sncRNAs in Epididymosomes: The Contribution to Embryonic Development and Offspring Health
Abstract
1. Introduction
2. Epididymosomes
3. The Mechanisms of Epididymosomal Cargoes Transfer to Spermatozoa
3.1. Delivery of Cargoes through Transient Fusion Pores
3.2. Lipid Raft-Mediated Cargoes Transfer
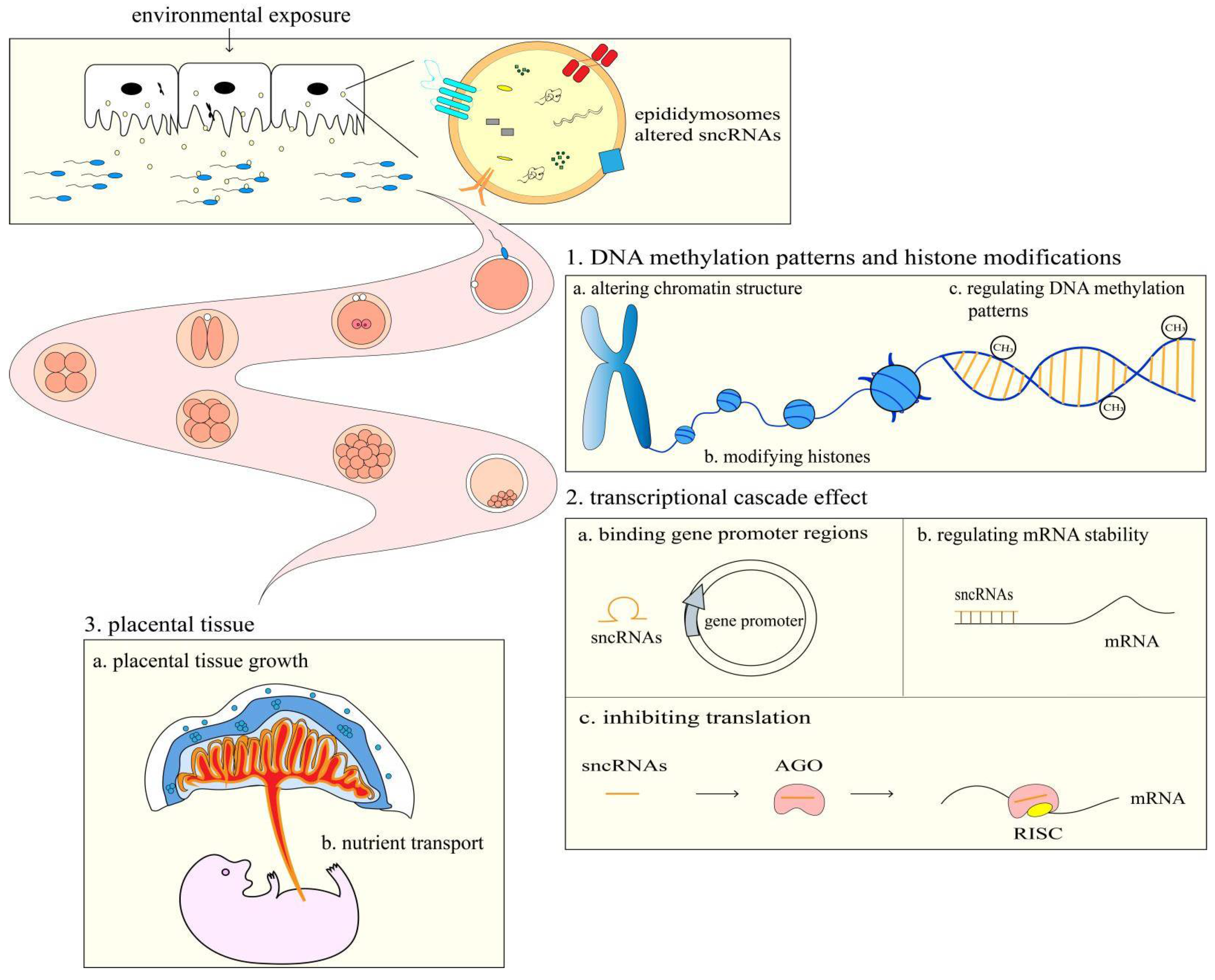
4. The Effects of Spermatozoa sncRANs in Embryonic Development and Offspring Health
4.1. The Role of Spermatozoa sncRANs in Embryonic Development
4.2. The Role of Spermatozoa sncRNAs in Offspring Health
4.3. The Contribution of Epididymosomes to the Alteration of Spermatozoa sncRNAs
5. The Possible Mechanisms by Which Spermatozoa sncRNAs Affect Embryonic Development and Offspring Health
6. Prospects and Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EVs | Extracellular vesicles |
| sncRANs | small noncoding RNAs |
| miRNAs | microRNAs |
| tsRNAs | transfer RNA-derived small RNAs |
| piRNAs | Piwi-interacting RNAs |
| snoRNAs | Small nucleolar RNAs |
| snRNAs | Small nuclear ribonucleic acids |
| ICSI | intracytoplasmic sperm injection |
| ART | Assisted Reproductive Technology |
| IVF | in vitro fertilization |
References
- Nieuwland, R.; Falcon-Perez, J.M.; Soekmadji, C.; Boilard, E.; Carter, D.; Buzas, E.I. Essentials of extracellular vesicles: Posters on basic and clinical aspects of extracellular vesicles. J. Extracell. Vesicles 2018, 7, 1548234. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, Y.; Tang, C.; Zhou, Z.; Wang, Z.; Li, Z.; Zheng, X.; Chen, S.; Zhou, Y.; Liang, A.; et al. Reassessment of the Proteomic Composition and Function of Extracellular Vesicles in the Seminal Plasma. Endocrinology 2022, 163, bqab214. [Google Scholar] [CrossRef] [PubMed]
- Nixon, B.; De Iuliis, G.N.; Hart, H.M.; Zhou, W.; Mathe, A.; Bernstein, I.R.; Anderson, A.L.; Stanger, S.J.; Skerrett-Byrne, D.A.; Jamaluddin, M.F.B.; et al. Proteomic Profiling of Mouse Epididymosomes Reveals their Contributions to Post-testicular Sperm Maturation. Mol. Cell. Proteom. 2019, 18, S91–S108. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, S.; Panner Selvam, M.K.; Agarwal, A. Chapter Four—Exosomes of male reproduction. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 95, pp. 149–163. [Google Scholar]
- Giacomini, E.; Makieva, S.; Murdica, V.; Vago, R.; Viganó, P. Extracellular vesicles as a potential diagnostic tool in assisted reproduction. Curr. Opin. Obstet. Gynecol. 2020, 32, 179–184. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, X.; Luo, Y.; Li, X.-K.; Li, X.-W. Research progress in sRNAs and functional proteins in epididymosomes. Yi Chuan 2018, 40, 197–206. [Google Scholar] [CrossRef]
- Paul, N.; Talluri, T.R.; Nag, P.; Kumaresan, A. Epididymosomes: A potential male fertility influencer. Andrologia 2021, 53, e14155. [Google Scholar] [CrossRef]
- Donkin, I.; Barrès, R. Sperm epigenetics and influence of environmental factors. Mol. Metab. 2018, 14, 1–11. [Google Scholar] [CrossRef]
- Bohacek, J.; Rassoulzadegan, M. Sperm RNA: Quo vadis? Semin. Cell Dev. Biol. 2020, 97, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Tamessar, C.T.; Trigg, N.A.; Nixon, B.; Skerrett-Byrne, D.A.; Sharkey, D.J.; Robertson, S.A.; Bromfield, E.G.; Schjenken, J.E. Roles of male reproductive tract extracellular vesicles in reproduction. Am. J. Reprod. Immunol. 2021, 85, e13338. [Google Scholar] [CrossRef]
- Nejabati, H.R.; Shahnazi, V.; Faridvand, Y.; Fathi-Maroufi, N.; Bahrami-Asl, Z.; Nikanfar, S.; Nouri, M. Epididymosomes: The black box of Darwin’s pangenesis? Mol. Hum. Reprod. 2021, 27, gaaa079. [Google Scholar] [CrossRef]
- Maciel, E.; Mansuy, I.M. Extracellular Vesicles and their miRNA Cargo: A Means of Communication between Soma and Germline in the Mammalian Reproductive System. Chimia 2019, 73, 356–361. [Google Scholar] [CrossRef] [PubMed]
- James, E.R.; Carrell, D.T.; Aston, K.I.; Jenkins, T.G.; Yeste, M.; Salas-Huetos, A. The Role of the Epididymis and the Contribution of Epididymosomes to Mammalian Reproduction. Int. J. Mol. Sci. 2020, 21, 5377. [Google Scholar] [CrossRef] [PubMed]
- Morgan, H.L.; Watkins, A.J. The influence of seminal plasma on offspring development and health. Semin. Cell Dev. Biol. 2020, 97, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Barrachina, F.; Battistone, M.A.; Castillo, J.; Mallofré, C.; Jodar, M.; Breton, S.; Oliva, R. Sperm acquire epididymis-derived proteins through epididymosomes. Hum. Reprod. 2022, 37, 651–668. [Google Scholar] [CrossRef]
- Sullivan, R.; Mieusset, R. The human epididymis: Its function in sperm maturation. Hum. Reprod. Update 2016, 22, 574–587. [Google Scholar] [CrossRef]
- Martin-DeLeon, P.A. Epididymosomes: Transfer of fertility-modulating proteins to the sperm surface. Asian J. Androl. 2015, 17, 720–725. [Google Scholar] [CrossRef]
- Jankovičová, J.; Sečová, P.; Michalková, K.; Antalíková, J. Tetraspanins, More than Markers of Extracellular Vesicles in Reproduction. Int. J. Mol. Sci. 2020, 21, 7568. [Google Scholar] [CrossRef]
- Faisal, K.; Akbarsha, M.A. Role of aposomes and epididymosomes in sperm quality control: A light and transmission electron microscopic study in an experimental rat model. Andrologia 2021, 53, e13862. [Google Scholar] [CrossRef]
- Zhou, W.; De Iuliis, G.N.; Dun, M.D.; Nixon, B. Characteristics of the Epididymal Luminal Environment Responsible for Sperm Maturation and Storage. Front. Endocrinol. 2018, 9, 59. [Google Scholar] [CrossRef]
- Zhou, W.; Stanger, S.J.; Anderson, A.L.; Bernstein, I.R.; De Iuliis, G.N.; McCluskey, A.; McLaughlin, E.A.; Dun, M.D.; Nixon, B. Mechanisms of tethering and cargo transfer during epididymosome-sperm interactions. BMC Biol. 2019, 17, 1–18. [Google Scholar] [CrossRef]
- Trigg, N.A.; Stanger, S.J.; Zhou, W.; Skerrett-Byrne, D.A.; Sipilä, P.; Dun, M.D.; Eamens, A.L.; De Iuliis, G.N.; Bromfield, E.G.; Roman, S.D.; et al. A novel role for milk fat globule-EGF factor 8 protein (MFGE8) in the mediation of mouse sperm-extracellular vesicle interactions. Proteomics 2021, 21, e2000079. [Google Scholar] [CrossRef]
- Nixon, B.; Stanger, S.J.; Mihalas, B.P.; Reilly, J.N.; Anderson, A.L.; Tyagi, S.; Holt, J.E.; McLaughlin, E.A. The microRNA signature of mouse spermatozoa is substantially modified during epididymal maturation. Biol. Reprod. 2015, 93, 91. [Google Scholar] [CrossRef] [PubMed]
- Trigg, N.A.; Eamens, A.L.; Nixon, B. The contribution of epididymosomes to the sperm small RNA profile. Reproduction 2019, 157, R209–R223. [Google Scholar] [CrossRef] [PubMed]
- Hutcheon, K.; McLaughlin, E.A.; Stanger, S.J.; Bernstein, I.R.; Dun, M.D.; Eamens, A.L.; Nixon, B. Analysis of the small non-protein-coding RNA profile of mouse spermatozoa reveals specific enrichment of piRNAs within mature spermatozoa. RNA Biol. 2017, 14, 1776–1790. [Google Scholar] [CrossRef]
- Conine, C.C.; Sun, F.; Song, L.; Rivera-Pérez, J.A.; Rando, O.J. Small RNAs Gained during Epididymal Transit of Sperm Are Essential for Embryonic Development in Mice. Dev. Cell 2018, 46, 470–480.e3. [Google Scholar] [CrossRef] [PubMed]
- Fernández-González, R.; Laguna, R.; Ramos-Ibeas, P.; Pericuesta, E.; Alcalde-Lopez, V.; Perez-Cerezales, S.; Gutierrez-Adan, A. Successful ICSI in Mice Using Caput Epididymal Spermatozoa. Front. Cell Dev. Biol. 2019, 7, 346. [Google Scholar] [CrossRef]
- Wang, Y.; Yamauchi, Y.; Wang, Z.; Zheng, H.; Yanagimachi, R.; Ward, M.A.; Yan, W. Both Cauda and Caput Epididymal Sperm Are Capable of Supporting Full-Term Development in FVB and CD-1 Mice. Dev. Cell 2020, 55, 675–676. [Google Scholar] [CrossRef]
- Zhou, D.; Suzuki, T.; Asami, M.; Perry, A.C.F. Caput Epididymidal Mouse Sperm Support Full Development. Dev. Cell 2019, 50, 5–6. [Google Scholar] [CrossRef]
- Conine, C.C.; Sun, F.; Song, L.; Rivera-Pérez, J.A.; Rando, O.J. Sperm Head Preparation and Genetic Background Affect Caput Sperm ICSI Embryo Viability: Cauda-Enriched miRNAs Only Essential in Specific Conditions. Dev. Cell 2020, 55, 677–678. [Google Scholar] [CrossRef] [PubMed]
- Nixon, B.; De Iuliis, G.N.; Dun, M.D.; Zhou, W.; Trigg, N.A.; Eamens, A.L. Profiling of epididymal small non-protein-coding RNAs. Andrology 2019, 7, 669–680. [Google Scholar] [CrossRef]
- Reza, A.M.M.T.; Choi, Y.-J.; Han, S.G.; Song, H.; Park, C.; Hong, K.; Kim, J.-H. Roles of microRNAs in mammalian reproduction: From the commitment of germ cells to peri-implantation embryos. Biol. Rev. 2019, 94, 415–438. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Blondin, P.; Vigneault, C.; Labrecque, R.; Sirard, M.-A. Sperm miRNAs- potential mediators of bull age and early embryo development. BMC Genom. 2020, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Schuster, A.; Tang, C.; Yu, T.; Ortogero, N.; Bao, J.; Zheng, H.; Yan, W. Sperm-borne miRNAs and endo-siRNAs are important for fertilization and preimplantation embryonic development. Development 2016, 143, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, X.; Wang, Z.; Li, J.; Xu, Z.; Miao, M.; Chen, G.; Lei, X.; Wu, J.; Shi, H.; et al. MicroRNA expression profile analysis in sperm reveals hsa-mir-191 as an auspicious omen of in vitro fertilization. BMC Genom. 2020, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Mudunuri, S.B.; Anaya, J.; Dutta, A. tRFdb: A database for transfer RNA fragments. Nucleic Acids Res. 2015, 43, D141–D145. [Google Scholar] [CrossRef]
- Nätt, D.; Kugelberg, U.; Casas, E.; Nedstrand, E.; Zalavary, S.; Henriksson, P.; Nijm, C.; Jäderquist, J.; Sandborg, J.; Flinke, E.; et al. Human sperm displays rapid responses to diet. PLoS Biol. 2019, 17, e3000559. [Google Scholar] [CrossRef]
- Sharma, U.; Conine, C.C.; Shea, J.M.; Boskovic, A.; Derr, A.G.; Bing, X.Y.; Belleannee, C.; Kucukural, A.; Serra, R.W.; Sun, F.; et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 2016, 351, 391–396. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, J.; Chen, Q. tsRNAs: New players in mammalian retrotransposon control. Cell Res. 2017, 27, 1307–1308. [Google Scholar] [CrossRef]
- Hua, M.; Liu, W.; Chen, Y.; Zhang, F.; Xu, B.; Liu, S.; Chen, G.; Shi, H.; Wu, L. Identification of small non-coding RNAs as sperm quality biomarkers for in vitro fertilization. Cell Discov. 2019, 5, 1–5. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, Y.; Lei, A.; Zhang, H.; Niu, H.; Li, X.; Zhang, P.; Liao, M.; Lv, Y.; Zhu, Z.; et al. Early cleavage of preimplantation embryos is regulated by tRNAGln-TTG-derived small RNAs present in mature spermatozoa. J. Biol. Chem. 2020, 295, 10885–10900. [Google Scholar] [CrossRef]
- Binder, N.K.; Beard, S.A.; Kaitu’u-Lino, T.J.; Tong, S.; Hannan, N.J.; Gardner, D.K. Paternal obesity in a rodent model affects placental gene expression in a sex-specific manner. Reproduction 2015, 149, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Fullston, T.; McPherson, N.O.; Owens, J.A.; Kang, W.X.; Sandeman, L.Y.; Lane, M. Paternal obesity induces metabolic and sperm disturbances in male offspring that are exacerbated by their exposure to an “obesogenic” diet. Physiol. Rep. 2015, 3, e12336. [Google Scholar] [CrossRef] [PubMed]
- Sales, V.M.; Ferguson-Smith, A.C.; Patti, M.-E. Epigenetic Mechanisms of Transmission of Metabolic Disease across Generations. Cell Metab. 2017, 25, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Finegersh, A.; Homanics, G.E. Paternal alcohol exposure reduces alcohol drinking and increases behavioral sensitivity to alcohol selectively in male offspring. PLoS ONE 2014, 9, e99078. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Choi, C.S.; Park, J.H.; Joo, S.H.; Kim, S.Y.; Ko, H.M.; Kim, K.C.; Jeon, S.J.; Park, S.H.; Han, S.-H.; et al. Chronic exposure to ethanol of male mice before mating produces attention deficit hyperactivity disorder-like phenotype along with epigenetic dysregulation of dopamine transporter expression in mouse offspring. J. Neurosci. Res. 2014, 92, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Rompala, G.R.; Finegersh, A.; Homanics, G.E. Paternal preconception ethanol exposure blunts hypothalamic-pituitary-adrenal axis responsivity and stress-induced excessive fluid intake in male mice. Alcohol 2016, 53, 19–25. [Google Scholar] [CrossRef]
- Gorini, G.; Nunez, Y.O.; Mayfield, R.D. Integration of miRNA and protein profiling reveals coordinated neuroadaptations in the alcohol-dependent mouse brain. PLoS ONE 2013, 8, e82565. [Google Scholar] [CrossRef]
- Donkin, I.; Versteyhe, S.; Ingerslev, L.R.; Qian, K.; Mechta, M.; Nordkap, L.; Mortensen, B.; Appel, E.V.R.; Jørgensen, N.; Kristiansen, V.B.; et al. Obesity and Bariatric Surgery Drive Epigenetic Variation of Spermatozoa in Humans. Cell Metab. 2016, 23, 369–378. [Google Scholar] [CrossRef]
- Fullston, T.; Ohlsson-Teague, E.M.C.; Print, C.G.; Sandeman, L.Y.; Lane, M. Sperm microRNA Content Is Altered in a Mouse Model of Male Obesity, but the Same Suite of microRNAs Are Not Altered in Offspring’s Sperm. PLoS ONE 2016, 11, e0166076. [Google Scholar] [CrossRef]
- de Castro Barbosa, T.; Ingerslev, L.R.; Alm, P.S.; Versteyhe, S.; Massart, J.; Rasmussen, M.; Donkin, I.; Sjögren, R.; Mudry, J.M.; Vetterli, L.; et al. High-fat diet reprograms the epigenome of rat spermatozoa and transgenerationally affects metabolism of the offspring. Mol. Metab. 2016, 5, 184–197. [Google Scholar] [CrossRef]
- Rompala, G.R.; Mounier, A.; Wolfe, C.M.; Lin, Q.; Lefterov, I.; Homanics, G.E. Heavy Chronic Intermittent Ethanol Exposure Alters Small Noncoding RNAs in Mouse Sperm and Epididymosomes. Front. Genet. 2018, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Blanco, S.; Dietmann, S.; Flores, J.V.; Hussain, S.; Kutter, C.; Humphreys, P.; Lukk, M.; Lombard, P.; Treps, L.; Popis, M.; et al. Aberrant methylation of tRNAs links cellular stress to neuro-developmental disorders. EMBO J. 2014, 33, 2020–2039. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.A.; Paulus, J.K.; Mensah, V.; Lem, J.; Saavedra-Rodriguez, L.; Gentry, A.; Pagidas, K.; Feig, L.A. Reduced levels of miRNAs 449 and 34 in sperm of mice and men exposed to early life stress. Transl. Psychiatry 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.C.; Morgan, C.P.; Adrian Leu, N.; Shetty, A.; Cisse, Y.M.; Nugent, B.M.; Morrison, K.E.; Jašarević, E.; Huang, W.; Kanyuch, N.; et al. Reproductive tract extracellular vesicles are sufficient to transmit intergenerational stress and program neurodevelopment. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Alshanbayeva, A.; Tanwar, D.K.; Roszkowski, M.; Manuella, F.; Mansuy, I.M. Early life stress affects the miRNA cargo of epididymal extracellular vesicles in mouse†. Biol. Reprod. 2021, 105, 593–602. [Google Scholar] [CrossRef]
- Grandjean, V.; Fourré, S.; De Abreu, D.A.F.; Derieppe, M.-A.; Remy, J.-J.; Rassoulzadegan, M. RNA-mediated paternal heredity of diet-induced obesity and metabolic disorders. Sci. Rep. 2015, 5, 18193. [Google Scholar] [CrossRef]
- Guo, Y.; Bai, D.; Liu, W.; Liu, Y.; Zhang, Y.; Kou, X.; Chen, J.; Wang, H.; Teng, X.; Zuo, J.; et al. Altered sperm tsRNAs in aged male contribute to anxiety-like behavior in offspring. Aging Cell 2021, 20, e13466. [Google Scholar] [CrossRef]
- Short, A.K.; Yeshurun, S.; Powell, R.; Perreau, V.M.; Fox, A.; Kim, J.H.; Pang, T.Y.; Hannan, A.J. Exercise alters mouse sperm small noncoding RNAs and induces a transgenerational modification of male offspring conditioned fear and anxiety. Transl. Psychiatry 2017, 7, e1114. [Google Scholar] [CrossRef]
- Stępień, E.Ł.; Durak-Kozica, M.; Kamińska, A.; Targosz-Korecka, M.; Libera, M.; Tylko, G.; Opalińska, A.; Kapusta, M.; Solnica, B.; Georgescu, A.; et al. Circulating ectosomes: Determination of angiogenic microRNAs in type 2 diabetes. Theranostics 2018, 8, 3874–3890. [Google Scholar] [CrossRef]
- Claycombe-Larson, K.G.; Bundy, A.N.; Roemmich, J.N. Paternal high-fat diet and exercise regulate sperm miRNA and histone methylation to modify placental inflammation, nutrient transporter mRNA expression and fetal weight in a sex-dependent manner. J. Nutr. Biochem. 2020, 81, 108373. [Google Scholar] [CrossRef]
- Short, A.K.; Fennell, K.A.; Perreau, V.M.; Fox, A.; O’Bryan, M.K.; Kim, J.H.; Bredy, T.W.; Pang, T.Y.; Hannan, A.J. Elevated paternal glucocorticoid exposure alters the small noncoding RNA profile in sperm and modifies anxiety and depressive phenotypes in the offspring. Transl. Psychiatry 2016, 6, e837. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yan, M.; Cao, Z.; Li, X.; Zhang, Y.; Shi, J.; Feng, G.; Peng, H.; Zhang, X.; Zhang, Y.; et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 2016, 351, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-M.; Tan, X.; Zhang, S.; Yao, J.; Li, H.-G. Transfer- or ‘transmission’-RNA fragments? The roles of tsRNAs in the reproductive system. Mol. Hum. Reprod. 2021, 27, gaab026. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Sun, F.; Conine, C.C.; Reichholf, B.; Kukreja, S.; Herzog, V.A.; Ameres, S.L.; Rando, O.J. Small RNAs are trafficked from the epididymis to developing mammalian sperm. Dev. Cell 2018, 46, 481–494.e6. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.N.; McLaughlin, E.A.; Stanger, S.J.; Anderson, A.L.; Hutcheon, K.; Church, K.; Mihalas, B.P.; Tyagi, S.; Holt, J.E.; Eamens, A.L.; et al. Characterisation of mouse epididymosomes reveals a complex profile of microRNAs and a potential mechanism for modification of the sperm epigenome. Sci. Rep. 2016, 6, 31794. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Li, Z.; Wang, G.; Wang, H.; Zhou, Y.; Zhao, X.; Cheng, C.Y.; Qiao, Y.; Sun, F. Sperm epigenetic alterations contribute to inter- and transgenerational effects of paternal exposure to long-term psychological stress via evading offspring embryonic reprogramming. Cell Discov. 2021, 7, 1–22. [Google Scholar] [CrossRef]
- Kumar, P.; Kuscu, C.; Dutta, A. Biogenesis and function of transfer RNA related fragments (tRFs). Trends Biochem. Sci. 2016, 41, 679–689. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Spadafora, C. Sperm-Mediated Transgenerational Inheritance. Front. Microbiol. 2017, 8, 2401. [Google Scholar] [CrossRef]
- Zhang, G.; Estève, P.-O.; Chin, H.G.; Terragni, J.; Dai, N.; Corrêa, I.R.; Pradhan, S. Small RNA-mediated DNA (cytosine-5) methyltransferase 1 inhibition leads to aberrant DNA methylation. Nucleic Acids Res. 2015, 43, 6112–6124. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Liu, X.; Jiang, S.; Zhao, C.; Shen, R.; Guo, X.; Ling, X.; Liu, C. Expression and Potential Role of microRNA-29b in Mouse Early Embryo Development. Cell. Physiol. Biochem. 2015, 35, 1178–1187. [Google Scholar] [CrossRef]
- Kiani, J.; Grandjean, V.; Liebers, R.; Tuorto, F.; Ghanbarian, H.; Lyko, F.; Cuzin, F.; Rassoulzadegan, M. RNA-mediated epigenetic heredity requires the cytosine methyltransferase Dnmt2. PLoS Genet. 2013, 9, e1003498. [Google Scholar] [CrossRef] [PubMed]
- Holoch, D.; Moazed, D. RNA-mediated epigenetic regulation of gene expression. Nat. Rev. Genet. 2015, 16, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qu, P.; Zhou, C.; Liu, X.; Ma, X.; Wang, M.; Wang, Y.; Su, J.; Liu, J.; Zhang, Y. MicroRNA-125b is a key epigenetic regulatory factor that promotes nuclear transfer reprogramming. J. Biol. Chem. 2017, 292, 15916–15926. [Google Scholar] [CrossRef] [PubMed]
- Krout, D.; Roemmich, J.N.; Bundy, A.; Garcia, R.A.; Yan, L.; Claycombe-Larson, K.J. Paternal exercise protects mouse offspring from high-fat-diet-induced type 2 diabetes risk by increasing skeletal muscle insulin signaling. J. Nutr. Biochem. 2018, 57, 35–44. [Google Scholar] [CrossRef]
- Vomhof-DeKrey, E.; Darland, D.; Ghribi, O.; Bundy, A.; Roemmich, J.; Claycombe, K. Maternal low protein diet leads to placental angiogenic compensation via dysregulated M1/M2 macrophages and TNFα expression in Sprague-Dawley rats. J. Reprod. Immunol. 2016, 118, 9–17. [Google Scholar] [CrossRef]
- Vaughan, O.R.; Maksym, K.; Silva, E.; Barentsen, K.; Anthony, R.V.; Brown, T.L.; Hillman, S.L.; Spencer, R.; David, A.L.; Rosario, F.J.; et al. Placenta-specific Slc38a2/SNAT2 knockdown causes fetal growth restriction in mice. Clin. Sci. 2021, 135, 2049–2066. [Google Scholar] [CrossRef]
- Wan, X.; He, X.; Liu, Q.; Wang, X.; Ding, X.; Li, H. Frequent and mild scrotal heat stress in mice epigenetically alters glucose metabolism in the male offspring. Am. J. Physiol. -Endocrinol. Metab. 2020, 319, E291–E304. [Google Scholar] [CrossRef]
| Title | Author | Date | ||
|---|---|---|---|---|
| Diet | Paternal obesity in a rodent model affects placental gene expression in a sex-specific manner | Binder et al. | 2015 | [42] |
| Paternal obesity induces metabolic and sperm disturbances in male offspring that are exacerbated by their exposure to an “obesogenic” diet | Fullston et al. | 2015 | [43] | |
| High-fat diet reprograms the epigenome of rat spermatozoa and transgenerationally affects metabolism of the offspring | de Castro Barbosa et al. | 2015 | [51] | |
| RNA-mediated paternal heredity of diet-induced obesity and metabolic disorders | Grandjean et al. | 2015 | [57] | |
| Obesity and Bariatric Surgery Drive Epigenetic Variation of Spermatozoa in Humans | Donkin et al. | 2016 | [49] | |
| Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals | Sharma et al. | 2016 | [38] | |
| Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder | Q. Chen et al. | 2016 | [63] | |
| Elevated paternal glucocorticoid exposure alters the small noncoding RNA profile in sperm and modifies anxiety and depressive phenotypes in the offspring | Short et al. | 2016 | [62] | |
| Epigenetic Mechanisms of Transmission of Metabolic Disease across Generations | Sales et al. | 2017 | [44] | |
| Exercise | Exercise alters mouse sperm small noncoding RNAs and induces a transgenerational modification of male offspring conditioned fear and anxiety | Short et al. | 2017 | [59] |
| Circulating ectosomes: Determination of angiogenic microRNAs in type 2 diabetes | Stępień et al. | 2018 | [60] | |
| Paternal high-fat diet and exercise regulate sperm miRNA and histone methylation to modify placental inflammation, nutrient transporter mRNA expression and fetal weight in a sex-dependent manner | Claycombe-Larson et al. | 2020 | [61] | |
| Stress | Reduced levels of miRNAs 449 and 34 in sperm of mice and men exposed to early life stress | Dickson et al. | 2018 | [54] |
| Reproductive tract extracellular vesicles are sufficient to transmit intergenerational stress and program neurodevelopment | Chan et al. | 2020 | [55] | |
| Early life stress affects the miRNA cargo of epididymal extracellular vesicles in mouse | Alshanbayeva et al. | 2021 | [56] | |
| Ethanol | Paternal preconception ethanol exposure blunts hypothalamic–pituitary–adrenal axis responsivity and stress-induced excessive fluid intake in male mice | Rompala et al. | 2016 | [47] |
| Heavy Chronic Intermittent Ethanol Exposure Alters Small Noncoding RNAs in Mouse Sperm and Epididymosomes | Rompala et al. | 2018 | [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Tan, X.; Li, H.; Ding, X. sncRNAs in Epididymosomes: The Contribution to Embryonic Development and Offspring Health. Int. J. Mol. Sci. 2022, 23, 10851. https://doi.org/10.3390/ijms231810851
Luo J, Tan X, Li H, Ding X. sncRNAs in Epididymosomes: The Contribution to Embryonic Development and Offspring Health. International Journal of Molecular Sciences. 2022; 23(18):10851. https://doi.org/10.3390/ijms231810851
Chicago/Turabian StyleLuo, Jingwen, Xia Tan, Honggang Li, and Xiaofang Ding. 2022. "sncRNAs in Epididymosomes: The Contribution to Embryonic Development and Offspring Health" International Journal of Molecular Sciences 23, no. 18: 10851. https://doi.org/10.3390/ijms231810851
APA StyleLuo, J., Tan, X., Li, H., & Ding, X. (2022). sncRNAs in Epididymosomes: The Contribution to Embryonic Development and Offspring Health. International Journal of Molecular Sciences, 23(18), 10851. https://doi.org/10.3390/ijms231810851






