Myofibrillar Lattice Remodeling Is a Structural Cytoskeletal Predictor of Diaphragm Muscle Weakness in a Fibrotic mdx (mdx Cmah−/−) Model
Abstract
1. Introduction
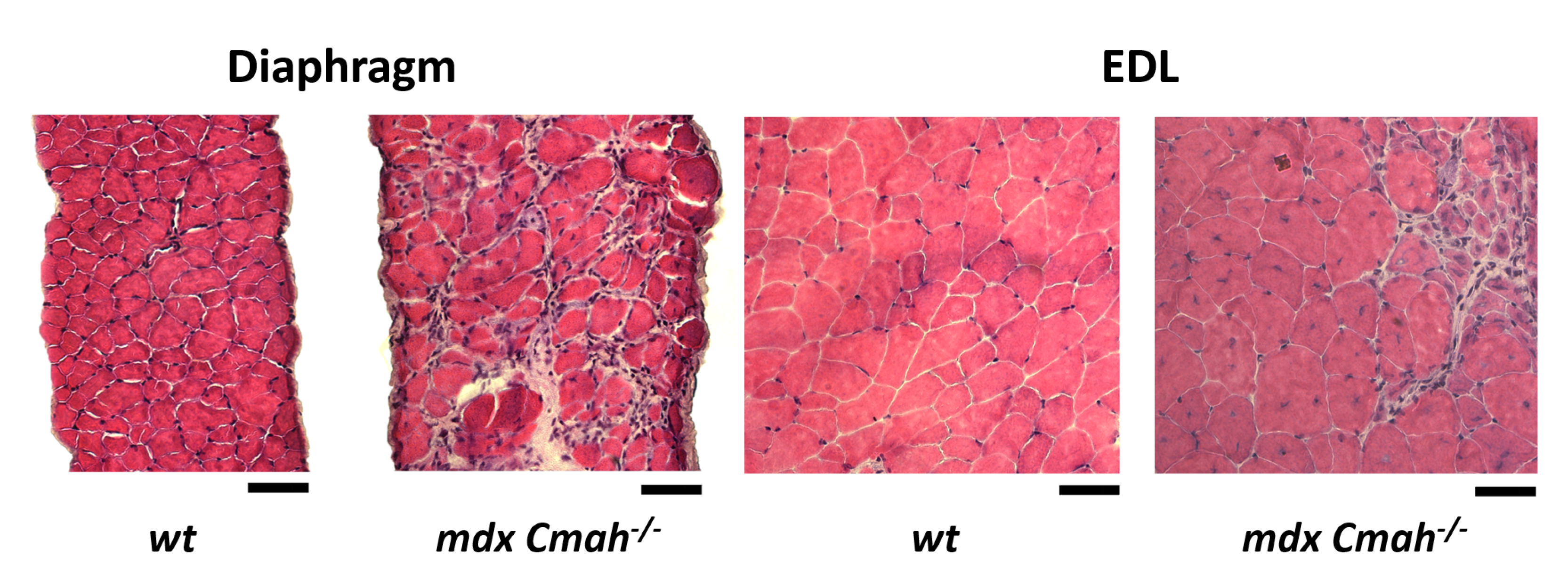
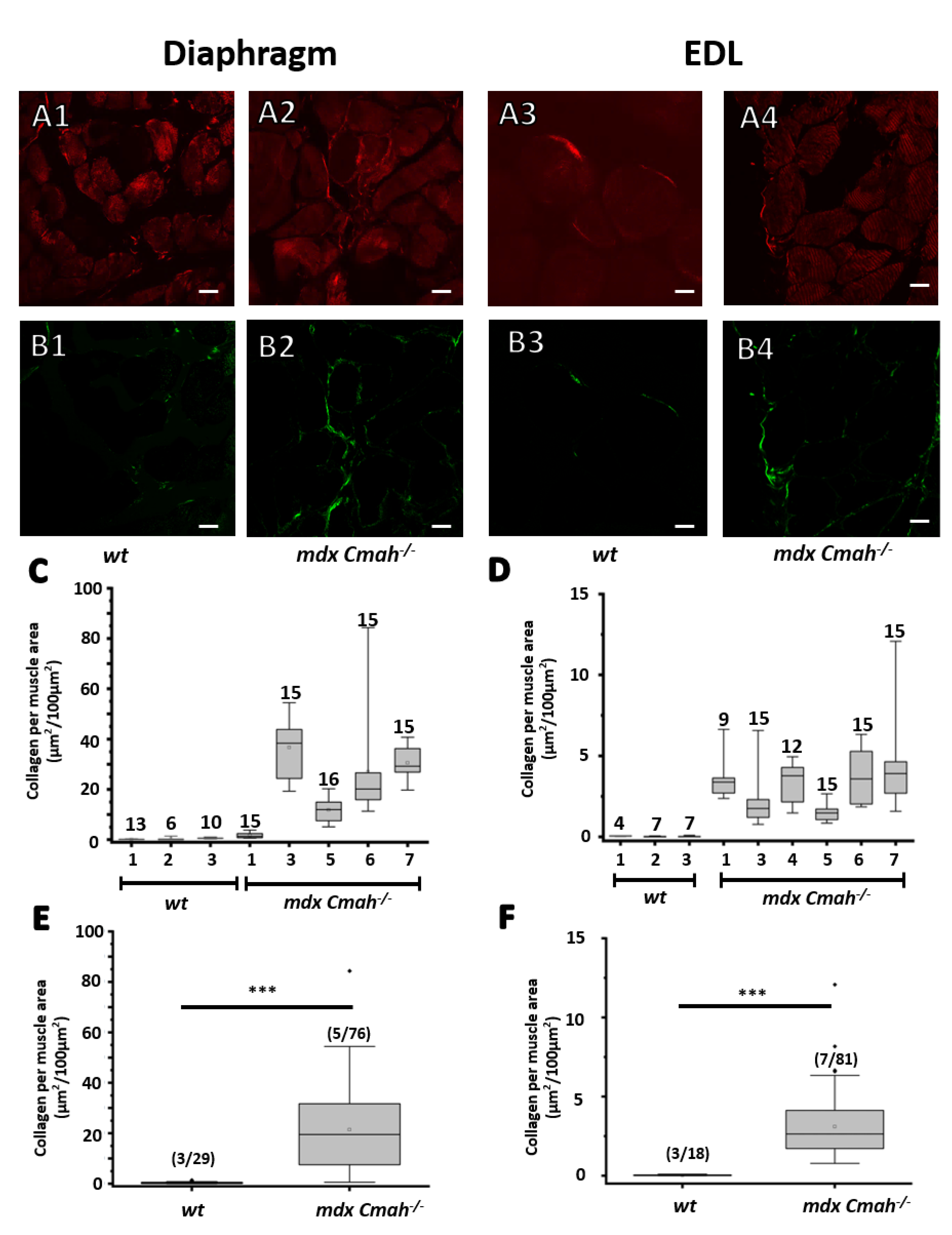
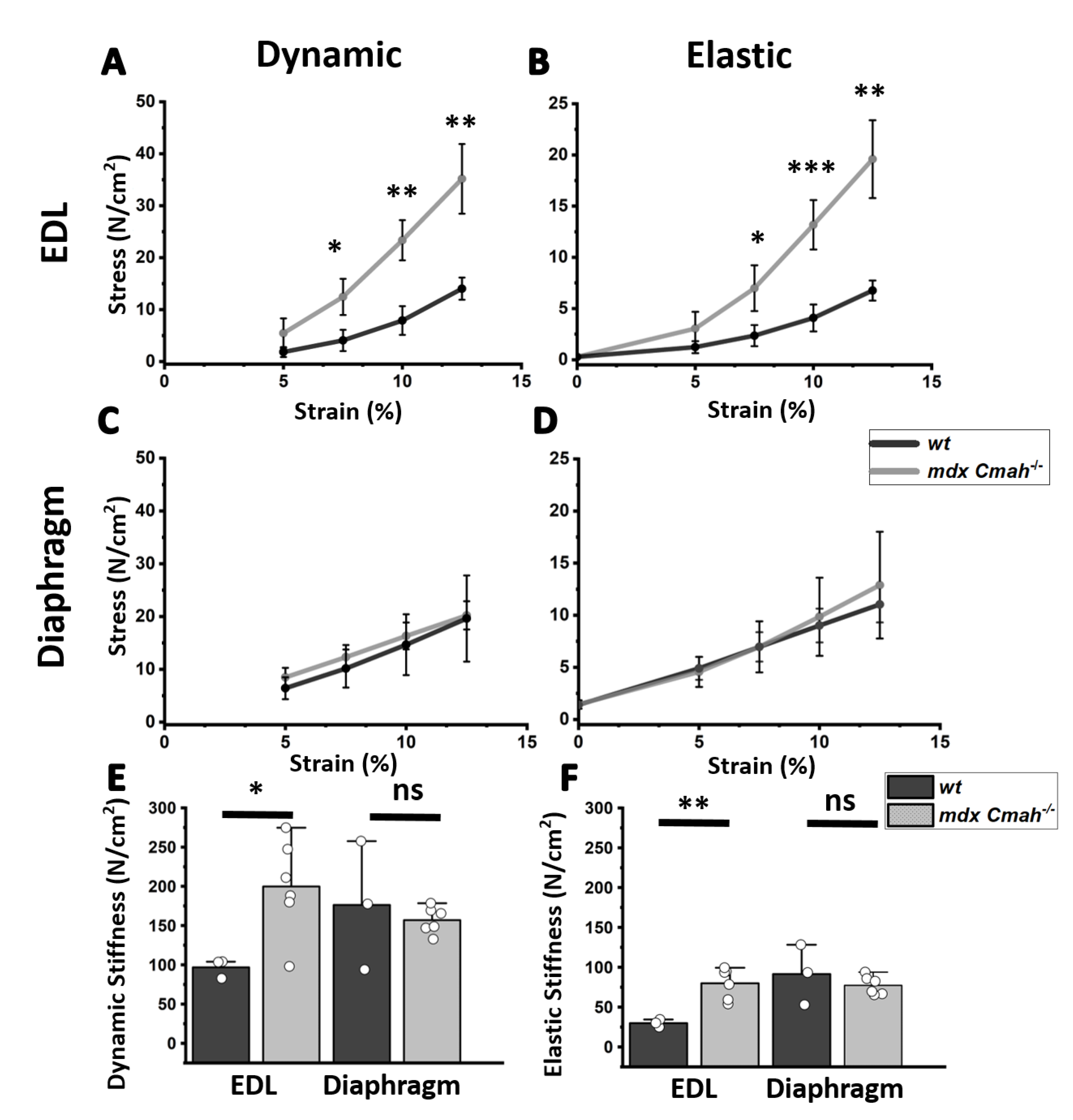
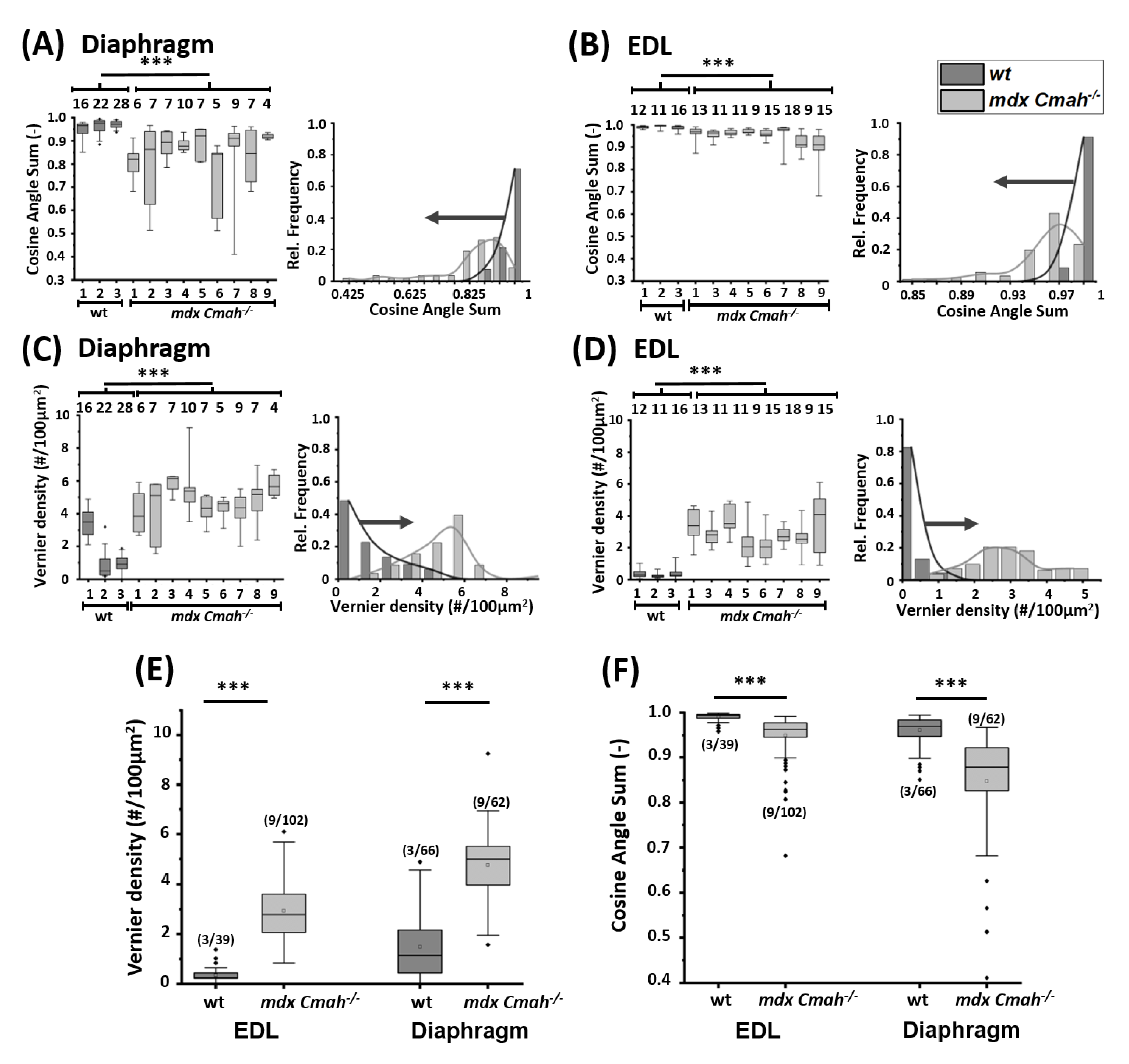
2. Results
3. Discussion
4. Materials and Methods
4.1. Animal Handling
4.2. Muscle Preparation and Multiphoton Imaging
4.3. Muscle Mechanics
4.4. Single Muscle Fiber Preparation and Cryosections
4.5. Feature Extraction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DMD | Duchenne Muscular Dystrophy |
| EDL | Extensor digitorum longus |
| SHG | Second harmonic generation |
| CAS | Cosine angle sum |
| VD | Verniers density |
References
- Campbell, K.P. Three muscular dystrophies: Review loss of cytoskeleton-extracellular matrix linkage. Cell 1995, 80, 675–679. [Google Scholar] [CrossRef]
- Head, S.I.; Houweling, P.J.; Chan, S.; Chen, G.; Hardeman, E.C. Properties of regenerated mouse extensor digitorum longus muscle following notexin injury. Exp. Physiol. 2014, 99, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Head, S.I. The role of branched fibres in the pathogenesis of Duchenne muscular dystrophy. Exp. Physiol. 2011, 96, 564–571. [Google Scholar] [CrossRef]
- Head, S.I. Branched fibres in old dystrophic mdx muscle are associated with mechanical weakening of the sarcolemma, abnormal Ca2+ transients and a breakdown of Ca2+ homeostasis during fatigue. Exp. Physiol. 2010, 95, 641–656. [Google Scholar] [CrossRef]
- Moxley, R.T.; Ashwal, S.; Pandya, S.; Connolly, A.; Florence, J.; Mathews, K.; Baumbach, L.; McDonald, C.; Sussman, M.; Wade, C. Practice parameter: Corticosteroid treatment of Duchenne dystrophy: Report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2005, 64, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Reitter, B. Deflazacort vs. prednisone in Duchenne muscular dystrophy: Trends of an ongoing study. Brain Dev. 1995, 17, 39–43. [Google Scholar] [CrossRef]
- Guiraud, S.; Aartsma-Rus, A.; Vieira, N.M.; Davies, K.E.; van Ommen, G.J.B.; Kunkel, L.M. The Pathogenesis and Therapy of Muscular Dystrophies. Annu. Rev. Genom. Hum. Genet. 2015, 16, 281–308. [Google Scholar] [CrossRef]
- Sicinski, P.; Geng, Y.; Ryder-Cook, A.S.; Barnard, E.A.; Darlison, M.G.; Barnard, P.J. The molecular basis of muscular dystrophy in the mdx mouse: A point mutation. Science 1989, 244, 1578–1580. [Google Scholar] [CrossRef]
- Banks, G.B.; Chamberlain, J.S. Chapter 9 The Value of Mammalian Models for Duchenne Muscular Dystrophy in Developing Therapeutic Strategies. In Mouse Models of Developmental Genetic Disease; Current Topics in Developmental Biology; Academic Press: Cambridge, MA, USA, 2008; Volume 84, pp. 431–453. [Google Scholar] [CrossRef]
- Tinsley, J.; Deconinck, N.; Fisher, R.; Kahn, D.; Phelps, S.; Gillis, J.M.; Davies, K. Expression of full-length utrophin prevents muscular dystrophy in mdx mice. Nat. Med. 1998, 4, 1441–1444. [Google Scholar] [CrossRef]
- Hammers, D.W.; Hart, C.C.; Matheny, M.K.; Wright, L.A.; Armellini, M.; Barton, E.R.; Sweeney, H.L. The D2.mdx mouse as a preclinical model of the skeletal muscle pathology associated with Duchenne muscular dystrophy. Sci. Rep. 2020, 10, 14070. [Google Scholar] [CrossRef]
- Deconinck, A.E.; Rafael, J.A.; Skinner, J.A.; Brown, S.C.; Potter, A.C.; Metzinger, L.; Watt, D.J.; Dickson, J.; Tinsley, J.M.; Davies, K.E. Utrophin-Dystrophin-Deficient Mice as a Model for Duchenne Muscular Dystrophy. Cell 1997, 90, 717–727. [Google Scholar] [CrossRef]
- Chandrasekharan, K.; Yoon, J.H.; Xu, Y.; de Vries, S.; Camboni, M.; Janssen, P.M.L.; Varki, A.; Martin, P.T. A human-specific deletion in mouse Cmah increases disease severity in the mdx model of Duchenne muscular dystrophy. Sci. Transl. Med. 2010, 2, 42ra54. [Google Scholar] [CrossRef]
- Okerblom, J.J.; Schwarz, F.; Olson, J.; Fletes, W.; Ali, S.R.; Martin, P.T.; Glass, C.K.; Nizet, V.; Varki, A. Loss of CMAH during Human Evolution Primed the Monocyte–Macrophage Lineage toward a More Inflammatory and Phagocytic State. J. Immunol. 2017, 198, 2366–2373. [Google Scholar] [CrossRef] [PubMed]
- Yucel, N.; Chang, A.C.; Day, J.W.; Rosenthal, N.; Blau, H.M. Humanizing the mdx mouse model of DMD: The long and the short of it. NPJ Regen. Med. 2018, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, S.V.; Kenny, A.M.; Walsh, S.J.; Joseph, B.Z.C.; Scranton, V.L.; Kuchel, G.A.; Dauser, D.; Pilbeam, M.X.C.C.; Adams, D.J.; Dougherty, R.P.; et al. Measurement of muscle disease by quantitative second-harmonic generation imaging. J. Biomed. Opt. 2008, 13. [Google Scholar] [CrossRef] [PubMed]
- Schneidereit, D.; Nübler, S.; Prölß, G.; Reischl, B.; Schürmann, S.; Müller, O.J.; Friedrich, O. Optical prediction of single muscle fiber force production using a combined biomechatronics and second harmonic generation imaging approach. Light. Sci. Appl. 2018, 7, 79. [Google Scholar] [CrossRef]
- Friedrich, O.; Both, M.; Weber, C.; Schürmann, S.; Teichmann, M.D.H.; von Wegner, F.; Fink, R.H.A.; Vogel, M.; Chamberlain, J.S.; Garbe, C. Microarchitecture is severely compromised but motor protein function is preserved in dystrophic mdx skeletal muscle. Biophys. J. 2010, 98, 606–616. [Google Scholar] [CrossRef][Green Version]
- Garbe, C.S.; Buttgereit, A.; Schürmann, S.; Friedrich, O. Automated multiscale morphometry of muscle disease from second harmonic generation microscopy using tensor-based image processing. IEEE Trans.-Bio-Med. Eng. 2012, 59, 39–44. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buttgereit, A.; Weber, C.; Garbe, C.S.; Friedrich, O. From chaos to split-ups - SHG microscopy reveals a specific remodelling mechanism in ageing dystrophic muscle. J. Pathol. 2013, 229, 477–485. [Google Scholar] [CrossRef]
- Smith, L.R.; Barton, E.R. Collagen content does not alter the passive mechanical properties of fibrotic skeletal muscle in mdx mice. Am. J. Physiol. Cell Physiol. 2014, 306, C889–C898. [Google Scholar] [CrossRef]
- Mann, C.J.; Perdiguero, E.; Kharraz, Y.; Aguilar, S.; Pessina, P.; Serrano, A.L.; Muñoz-Cánoves, P. Aberrant repair and fibrosis development in skeletal muscle. Skelet. Muscle 2011, 1, 21. [Google Scholar] [CrossRef] [PubMed]
- Law, D.J.; Caputo, A.; Tidball, J.G. Site and mechanics of failure in normal and dystrophin-deficient skeletal muscle. Muscle Nerve 1995, 18, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Hakim, C.H.; Grange, R.W.; Duan, D. The passive mechanical properties of the extensor digitorum longus muscle are compromised in 2- to 20-mo-old mdx mice. J. Appl. Physiol. 2011, 110, 1656–1663. [Google Scholar] [CrossRef] [PubMed]
- Brashear, S.E.; Wohlgemuth, R.P.; Gonzalez, G.; Smith, L.R. Passive stiffness of fibrotic skeletal muscle in mdx mice relates to collagen architecture. J. Physiol. 2021, 599, 943–962. [Google Scholar] [CrossRef] [PubMed]
- Coirault, C.; Lambert, F.; Marchand-Adam, S.; Attal, P.; Chemla, D.; Lecarpentier, Y. Myosin molecular motor dysfunction in dystrophic mouse diaphragm. Am. J. Physiol. 1999, 277, C1170–C1176. [Google Scholar] [CrossRef] [PubMed]
- Klingler, W.; Jurkat-Rott, K.; Lehmann-Horn, F.; Schleip, R. The role of fibrosis in Duchenne muscular dystrophy. Acta Myol. Myopathies Cardiomyopathies Off. J. Mediterr. Soc. Myol. 2012, 31, 184–195. [Google Scholar]
- Kharraz, Y.; Guerra, J.; Pessina, P.; Serrano, A.L.; Muñoz-Cánoves, P. Understanding the process of fibrosis in Duchenne muscular dystrophy. BioMed Res. Int. 2014, 2014, 965631. [Google Scholar] [CrossRef]
- Louboutin, J.P.; Fichter-Gagnepain, V.; Thaon, E.; Fardeau, M. Morphometric analysis of mdx diaphragm muscle fibres. Comparison with hindlimb muscles. Neuromuscul. Disord. 1993, 3, 463–469. [Google Scholar] [CrossRef]
- Stedman, H.H.; Sweeney, H.L.; Shrager, J.B.; Maguire, H.C.; Panettieri, R.A.; Petrof, B.; Narusawa, M.; Leferovich, J.M.; Sladky, J.T.; Kelly, A.M. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature 1991, 352, 536–539. [Google Scholar] [CrossRef]
- Coulton, G.R.; Morgan, J.E.; Partridge, T.A.; Sloper, J.C. The mdx mouse skeletal muscle myopathy: I. A histological, morphometric and biochemical investigation. Neuropathol. Appl. Neurobiol. 1988, 14, 53–70. [Google Scholar] [CrossRef]
- Vielreicher, M.; Gellner, M.; Rottensteiner, U.; Horch, R.E.; Arkudas, A.; Friedrich, O. Multiphoton microscopy analysis of extracellular collagen I network formation by mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2017, 11, 2104–2115. [Google Scholar] [CrossRef] [PubMed]
- Hakim, C.H.; Duan, D. Truncated dystrophins reduce muscle stiffness in the extensor digitorum longus muscle of mdx mice. J. Appl. Physiol. 2013, 114, 482–489. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Diermeier, S.; Iberl, J.; Vetter, K.; Haug, M.; Pollmann, C.; Reischl, B.; Buttgereit, A.; Schürmann, S.; Spörrer, M.; Goldmann, W.H.; et al. Early signs of architectural and biomechanical failure in isolated myofibers and immortalized myoblasts from desmin-mutant knock-in mice. Sci. Rep. 2017, 7, 1391. [Google Scholar] [CrossRef] [PubMed]
- Mendez, J.; Keys. Density and composition of mammalian muscle. Metabolism 1960, 9, 184–188. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ritter, P.; Nübler, S.; Buttgereit, A.; Smith, L.R.; Mühlberg, A.; Bauer, J.; Michael, M.; Kreiß, L.; Haug, M.; Barton, E.; et al. Myofibrillar Lattice Remodeling Is a Structural Cytoskeletal Predictor of Diaphragm Muscle Weakness in a Fibrotic mdx (mdx Cmah−/−) Model. Int. J. Mol. Sci. 2022, 23, 10841. https://doi.org/10.3390/ijms231810841
Ritter P, Nübler S, Buttgereit A, Smith LR, Mühlberg A, Bauer J, Michael M, Kreiß L, Haug M, Barton E, et al. Myofibrillar Lattice Remodeling Is a Structural Cytoskeletal Predictor of Diaphragm Muscle Weakness in a Fibrotic mdx (mdx Cmah−/−) Model. International Journal of Molecular Sciences. 2022; 23(18):10841. https://doi.org/10.3390/ijms231810841
Chicago/Turabian StyleRitter, Paul, Stefanie Nübler, Andreas Buttgereit, Lucas R. Smith, Alexander Mühlberg, Julian Bauer, Mena Michael, Lucas Kreiß, Michael Haug, Elisabeth Barton, and et al. 2022. "Myofibrillar Lattice Remodeling Is a Structural Cytoskeletal Predictor of Diaphragm Muscle Weakness in a Fibrotic mdx (mdx Cmah−/−) Model" International Journal of Molecular Sciences 23, no. 18: 10841. https://doi.org/10.3390/ijms231810841
APA StyleRitter, P., Nübler, S., Buttgereit, A., Smith, L. R., Mühlberg, A., Bauer, J., Michael, M., Kreiß, L., Haug, M., Barton, E., & Friedrich, O. (2022). Myofibrillar Lattice Remodeling Is a Structural Cytoskeletal Predictor of Diaphragm Muscle Weakness in a Fibrotic mdx (mdx Cmah−/−) Model. International Journal of Molecular Sciences, 23(18), 10841. https://doi.org/10.3390/ijms231810841




