Abstract
Heat stress during the preflowering panicle initiation stage seriously decreases rice grain weight in an invisible way and has not been given enough attention. The current review aims to (i) specify the heat effects on rice grain weight during the panicle initiation stage compared with the most important grain-filling stage; and (ii) discuss the physiological mechanisms of the decreased rice grain weight induced by heat during panicle initiation in terms of assimilate supply and phytohormone regulation, which are key physiological processes directly regulating rice grain weight. We emphasize that the effect of heat during the panicle initiation stage on rice grain weight is more serious than that during the grain-filling stage. Heat stress during the panicle initiation stage induces alterations in endogenous phytohormones, leading to the inhibition of the photosynthesis of functional leaves (source) and the formation of vascular bundles (flow), thus reducing the accumulation and transport of nonstructural carbohydrates and the growth of lemmata and paleae. The disruptions in the “flow” and restrictions in the preanthesis “source” tissue reduce grain size directly and decrease grain plumpness indirectly, resulting in a reduction in the final grain weight, which could be the direct physiological causes of the lower rice grain weight induced by heat during the panicle initiation stage. We highlight the seriousness of preflowering heat stress on rice grain weight, which can be regarded as an invisible disaster. The physiological mechanisms underlying the lower grain weight induced by heat during panicle initiation show a certain novelty because they distinguish this stage from the grain-filling stage. Additionally, a number of genes that control grain size through phytohormones have been summarized, but their functions have not yet been fully tested under heat conditions, except for the Grain Size and Abiotic stress tolerance 1 (GSA1) and BRASSINOSTEROID INSENSITIVE1 (OsBRI1) genes, which are reported to respond rapidly to heat stress. The mechanisms of reduced rice grain weight induced by heat during the panicle initiation stage should be studied in more depth in terms of molecular pathways.
1. Introduction
Global warming has raised the Earth’s average surface temperature, and extreme heat events have become more frequent worldwide []. On a regional scale, significant warming trends are observed in China (0.4 °C/decade), India (0.45 °C/decade), Spain (0.5 °C/decade), and Iran (0.6 °C/decade) [,,,]. As one of the most important staple crops, rice has experienced severe yield losses due to frequent heat events [,]. Recently, the occurrence of heat events has shifted to an earlier date. For example, heat events have been occurring in the rice growing regions of the Yangtze River basin in China in mid-July or even earlier []. In most rice paddy regions in China, the panicle initiation stage of middle rice often occurs in July, which begins when the panicle primordia have differentiated (approximately 30 days before heading) and ends when the pollen is fully matured []. The optimum and maximum temperatures for panicle initiation in rice are 26.7 °C and 33.1 °C, respectively []. However, the average maximum temperature in July in most rice growing regions in the Yangtze River basin has frequently surpassed 35 °C in recent years. The significance of heat stress during the preflowering periods in rice plants [], especially the heat effects on meristem initiation and development of rice [], has attracted attention and is emphasized as a key area for understanding plant heat stress responses in future studies.
Heat stress, which appears as an increase in temperature beyond a critical threshold (33.1 °C for panicle initiation) for a certain duration [], occurs during the panicle initiation stage and leads to marked declines in the number of spikelets per panicle, spikelet fertility, and grain weight []. The effects of heat stress during the reproductive stage of rice on yield components as well as the underlying mechanism have been studied in depth [,]. However, studies of the heat effect on grain weight have focused mostly on the middle and late reproductive growth stages, i.e., the flowering to grain-filling stage [], while the influence of heat stress on the grain weight of rice during the early reproductive growth stage, i.e., the panicle initiation stage, has not attracted much attention and is thus only superficially understood []. Rice grain weight depends on grain size (width, length, grain thickness) and grain plumpness (degree of filling) [], both of which require assimilates as basic materials that are regulated by phytohormones []. In the current review, the effects of heat stress during the panicle initiation stage on the grain weight of rice are discussed with respect to two components: grain size and grain plumpness. Furthermore, the physiological mechanism through which heat stress during the panicle initiation stage influences the grain weight of rice is analyzed in terms of assimilate supply and phytohormone regulation.
2. Heat Effects on Rice Grain Weight during the Panicle Initiation Stage
The grain weight of rice is severely decreased by heat stress during the panicle initiation stage, during which the heat effects on grain weight are even more severe than those in the grain-filling stage, according to Aghamolki et al. (2014) [] and the observations of our 5-year case studies (Figure 1). Similarly, high soil temperature treatment significantly reduces the grain weight of rice during the panicle initiation stage but has a small or null effect on rice grain weight during the grain-filling stage []. It is estimated that the grain weight of rice is decreased by an average of 11.7% (5.4–17.1%) by heat during the panicle initiation stage [,,,]. Thus, the panicle initiation stage is one of the critical periods for rice grain weight under heat conditions, in addition to the grain-filling stage.
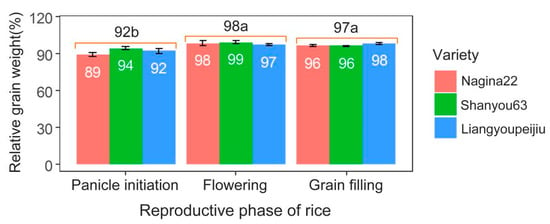
Figure 1.
Effects of heat stress on rice grain weight during the panicle initiation, flowering, and grain-filling stages. Data originated from a location experiment repeated for 5 years [] at Huazhong Agricultural University, Wuhan, China (30°29′ N, 114°22′ E). Heat treatments were imposed at panicle initiation, flowering, and grain-filling for 15, 7, and 30 days, respectively.
Grain size, which is established during the panicle initiation stage (the first critical stage), determines the maximum potential grain weight, and grain plumpness, which is established during the grain-filling stage (the second critical stage), determines the final grain weight []. Previous studies on the effects of heat stress on grain weight have mostly concentrated on the grain-filling stage, during which heat stress reduced grain weight by decreasing grain plumpness but not through grain size, and the reduced grain plumpness was mainly attributed to the affected physiological and biochemical processes such as sucrose unloading, conversion, and starch synthesis under heat stress during the grain-filling stage []. It is concluded that heat stress during the panicle initiation stage has serious adverse effects on rice grain weight, but the reasons for the effects of heat stress during the panicle initiation stage on the grain formation of rice are somewhat different from those during the grain-filling stage [,].
3. Heat-Induced Changes in Assimilate Supply Explain the Reduced Rice Grain Weight during the Panicle Initiation Stage
The reduced grain weight of rice induced by heat during the panicle initiation stage is associated with decreased grain size and grain plumpness, both of which require sufficient amounts of nonstructural carbohydrates (NSCs) as basic materials []. Heat stress inhibits the photosynthesis of functional leaves and thus reduces the preflowering production and accumulation of NSCs during the panicle initiation stage []. If combined with a high nighttime temperature, additional respiration-based carbohydrate consumption will be induced [], resulting in further reduction of the preflowering accumulation of NSCs in the stems. Heat stress during the panicle initiation stage results in an inadequate accumulation and supply of NSCs [] and further restricts the growth of lemmata and paleae [], manifesting as a reduced grain size, which cannot be discerned until heading [].
Heat stress during the panicle initiation stage reduces the number of vascular bundles and the area of the large and small vascular bundles in panicles, thus restricting the transport of NSCs toward the young panicles during the panicle initiation stage and hindering the transport of assimilates to the spikelets during the grain-filling period []. In fact, the panicle initiation stage is a critical period for the development of young panicles, the formation of vascular bundles, and the accumulation of NSCs in panicles prior to flowering under heat conditions [,,,]. As a result, insufficient and disrupted NSC accumulation prior to flowering restricts post-flowering grain-filling (endosperm proliferation and plumpness) and reduces grain plumpness, ultimately reducing rice grain weight.
Most studies on the influence of heat stress on grain weight have focused on the grain-filling stage. Rice glumes reach their final size at anthesis, and heat treatment during the grain-filling stage thus has no obvious influence on the length and width of rice grains [,]. However, heat stress during the panicle initiation stage directly reduces the length and width of grain [] and indirectly reduces post-flowering grain plumpness, thereby resulting in decreased grain weight. Hence, the underlying mechanism through which heat stress induces a decrease in rice grain weight during the panicle initiation stage is different from that during the grain-filling stage. In summary, heat stress during the panicle initiation stage may simultaneously decrease both grain size and plumpness, which are associated with the assimilate supply (Figure 2). However, it is not clear exactly how heat stress reduces rice grain size and grain plumpness by regulating the supply of assimilates during the panicle initiation stage. The relationships between grain size, grain plumpness, and grain weight from the aspects of the assimilate supply and distribution are discussed as follows.
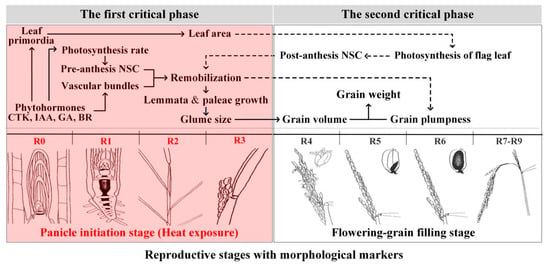
Figure 2.
Proposed mechanisms of reduced rice grain weight in terms of phytohormonal regulation and assimilate supply under heat during the panicle initiation stage. R0: initiation of panicle development; R1: formation of panicle branches; R2: formation of the flag leaf collar; R3: panicle emergence from the boot; R4: at least one floret on the main panicle reach anthesis; R5–R6: one or more caryopsis on the main panicle elongate to the end of the hull; R7–R9: grains on the main stem panicle show yellow or brown hulls. (the illustrations of the reproductive stages with morphological markers are adapted from those by Wu et al. []).
(i) Grain plumpness. The amount of NSCs in the stems decreased during the panicle initiation stage when plants were subjected to heat stress, but the heat-stressed plants showed a compensatory response after the heat stress was removed [], which was reflected by a greater accumulation of aboveground biomass compared with that of the plants under control conditions. Deficit irrigation is usually adopted to ensure high and stable grain yields [], which benefit mainly from the compensatory effect induced by regulating water availability []. However, how the compensatory effect induced by heat stress during the preflowering panicle initiation stage influences grain plumpness has rarely been evaluated. If heat stress during the panicle initiation stage exerts any effect on grain plumpness, is the outcome negative (zero or partial compensation) or positive (overcompensation)? If the effect is negative, what are the main reasons for the reduction in grain weight caused by heat stress? Furthermore, it is unclear whether the NSC/floret ratio (NSCs/number of florets) at heading decreases [] and whether the quantity of NSCs increases due to the compensatory effect under heat conditions during the panicle initiation stage []. It is speculated that the amount of photosynthesis may not be the primary reason why heat stress restricts grain plumpness. Heat stress during the panicle initiation stage may affect grain plumpness by influencing the post-flowering remobilization of NSCs.
(ii) Grain size. To obtain heavy grain, breeding scientists usually aim to increase grain size, i.e., sink expansion, through genetic improvements [], and cultivation experts generally advocate for “increasing the source and improving the flow”, in addition to “sink expansion”, to improve grain plumpness []. In fact, the variation in grain size resulting from cultural practices can cause a variation as high as 10% in grain weight []. In recent years, scholars have suggested increasing the grain weight by regulating the grain size using cultivation techniques []. Statistical analysis of existing data shows that heat events during the panicle initiation stage induce more than a 10% variation in grain weight [,,], imposing a great impact on rice grain yield. Heat stress during the panicle initiation stage reduces the preflowering assimilate supply and thus decreases grain size, which restricts the sink capacity. Further studies should be performed to elucidate exactly how heat stress dominates rice grain weight during the panicle initiation stage.
4. Effect of Phytohormones on Grain Weight under Heat during the Panicle Initiation Stage
Rice grain weight is directly regulated by phytohormones such as cytokinins (CTKs), indoleacetic acid (IAA), gibberellic acids (GAs), and brassinosteroids (BRs) to a considerable degree [,,,]. IAA in the shoot apical meristem regulates leaf differentiation and further influences leaf area [], CTKs affect the net photosynthetic rate of leaves [] and aboveground biomass [], and CTK, GA, and IAA regulate the development of vascular bundles in rice [,]. The physiological activities directly determine the preflowering accumulation and transport of NSCs and thus influence grain size and grain plumpness. IAA interacts with salicylic acid to mitigate injury during the differentiation and growth of spikelets under heat conditions [], and the changes in endogenous CTK, IAA, and GA1 levels induced by heat are significantly correlated with heat-induced changes in grain weight during the panicle initiation stage []. Path analysis revealed that CTKs were most strongly correlated with grain weight (coefficient of determination of 0.91), followed by IAA (0.27), GA1 (0.25), and ABA (−0.08) (unpublished data). These results indicate that phytohormones, particularly CTKs, are strongly related to rice grain weight under heat stress during the panicle initiation stage.
Previous studies have shown that many genes or quantitative trait loci controlling rice grain weight also regulate grain size by influencing phytohormones (Table 1). For example, the Cytokinin oxidase (OsCKX), PURINE PERMEASE (OsPUP), MIKCC class type II MADS-box gene (OsMADS29), and DROUGHT AND SALT TOLERANCE (DST) genes regulate grain size by influencing CTK contents. The OsCKX gene regulates the oxidative degradation of CTKs [], the DST gene regulates CTK accumulation in the shoot apical meristem by interacting with OsCKX2 directly [], the OsPUP4 gene regulates the long-distance transport of CTK [], and the OsMADS29 gene affects grain size and grain weight by regulating carbohydrate metabolism via CTKs []. The genes GRAIN-LENGTH-ASSOCIATED 2 (GL2), GRAIN WIDTH 5 (GW5), and SLENDER GRAIN ON CHROMOSOME (SLG) regulate grain size through BRs that possess antistress activity [,,]. Among the genes regulating rice grain weight (Table 1), the OsCKX2, DST, a member of the CYP450 gene cluster (DSS1/CYP96B), calcium-dependent protein kinase 1 (OsCDPK1), Grain Size and Abiotic stress tolerance 1 (GSA1), and BRASSINOSTEROID INSENSITIVE1 (OsBRI1) genes showed rapid and strong changes in their expression under abiotic stresses, such as heat, high light, salinity, and drought. However, the functions of the other genes in Table 1 have not yet been tested under abiotic stress and should be studied in more depth to clarify the molecular mechanisms for reducing rice grain weight under heat during the panicle initiation stage.

Table 1.
The genes controlling grain size.
5. Conclusions and Perspective
Heat stress during the panicle initiation stage negatively affects grain in seemingly unapparent ways. The mechanism through which heat stress during the panicle initiation stage reduces grain size and grain plumpness is summarized in terms of assimilates and endogenous phytohormones. (i) Heat stress during the panicle initiation stage prevents the accumulation and sufficient supply of NSCs in young panicles, thus restricting spikelet growth and leading to reduced grain size. Moreover, the inadequate accumulation and impaired translocation of NSCs in the culm during the panicle initiation stage and the reduced sink size may restrict post-flowering grain plumpness. (ii) Heat stress during the panicle initiation stage influences the levels of endogenous phytohormones (CTKs, BRs, IAA, and GAs), thus affecting glume enlargement and the accumulation and transport of NSCs, further hindering preflowering glume growth and reducing post-flowering grain plumpness. There is no doubt that heat stress during the panicle initiation stage can reduce grain size and grain plumpness simultaneously, but it is still not clear exactly how heat stress dominates rice grain weight during the panicle initiation stage, which should be elucidated in further studies.
Phytohormone homeostasis (biosynthesis, catabolism, deactivation, and transport) is pivotal in regulating plant acclimation to environmental stress []. However, there is still a lack of comprehensive analysis and an overall understanding of the effects of heat stress on the processes involved in phytohormones homeostasis. The possible mechanisms of reduced rice grain weight in terms of CTK homeostasis under heat during the panicle initiation stage are proposed (Figure 3) based on the discussion above and our previous study []. Future studies on the physiological mechanisms of the reduced rice grain weight induced by heat stress during the panicle initiation stage should focus on the responses of processes involved in the homeostasis of other phytohormones, especially the anti-stress hormone BRs. Additionally, a number of genes have recently been identified to regulate grain weight through phytohormones (Table 1). However, most of the currently identified genes that play a role in grain weight are associated with grain size, but the genes that regulate grain plumpness have rarely been identified. The genes involved in grain size and grain plumpness should be further explored, and their functions should be given more attention under heat conditions during the panicle initiation stage.
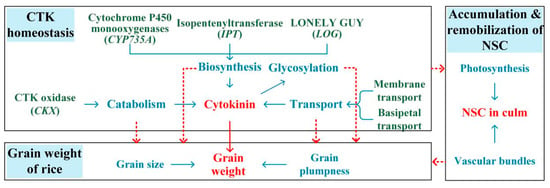
Figure 3.
Proposed mechanisms underlying grain weight changes mediated by CTK under heat during the panicle initiation stage.
Notably, the panicle initiation stage is the key stage for top-dressing fertilizers such as nitrogen, potassium, and phosphorus, which regulate source–sink relations and thus contribute to high grain yield []. Heat stress inhibited the remobilization of nitrogen, potassium, and phosphorus to panicles [,], and high levels of nitrogen and/or silicon application significantly impacted the aboveground biomass and rice grain weight under heat conditions during the panicle initiation stage [,]. In fact, fertilizer application usually interacts with heat treatments in terms of source–sink relations and yield formation []. Nitrogen and silicon may protect rice plants against heat injury because fertilizers delay senescence and enhance the synthesis of cytokinins [,], which tightly regulate source–sink relations and grain weight in rice plants (Figure 3). Furthermore, silicon fertilizer positively regulated the translocation efficiencies and allocation rates of nitrogen and potassium under heat conditions []. Thus, the effects of fertilizers on rice grain weight under heat stress during the panicle initiation stage should also be emphasized in further studies.
Author Contributions
Conceptualization, C.W. and K.C.; methodology, C.W.; software, C.W.; validation, C.W., K.C. and S.F.; formal analysis, C.W.; investigation, C.W.; resources, C.W. and K.C.; data curation, C.W.; writing—original draft preparation, C.W.; writing—review and editing, K.C. and S.F.; visualization, K.C.; supervision, K.C.; project administration, C.W.; funding acquisition, C.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 32001471, 32160504), the Guangxi Natural Science Foundation (grant no. 2020GXNSFAA297027), the Basic Research Fund of Guangxi Academy of Sciences (grant no. CQZ-C-1901), and the Guilin Innovation Platform and Talent Plan (20210102-3).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021; pp. 56–73. [Google Scholar]
- Wang, X.; Li, Y.; Wang, M.; Li, Y.; Gong, X.; Chen, Y.; Chen, Y.; Cao, W. Changes in daily extreme temperature and precipitation events in mainland China from 1960 to 2016 under global warming. Int. J. Climatol. 2021, 41, 1465–1483. [Google Scholar] [CrossRef]
- Dhorde, A.; Wakhare, A. Evidence of long-term climate change at major cities of India during the twentieth century. Int. J. Clim. Chang. Imp. Res. 2009, 1, 15–41. [Google Scholar] [CrossRef]
- Martínez, M.; Serra, C.; BurgueñO, A.; Lana, X. Time trends of daily maximum and minimum temperatures in Catalonia (ne Spain) for the period 1975–2004. Int. J. Climatol. 2010, 30, 267–290. [Google Scholar] [CrossRef]
- Tabari, H.; Talaee, P.H. Recent trends of mean maximum and minimum air temperatures in the western half of Iran. Meteorol. Atmos. Phys. 2011, 111, 121–131. [Google Scholar] [CrossRef]
- Wu, C.; Cui, K.; Li, Q.; Li, L.; Wang, W.; Hu, Q.; Ding, Y.; Li, G.; Fahad, S.; Huang, J.; et al. Estimating the yield stability of heat-tolerant rice genotypes under various heat conditions across reproductive stages: A 5-year case study. Sci. Rep. 2021, 11, 13604. [Google Scholar] [CrossRef]
- Fahad, S.; Ihsan, M.Z.; Khaliq, A.; Daur, I.; Saud, S.; Alzamanan, S.; Nasim, W.; Abdullah, M.; Khan, I.A.; Wu, C.; et al. Consequences of high temperature under changing climate optima for rice pollen characteristics-concepts and perspectives. Arch. Agron. Soil Sci. 2018, 64, 1473–1488. [Google Scholar] [CrossRef]
- Lin, M.; Wang, C.; Zhang, J. Heat injury risk assessment for single-cropping rice in the middle and lower reaches of the Yangtze river under climate change. J. Meteorol. Res. 2016, 30, 426–443. [Google Scholar]
- Wang, Y.; Li, J. The plant architecture of rice (Oryza sativa). Plant Mol. Biol. 2005, 59, 75–84. [Google Scholar] [CrossRef]
- Sánchez, B.; Rasmussen, A.; Porter, J.R. Temperatures and the growth and development of maize and rice: A review. Global Chang. Biol. 2014, 20, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Impa, S.M.; Raju, B.; Hein, N.T.; Sandhu, J.; Prasad, P.V.V.; Walia, H.; Jagadish, S.V.K. High night temperature effects on wheat and rice: Current status and way forward. Plant Cell Environ. 2021, 44, 2049–2065. [Google Scholar] [CrossRef] [PubMed]
- Jagadish, K.S.; Way, D.A.; Sharkey, T.D. Plant heat stress: Concepts directing future research. Plant Cell Environ. 2021, 44, 1992–2005. [Google Scholar] [CrossRef]
- Wu, C.; Cui, K.; Wang, W.; Li, Q.; Fahad, S.; Hu, Q.; Huang, J.; Nie, L.; Peng, S. Heat-induced phytohormone changes are associated with disrupted early reproductive development and reduced yield in rice. Sci. Rep. 2016, 6, 34978. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chu, C.; Yao, S. The impact of high-temperature stress on rice: Challenges and solutions. Crop J. 2021, 9, 963–976. [Google Scholar] [CrossRef]
- Qi, B.; Wu, C. Potential roles of stigma exsertion on spikelet fertility in rice (Oryza sativa L.) under heat stress. Front. Plant Sci. 2022, 13, 983070. [Google Scholar]
- Ren, Y.; Huang, Z.; Jiang, H.; Wang, Z.; Wu, F.; Xiong, Y.; Yao, J. A heat stress responsive NAC transcription factor heterodimer plays key roles in rice grain filling. J. Exp. Bot. 2021, 72, 2947–2964. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Zhang, Q. Genetic and molecular bases of rice yield. Annu. Rev. Plant Biol. 2010, 61, 421–442. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Tang, S.; Li, G.; Wang, S.; Fahad, S.; Ding, Y. Roles of phytohormone changes in the grain yield of rice plants exposed to heat: A review. PeerJ 2019, 7, e7792. [Google Scholar] [CrossRef] [PubMed]
- Aghamolki, M.T.K.; Yusop, M.K.; Oad, F.C.; Zakikhani, H.; Jaafar, H.Z.; Kharidah, S.; Musa, M.H. Heat stress effects on yield parameters of selected rice cultivars at reproductive growth stages. J. Food Agric. Environ. 2014, 12, 741–746. [Google Scholar]
- Arai-Sanoh, Y.; Ishimaru, T.; Ohsumi, A.; Kondo, M. Effects of soil temperature on growth and root function in rice. Plant Prod. Sci. 2010, 13, 235–242. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhang, Y.P.; Zeng, Y.H.; Wu, H.; Xiang, J.; Chen, H.Z.; Zhang, Y.K.; Zhu, D.F. Effect of high temperature stress on rice spikelet differentiation and degeneration during panicle initiation stage. Chin. J. Agrometeorol. 2015, 36, 724–731. [Google Scholar]
- Luo, Z.Q.; Shi, C.L.; Jiang, M.; Liu, Y.; Xuan, S.L.; Jin, Z.Q. Effect of high temperature on rice dry matter partition and yield component during booting stage. Chin. J. Agrometeorol. 2016, 37, 326–334. [Google Scholar]
- Zhang, C.X.; Feng, B.H.; Chen, T.T.; Zhang, X.F.; Tao, L.X.; Fu, G.F. Sugars, antioxidant enzymes and IAA mediate salicylic acid to prevent rice spikelet degeneration caused by heat stress. Plant Growth Regul. 2017, 83, 313–323. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, J.; Chen, L.; Huang, X.; Cheng, Z.; Han, B.; Zhang, Q.; Wu, C. Rice functional genomics research: Past decade and future. Mol. Plant 2018, 11, 359–380. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Henry, A.; Sreenivasulu, N. Rice yield formation under high day and night temperatures—A prerequisite to ensure future food security. Plant Cell Environ. 2020, 43, 1595–1608. [Google Scholar] [CrossRef]
- Wu, C.; Song, Y.; Qi, B.; Fahad, S. Effects of asymmetric heat on grain quality during the panicle initiation stage in contrasting rice genotypes. J. Plant Growth Regul. 2022, 1–7. [Google Scholar] [CrossRef]
- Pan, J.; Cui, K.; Wei, D.; Huang, J.; Xiang, J.; Nie, L. Relationships of non-structural carbohydrates accumulation and translocation with yield formation in rice recombinant inbred lines under two nitrogen levels. Physiol. Plantarum 2010, 141, 321–331. [Google Scholar] [CrossRef]
- Hermann, R.-D.; Gabriel, G.-V. Response of rice plants to heat stress during initiation of panicle primordia or grain-filling phases. J. Stress Physiol. Biochem. 2013, 9, 318–325. [Google Scholar]
- Laza, M.R.C.; Sakai, H.; Cheng, W.; Tokida, T.; Peng, S.; Hasegawa, T. Differential response of rice plants to high night temperatures imposed at varying developmental phases. Agric. Forest Meteorol. 2015, 209–210, 69–77. [Google Scholar] [CrossRef]
- Zhen, F.; Zhou, J.; Mahmood, A.; Wang, W.; Chang, X.; Liu, B.; Liu, L.; Cao, W.; Zhu, Y.; Tang, L. Quantifying the effects of short-term heat stress at booting stage on nonstructural carbohydrates remobilization in rice. Crop J. 2020, 8, 194–212. [Google Scholar] [CrossRef]
- Takeoka, Y.; Hiroi, K.; Kitano, H.; Wada, T. Pistil hyperplasia in rice spikelets as affected by heat stress. Sexual Plant Reprod. 1991, 4, 39–43. [Google Scholar] [CrossRef]
- Zhang, G.L.; Chen, L.Y.; Zhang, S.T.; Zheng, H.; Liu, G.H. Effects of high temperature stress on microscopic and ultrastructural characteristics of mesophyll cells in flag leaves of rice. Rice Sci. 2009, 16, 65–71. [Google Scholar] [CrossRef]
- Hu, Q.; Wencheng, W.; Lu, Q.; Huang, J.; Peng, S.; Cui, K. Abnormal anther development leads to lower spikelet fertility in rice (Oryza sativa L.) under high temperature during the panicle initiation stage. BMC Plant Biol. 2021, 21, 428. [Google Scholar] [CrossRef] [PubMed]
- Counce, P.A.; Bryant, R.J.; Bergman, C.J.; Bautista, R.C.; Wang, Y.J.; Siebenmorgen, T.J.; Kak, M.; Jfc, M. Rice milling quality, grain dimensions, and starch branching as affected by high night temperatures. Cereal Chem 2005, 82, 645–648. [Google Scholar] [CrossRef]
- Morita, S.; Yonemaru, J.I.; Takanashi, J.I. Grain growth and endosperm cell size under high night temperatures in rice (Oryza sativa L.). Ann. Bot. 2005, 95, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Y. Effects of High Temperature Treatments at Different Growth Stages on the Growth and Yield Formation of Rice. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2015. [Google Scholar]
- Kumar, A.; Nayak, A.K.; Pani, D.R.; Das, B.S. Physiological and morphological responses of four different rice cultivars to soil water potential based deficit irrigation management strategies. Field Crops Res. 2017, 205, 78–94. [Google Scholar] [CrossRef]
- Zhang, B.; Li, F.M.; Huang, G.; Cheng, Z.Y.; Zhang, Y. Yield performance of spring wheat improved by regulated deficit irrigation in an arid area. Agric. Water Manag. 2006, 79, 28–42. [Google Scholar] [CrossRef]
- Wu, C.; Cui, K.; Wang, W.; Li, Q.; Fahad, S.; Hu, Q.; Huang, J.; Nie, L.; Mohapatra, P.K.; Peng, S. Heat-induced cytokinin transportation and degradation are associated with reduced panicle cytokinin expression and fewer spikelets per panicle in rice. Front. Plant Sci. 2017, 8, 371. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C. Mechanism and regulation in the filling of inferior spikelets of rice. Acta Agron. Sin. 2010, 36, 2011–2019. [Google Scholar]
- Yang, L.X.; Wang, Y.L.; Huang, J.Y.; Zhou, X.D.; Xu, J.K.; Gu, H.; Yang, J.H.; Dong, G.C. Dynamic change and influencing factors of hull size in Yangdao 6, a rice variety. Acta Agron. Sin. 2003, 29, 853–859. [Google Scholar]
- Castillo, F.M.; Vásquez, S.C.; Calderini, D.F. Does the pre-flowering period determine the potential grain weight of sunflower? Field Crops Res. 2017, 212, 23–33. [Google Scholar] [CrossRef]
- Ishimaru, K.; Hirotsu, N.; Madoka, Y.; Murakami, N.; Hara, N.; Onodera, H.; Kashiwagi, T.; Ujiie, K.; Shimizu, B.; Onishi, A. Loss of function of the IAA-glucose hydrolase gene TGW6 enhances rice grain weight and increases yield. Nat. Genet. 2013, 45, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, B.; Yuan, D.; Duan, M.; Li, C. Rice zinc finger protein DST enhances grain production through controlling Gn1a/OsCKX2 expression. Proc. Natl. Acad. Sci. USA 2013, 110, 3167–3172. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Shen, Y.; Zheng, M.; Yang, C.; Chen, Y.; Feng, Z.; Liu, X.; Liu, S.; Chen, Z.; Lei, C. Gene SGL, encoding a kinesin-like protein with transactivation activity, is involved in grain length and plant height in rice. Plant Cell Rep. 2014, 33, 235–244. [Google Scholar] [CrossRef]
- Itoh, J.I.; Hibara, K.I.; Sato, Y.; Nagato, Y. Developmental role and auxin responsiveness of class III homeodomain leucine zipper gene family members in rice. Plant Physiol. 2008, 147, 1960–1975. [Google Scholar] [CrossRef] [PubMed]
- Mangieri, M.A.; Hall, A.J.; Striker, G.G.; Chimenti, C.A. Cytokinins: A key player in determining differences in patterns of canopy senescence in Stay-Green and Fast Dry-Down sunflower (Helianthus annuus L.) hybrids. Eur. J. Agron. 2017, 86, 60–70. [Google Scholar] [CrossRef]
- Dawood, M.F.A.; Abu-Elsaoud, A.M.; Sofy, M.R.; Mohamed, H.I.; Soliman, M.H. Appraisal of kinetin spraying strategy to alleviate the harmful effects of UVC stress on tomato plants. Environ. Sci. Pollut. R. 2022, 29, 52378–52398. [Google Scholar] [CrossRef]
- Groover, A.T.; Pattishall, A.; Jones, A.M. IAA8 expression during vascular cell differentiation. Plant Mol. Biol. 2003, 51, 427–435. [Google Scholar] [CrossRef]
- Mähönen, A.P.; Bishopp, A.; Higuchi, M.; Nieminen, K.M.; Kinoshita, K.; Törmäkangas, K.; Ikeda, Y.; Oka, A.; Kakimoto, T.; Helariutta, Y. Cytokinin signaling and its inhibitor AHP6 regulate cell fate during vascular development. Science 2006, 311, 94–98. [Google Scholar] [CrossRef]
- Joshi, R.; Sahoo, K.K.; Tripathi, A.K.; Kumar, R.; Gupta, B.K.; Pareek, A.; Singla-Pareek, S.L. Knockdown of an inflorescence meristem-specific cytokinin oxidase—OsCKX2 in rice reduces yield penalty under salinity stress condition. Plant Cell Environ. 2017, 41, 936–946. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, D.; Zhang, G.; Gao, S.; Liu, L.; Xu, F.; Che, R.; Wang, Y.; Tong, H.; Chu, C. Big Grain3, encoding a purine permease, regulates grain size via modulating cytokinin transport in rice. J. Integr. Plant Biol. 2019, 61, 581–597. [Google Scholar] [CrossRef]
- Nayar, S.; Sharma, R.; Tyagi, A.K.; Kapoor, S. Functional delineation of rice MADS29 reveals its role in embryo and endosperm development by affecting hormone homeostasis. J. Exp. Bot. 2013, 64, 4239–4253. [Google Scholar] [CrossRef] [PubMed]
- Che, R.; Tong, H.; Shi, B.; Liu, Y.; Fang, S.; Liu, D.; Xiao, Y.; Hu, B.; Liu, L.; Wang, H. Control of grain size and rice yield by GL2-mediated brassinosteroid responses. Nat. Plants 2015, 2, 15195. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Wu, C.; Wang, C.; Roh, J.; Zhang, L.; Chen, J.; Zhang, S.; Zhang, H.; Yang, C.; Hu, J. SLG controls grain size and leaf angle by modulating brassinosteroid homeostasis in rice. J. Exp. Bot. 2016, 67, 4241–4253. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, J.; Zheng, X.; Wu, F.; Lin, Q.; Heng, Y.; Tian, P.; Cheng, Z.; Yu, X.; Zhou, K. GW5 acts in the brassinosteroid signalling pathway to regulate grain width and weight in rice. Nat. Plants 2017, 3, 17043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, M.; Wang, Y.; Liang, J. RGB1 regulates rice panicle architecture and grain filling through monitoring cytokinin level in inflorescence meristem and grain abscisic acid level during filling stage. Rice Sci. 2021, 28, 317–321. [Google Scholar]
- Guo, T.; Lu, Z.Q.; Shan, J.X.; Ye, W.W.; Lin, H.X. ERECTA1 acts upstream of the OsMKKK10-OsMKK4-OsMPK6 cascade to control spikelet number by regulating cytokinin metabolism in rice. Plant Cell 2020, 32, 2763–2779. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, J.; Yu, G.; Lu, X.; Tang, W. Endoplasmic reticulum-localized PURINE PERMEASE1 regulates plant height and grain weight by modulating cytokinin distribution in rice. Front. Plant Sci. 2020, 11, 618560. [Google Scholar] [CrossRef]
- Yin, W.; Xiao, Y.; Niu, M.; Meng, W.; Tong, H. ARGONAUTE2 enhances grain length and salt tolerance by activating BIG GRAIN3 to modulate cytokinin distribution in rice. Plant Cell 2020, 32, 2292–2306. [Google Scholar] [CrossRef]
- Tsago, Y.; Chen, Z.; Cao, H.; Sunusi, M.; Khan, A.U.; Shi, C.; Jin, X. Rice gene, OsCKX2-2, regulates inflorescence and grain size by increasing endogenous cytokinin content. Plant Growth Regul. 2020, 92, 283–294. [Google Scholar] [CrossRef]
- Liu, K.; Li, M.; Zhang, B.; Cui, Y.; Wang, M. Poaceae orthologs of rice OsSGL, DUF1645 domain-containing genes, positively regulate drought tolerance, grain length and weight in rice. Res. Sq. 2020, 29, 257–267. [Google Scholar] [CrossRef]
- Wang, M.; Lu, X.; Xu, G.; Yin, X.; Cui, Y.; Huang, L.; Rocha, P.S.C.F.; Xia, X. OsSGL, a novel pleiotropic stress-related gene enhances grain length and yield in rice. Sci. Rep. 2016, 6, 38157. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Mi, X.F.; Shan, J.X.; Lin, H.X. The QTL GNP1 encodes GA20ox1, which increases grain number and yield by increasing cytokinin activity in rice panicle meristems. PLoS Genet. 2016, 12, e1006386. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Xiong, L. Characterization of a purine permease family gene OsPUP7 involved in growth and development control in rice. J. Integr. Plant Biol. 2013, 55, 1119–1135. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.L.; Dong, N.Q.; Guo, T.; Ye, W.W.; Shan, J.X.; Lin, H.X. A quantitative trait locus GW6 controls rice grain size and yield through the gibberellin pathway. Plant J. 2020, 103, 1174–1188. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Cheng, Z.; Liu, L.; Wang, M.; You, X.; Wang, J.; Zhang, F.; Zhou, C.; Zhang, Z.; Zhang, H.; et al. Small Grain and Dwarf 2, encoding an HD-Zip II family transcription factor, regulates plant development by modulating gibberellin biosynthesis in rice. Plant Sci. 2019, 288, 110208. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, S.; Tao, Q.; Tao, Y.; Miao, J.; Peng, X.; Li, C.; Yang, Z.; Zhou, Y.; Liang, G. OsGASR9 positively regulates grain size and yield in rice (Oryza sativa). Plant Sci. 2019, 286, 17–27. [Google Scholar] [CrossRef]
- Tamiru, M.; Undan, J.R.; Takagi, H.; Abe, A.; Yoshida, K.; Undan, J.Q.; Natsume, S.; Uemura, A.; Saitoh, H.; Matsumura, H.; et al. A cytochrome P450, OsDSS1, is involved in growth and drought stress responses in rice (Oryza sativa L.). Plant Mol. Biol. 2015, 88, 85–99. [Google Scholar] [CrossRef]
- Ho, S.L.; Huang, L.F.; Lu, C.A.; He, S.L.; Wang, C.C.; Yu, S.P.; Chen, J.; Yu, S.M. Sugar starvation- and GA-inducible calcium-dependent protein kinase 1 feedback regulates GA biosynthesis and activates a 14-3-3 protein to confer drought tolerance in rice seedlings. Plant Mol. Biol. 2013, 81, 347–361. [Google Scholar] [CrossRef]
- Qiao, J.; Jiang, H.; Lin, Y.; Shang, L.; Qian, Q. A novel miR167a-OsARF6-OsAUX3 module regulates grain length and weight in rice. Mol. Plant 2021, 14, 1683–1698. [Google Scholar] [CrossRef]
- Dong, N.Q.; Sun, Y.; Guo, T.; Shi, C.L.; Lin, H.X. UDP-glucosyltransferase regulates grain size and abiotic stress tolerance associated with metabolic flux redirection in rice. Nat. Commun. 2020, 11, 2629. [Google Scholar] [CrossRef]
- Guo, T.; Chen, K.; Dong, N.-Q.; Ye, W.-W.; Shan, J.-X.; Lin, H.-X. TILLERING AND SMALL GRAIN 1 dominates the tryptophan aminotransferase family required for local auxin biosynthesis in rice. J. Integr. Plant Biol. 2020, 62, 581–600. [Google Scholar] [CrossRef]
- Hu, Z.; Lu, S.J.; Wang, M.J.; He, H.; Le, S.; Wang, H.; Liu, X.H.; Ling, J.; Sun, J.L.; Xin, X. A novel QTL qTGW3 encodes the GSK3/SHAGGY-like kinase OsGSK5/OsSK41 that interacts with OsARF4 to negatively regulate grain size and weight in rice. Mol. Plant 2018, 11, 736–749. [Google Scholar] [CrossRef]
- Liu, X.; Yang, C.Y.; Miao, R.; Zhou, C.L.; Cao, P.H.; Lan, J.; Zhu, X.J.; Mou, C.L.; Huang, Y.S.; Liu, S.J.; et al. DS1/OsEMF1 interacts with OsARF11 to control rice architecture by regulation of brassinosteroid signaling. Rice 2018, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, J.; Tang, Z.; Sun, X.; Zhang, H.; Yu, J.; Yao, G.; Li, G.; Guo, H.; Li, J.; et al. Gnp4/LAX2, a RAWUL protein, interferes with the OsIAA3–OsARF25 interaction to regulate grain length via the auxin signaling pathway in rice. J. Exp. Bot. 2018, 69, 4723–4737. [Google Scholar] [CrossRef]
- Aya, K.; Hobo, T.; Sato-Izawa, K.; Ueguchi-Tanaka, M.; Kitano, H.; Matsuoka, M. A novel AP2-type transcription factor, SMALL ORGAN SIZE1, controls organ size downstream of an auxin signaling pathway. Plant Cell Physiol. 2014, 55, 897–912. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Yoshida, H.; Aya, K.; Kawamura, M.; Hayashi, M.; Hobo, T.; Sato-Izawa, K.; Kitano, H.; Ueguchi-Tanaka, M.; Matsuoka, M. SMALL ORGAN SIZE 1 and SMALL ORGAN SIZE 2/DWARF and LOW-TILLERING form a complex to integrate auxin and brassinosteroid signaling in rice. Mol. Plant 2017, 10, 590–604. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Tong, H.; Xiao, Y.; Che, R.; Xu, F.; Hu, B.; Liang, C.; Chu, J.; Li, J.; Chu, C. Activation of Big Grain1 significantly improves grain size by regulating auxin transport in rice. Proc. Natl. Acad. Sci. USA 2015, 112, 11102. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Fang, J.; Ou, S.; Gao, S.; Zhang, F.; Du, L.; Xiao, Y.; Wang, H.; Sun, X.; Chu, J.; et al. Variations in CYP78A13 coding region influence grain size and yield in rice. Plant Cell Environ. 2015, 38, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-H.; Yoo, S.-C.; Zhang, H.; Pandeya, D.; Koh, H.-J.; Hwang, J.-Y.; Kim, G.-T.; Paek, N.-C. The rice narrow leaf2 and narrow leaf3 loci encode WUSCHEL-related homeobox 3A (OsWOX3A) and function in leaf, spikelet, tiller and lateral root development. New Phytol. 2013, 198, 1071–1084. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fan, X.; Song, W.; Zhang, Y.; Xu, G. Over-expression of OsPIN2 leads to increased tiller numbers, angle and shorter plant height through suppression of OsLAZY1. Plant Biotechnol. J. 2012, 10, 139–149. [Google Scholar] [CrossRef]
- Li, Y.; Huang, R.; Li, J.; Huang, X.; Zeng, X.; Zhao, D. DWARF and SMALL SEED1, a novel allele of OsDWARF, controls rice plant architecture, seed size, and chlorophyll biosynthesis. Phyton 2021, 90, 111–127. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, R.; Xing, Y.; Xu, Y.; Xiong, D.; Wang, Y.; Yao, S. Separable regulation of POW1 in grain size and leaf angle development in rice. Plant Biotechnol. J. 2021, 19, 2517–2531. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Xu, H.; Li, B.; Shang, Y.; Wei, M.; Zhang, S.; Zhao, C.; Qin, R.; Cui, F.; Wu, Y. The brassinosteroid biosynthesis gene, ZmD11, increases seed size and quality in rice and maize. Plant Physiol. Biochem. 2021, 160, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Morinaka, Y.; Sakamoto, T.; Inukai, Y.; Agetsuma, M.; Kitano, H.; Ashikari, M.; Matsuoka, M. Morphological alteration caused by brassinosteroid insensitivity increases the biomass and grain production of rice. Plant Physiol. 2006, 141, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhu, W.; Tong, Y. Knock-down the expression of brassinosteroid receptor TaBRI1 reduces photosynthesis, tolerance to high light and high temperature stresses and grain yield in wheat. Plants 2020, 9, 840. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Yan, J.; Su, J.; Liu, H.; Hu, C.; Li, G.; Wang, F.; Lin, Y. Novel OsGRAS19 mutant, D26, positively regulates grain shape in rice (Oryza sativa). Funct. Plant Biol. 2019, 46, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qin, P.; Peng, Y.; Ma, B.; Hu, J.; Fan, S.; Hu, B.; Zhang, G.; Yuan, H.; Yan, W. A single nucleotide substitution at 5’-UTR of GSN1 represses its translation and leads to an increase of grain length in rice. J. Genet. Genom. 2019, 46, 105–108. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, Y.; Liu, R.; Xue, H.W.; Yang, Z. The Rho-family GTPase OsRac1 controls rice grain size and yield by regulating cell division. Proc. Natl. Acad. Sci. USA 2019, 116, 16121–16126. [Google Scholar] [CrossRef] [PubMed]
- Qin, R.; Zeng, D.; Yang, C.; Akhter, D.; Alamin, M.; Jin, X.; Shi, C. LTBSG1, a new allele of BRD2, regulates panicle and grain development in rice by brassinosteroid biosynthetic pathway. Genes 2018, 9, 292. [Google Scholar] [CrossRef] [PubMed]
- Segami, S.; Takehara, K.; Yamamoto, T.; Kido, S.; Kondo, S.; Iwasaki, Y.; Miura, K. Overexpression of SRS5 improves grain size of brassinosteroid-related dwarf mutants in rice (Oryza sativa L.). Breed. Sci. 2017, 67, 393–397. [Google Scholar] [CrossRef]
- Wang, N.; Xing, Y.; Lou, Q.; Feng, P.; Liu, S.; Zhu, M.; Yin, W.; Fang, S.; Lin, Y.; Zhang, T.; et al. Dwarf and short grain 1, encoding a putative U-box protein regulates cell division and elongation in rice. J. Plant Physiol. 2017, 209, 84–94. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, D.; Zhang, G.; Tong, H.; Chu, C. Brassinosteroids regulate OFP1, a DLT interacting protein, to modulate plant architecture and grain morphology in rice. Front. Plant Sci. 2017, 8, 1698. [Google Scholar] [CrossRef]
- Yu, H.; Banpu, R.; Wang, Z.; Ren, D.; Zhang, Y.; Leng, Y.; Zeng, D.; Hu, J.; Zhang, G.; Zhu, L. Fine mapping of a novel defective glume 1 (dg1) mutant, which affects vegetative and spikelet development in rice. Front. Plant Sci. 2017, 8, 486. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Wu, C.; Fu, Y.; Hu, G.; He, Z.; Liu, W. Novel rice mutants overexpressing the brassinosteroid catabolic gene CYP734A4. Plant Mol. Biol. 2017, 93, 197–208. [Google Scholar] [CrossRef]
- Fang, N.; Xu, R.; Huang, L.; Zhang, B.; Duan, P.; Li, N.; Luo, Y.; Li, Y. SMALL GRAIN 11 controls grain size, grain number and grain yield in rice. Rice 2016, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Feng, Z.M.; Zhou, C.L.; Ren, Y.K.; Mou, C.L.; Wu, T.; Yang, C.Y.; Liu, S.J.; Jiang, L.; Wan, J.M. Brassinosteroid (BR) biosynthetic gene lhdd10 controls late heading and plant height in rice (Oryza sativa L.). Plant Cell Rep. 2016, 35, 357–368. [Google Scholar] [CrossRef]
- Wu, Y.; Fu, Y.; Zhao, S.; Gu, P.; Zhu, Z.; Sun, C.; Tan, L. CLUSTERED PRIMARY BRANCH 1, a new allele of DWARF11, controls panicle architecture and seed size in rice. Plant Biotechnol. J. 2016, 14, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hua, L.; Dong, S.; Chen, H.; Zhu, X.; Jiang, J.E.; Zhang, F.; Li, Y.; Fang, X.; Chen, F. OsMAPK6, a mitogen-activated protein kinase, influences rice grain size and biomass production. Plant J. 2015, 84, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Rao, Y.; Xu, J.; Hu, S.; Fang, Y.; Yu, H.; Pan, J.; Liu, R.; Ren, D.; Wang, X.; et al. Characterization and cloning of SMALL GRAIN 4, a novel DWARF11 allele that affects brassinosteroid biosynthesis in rice. Sci. Bull. 2015, 60, 905–915. [Google Scholar] [CrossRef]
- Xu, C.J.; Liu, Y.; Li, Y.B.; Xu, X.D.; Xu, C.G.; Li, X.H.; Xiao, J.H.; Zhang, Q.F. Differential expression of GS5 regulates grain size in rice. J. Exp. Bot. 2015, 66, 2611–2623. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Rao, Y.; Zeng, D.; Yang, Y.; Xu, R.; Zhang, B.; Dong, G.; Qian, Q.; Li, Y. SMALL GRAIN 1, which encodes a mitogen-activated protein kinase kinase 4, influences grain size in rice. Plant J. 2014, 77, 547–557. [Google Scholar] [CrossRef]
- Kitagawa, K.; Kurinami, S.; Oki, K.; Abe, Y.; Ando, T.; Kono, I.; Yano, M.; Kitano, H.; Iwasaki, Y. A novel kinesin 13 protein regulating rice seed length. Plant Cell Physiol. 2010, 51, 1315–1329. [Google Scholar] [CrossRef]
- Xu, F.X.; Xiong, H.; Zhang, L.; Zhu, Y.; Liu, M.; Jiang, P.; Guo, X.; Zhou, X. Research progresses and prospects of sink-source structures and optimal regulation of high-yield varieties of hybrid rice in China. Chin. J. Eco-Agric. 2016, 24, 1285–1299. [Google Scholar]
- Zhen, F.; Liu, Y.; Ali, I.; Liu, B.; Zhu, Y. Short-term heat stress at booting stage inhibited nitrogen remobilization to grain in rice. J. Agric. Food Res. 2020, 2, 100066. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, H.; Sun, Z.; Lin, X.; Zhou, X. Translocation efficiencies and allocation of nitrogen, phosphorous and potassium in rice as affected by silicon fertilizer under high daytime temperature. J. Agron. Crop Sci. 2019, 205, 188–201. [Google Scholar] [CrossRef]
- Yang, J.; Chen, X.R.; Zhu, C.L.; Peng, X.S.; He, X.P.; Fu, J.R.; Ouyang, L.J.; Bian, J.M.; Hu, L.F.; He, H.H. Effects of nitrogen level and high temperature at late booting stage on yield and physiological characteristics of two early rice cultivars. Chin. J. Rice Sci. 2014, 28, 523–533. [Google Scholar]
- Liu, Q.; Sun, Z.; Xin, C.; Ma, J.Q. Effects of silicon on dry matter remobilization, distribution and grain yield of rice under high air temperature. J. Nucl. Agric. Sci. 2016, 30, 1833–1839. [Google Scholar]
- Gunawardena, T.; Fukai, S. The interaction of nitrogen application and temperature during reproductive stage on spikelet sterility in field-grown rice. Aust. J. Agric. Res. 2005, 56, 625–636. [Google Scholar] [CrossRef]
- Ding, C.Q.; You, J.; Chen, L.; Wang, S.H.; Ding, Y.F. Nitrogen fertilizer increases spikelet number per panicle by enhancing cytokinin synthesis in rice. Plant Cell Rep. 2014, 33, 363–371. [Google Scholar] [CrossRef]
- Markovich, O.; Steiner, E.; Kouřil, Š.; Tarkowski, P.; Aharoni, A.; Elbaum, R. Silicon promotes cytokinin biosynthesis and delays senescence in Arabidopsis and Sorghum. Plant Cell Environ. 2017, 40, 1189–1196. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).