Serum Collected from Preeclamptic Pregnancies Drives Vasoconstriction of Human Omental Arteries—A Novel Ex Vivo Model of Preeclampsia for Therapeutic Development
Abstract
1. Introduction
2. Results
2.1. Antiangiogenic Factors sFlt-1 and sENG, and the Vasoconstrictor ET-1, Are Increased in Serum from Pregnancies Complicated by Preeclampsia
 ) or (B) term preeclampsia (
) or (B) term preeclampsia ( ) and from patients with uncomplicated pregnancies at matched gestation (preterm and term controls;
) and from patients with uncomplicated pregnancies at matched gestation (preterm and term controls;  ) are shown. Circulating sENG concentration in (C) preterm and (D) term preeclamptic serum in comparison to gestation-matched controls are also presented, as well as ET-1 levels in serum collected from (E) preterm and (F) term preeclamptic pregnancies and gestation-matched controls. Individual points represent individual patients, and the data are expressed as mean ± SEM (n = 4–10). p-values indicated as follows: ** p < 0.01, **** p < 0.0001.
) are shown. Circulating sENG concentration in (C) preterm and (D) term preeclamptic serum in comparison to gestation-matched controls are also presented, as well as ET-1 levels in serum collected from (E) preterm and (F) term preeclamptic pregnancies and gestation-matched controls. Individual points represent individual patients, and the data are expressed as mean ± SEM (n = 4–10). p-values indicated as follows: ** p < 0.01, **** p < 0.0001.
 ) or (B) term preeclampsia (
) or (B) term preeclampsia ( ) and from patients with uncomplicated pregnancies at matched gestation (preterm and term controls;
) and from patients with uncomplicated pregnancies at matched gestation (preterm and term controls;  ) are shown. Circulating sENG concentration in (C) preterm and (D) term preeclamptic serum in comparison to gestation-matched controls are also presented, as well as ET-1 levels in serum collected from (E) preterm and (F) term preeclamptic pregnancies and gestation-matched controls. Individual points represent individual patients, and the data are expressed as mean ± SEM (n = 4–10). p-values indicated as follows: ** p < 0.01, **** p < 0.0001.
) are shown. Circulating sENG concentration in (C) preterm and (D) term preeclamptic serum in comparison to gestation-matched controls are also presented, as well as ET-1 levels in serum collected from (E) preterm and (F) term preeclamptic pregnancies and gestation-matched controls. Individual points represent individual patients, and the data are expressed as mean ± SEM (n = 4–10). p-values indicated as follows: ** p < 0.01, **** p < 0.0001.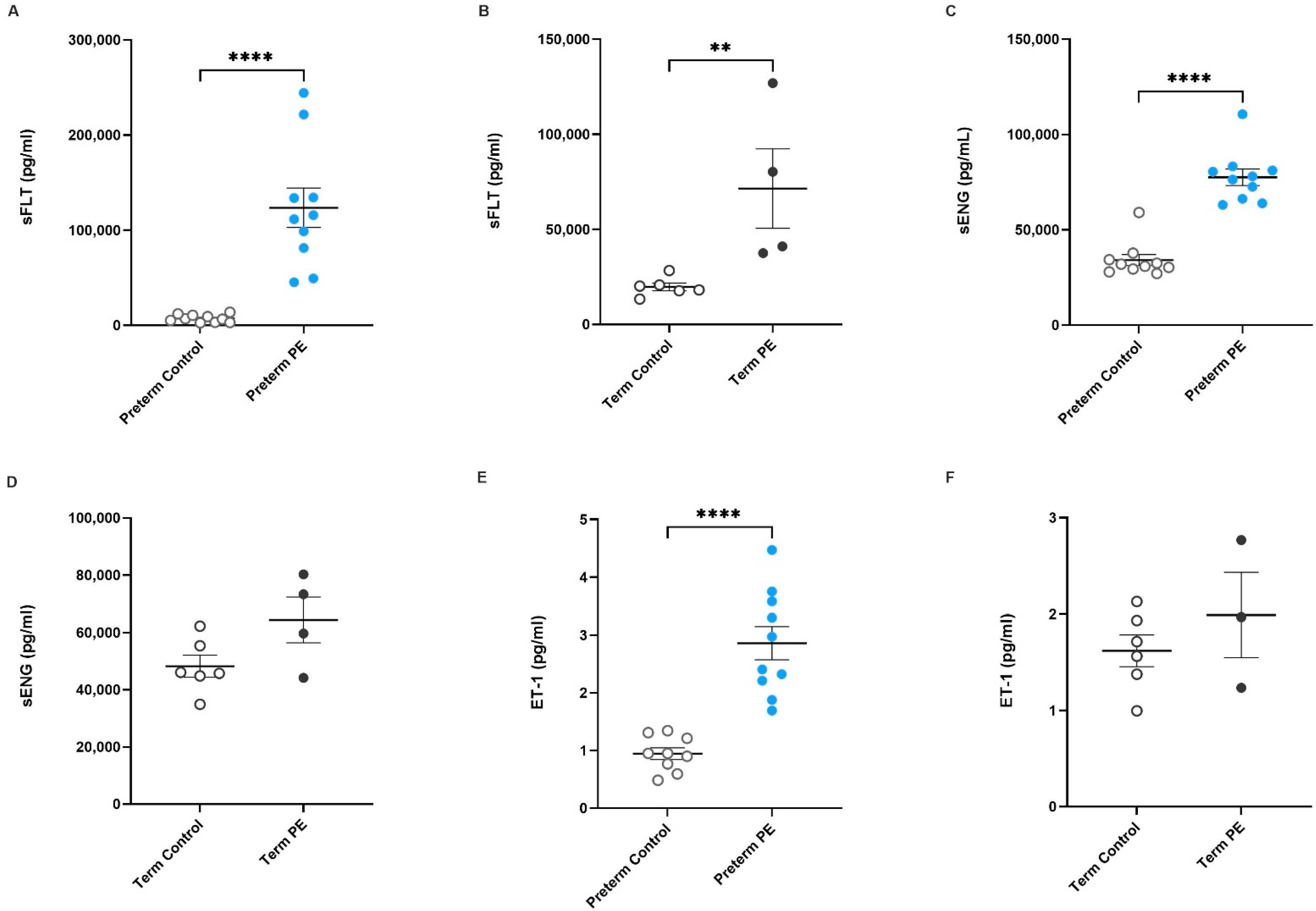
2.2. Serum from Pregnancies Complicated by Preeclampsia Did Not Induce Greater Constriction of Human Omental Arteries Compared to Serum from Gestation-Matched Controls
2.3. Esomeprazole Treatment Induced Vasodilation of Pregnant Human Omental Arteries Pre-Constricted with Serum Collected from Preterm Preeclamptic Patients
 ) and (B) term preeclampsia (
) and (B) term preeclampsia ( ) are compared to gestation-matched preterm and term controls (
) are compared to gestation-matched preterm and term controls (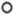 ), respectively. The percentage of constriction was normalised to the maximum constriction induced by 50mM potassium physiological salt solution (KPSS). Analysis of the area under the curve in (C) preterm and (D) term constriction curves is shown; (E) preterm EC50 and (F) term EC50 values are derived; and maximum constriction (Emax) in response to (G) preterm serum and (H) term serum compared to gestation-matched controls is presented. Individual points represent individual patients (n = 4–6). The data are expressed as mean ± SEM.
), respectively. The percentage of constriction was normalised to the maximum constriction induced by 50mM potassium physiological salt solution (KPSS). Analysis of the area under the curve in (C) preterm and (D) term constriction curves is shown; (E) preterm EC50 and (F) term EC50 values are derived; and maximum constriction (Emax) in response to (G) preterm serum and (H) term serum compared to gestation-matched controls is presented. Individual points represent individual patients (n = 4–6). The data are expressed as mean ± SEM.
 ) and (B) term preeclampsia (
) and (B) term preeclampsia ( ) are compared to gestation-matched preterm and term controls (
) are compared to gestation-matched preterm and term controls ( ), respectively. The percentage of constriction was normalised to the maximum constriction induced by 50mM potassium physiological salt solution (KPSS). Analysis of the area under the curve in (C) preterm and (D) term constriction curves is shown; (E) preterm EC50 and (F) term EC50 values are derived; and maximum constriction (Emax) in response to (G) preterm serum and (H) term serum compared to gestation-matched controls is presented. Individual points represent individual patients (n = 4–6). The data are expressed as mean ± SEM.
), respectively. The percentage of constriction was normalised to the maximum constriction induced by 50mM potassium physiological salt solution (KPSS). Analysis of the area under the curve in (C) preterm and (D) term constriction curves is shown; (E) preterm EC50 and (F) term EC50 values are derived; and maximum constriction (Emax) in response to (G) preterm serum and (H) term serum compared to gestation-matched controls is presented. Individual points represent individual patients (n = 4–6). The data are expressed as mean ± SEM.
 ), are presented. The percentage of relaxation was normalised to the maximum relaxation induced by bradykinin. Analysis of the area under the curve (AUC) (B) and maximum relaxation (C) in response to esomeprazole (
), are presented. The percentage of relaxation was normalised to the maximum relaxation induced by bradykinin. Analysis of the area under the curve (AUC) (B) and maximum relaxation (C) in response to esomeprazole ( ) or the vehicle (
) or the vehicle ( ) showed arteries treated with esomeprazole had enhanced relaxation when constricted with serum from preterm preeclampsia. Omental arteries constricted with preterm control (gestation-matched) serum: relaxation curves (D), as well as AUC (E) and maximum relaxation plots (F), demonstrate there was no significant effect with esomeprazole treatment (
) showed arteries treated with esomeprazole had enhanced relaxation when constricted with serum from preterm preeclampsia. Omental arteries constricted with preterm control (gestation-matched) serum: relaxation curves (D), as well as AUC (E) and maximum relaxation plots (F), demonstrate there was no significant effect with esomeprazole treatment ( ) compared to vehicle control (□). Individual points represent individual patients (n = 3). Data are expressed as mean ± SEM; p-values indicated as follows: * p < 0.05.
) compared to vehicle control (□). Individual points represent individual patients (n = 3). Data are expressed as mean ± SEM; p-values indicated as follows: * p < 0.05.
 ), are presented. The percentage of relaxation was normalised to the maximum relaxation induced by bradykinin. Analysis of the area under the curve (AUC) (B) and maximum relaxation (C) in response to esomeprazole (
), are presented. The percentage of relaxation was normalised to the maximum relaxation induced by bradykinin. Analysis of the area under the curve (AUC) (B) and maximum relaxation (C) in response to esomeprazole ( ) or the vehicle (
) or the vehicle ( ) showed arteries treated with esomeprazole had enhanced relaxation when constricted with serum from preterm preeclampsia. Omental arteries constricted with preterm control (gestation-matched) serum: relaxation curves (D), as well as AUC (E) and maximum relaxation plots (F), demonstrate there was no significant effect with esomeprazole treatment (
) showed arteries treated with esomeprazole had enhanced relaxation when constricted with serum from preterm preeclampsia. Omental arteries constricted with preterm control (gestation-matched) serum: relaxation curves (D), as well as AUC (E) and maximum relaxation plots (F), demonstrate there was no significant effect with esomeprazole treatment ( ) compared to vehicle control (□). Individual points represent individual patients (n = 3). Data are expressed as mean ± SEM; p-values indicated as follows: * p < 0.05.
) compared to vehicle control (□). Individual points represent individual patients (n = 3). Data are expressed as mean ± SEM; p-values indicated as follows: * p < 0.05.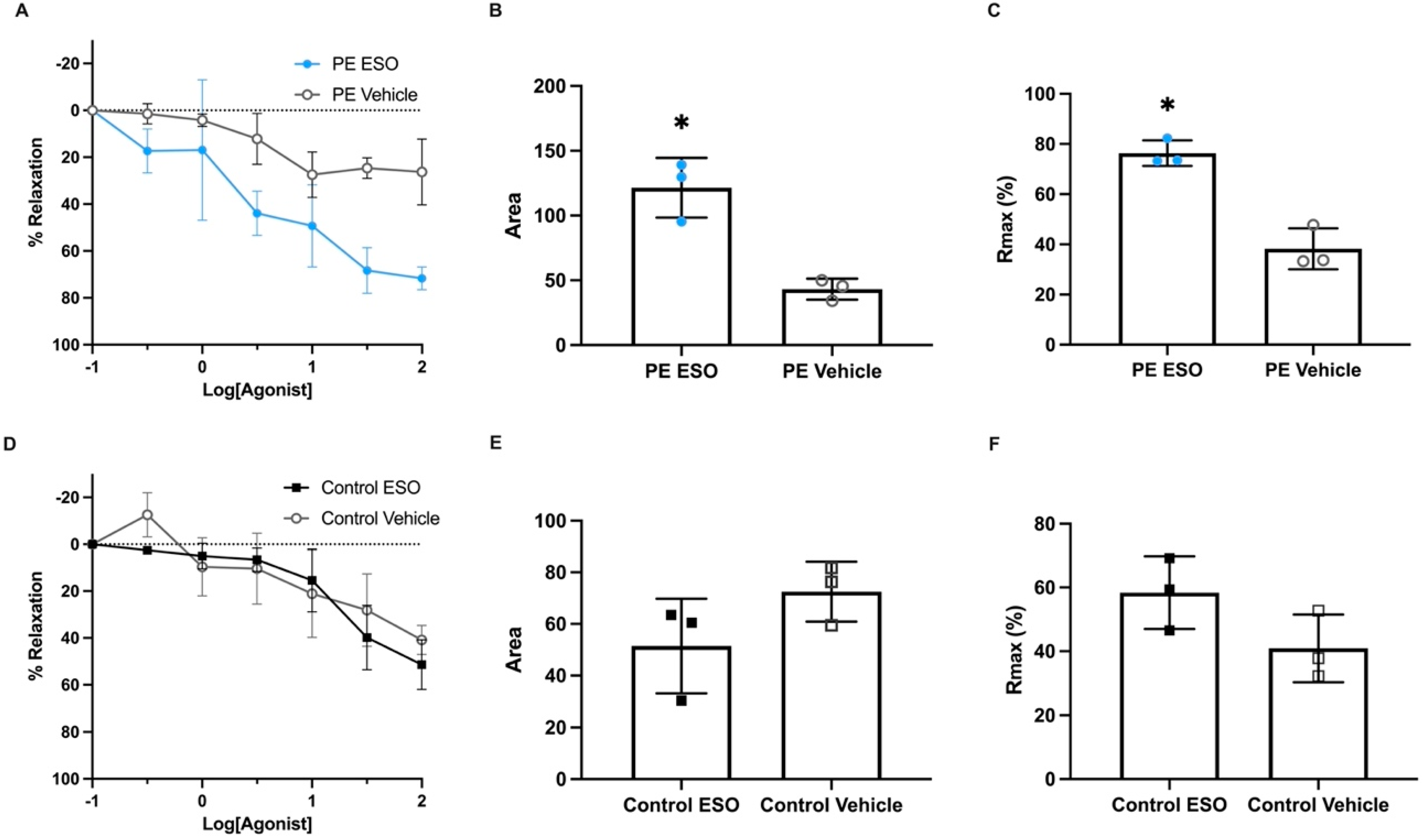
3. Discussion
4. Materials and Methods
4.1. Tissue Collection
4.1.1. Serum Collection
4.1.2. Omental Fat Tissue Collection
4.2. Enzyme-Linked Immunosorbent Assay (ELISA)
4.3. Assessment of Vascular Reactivity
4.4. Serum-Induced Vasoconstriction
4.5. Treatment with the Proton Pump Inhibitor Esomeprazole to Determine Effects on Vasorelaxation
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mol, B.W.J.; Roberts, C.T.; Thangaratinam, S.; Magee, L.A.; de Groot, C.J.M.; Hofmeyr, G.J. Pre-eclampsia. Lancet 2016, 387, 999–1011. [Google Scholar] [CrossRef]
- Khan, K.S.; Wojdyla, D.; Say, L.; Gülmezoglu, A.M.; Van Look, P.F. WHO analysis of causes of maternal death: A systematic review. Lancet 2006, 367, 1066–1074. [Google Scholar] [CrossRef]
- Magee, L.A.; Nicolaides, K.H.; von Dadelszen, P. Preeclampsia. N. Engl. J. Med. 2022, 386, 1817–1832. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. Hypertensive Disorders of Pregnancy. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef]
- Bosio, P.M.; McKenna, P.J.; Conroy, R.; O’Herlihy, C. Maternal central hemodynamics in hypertensive disorders of pregnancy. Obs. Gynecol. 1999, 94, 978–984. [Google Scholar]
- Tihtonen, K.M.; Kööbi, T.; Uotila, J.T. Arterial stiffness in preeclamptic and chronic hypertensive pregnancies. Eur. J. Obs. Gynecol. Reprod. Biol. 2006, 128, 180–186. [Google Scholar] [CrossRef]
- Robb, A.O.; Mills, N.L.; Din, J.N.; Smith, I.B.; Paterson, F.; Newby, D.E.; Denison, F.C. Influence of the menstrual cycle, pregnancy, and preeclampsia on arterial stiffness. Hypertension 2009, 53, 952–958. [Google Scholar] [CrossRef]
- Tranquilli, A.L.; Brown, M.A.; Zeeman, G.G.; Dekker, G.; Sibai, B.M. The definition of severe and early-onset preeclampsia. Statements from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Pregnancy Hypertens. 2013, 3, 44–47. [Google Scholar] [CrossRef]
- Lisonkova, S.; Joseph, K.S. Incidence of preeclampsia: Risk factors and outcomes associated with early- versus late-onset disease. Am. J. Obs. Gynecol. 2013, 209, 544.e1–544.e12. [Google Scholar] [CrossRef]
- Steegers, E.A.P.; von Dadelszen, P.; Duvekot, J.J.; Pijnenborg, R. Pre-eclampsia. Lancet 2010, 376, 631–644. [Google Scholar] [CrossRef]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef] [PubMed]
- Maynard, S.E.; Min, J.-Y.; Merchan, J.; Lim, K.-H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Vitoratos, N.; Economou, E.; Iavazzo, C.; Panoulis, K.; Creatsas, G. Maternal serum levels of TNF-alpha and IL-6 long after delivery in preeclamptic and normotensive pregnant women. Mediat. Inflamm. 2010, 2010, 908649. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.N.; Varma, M.; Teng, N.N.; Roberts, J.M. Women with preeclampsia have higher plasma endothelin levels than women with normal pregnancies. J. Clin. Endocrinol. Metab. 1990, 71, 1675–1677. [Google Scholar] [CrossRef]
- Aggarwal, P.K.; Chandel, N.; Jain, V.; Jha, V. The relationship between circulating endothelin-1, soluble fms-like tyrosine kinase-1 and soluble endoglin in preeclampsia. J. Hum. Hypertens. 2012, 26, 236–241. [Google Scholar] [CrossRef]
- Onda, K.; Tong, S.; Beard, S.; Binder, N.; Muto, M.; Senadheera, S.N.; Parry, L.; Dilworth, M.; Renshall, L.; Brownfoot, F.; et al. Proton Pump Inhibitors Decrease Soluble fms-Like Tyrosine Kinase-1 and Soluble Endoglin Secretion, Decrease Hypertension, and Rescue Endothelial Dysfunction. Hypertension 2017, 69, 457–468. [Google Scholar] [CrossRef]
- Redman, C. Pre-eclampsia: A complex and variable disease. Pregnancy Hypertens. 2014, 4, 241–242. [Google Scholar] [CrossRef]
- Zheng, S.; Shi, A.; Hill, S.; Grant, C.; Kokkinos, M.I.; Murthi, P.; Georgiou, H.M.; Brennecke, S.P.; Kalionis, B. Decidual mesenchymal stem/stromal cell-derived extracellular vesicles ameliorate endothelial cell proliferation, inflammation, and oxidative stress in a cell culture model of preeclampsia. Pregnancy Hypertens. 2020, 22, 37–46. [Google Scholar] [CrossRef]
- Tong, S.; Kaitu’u-Lino, T.J.; Onda, K.; Beard, S.; Hastie, R.; Binder, N.K.; Cluver, C.; Tuohey, L.; Whitehead, C.; Brownfoot, F.; et al. Heme Oxygenase-1 Is Not Decreased in Preeclamptic Placenta and Does Not Negatively Regulate Placental Soluble fms-Like Tyrosine Kinase-1 or Soluble Endoglin Secretion. Hypertension 2015, 66, 1073–1081. [Google Scholar] [CrossRef]
- Hayman, R.; Warren, A.; Johnson, I.; Baker, P. Inducible change in the behavior of resistance arteries from circulating factor in preeclampsia: An effect specific to myometrial vessels from pregnant women. Am. J. Obstet. Gynecol. 2001, 184, 420–426. [Google Scholar] [CrossRef]
- Myers, J.; Mires, G.; Macleod, M.; Baker, P. In Preeclampsia, the Circulating Factors Capable of Altering In Vitro Endothelial Function Precede Clinical Disease. Hypertension 2005, 45, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Hayman, R.; Warren, A.; Brockelsby, J.; Johnson, I.; Baker, P. Plasma from women with pre-eclampsia induces an in vitro alteration in the endothelium-dependent behaviour of myometrial resistance arteries. Bjog Int. J. Obstet. Gynaecol. 2000, 107, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Portelli, M.; Baron, B. Clinical Presentation of Preeclampsia and the Diagnostic Value of Proteins and Their Methylation Products as Biomarkers in Pregnant Women with Preeclampsia and Their Newborns. J. Pregnancy 2018, 2018, 2632637. [Google Scholar] [CrossRef]
- Yu, Z.; Kastenmüller, G.; He, Y.; Belcredi, P.; Möller, G.; Prehn, C.; Mendes, J.; Wahl, S.; Roemisch-Margl, W.; Ceglarek, U.; et al. Differences between human plasma and serum metabolite profiles. PLoS ONE 2011, 6, e21230. [Google Scholar] [CrossRef] [PubMed]
- Lominadze, D.; Dean, W.L.; Tyagi, S.C.; Roberts, A.M. Mechanisms of fibrinogen-induced microvascular dysfunction during cardiovascular disease. Acta Physiol. 2010, 198, 1–13. [Google Scholar] [CrossRef]
- Levine, R.J.; Qian, C.; Maynard, S.E.; Yu, K.F.; Epstein, F.H.; Karumanchi, S.A. Serum sFlt1 concentration during preeclampsia and mid trimester blood pressure in healthy nulliparous women. Am. J. Obstet. Gynecol. 2006, 194, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.; Lim, K.H.; Karumanchi, S.A. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension 2005, 46, 1077–1085. [Google Scholar] [CrossRef]
- Levine, R.J.; Maynard, S.E.; Qian, C.; Lim, K.H.; England, L.J.; Yu, K.F.; Schisterman, E.F.; Thadhani, R.; Sachs, B.P.; Epstein, F.H.; et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004, 350, 672–683. [Google Scholar] [CrossRef]
- Than, N.G.; Romero, R.; Hillermann, R.; Cozzi, V.; Nie, G.; Huppertz, B. Prediction of preeclampsia—A workshop report. Placenta 2008, 29 (Suppl. A), S83–S85. [Google Scholar] [CrossRef]
- Vural, P. Nitric oxide/endothelin-1 in preeclampsia. Clin. Chim. Acta 2002, 317, 65–70. [Google Scholar] [CrossRef]
- Binder, N.K.; Beard, S.; de Alwis, N.; Kaitu’u-Lino, T.J.; MacDonald, T.M.; Myers, J.E.; Keenan, E.; Brownfoot, F.; Pritchard, N.; Walker, S.P.; et al. Endothelin-1 is exacerbated in pregnancies complicated by early onset preeclampsia and is also elevated prior to the development of preeclampsia and detection of poor fetal growth. Am. J. Obstet. Gynecol. 2022. under review. [Google Scholar]
- Crews, J.K.; Herrington, J.N.; Granger, J.P.; Khalil, R.A. Decreased endothelium-dependent vascular relaxation during reduction of uterine perfusion pressure in pregnant rat. Hypertension 2000, 35 Pt 2, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Savvidou, M.D.; Hingorani, A.D.; Tsikas, D.; Frölich, J.C.; Vallance, P.; Nicolaides, K.H. Endothelial dysfunction and raised plasma concentrations of asymmetric dimethylarginine in pregnant women who subsequently develop pre-eclampsia. Lancet 2003, 361, 1511–1517. [Google Scholar] [CrossRef]
- Levine, R.J.; Lam, C.; Qian, C.; Yu, K.F.; Maynard, S.E.; Sachs, B.P.; Sibai, B.M.; Epstein, F.H.; Romero, R.; Thadhani, R.; et al. Soluble Endoglin and Other Circulating Antiangiogenic Factors in Preeclampsia. N. Engl. J. Med. 2006, 355, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Harmon, A.C.; Cornelius, D.C.; Amaral, L.M.; Faulkner, J.L.; Cunningham, M.W., Jr.; Wallace, K.; LaMarca, B. The role of inflammation in the pathology of preeclampsia. Clin. Sci. 2016, 130, 409–419. [Google Scholar] [CrossRef]
- Saleh, L.; Samantar, R.; Garrelds, I.M.; van den Meiracker, A.H.; Visser, W.; Danser, A.H.J. Low Soluble Fms-Like Tyrosine Kinase-1, Endoglin, and Endothelin-1 Levels in Women With Confirmed or Suspected Preeclampsia Using Proton Pump Inhibitors. Hypertension 2017, 70, 594–600. [Google Scholar] [CrossRef]
| Preterm Control | Preterm Preeclamptic | p Test | Term Control | Term Preeclamptic | p Test | |
|---|---|---|---|---|---|---|
| n | 10 | 10 | 7 | 4 | ||
| Maternal age (years) (SD) | 28.70 (4.42) | 31.10 (3.21) | 0.182 | 33.29 (5.88) | 33.75 (2.63) | 0.886 |
| Body mass index; BMI (kg/m2) [IQR] | 24.00 [23.25, 25.75] | 31.45 [27.25, 36.77] | 0.004 | 32.60 [26.70, 34.00] | 29.45 [24.87, 34.08] | 0.849 |
| Gestation at delivery (weeks) [IQR] | 39.43 [39.00, 40.11] | 28.64 [26.93, 30.79] | <0.001 | 39.00 [38.86, 39.07] | 37.22 [37.11, 37.29] | 0.008 |
| Gestation at blood collection (weeks) (SD) | 28.81 (2.49) | 28.52 (2.57) | 0.794 | 38.80 (0.38) | 37.18 (0.14) | <0.001 |
| Highest systolic blood pressure during admission, including postpartum (mmHg) (SD) | 125.33 (8.47) ^ | 170.60 (14.03) | <0.001 | 117.14 (14.10) | 152.50 (22.17) | 0.010 |
| Highest diastolic blood pressure during admission, including postpartum (mmHg) (SD) | 79.11 (4.86) ^ | 97.20 (9.50) | <0.001 | 71.43 (10.29) | 96.50 (12.61) | 0.006 |
| Birth weight (g) (SD) | 3454.50 (470.51) | 1049.60 (415.02) | <0.001 | 3728.57 (483.99) | 2567.50 (422.64) | 0.003 |
| Parity no (%) | ||||||
| 0 | 5 (50.0) | 9 (90.0) | 1 (14.3) | 2 (50.0) | ||
| 1 | 3 (30.0) | 1 (10.0) | 4 (57.1) | 2 (50.0) | ||
| 2 | 2 (20.0) | 0 (0.0) | 2 (28.6) | 0 (0.0) | ||
| Mode of delivery (%) | ||||||
| Vaginal | 8 (80.0) | 0 (0.0) | 0.001 | 0 (0.0) | 0 (0.0) | |
| Caesarean section | 2 (20.0) | 10 (100.0) | 7 (100.0) | 4 (100.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fato, B.R.; de Alwis, N.; Beard, S.; Binder, N.K.; Pritchard, N.; Tong, S.; Kaitu’u-Lino, T.J.; Hannan, N.J. Serum Collected from Preeclamptic Pregnancies Drives Vasoconstriction of Human Omental Arteries—A Novel Ex Vivo Model of Preeclampsia for Therapeutic Development. Int. J. Mol. Sci. 2022, 23, 10852. https://doi.org/10.3390/ijms231810852
Fato BR, de Alwis N, Beard S, Binder NK, Pritchard N, Tong S, Kaitu’u-Lino TJ, Hannan NJ. Serum Collected from Preeclamptic Pregnancies Drives Vasoconstriction of Human Omental Arteries—A Novel Ex Vivo Model of Preeclampsia for Therapeutic Development. International Journal of Molecular Sciences. 2022; 23(18):10852. https://doi.org/10.3390/ijms231810852
Chicago/Turabian StyleFato, Bianca R., Natasha de Alwis, Sally Beard, Natalie K. Binder, Natasha Pritchard, Stephen Tong, Tu’uhevaha J. Kaitu’u-Lino, and Natalie J. Hannan. 2022. "Serum Collected from Preeclamptic Pregnancies Drives Vasoconstriction of Human Omental Arteries—A Novel Ex Vivo Model of Preeclampsia for Therapeutic Development" International Journal of Molecular Sciences 23, no. 18: 10852. https://doi.org/10.3390/ijms231810852
APA StyleFato, B. R., de Alwis, N., Beard, S., Binder, N. K., Pritchard, N., Tong, S., Kaitu’u-Lino, T. J., & Hannan, N. J. (2022). Serum Collected from Preeclamptic Pregnancies Drives Vasoconstriction of Human Omental Arteries—A Novel Ex Vivo Model of Preeclampsia for Therapeutic Development. International Journal of Molecular Sciences, 23(18), 10852. https://doi.org/10.3390/ijms231810852






