Analysis of Circulating Tumor and Cancer Stem Cells Provides New Opportunities in Diagnosis and Treatment of Small Cell Lung Cancer
Abstract
1. Introduction
2. Results
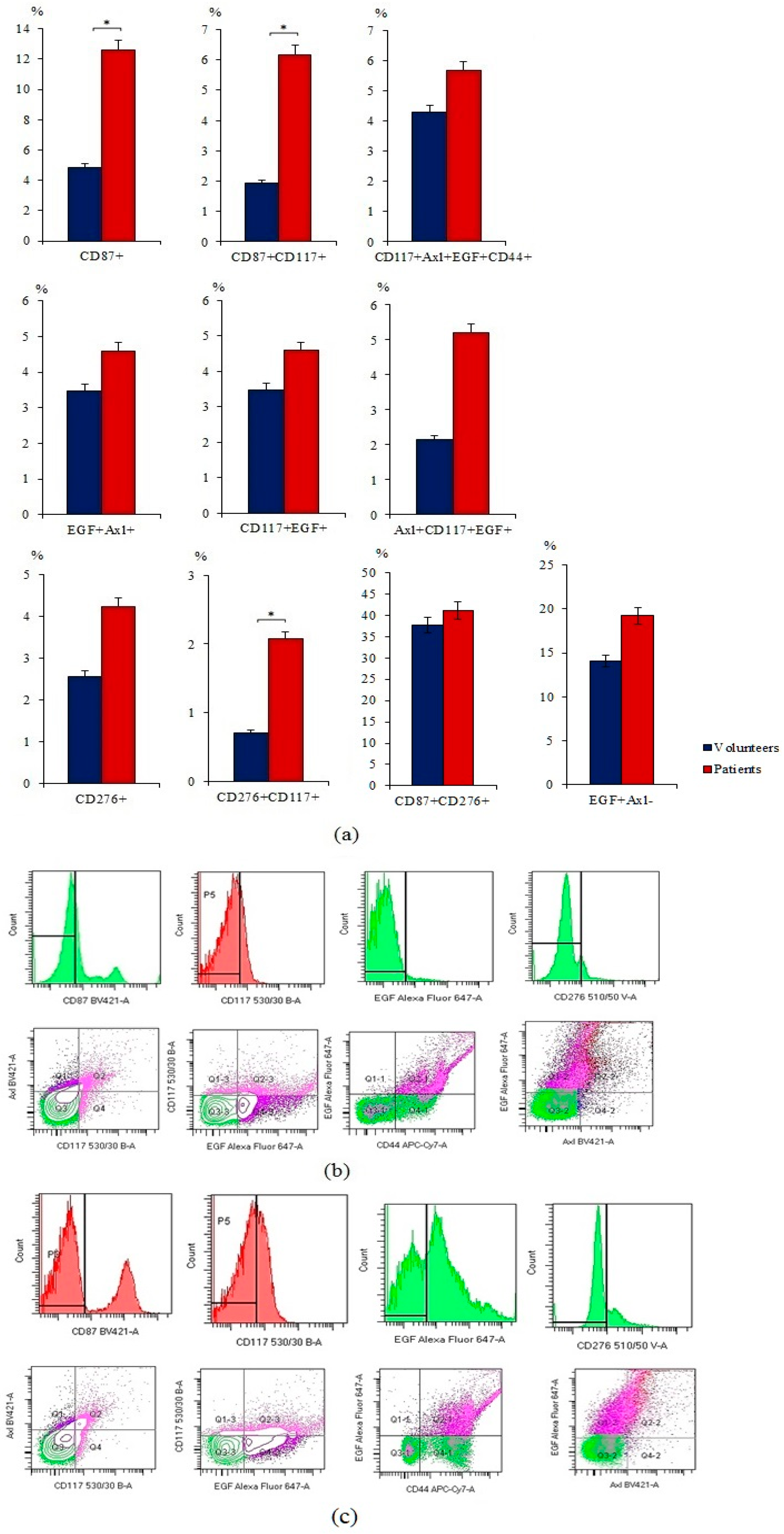
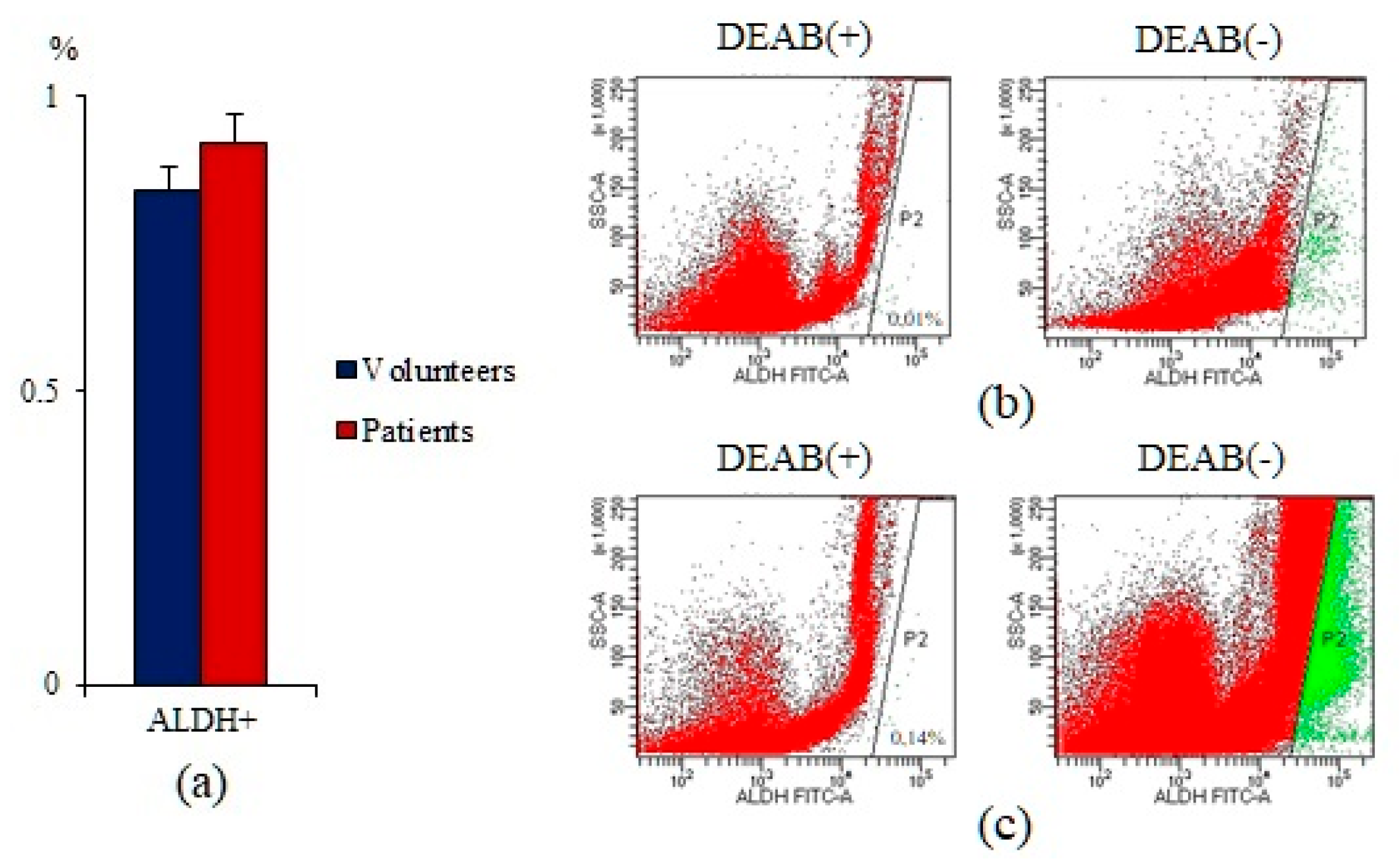
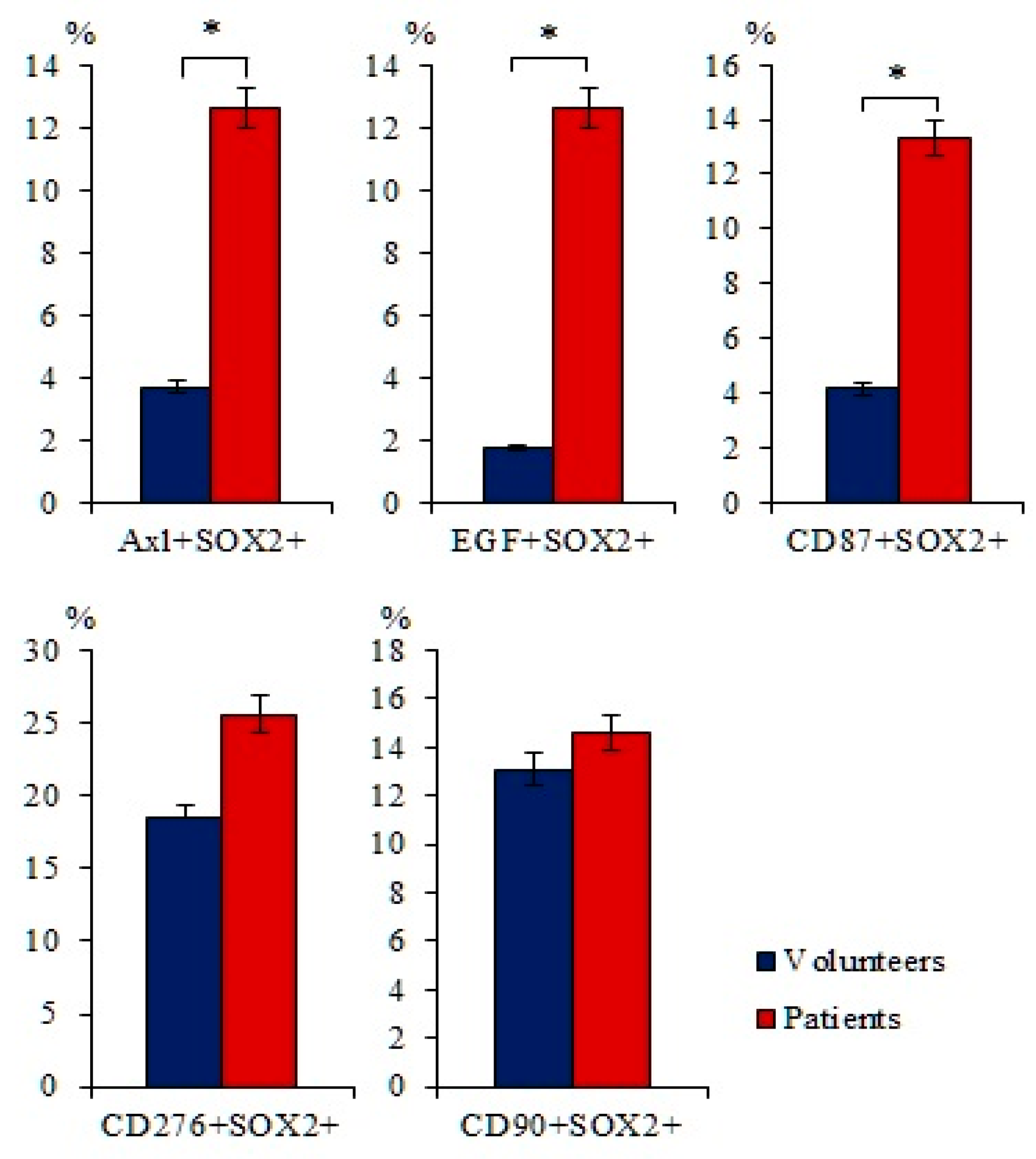
2.1. A Significant Number of Different Subpopulations of CTCs and CSCs Are Present the Blood of Patients with SCLC
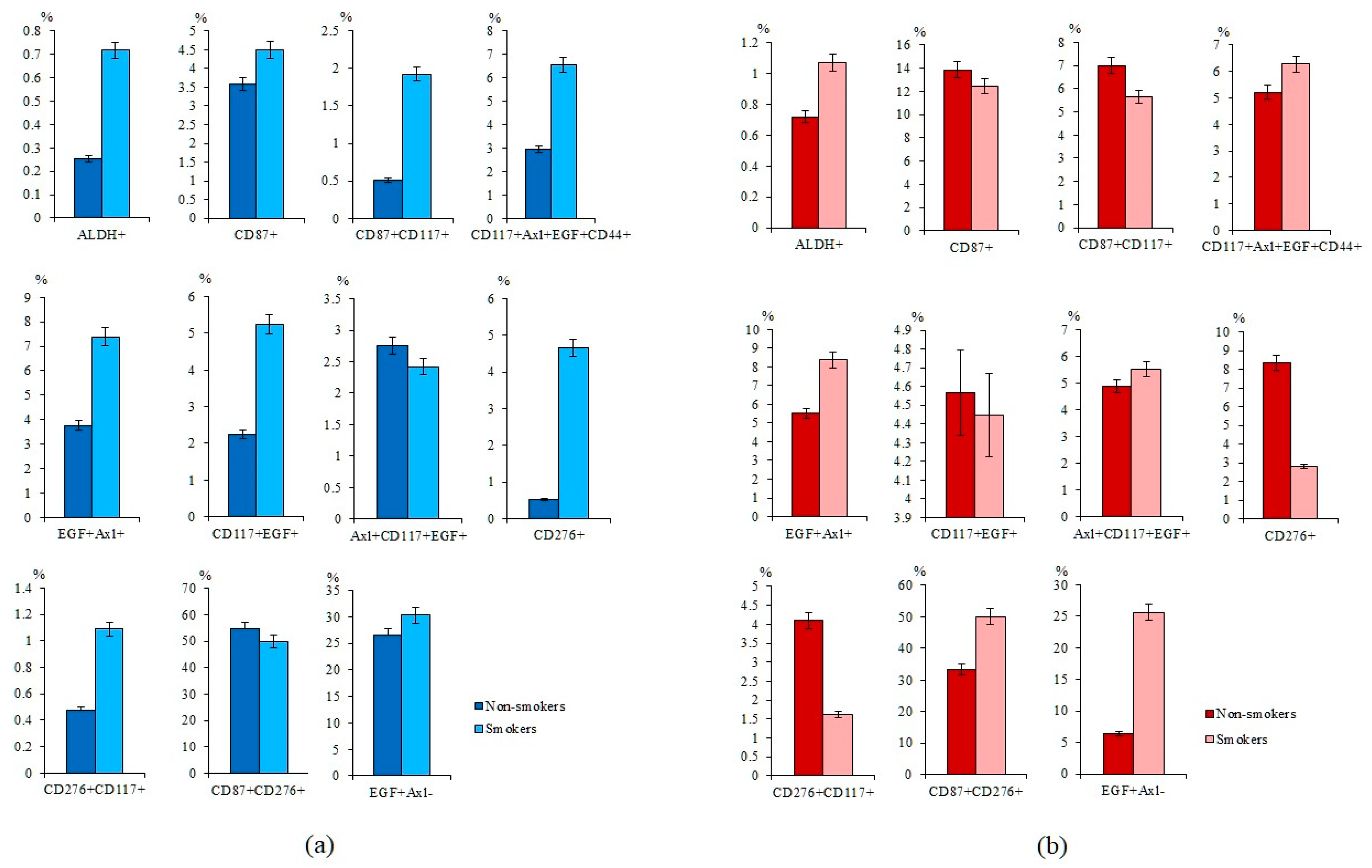
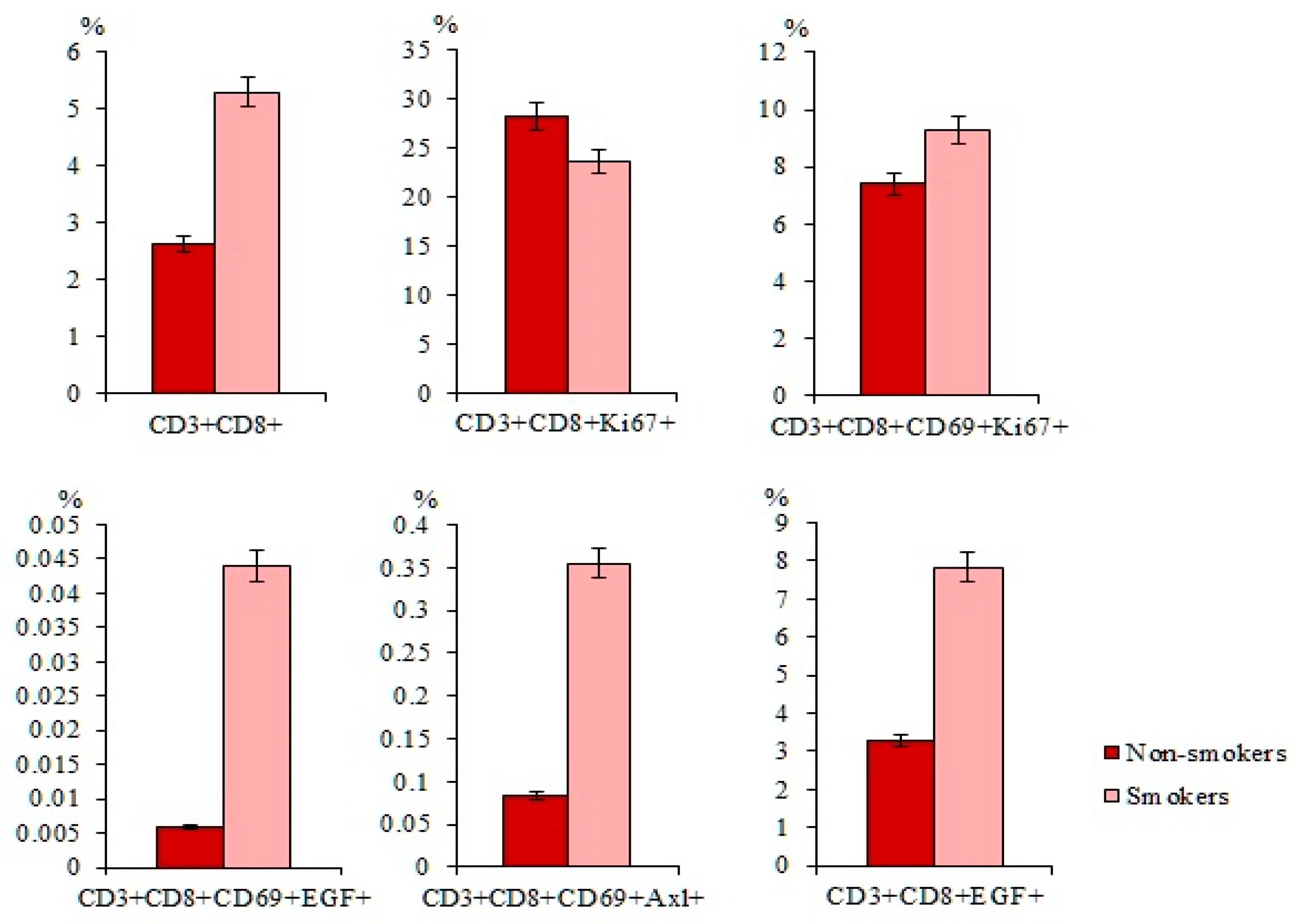
2.2. Smoking Increases the Expression of Tumor Markers in Healhy Volunteers and the Number of CTCs in Patients with SCLC
2.3. The Number of CD3+CD8+ T-Lymphocytes was Decreased in the Blood of Patients with SCLC
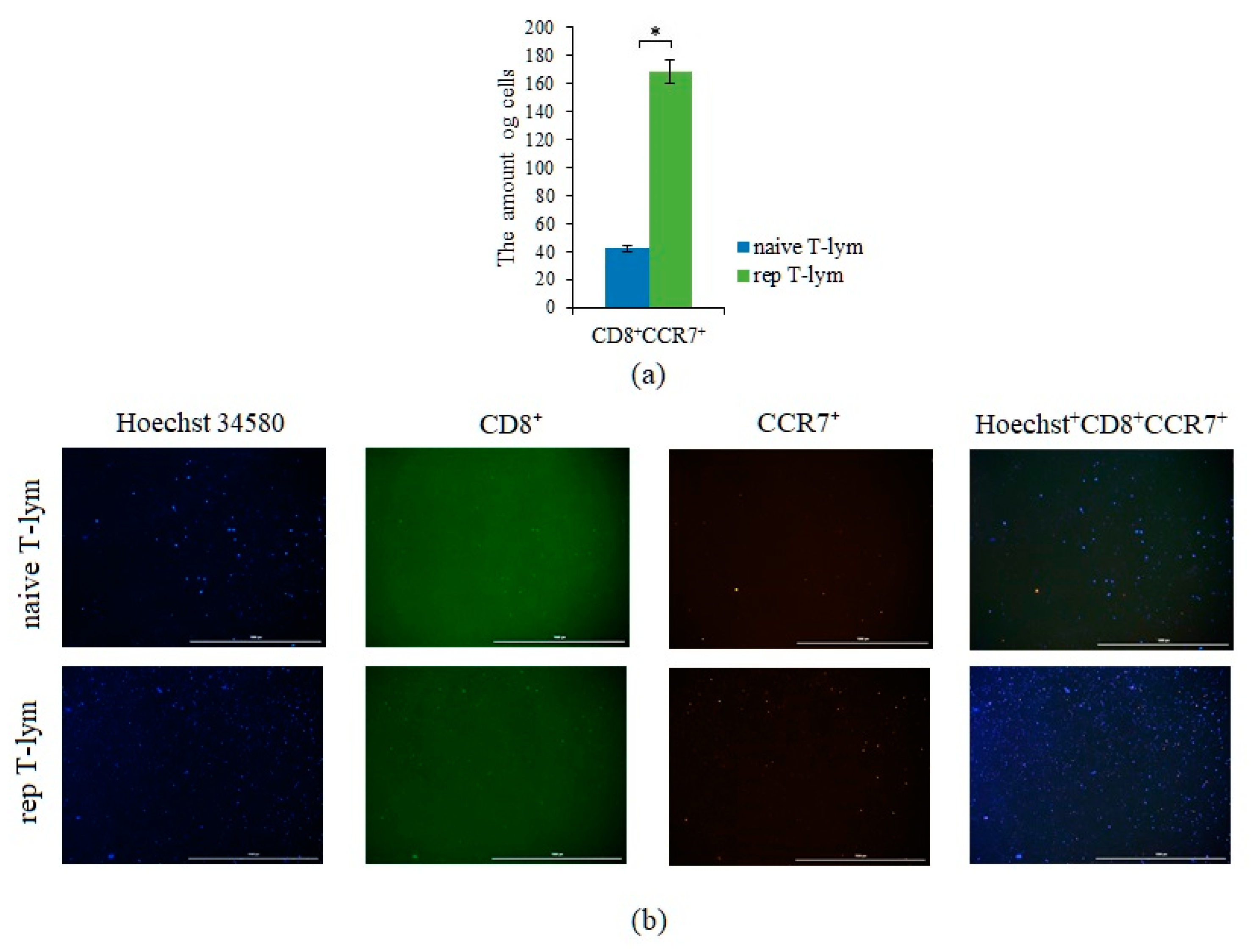
2.4. Smoking Increases the Number of CD3+CD8+ T-Lymphocytes in Patients with SCLC
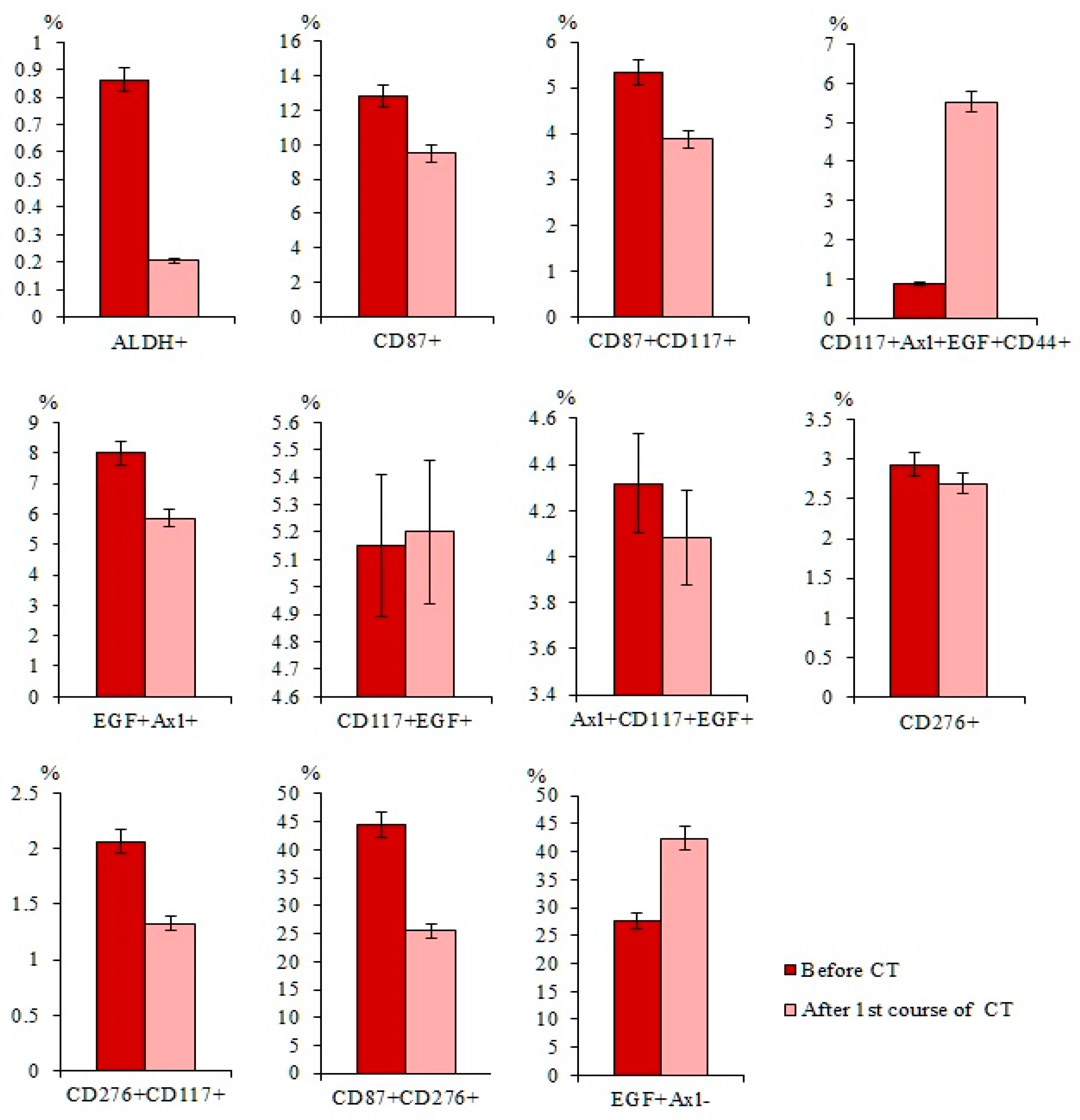
2.5. Cytostatic-Resistant CTCs Are Present in Patients with SCLC after Chemotherapy
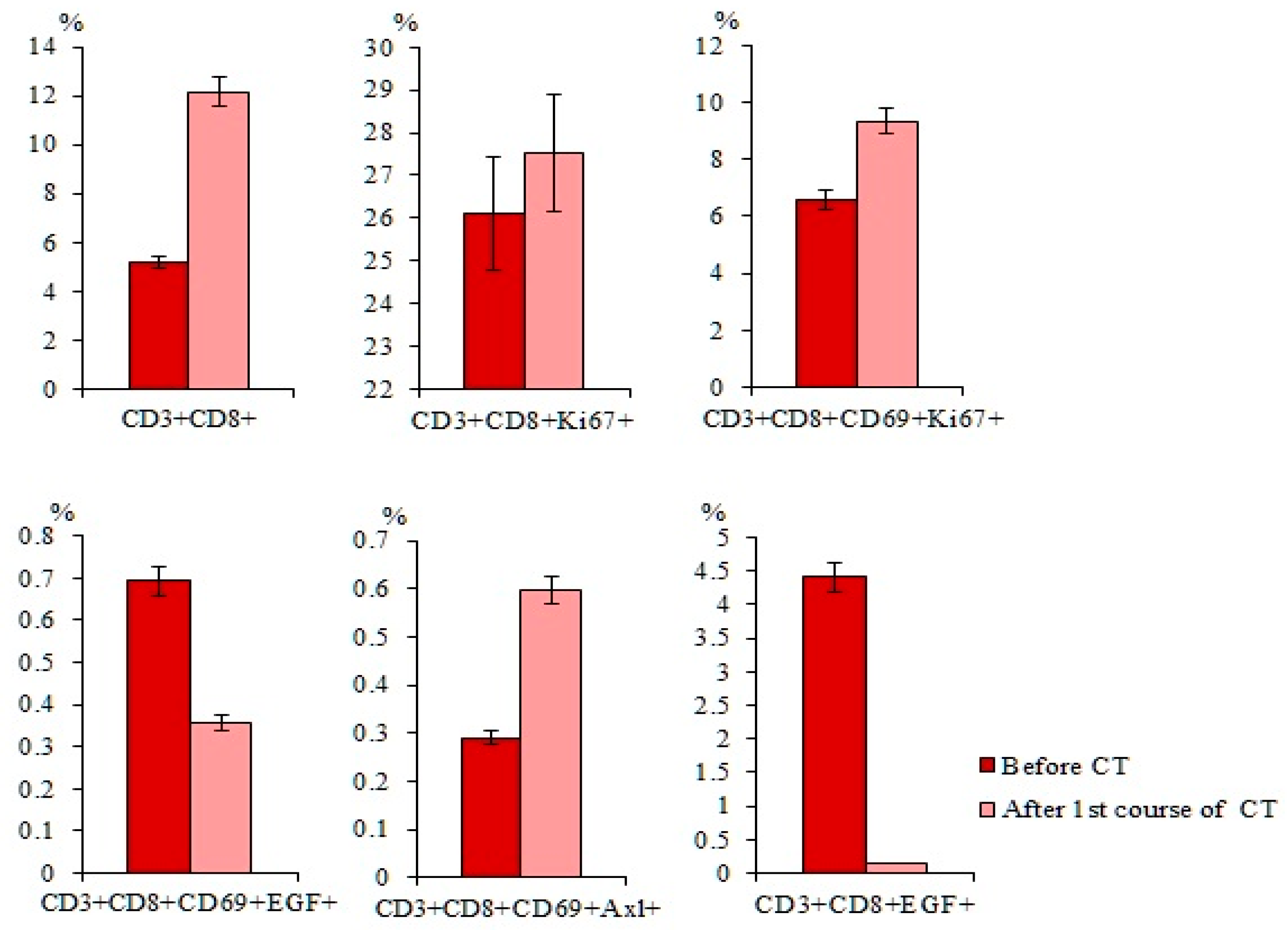
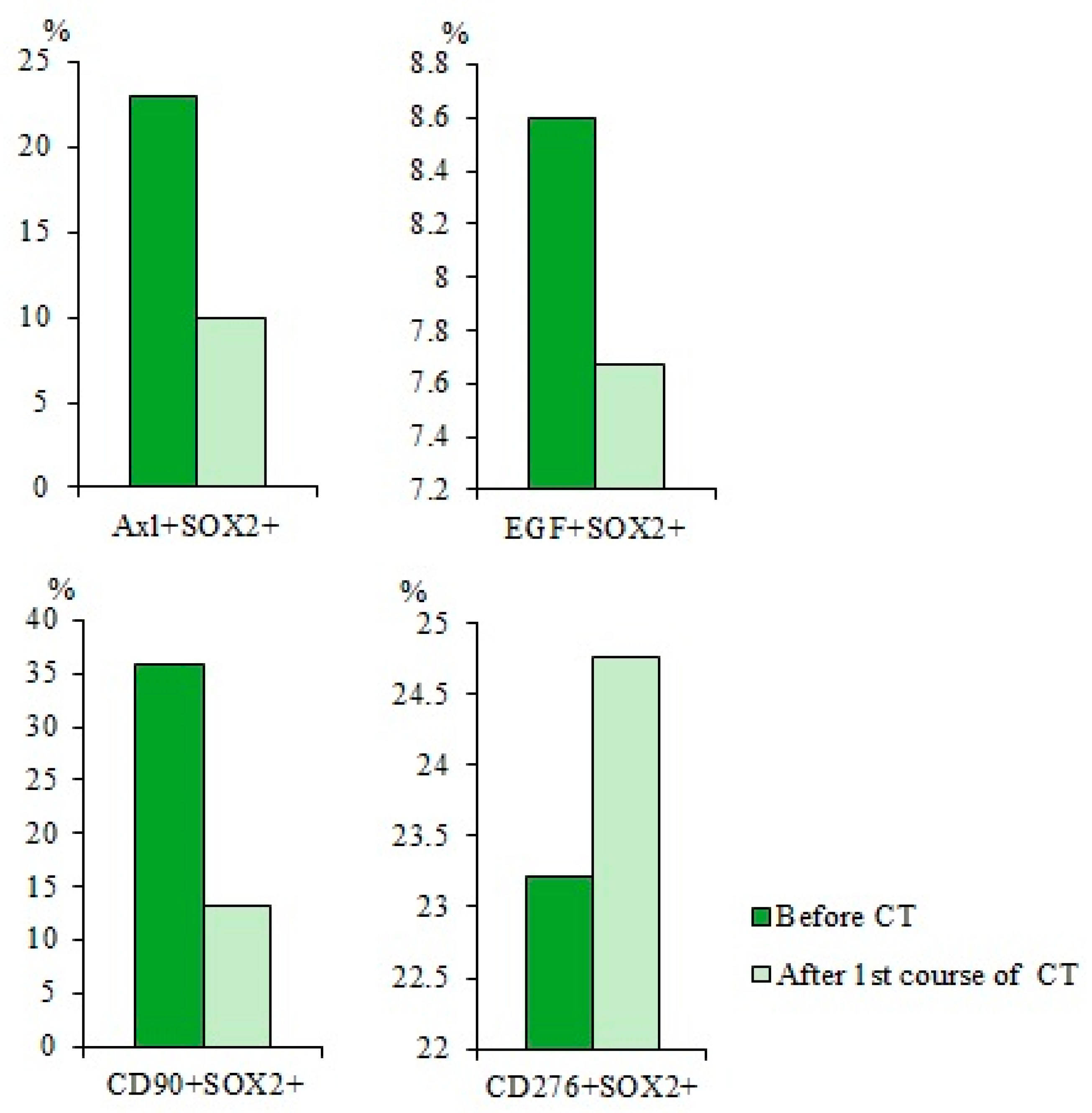
2.6. Chemotherapy had Differential Effects on CD3+CD8+ T-Lymphocytes in Patients with SCLC
2.7. Characteristics of CTCs and CD3+CD8+ T-Lymphocytes in Smoking Patient G with SCLC
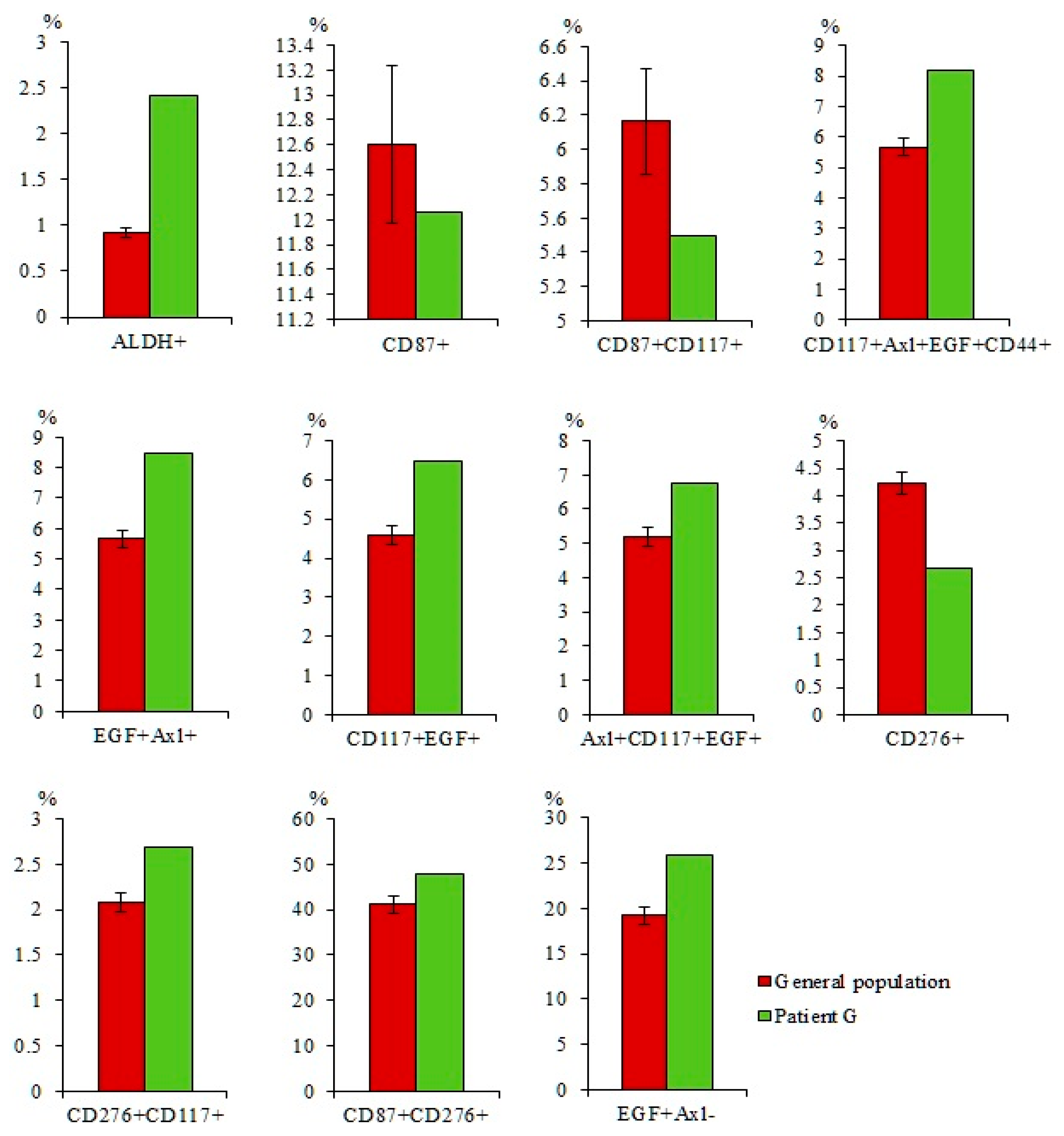
2.7.1. A Larger Number of CTCs were Found in the Blood of Patient G Than in the General Group of Patients with SCLC
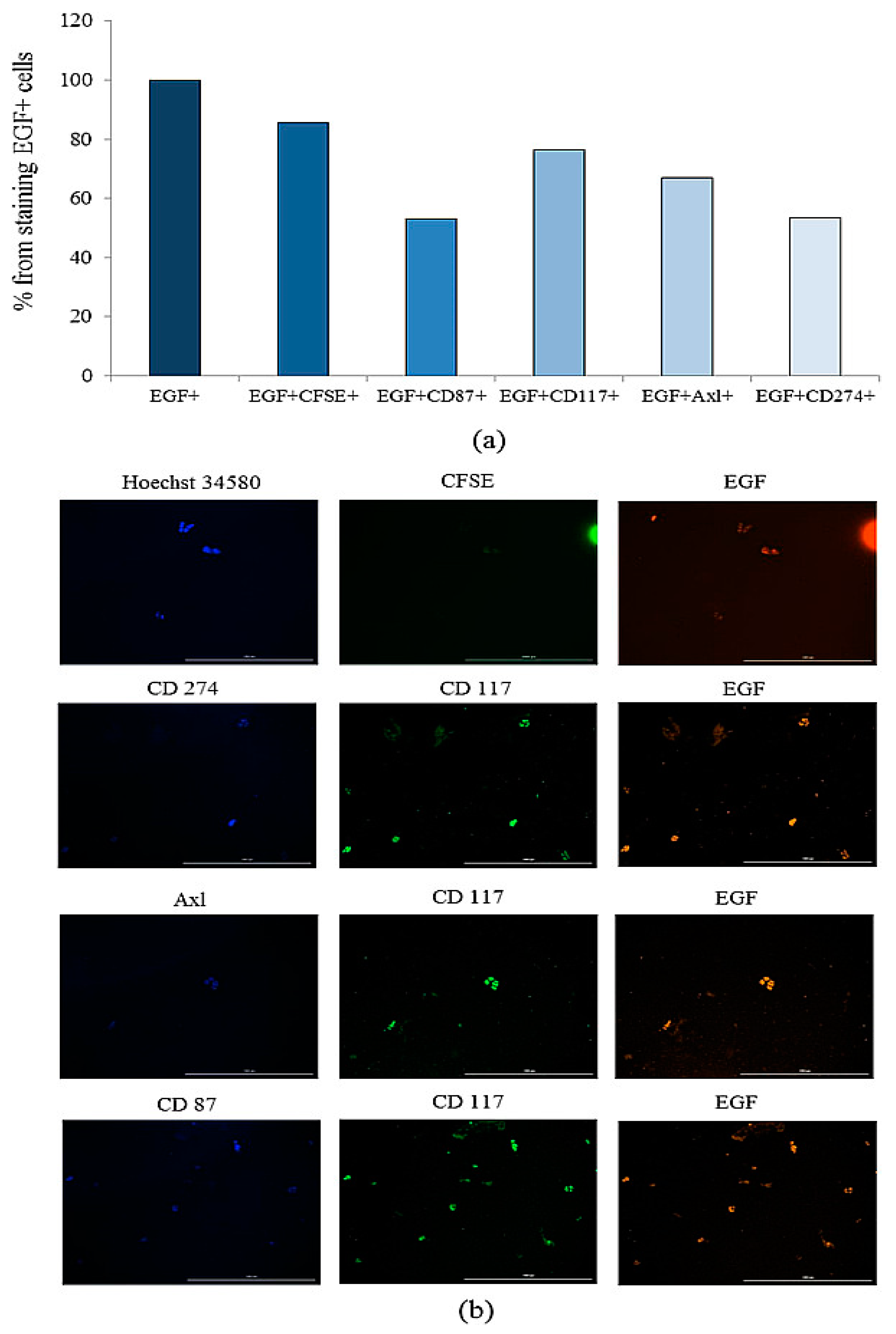
2.7.2. A Larger Number of CD3+CD8+CD69+Axl+ and CD3+CD8+EGF+ T-Lymphocytes were Found in the Blood of Patient G compared to the General Group of Patients with SCLC
2.7.3. A Higher Number of Subpopulations of CTCs Resistant to Cytostatic were Detected in the Blood of Patient G
2.7.4. Differences in the Effect of Cytostatics on CD3+CD8+ T-Lymphocytes of Patient G were Revealed Relative to the General Group of Patients with SCLC
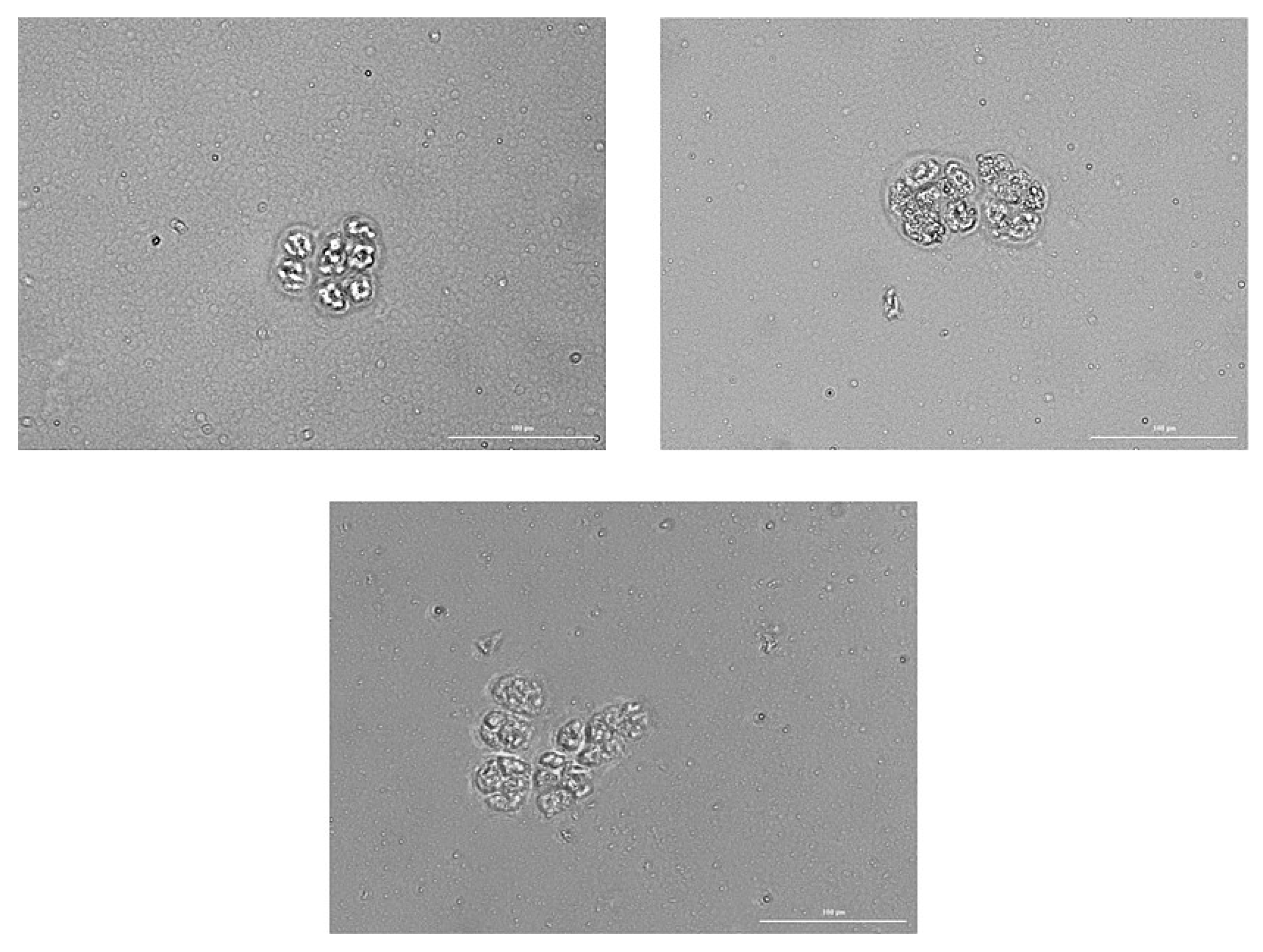
2.7.5. Tumor Cells Isolated from the Blood of Patient G Form Spheroids In Vitro, Which Included Cells Expressing CD87, CD117, CD274, EGF, and Axl
2.8. Reprogrammed CD3+CD8+ T-Lymphocytes of Volunteer K Have Cytotoxic Effects on CSC of Patient G In Vitro
- −
- Spheroids without apoptotic changes without the inclusion of rTcells;
- −
- Spheroids with apoptotic changes with the inclusion of rTcells;
- −
- Spheroids with apoptotic changes without the inclusion of rTcells.
3. Discussion
4. Material and Methods
4.1. Patients
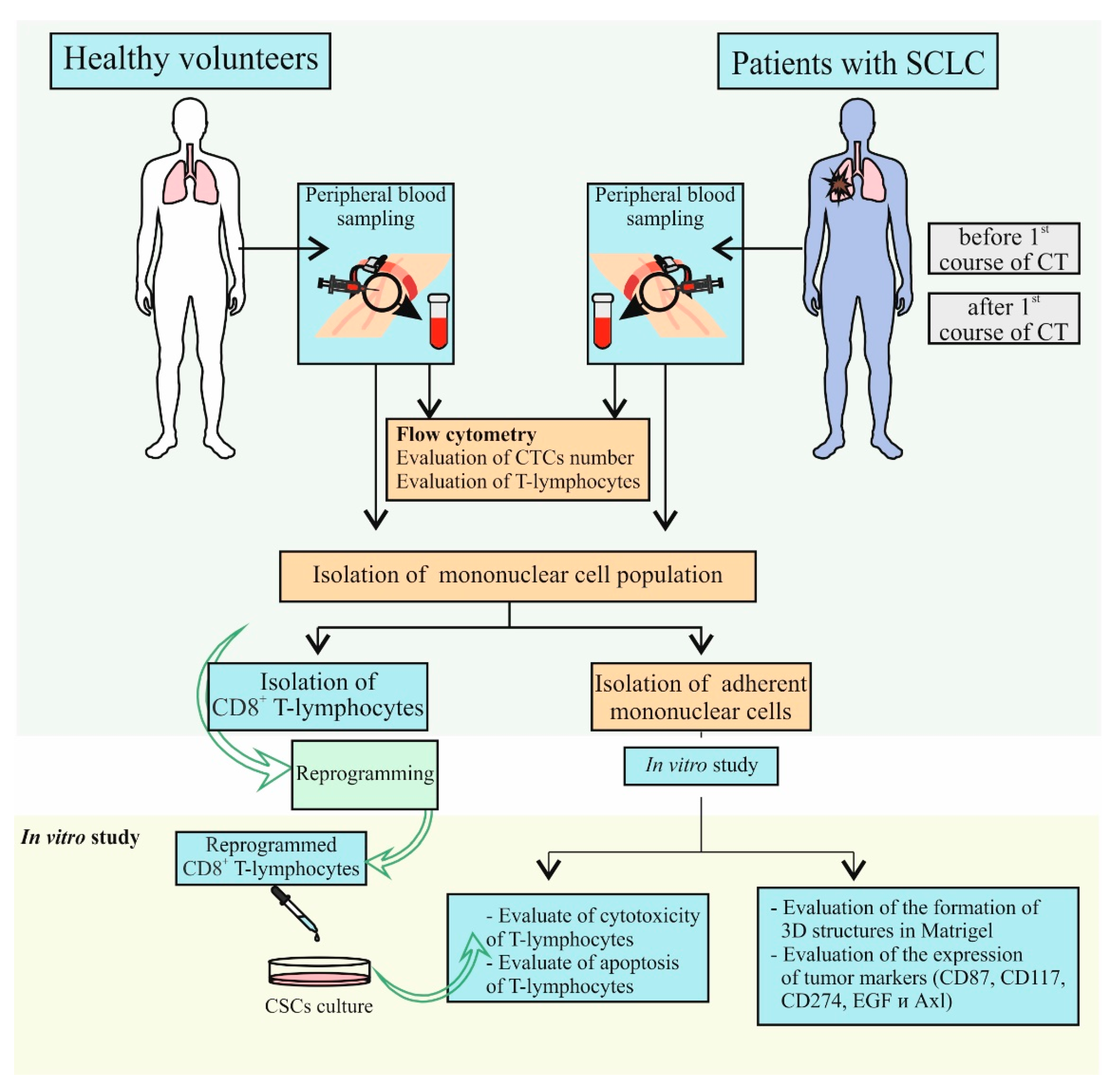
4.2. Design of Investigation
4.3. Isolation of Blood Mononuclear Cells
4.4. Cryopreservation of Mononuclear Cells Obtained from Blood of Patients with SCLC
4.5. Magnetic Separation of Human CD8+ T-lymphocytes
4.6. Flow Cytometry
4.7. Measurement of ALDH Activity
4.8. Cultivation of CSCs Obtained from Blood of Patients with SCLC
4.9. Reprogramming of Human CD8+ T-Lymphocytes
4.10. Detection of the CCR7 Expression on Reprogrammed Human CD8+ T-Lymphocytes In Vitro
4.11. Detection of the Cytotoxicity and Apoptosis of Reprogrammed Human CD8+ T-Lymphocytes In Vitro
4.12. Detection of the CSCs
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bade, B.C.; Dela Cruz, C.S. Lung cancer 2020: Epidemiology, etiology, and prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 1 August 2022).
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- American Cancer Society. Available online: https://www.cancer.org/cancer/lung-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 1 August 2022).
- Codony-Servat, J.; Verlicchi, A.; Rosell, R. Cancer stem cells in small cell lung cancer. Transl. Lung Cancer Res. 2016, 5, 16–25. [Google Scholar] [CrossRef]
- Prabavathy, D.; Ramadoss, N. Heterogeneity of small cell lung cancer stem cells. Adv. Exp. Med. Biol. 2019, 1139, 41–57. [Google Scholar] [CrossRef]
- An, N.; Wang, H.; Jia, W.; Jing, W.; Liu, C.; Zhu, H.; Yu, J. The prognostic role of circulating CD8+ T cell proliferation in patients with untreated extensive stage small cell lung cancer. J. Transl. Med. 2019, 17, 402. [Google Scholar] [CrossRef]
- Skurikhin, E.; Pershina, O.; Zhukova, M.; Widera, D.; Ermakova, N.; Pan, E.; Pakhomova, A.; Morozov, S.; Kubatiev, A.; Dygai, A. Potential of stem cells and CART as a potential polytherapy for small cell lung cancer. Front. Cell Dev. Biol. 2021, 9, 778020. [Google Scholar] [CrossRef]
- Korde, R.; Veluswamy, R.; Barnes, G. Small cell lung cancer patients treated with immune checkpoint inhibitor: A systematic literature review of treatment efficacy, safety, and quality of life. Curr. Med. Res. Opin. 2022, 14, 1361–1368. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, M.; Ding, Y.; Guo, F.; Chen, M.; Lu, T. The efficacy and safety of immune checkpoint inhibitor in patients with relapsed small-cell lung cancer: A systematic review and meta-analysis. J. Clin. Pharm. Ther. 2022, 47, 421–429. [Google Scholar] [CrossRef]
- Schmid, S.; Früh, M. Immune checkpoint inhibitors and small cell lung cancer: What’s new? J. Thorac. Dis. 2018, 10, S1503–S1508. [Google Scholar] [CrossRef] [PubMed]
- Hochmair, M.; Rath, B.; Klameth, L.; Ulsperger, E.; Weinlinger, C.; Fazekas, A.; Plangger, A.; Zeillinger, R.; Hamilton, G. Effects of salinomycin and niclosamide on small cell lung cancer and small cell lung cancer circulating tumor cell lines. Investig. New Drugs 2020, 38, 946–955. [Google Scholar] [CrossRef]
- Maiuthed, A.; Chantarawong, W.; Chanvorachote, P. Lung cancer stem cells and cancer stem cell-targeting natural compounds. Anticancer Res. 2018, 38, 3797–3809. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J. Tumor cell plasticity and intrinsic immunogenicity: Implications for immunotherapy resistance in small-cell lung cancer. Thorac. Cancer 2021, 12, 2523–2525. [Google Scholar] [CrossRef]
- Pershina, O.; Ermakova, N.; Pakhomova, A.; Widera, D.; Pan, E.; Zhukova, M.; Slonimskaya, E.; Morozov, S.G.; Kubatiev, A.; Dygai, A.; et al. Cancer stem cells and somatic stem cells as potential new drug targets, prognosis markers, and therapy efficacy predictors in breast cancer treatment. Biomedicines 2021, 9, 1223. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, G.; Rath, B. Role of circulating tumor cell spheroids in drug resistance. Cancer Drug Resist. 2019, 2, 762–772. [Google Scholar] [CrossRef]
- Ishiguro, T.; Ohata, H.; Sato, A.; Yamawaki, K.; Enomoto, T.; Okamoto, K. Tumor-derived spheroids: Relevance to cancer stem cells and clinical applications. Cancer Sci. 2017, 108, 283–289. [Google Scholar] [CrossRef]
- Prado-Garcia, H.; Romero-Garcia, S.; Aguilar-Cazares, D.; Meneses-Flores, M.; Lopez-Gonzalez, J.S. Tumor-induced CD8+ T-cell dysfunction in lung cancer patients. Clin. Dev. Immunol. 2012, 2012, 741741. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Zhu, B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015, 6, e1792. [Google Scholar] [CrossRef]
- Kim, C.G.; Hong, M.H.; Kim, K.H.; Seo, I.H.; Ahn, B.C.; Pyo, K.H.; Synn, C.B.; Yoon, H.I.; Shim, H.S.; Lee, Y.I.; et al. Dynamic changes in circulating PD-1+CD8+ T lymphocytes for predicting treatment response to PD-1 blockade in patients with non-small-cell lung cancer. Eur. J. Cancer 2021, 143, 113–126. [Google Scholar] [CrossRef]
- Gattinoni, L.; Klebanoff, C.A.; Restifo, N.P. Paths to stemness: Building the ultimate antitumour T cell. Nat. Rev. Cancer 2012, 12, 671–684. [Google Scholar] [CrossRef]
- Kim, W.T.; Ryu, C.J. Cancer stem cell surface markers on normal stem cells. BMB Rep. 2017, 50, 285–298. [Google Scholar] [CrossRef]
- Liu, X.; Qiao, B.; Zhao, T.; Hu, F.; Lam, A.K.; Tao, Q. Sox2 promotes tumor aggressiveness and epithelial-mesenchymal transition in tongue squamous cell carcinoma. Int. J. Mol. Med. 2018, 42, 1418–1426. [Google Scholar] [CrossRef]
- Sodja, E.; Rijavec, M.; Koren, A.; Sadikov, A.; Korošec, P.; Cufer, T. The prognostic value of whole blood SOX2, NANOG and OCT4 mRNA expression in advanced small-cell lung cancer. Radiol. Oncol. 2016, 50, 188–196. [Google Scholar] [CrossRef]
- Karachaliou, N.; Rosell, R.; Viteri, S. The role of SOX2 in small cell lung cancer, lung adenocarcinoma and squamous cell carcinoma of the lung. Transl. Lung Cancer Res. 2013, 2, 172–179. [Google Scholar] [CrossRef]
- Bernhardt, E.B.; Jalal, S.I. Small cell lung cancer. Cancer Treat. Res. 2016, 170, 301–322. [Google Scholar] [CrossRef]
- Lozano, T.; Chocarro, S.; Martin, C.; Lasarte-Cia, A.; Del Valle, C.; Gorraiz, M.; Sarrión, P.; de Galarreta, M.R.; Lujambio, A.; Hervás-Stubbs, S.; et al. Genetic modification of CD8+ T cells to express EGFR: Potential application for adoptive T cell therapies. Front. Immunol. 2019, 10, 2990. [Google Scholar] [CrossRef]
- Saygin, C.; Matei, D.; Majeti, R.; Reizes, O.; Lathia, J.D. Targeting cancer stemness in the clinic: From hype to hope. Cell Stem Cell 2019, 24, 25–40. [Google Scholar] [CrossRef]
- Lu, L.; Liang, Q.; Shen, S.; Feng, L.; Jin, L.; Liang, Z.F. Tobacco smoke plays an important role in initiation and development of lung cancer by promoting the characteristics of cancer stem cells. Cancer Manag. Res. 2020, 12, 9735–9739. [Google Scholar] [CrossRef]
- Xu, L.; Chen, D.; Lu, C.; Liu, X.; Wu, G.; Zhang, Y. Advanced lung cancer is associated with decreased expression of perforin, CD95, CD38 by circulating CD3+CD8+ T lymphocytes. Ann. Clin. Lab. Sci. 2015, 45, 528–532. [Google Scholar]
- De Sousa, V.M.L.; Carvalho, L. Heterogeneity in lung cancer. Pathobiology 2018, 85, 96–107. [Google Scholar] [CrossRef]
- Chmielik, E.; Rusinek, D.; Oczko-Wojciechowska, M.; Jarzab, M.; Krajewska, J.; Czarniecka, A.; Jarzab, B. Heterogeneity of thyroid cancer. Pathobiology 2018, 85, 117–129. [Google Scholar] [CrossRef]
- Zheng, H.; Pomyen, Y.; Hernandez, M.O.; Li, C.; Livak, F.; Tang, W.; Dang, H.; Greten, T.F.; Davis, J.L.; Zhao, Y.; et al. Single-cell analysis reveals cancer stem cell heterogeneity in hepatocellular carcinoma. Hepatology 2018, 68, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.A.; Gay, C.M.; Xi, Y.; Sivajothi, S.; Sivakamasundari, V.; Fujimoto, J.; Bolisetty, M.; Hartsfield, P.M.; Balasubramaniyan, V.; Chalishazar, M.D.; et al. Single-cell analyses reveal increased intratumoral heterogeneity after the onset of therapy resistance in small-cell lung cancer. Nat. Cancer 2020, 1, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Pisco, A.O.; Huang, S. Non-genetic cancer cell plasticity and therapy-induced stemness in tumour relapse: ‘What does not kill me strengthens me’. Br. J. Cancer 2015, 112, 1725–1732. [Google Scholar] [CrossRef]
- Greenbaum, A.; Martin, D.R.; Bocklage, T.; Lee, J.H.; Ness, S.A.; Rajput, A. Tumor heterogeneity as a predictor of response to neoadjuvant chemotherapy in locally advanced rectal cancer. Clin. Colorectal Cancer 2019, 18, 102–109. [Google Scholar] [CrossRef]
- Janiszewska, M.; Stein, S.; Filho, O.M.; Eng, J.; Kingston, N.L.; Harper, N.W.; Rye, I.H.; Alečković, M.; Trinh, A.; Murphy, K.C.; et al. The impact of tumor epithelial and microenvironmental heterogeneity on treatment responses in HER2+ breast cancer. JCI Insight 2021, 6, e147617. [Google Scholar] [CrossRef]
- Hiltermann, T.J.N.; Pore, M.M.; van den Berg, A.; Timens, W.; Boezen, H.M.; Liesker, J.J.W.; Schouwink, J.H.; Wijnands, W.J.A.; Kerner, G.S.M.A.; Kruyt, F.A.E.; et al. Circulating tumor cells in small-cell lung cancer: A predictive and prognostic factor. Ann. Oncol. 2012, 23, 2937–2942. [Google Scholar] [CrossRef]
- Messaritakis, I.; Politaki, E.; Plataki, M.; Karavassilis, V.; Kentepozidis, N.; Koinis, F.; Samantas, E.; Georgoulias, V.; Kotsakis, A. Heterogeneity of circulating tumor cells (CTCs) in patients with recurrent small cell lung cancer (SCLC) treated with pazopanib. Lung Cancer 2017, 104, 16–23. [Google Scholar] [CrossRef]
- Shen, J.; Zhao, J.; Jiang, T.; Li, X.; Zhao, C.; Su, C.; Zhou, C. Predictive and prognostic value of folate receptor-positive circulating tumor cells in small cell lung cancer patients treated with first-line chemotherapy. Oncotarget 2017, 8, 49044–49052. [Google Scholar] [CrossRef]
- Skurikhin, E.G.; Pershina, O.; Ermakova, N.; Pakhomova, A.; Widera, D.; Zhukova, M.; Pan, E.; Sandrikina, L.; Kogai, L.; Kushlinskii, N.; et al. Reprogrammed CD8+ T-lymphocytes isolated from bone marrow have anticancer potential in lung cancer. Biomedicines 2022, 10, 1450. [Google Scholar] [CrossRef]
- Eke, I.; Hehlgans, S.; Sandfort, V.; Cordes, N. 3D matrix-based cell cultures: Automated analysis of tumor cell survival and proliferation. Int. J. Oncol. 2016, 48, 313–321. [Google Scholar] [CrossRef]
- Roudi, R.; Madjd, Z.; Korourian, A.; Mehrazma, M.; Molanae, S.; Sabet, M.N.; Shariftabrizi, A. Clinical significance of putative cancer stem cell marker CD44 in different histological subtypes of lung cancer. Cancer Biomark. 2014, 14, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Masciale, V.; Grisendi, G.; Banchelli, F.; D’Amico, R.; Maiorana, A.; Sighinolfi, P.; Pinelli, M.; Lovati, E.; Stefani, A.; Morandi, U.; et al. Correlating tumor-infiltrating lymphocytes and lung cancer stem cells: A cross-sectional study. Ann. Transl. Med. 2019, 7, 619. [Google Scholar] [CrossRef] [PubMed]
- Ling, M.; Yang, X. Correlation between cancer stem cells (CSCs) and tumor-infiltrating lymphocytes (TILs): Do TILs interact with CSCs in non-small cell lung cancer? Ann. Transl. Med. 2020, 8, 914. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, S.; Zernecke, A. CD8+ T cells in atherosclerosis. Cells 2020, 10, 37. [Google Scholar] [CrossRef]
- Del Vecchio, F.; Martinez-Rodriguez, V.; Schukking, M.; Cocks, A.; Broseghini, E.; Fabbri, M. Professional killers: The role of extracellular vesicles in the reciprocal interactions between natural killer, CD8+ cytotoxic T-cells and tumour cells. J. Extracell. Vesicles 2021, 10, e12075. [Google Scholar] [CrossRef]
- Soares, A.; Govender, L.; Hughes, J.; Mavakla, W.; de Kock, M.; Barnard, C.; Pienaar, B.; van Rensburg, E.J.; Jacobs, G.; Khomba, G.; et al. Novel application of Ki67 to quantify antigen-specific in vitro lymphoproliferation. J. Immunol. Methods 2010, 362, 43–50. [Google Scholar] [CrossRef]
- Buggert, M.; Nguyen, S.; Salgado-Montes de Oca, G.; Bengsch, B.; Darko, S.; Ransier, A.; Roberts, E.R.; Del Alcazar, D.; Brody, I.B.; Vella, L.A.; et al. Identification and characterization of HIV-specific resident memory CD8+ T cells in human lymphoid tissue. Sci. Immunol. 2018, 3, eaar4526. [Google Scholar] [CrossRef]
- Berard, M.; Tough, D.F. Qualitative differences between naïve and memory T cells. Immunology 2002, 106, 127–138. [Google Scholar] [CrossRef]
- Guo, Z.; Li, Y.; Zhang, D.; Ma, J. Axl inhibition induces the antitumor immune response which can be further potentiated by PD-1 blockade in the mouse cancer models. Oncotarget 2017, 8, 89761–89774. [Google Scholar] [CrossRef]
- Yan, S.; Vandewalle, N.; De Beule, N.; Faict, S.; Maes, K.; De Bruyne, E.; Menu, E.; Vanderkerken, K.; De Veirman, K. AXL receptor tyrosine kinase as a therapeutic target in hematological malignancies: Focus on multiple myeloma. Cancers 2019, 11, 1727. [Google Scholar] [CrossRef]
- Colavito, S.A. AXL as a Target in Breast Cancer Therapy. J. Oncol. 2020, 2020, 5291952. [Google Scholar] [CrossRef] [PubMed]
- Romer, J.; Nielsen, B.; Ploug, M. The urokinase receptor as a potential target in cancer therapy. Curr. Pharm. Des. 2004, 10, 2359–2376. [Google Scholar] [CrossRef] [PubMed]
- Gutova, M.; Najbauer, J.; Gevorgyan, A.; Metz, M.Z.; Weng, Y.; Shih, C.-C.; Aboody, K.S. Identification of uPAR-positive chemoresistant cells in small cell lung cancer. PLoS ONE 2007, 2, e243. [Google Scholar] [CrossRef] [PubMed]
- Almasi, C.E.; Drivsholm, L.; Pappot, H.; Høyer-Hansen, G.; Christensen, I.J. The liberated domain i of urokinase plasminogen activator receptor—A new tumour marker in small cell lung cancer. APMIS 2013, 121, 189–196. [Google Scholar] [CrossRef]
- Kubo, T.; Takigawa, N.; Osawa, M.; Harada, D.; Ninomiya, T.; Ochi, N.; Ichihara, E.; Yamane, H.; Tanimoto, M.; Kiura, K. Subpopulation of small-cell lung cancer cells expressing CD133 and CD87 show resistance to chemotherapy. Cancer Sci. 2013, 104, 78–84. [Google Scholar] [CrossRef]
- MacDonagh, L.; Gray, S.G.; Breen, E.; Cuffe, S.; Finn, S.P.; O’Byrne, K.J.; Barr, M.P. Lung cancer stem cells: The root of resistance. Cancer Lett. 2016, 372, 147–156. [Google Scholar] [CrossRef]
- Miettinen, M.; Lasota, J. KIT (CD117): A review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl. Immunohistochem. Mol. Morphol. 2005, 13, 205–220. [Google Scholar] [CrossRef]
- Walcher, L.; Kistenmacher, A.K.; Suo, H.; Kitte, R.; Dluczek, S.; Strauß, A.; Blaudszun, A.R.; Yevsa, T.; Fricke, S.; Kossatz-Boehlert, U. Cancer stem cells-origins and biomarkers: Perspectives for targeted personalized therapies. Front. Immunol. 2020, 11, 1280. [Google Scholar] [CrossRef]
- Jones, R.J.; Barber, J.P.; Vala, M.S.; Collector, M.I.; Kaufmann, S.H.; Ludeman, S.M.; Colvin, O.M.; Hilton, J. Assessment of aldehyde dehydrogenase in viable cells. Blood 1995, 85, 2742–2746. [Google Scholar] [CrossRef]
- Ginestier, C.; Hur, M.H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Jacquemier, J.; Viens, P.; Kleer, C.G.; Liu, S.; et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007, 1, 555–567. [Google Scholar] [CrossRef]
- Huang, E.H.; Hynes, M.J.; Zhang, T.; Ginestier, C.; Dontu, G.; Appelman, H.; Fields, J.Z.; Wicha, M.S.; Boman, B.M. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009, 69, 3382–3389. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Qiu, Q.; Khanna, A.; Todd, N.W.; Deepak, J.; Xing, L.; Wang, H.; Liu, Z.; Su, Y.; Stass, S.A.; et al. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol. Cancer Res. 2009, 7, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Okudela, K.; Woo, T.; Mitsui, H.; Suzuki, T.; Tajiri, M.; Sakuma, Y.; Miyagi, Y.; Tateishi, Y.; Umeda, S.; Masuda, M.; et al. Downregulation of ALDH1A1 expression in non-small cell lung carcinomas—Its clinicopathologic and biological significance. Int. J. Clin. Exp. Pathol. 2013, 6, 1–12. [Google Scholar]
- MacDonagh, L.; Gallagher, M.F.; Ffrench, B.; Gasch, C.; Breen, E.; Gray, S.G.; Nicholson, S.; Leonard, N.; Ryan, R.; Young, V.; et al. Targeting the cancer stem cell marker, aldehyde dehydrogenase 1, to circumvent cisplatin resistance in NSCLC. Oncotarget 2017, 8, 72544–72563. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liang, J.; Liu, Z.; Zhang, C.; Wang, Y.; Watson, A.H.; Zhou, C.; Zhang, F.; Wu, K.; Zhang, F.; et al. The role of CD276 in cancers. Front. Oncol. 2021, 11, 654684. [Google Scholar] [CrossRef] [PubMed]




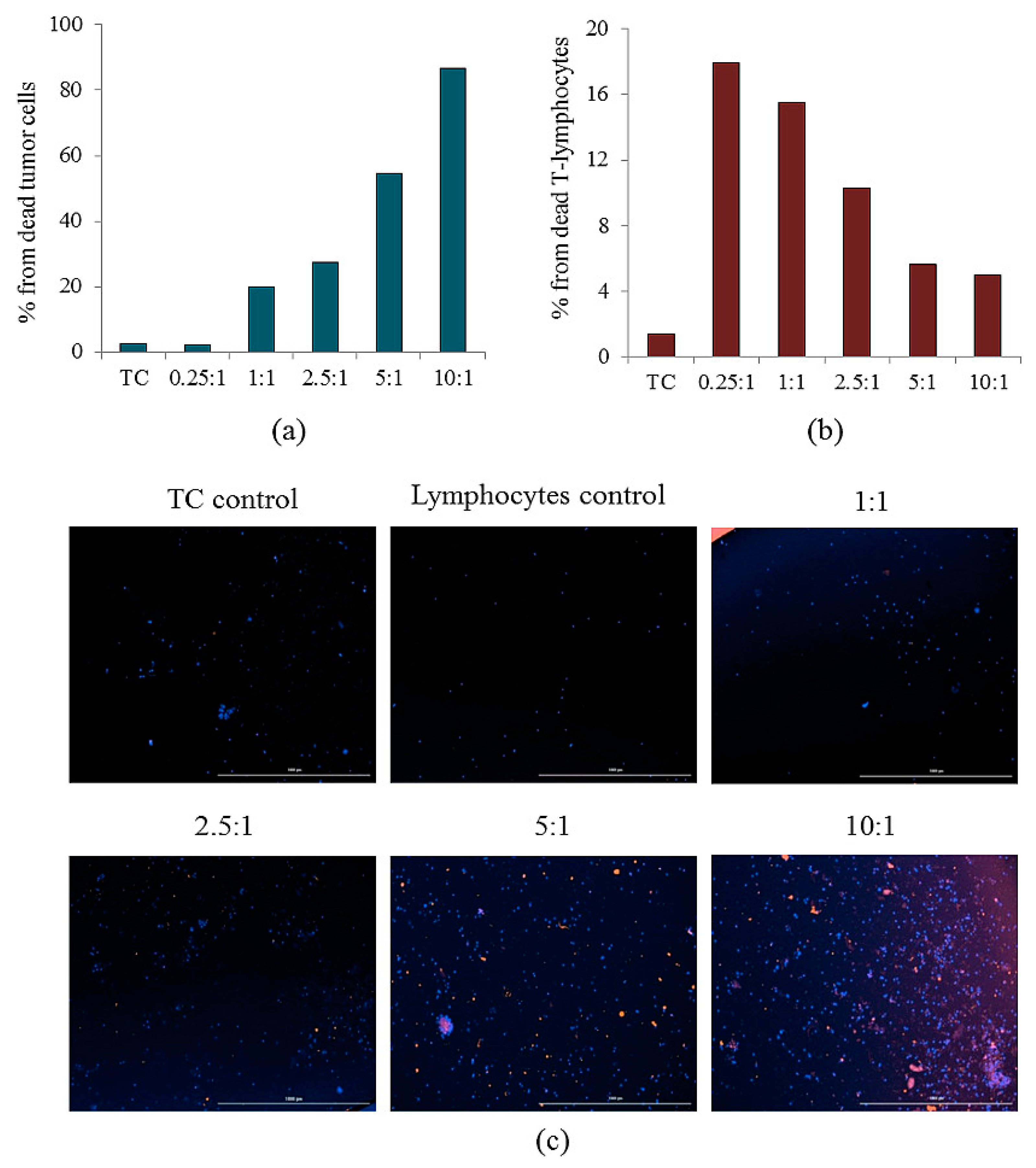

| Number of Cells | Number of Spheroids | % of the Total Number of Spheroids |
|---|---|---|
| from 10 to 14 | 64 | 63.37 |
| from 15 to 19 | 31 | 30.69 |
| from 20 to 24 | 3 | 2.97 |
| from 25 to 29 | 2 | 1.98 |
| from 20 to 34 | 1 | 0.99 |
| CD3+CD8+ T-Lymphocytes | Ratio of Reprogrammed and Naive CD3+CD8+ T-Lymphocytes to Tumor Cells (CSCs) (rTcells/CSCs and nTcells/CSCs) | |||||
|---|---|---|---|---|---|---|
| 0:1 (Tumor Control) | 0.25:1 | 1:1 | 2.5:1 | 5:1 | 10:1 | |
| Naive | 2.68 ± 0.21 | 6.07 ± 0.79 | 15.54 ± 1.53 | 24.19 ± 0.82 | 27.24 ± 3.26 | 31.36 ± 2.2 |
| Reprogrammed | 2.68 ± 0.21 | 5.42 ± 0.61 | 12.30 ± 2.12 | 30.21 ± 2.61 | 46.28 ± 3.59 | 70.06 ± 2.84 |
| Ratio rTcells/CSCs | Spheroids without Apoptosis and Inclusion rTcells | Spheroids with Apoptosis and Inclusion rTcells | Spheroids with Apoptosis and without Inclusion |
|---|---|---|---|
| 0:1 (tumor control) | 100 | 100 | 100 |
| 0.25:1 | 90.47 ± 1.68 | 6.06 ± 0.96 | 3.07 ± 0.97 |
| 1:1 | 76.50 ± 1.66 | 20.39 ± 2.13 | 3.11 ± 0.99 |
| 2.5:1 | 61.62 ± 2.68 | 32.43 ± 2.30 | 5.98 ± 0.82 |
| 5:1 | 30.98 ± 2.01 | 58.73 ± 2.18 | 10.30 ± 0.92 |
| 10:1 | 2.28 ± 1.02 | 24.32 ± 2.31 | 73.41 ± 2.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skurikhin, E.G.; Ermakova, N.; Zhukova, M.; Pershina, O.; Pan, E.; Pakhomova, A.; Kogai, L.; Goldberg, V.; Simolina, E.; Skurikhina, V.; et al. Analysis of Circulating Tumor and Cancer Stem Cells Provides New Opportunities in Diagnosis and Treatment of Small Cell Lung Cancer. Int. J. Mol. Sci. 2022, 23, 10853. https://doi.org/10.3390/ijms231810853
Skurikhin EG, Ermakova N, Zhukova M, Pershina O, Pan E, Pakhomova A, Kogai L, Goldberg V, Simolina E, Skurikhina V, et al. Analysis of Circulating Tumor and Cancer Stem Cells Provides New Opportunities in Diagnosis and Treatment of Small Cell Lung Cancer. International Journal of Molecular Sciences. 2022; 23(18):10853. https://doi.org/10.3390/ijms231810853
Chicago/Turabian StyleSkurikhin, Evgenii G., Natalia Ermakova, Mariia Zhukova, Olga Pershina, Edgar Pan, Angelina Pakhomova, Lena Kogai, Victor Goldberg, Elena Simolina, Victoria Skurikhina, and et al. 2022. "Analysis of Circulating Tumor and Cancer Stem Cells Provides New Opportunities in Diagnosis and Treatment of Small Cell Lung Cancer" International Journal of Molecular Sciences 23, no. 18: 10853. https://doi.org/10.3390/ijms231810853
APA StyleSkurikhin, E. G., Ermakova, N., Zhukova, M., Pershina, O., Pan, E., Pakhomova, A., Kogai, L., Goldberg, V., Simolina, E., Skurikhina, V., Widera, D., Kubatiev, A., Morozov, S. G., Kushlinskii, N., & Dygai, A. (2022). Analysis of Circulating Tumor and Cancer Stem Cells Provides New Opportunities in Diagnosis and Treatment of Small Cell Lung Cancer. International Journal of Molecular Sciences, 23(18), 10853. https://doi.org/10.3390/ijms231810853







