerance and scavenging and compatible solute accumulation | [108] |
| Wheat (Triticum aestivum) | Arabidopsis | TaWRKY75-A | TraesCS4A01G193600.1 ** | Involved in JA pathway | [109] |
| Wheat (Triticum aestivum) | Arabidopsis | TaWRKY79 | AFN44008.1 * | OE enhanced hormone signaling and osmotic response pathways | [110] |
| Wheat (Triticum aestivum L.) | Arabidopsis | TaWRKY93 | AFW98256.1 * | OE enhanced osmotic and hormone signaling pathways | [111] |
| Bog bilberry (Vaccinium uliginosum) | Arabidopsis | VuWRKY | n.a. | OE enhanced ROS scavenging and osmotic response pathways | [112] |
| Grape (Vitis vinifera L.) | Arabidopsis | VvWRKY30 | ALM96663.1 * | OE enhanced osmotic response in proline accumulation and oxidative stress response activities | [113] |
| Maize (Zea mays) | Maize | ZmWRKY104 | Zm00001d020495 *** | OE enhanced ROS scavenging response | [114] |
| WRKY Gene | New Name | Issues with Naming and Comments |
|---|---|---|
| AhWRKY75 | AhWRKY_IIc1 | No protein ID available to check |
| AtWRKY33 | AtWRKY_I1 | – |
| AtWRKY8 | AtWRKY_IIc1 | No subgroup in the paper and thus deduced from phylogenetic analysis |
| CaWRKY27 | – | No information on grouping and no accession available |
| CdWRKY50 | – | Classed as group II but without subgroup and accession |
| CmWRKY17 | CmWRKY_IId | No subgroup in the paper and thus deduced from phylogenetic analysis |
| DgWRKY1 | DgWRKY_Iic | – |
| DgWRKY3 | DgWRKY_III1 | – |
| DgWRKY4 | DgWRKY_I1 | No protein ID available to check |
| DgWRKY5 | DgWRKY_I2 | No protein ID available to check |
| FcWRKY40 | FcWRKY_Iia | No protein ID available to check |
| FtWRKY46 | FtWRKY_III1 | – |
| GbWRKY1 | GbWRKY_IIc1 | No protein ID available to check |
| GhWRKY17 | GhWRKY_IId1 | No subgroup in the paper and thus deduced from phylogenetic analysis |
| GhWRKY34 | GhWRKY_III1 | No protein ID available to check |
| GhWRKY39-1 | GhWRKY_IId2 | No subgroup in the paper and thus deduced from phylogenetic analysis |
| GhWRKY46 | GhWRKY_IIc1 | No protein ID available to check |
| GhWRKY6 | – | No information on grouping and no accession available |
| GhWRKY6-like | – | No information on grouping and no accession available |
| HbWRKY82 | HbWRKY_IIc1 | No protein ID available to check |
| IbWRKY2 | IbWRKY_I1 | No protein ID available to check |
| JcWRKY | JcWRKY_III1 | Classed as a group, but phylogenetic analysis deduced JcWRKY as group III |
| MbWRKY4 | – | No information on grouping and no accession available |
| MdWRKY30 | MdWRKY_IIa1 | – |
| MbWRKY5 | MbWRKY_I1 | – |
| MfWRKY70 | MfWRKY_IIa1 | No protein ID available to check |
| MxWRKY53 | MxWRKY_IIc1 | No protein ID available to check |
| MxWRKY55 | – | No information on grouping and no accession available |
| OsWRKY72 | OsWRKY_IIc1 | No subgroup in the paper and thus deduced from phylogenetic analysis |
| OsWRKY87 | – | No information on grouping and no accession available |
| PalWRKY77 | PaWRKY_IIa1 | – |
| PbWRKY40 | PbWRKY_IIa1 | No subgroup in the paper and thus deduced from phylogenetic analysis |
| PcWRKY11 | PcWRKY_IId1 | No protein ID available to check |
| PcWRKY33 | PcWRKY_I1 | – |
| PeWRKY83 | PeWRKY_IIc1 | No subgroup in the paper and thus deduced from phylogenetic analysis |
| SbWRKY50 | SbWRKY_IIc1 | Grouped within class III but determined group II subgroup c from phylogenetic analysis |
| SlWRKY3 | SlWRKY_III1 | – |
| SlWRKY8 | SlWRKY_IId1 | – |
| TaWRKY10 | TaWRKY_IIc1 | Grouped within class I but determined to be group II subgroup c from phylogenetic analysis |
| TaWRKY13 | TaWRKY_III1 | Grouped within class II without subgrouping, but phylogenetic analysis determined to be group III |
| TaWRKY19 | TaWRKY_I1 | – |
| TaWRKY2 | TaWRKY_I2 | Grouped as group II but analysis determined closer to group I |
| TaWRKY44 | TaWRKY_IIa1 | Grouped as a class I protein, but analysis determined group II subgroup a |
| TaWRKY75-A | TaWRKY_III2-A | – |
| TaWRKY79 | TaWRKY_IIa2 | No subgroup in the paper and thus deduced from phylogenetic analysis |
| TaWRKY93 | TaWRKY_IIa3 | No subgroup in the paper and thus deduced from phylogenetic analysis |
| VuWRKY | VuWRKY_I1 | No protein ID available to check |
| VvWRKY13 | – | No information on grouping and no accession available |
| VvWRKY30 | VvWRKY_III1 | – |
| ZmWRKY104 | ZmWRKY_IIa1 | No subgroup in the paper and thus deduced from phylogenetic analysis |
| ZmWRKY17 | ZmWRKY_IId1 | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Price, L.; Han, Y.; Angessa, T.; Li, C. Molecular Pathways of WRKY Genes in Regulating Plant Salinity Tolerance. Int. J. Mol. Sci. 2022, 23, 10947. https://doi.org/10.3390/ijms231810947
Price L, Han Y, Angessa T, Li C. Molecular Pathways of WRKY Genes in Regulating Plant Salinity Tolerance. International Journal of Molecular Sciences. 2022; 23(18):10947. https://doi.org/10.3390/ijms231810947
Chicago/Turabian StylePrice, Lewis, Yong Han, Tefera Angessa, and Chengdao Li. 2022. "Molecular Pathways of WRKY Genes in Regulating Plant Salinity Tolerance" International Journal of Molecular Sciences 23, no. 18: 10947. https://doi.org/10.3390/ijms231810947
APA StylePrice, L., Han, Y., Angessa, T., & Li, C. (2022). Molecular Pathways of WRKY Genes in Regulating Plant Salinity Tolerance. International Journal of Molecular Sciences, 23(18), 10947. https://doi.org/10.3390/ijms231810947








