Mixture Effects of Tryptophan Intestinal Microbial Metabolites on Aryl Hydrocarbon Receptor Activity
Abstract
:1. Introduction
2. Results
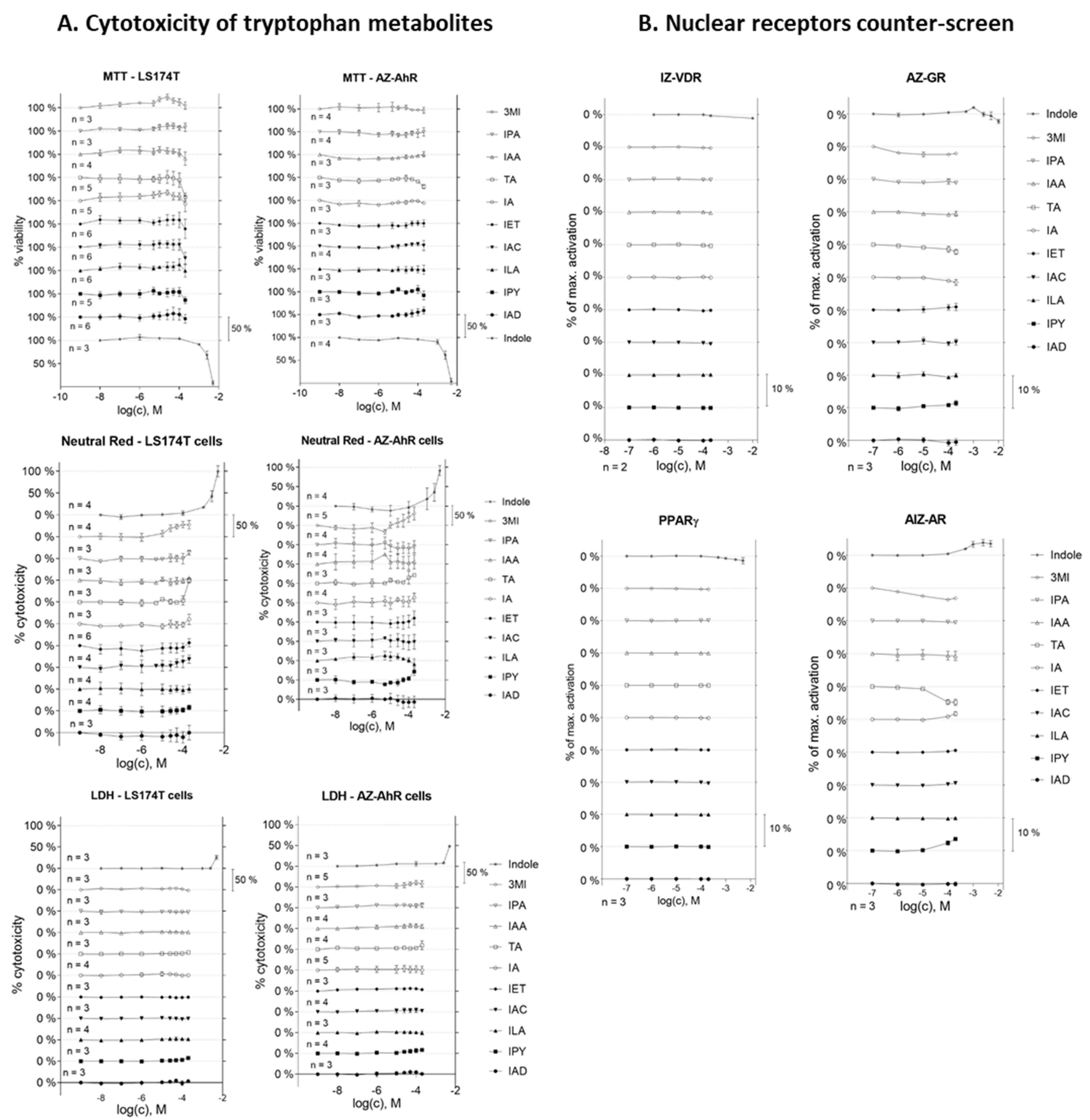
2.1. Cell Viability Assay and Nuclear Receptor Counter-Screen of MICTs
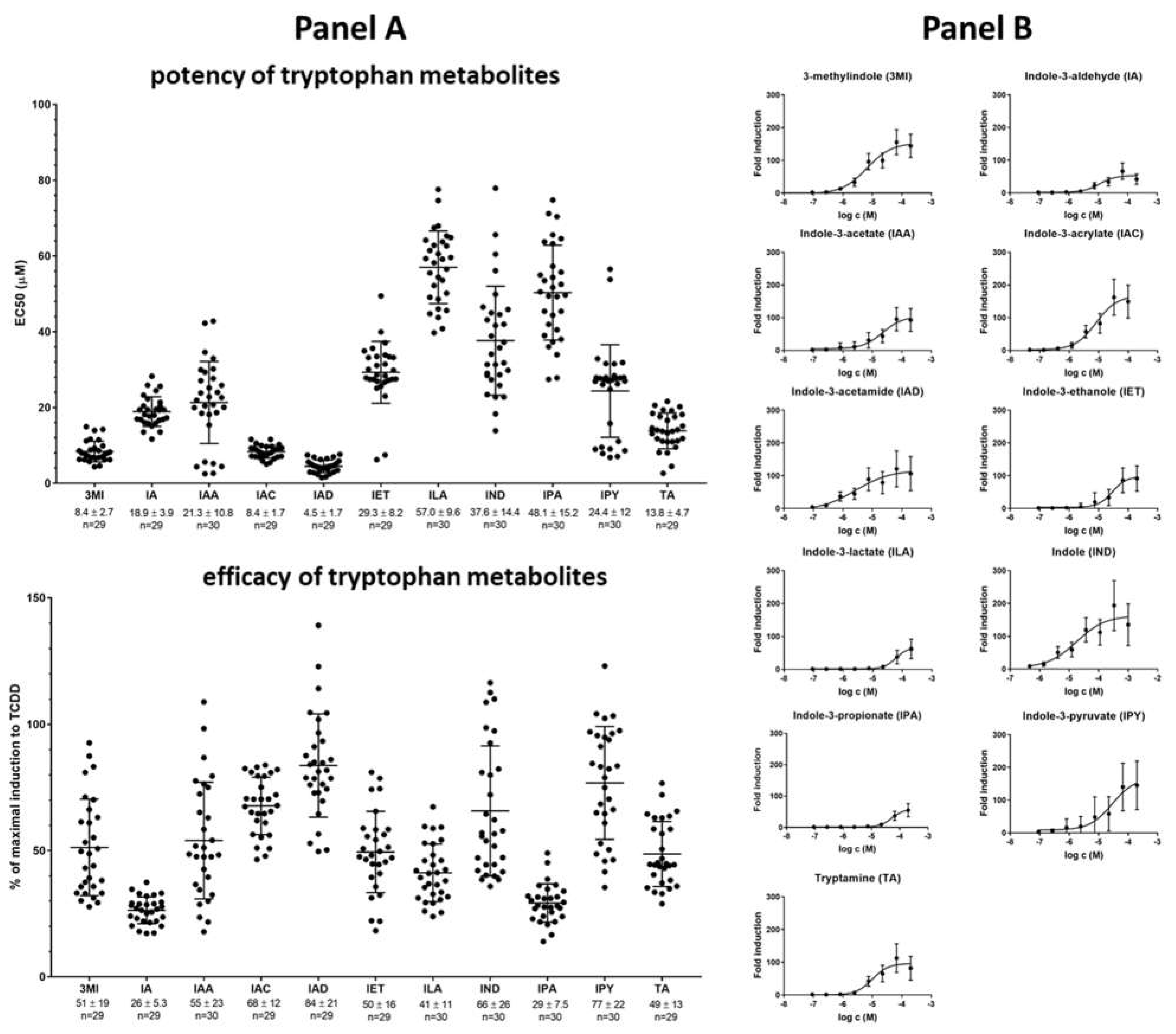
2.2. High-Throughput Screening (HTS) of AHR Receptor Activity by Individual MICTs
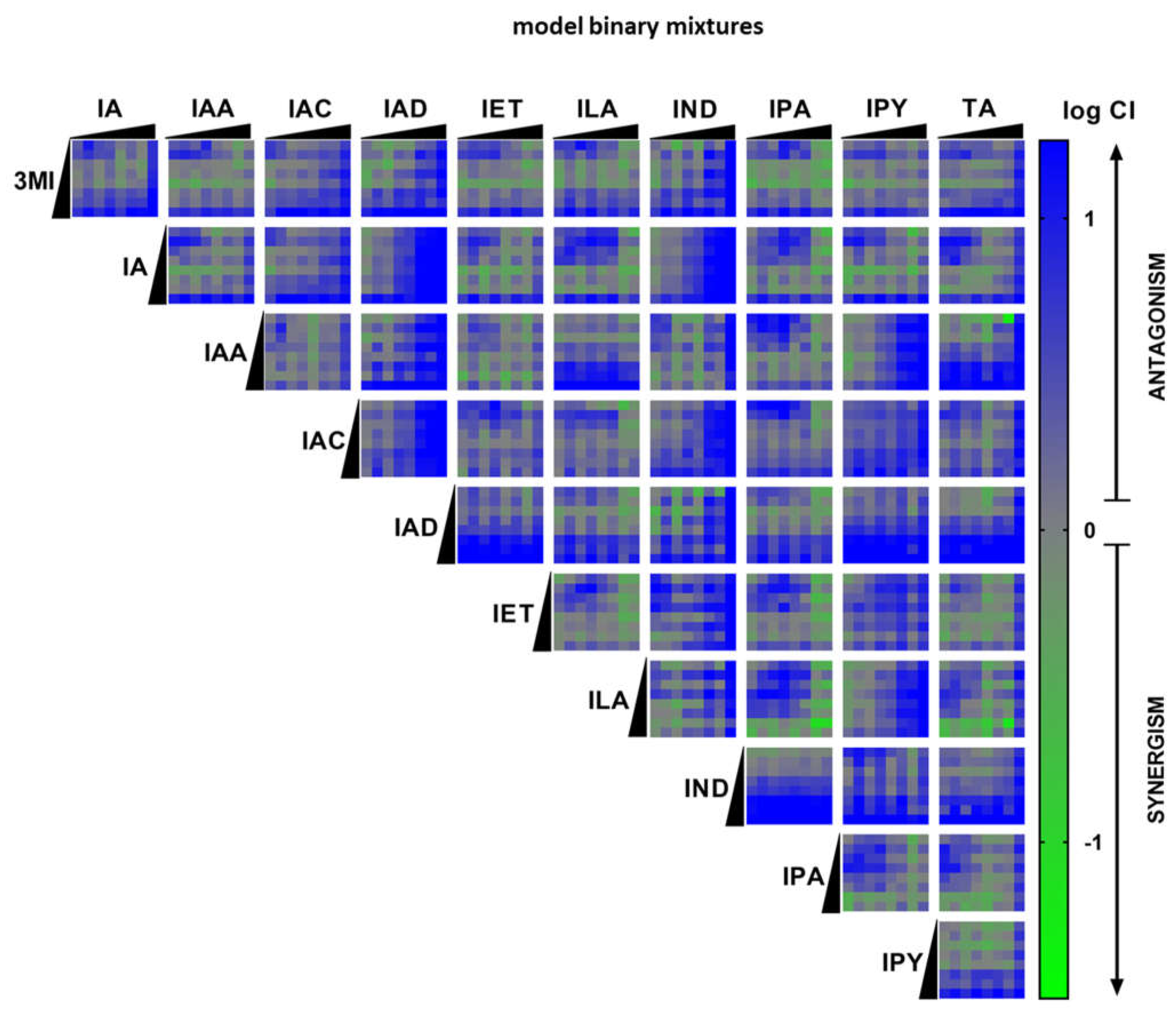
2.3. High-Throughput Screening (HTS) of AHR Receptor Activity by Binary Mixtures of MICTs
2.4. Binary Mixtures of ILA and IPA Synergistically Induce CYP1A1
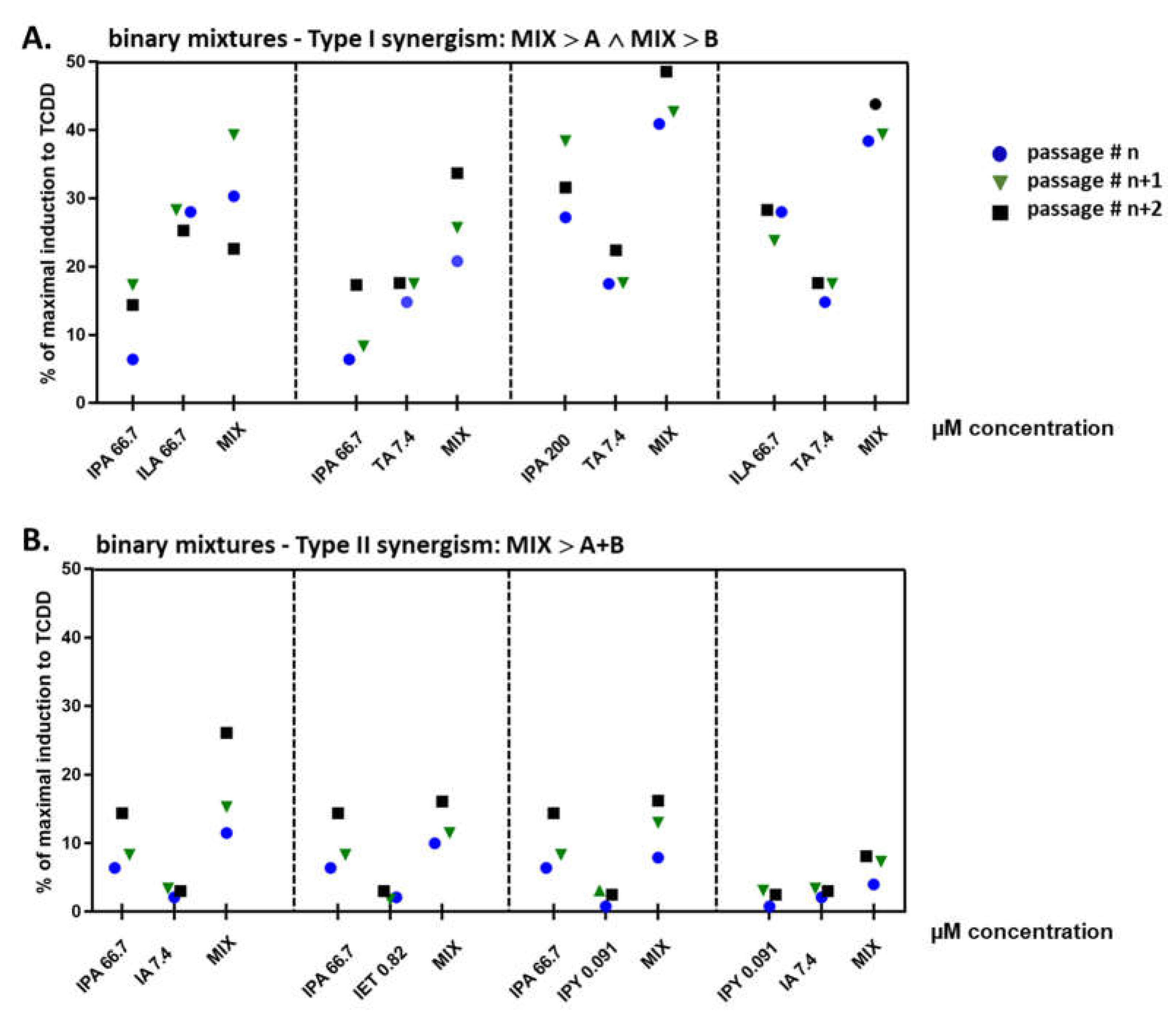
2.5. Effect of Multicomponent Mimic Fecal Mixtures of MICTs on the AHR Activity
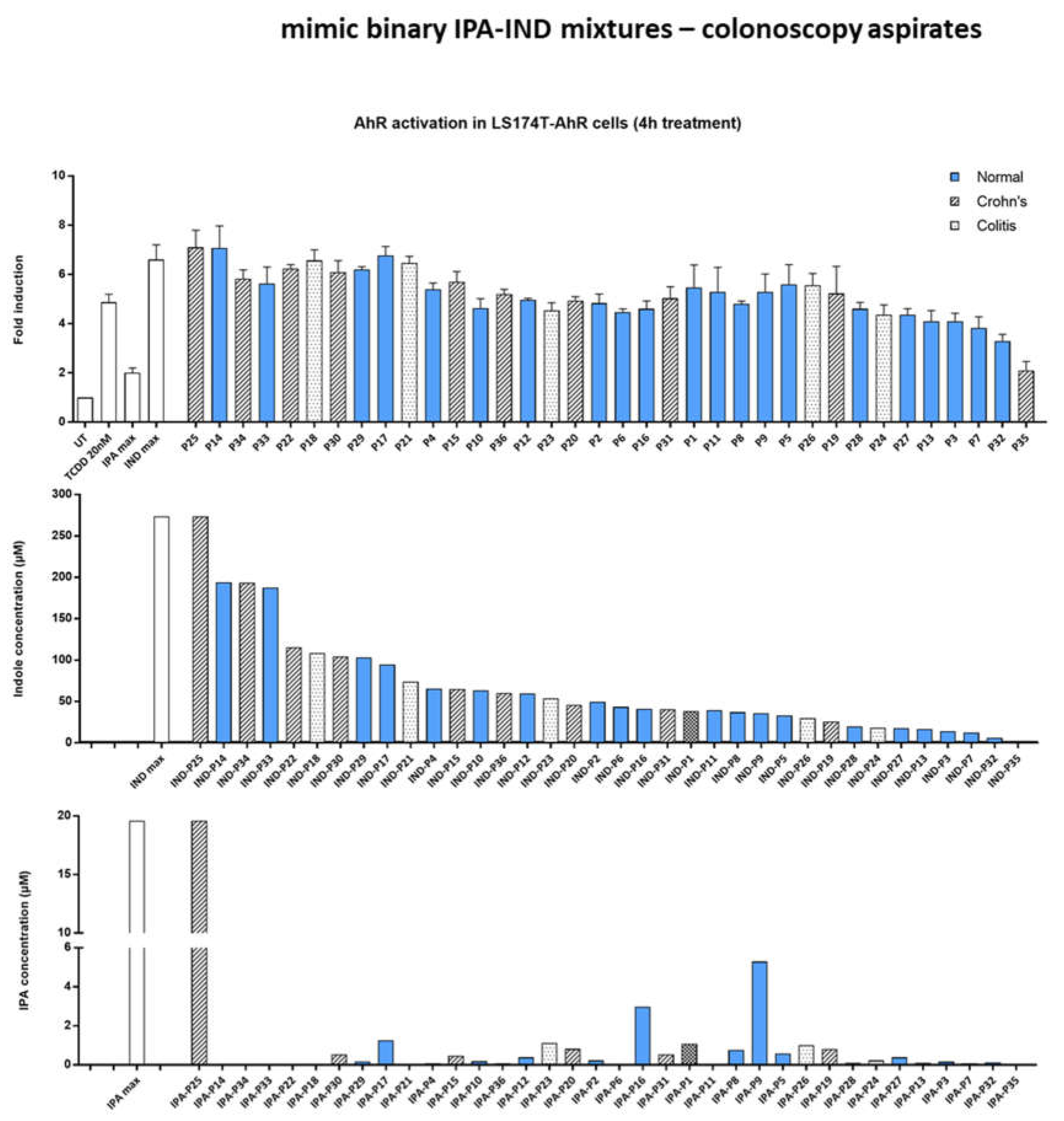
2.6. Effect of IND and IPA Binary Mimic Mixtures on AHR Activity
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Cultures
4.3. Human Studies
4.4. Cell Viability Assays—MTT, Neutral Red, and LDH
4.5. Luciferase Reporter Assay
4.6. High-Throughput AhR Reporter Gene Screening Assay
4.7. Isolation of RNA and Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
4.8. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698–3703. [Google Scholar] [CrossRef] [PubMed]
- Sittipo, P.; Shim, J.W.; Lee, Y.K. Microbial metabolites determine host health and the Status of some diseases. Int. J. Mol. Sci 2019, 20, 5296. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Quinn, R.A.; Debelius, J.; Xu, Z.Z.; Morton, J.; Garg, N.; Jansson, J.K.; Dorrestein, P.C.; Knight, R. Microbiome-wide association studies link dynamic microbial consortia to disease. Nature 2016, 535, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.B.; Tolonen, A.C.; Xavier, R.J. Human genetic variation and the gut microbiome in disease. Nat. Rev. Genet. 2017, 18, 690–699. [Google Scholar] [CrossRef]
- Modoux, M.; Rolhion, N.; Mani, S.; Sokol, H. Tryptophan metabolism as a pharmacological target. Trends Pharmacol. Sci. 2021, 42, 60–73. [Google Scholar] [CrossRef]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef]
- Hubbard, T.D.; Murray, I.A.; Bisson, W.H.; Lahoti, T.S.; Gowda, K.; Amin, S.G.; Patterson, A.D.; Perdew, G.H. Adaptation of the human aryl hydrocarbon receptor to sense microbiota-derived indoles. Sci. Rep. 2015, 5, 12689. [Google Scholar] [CrossRef]
- Hubbard, T.D.; Murray, I.A.; Perdew, G.H. Indole and tryptophan metabolism: Endogenous and dietary routes to ah receptor activation. Drug Metab. Dispos. 2015, 43, 1522–1535. [Google Scholar] [CrossRef]
- Vyhlidalova, B.; Krasulova, K.; Pecinkova, P.; Marcalikova, A.; Vrzal, R.; Zemankova, L.; Vanco, J.; Travnicek, Z.; Vondracek, J.; Karasova, M.; et al. Gut microbial catabolites of tryptophan are ligands and agonists of the aryl hydrocarbon receptor: A detailed characterization. Int. J. Mol. Sci. 2020, 21, 2614. [Google Scholar] [CrossRef] [Green Version]
- Dvorak, Z.; Sokol, H.; Mani, S. Drug mimicry: Promiscuous receptors PXR and AhR, and microbial metabolite interactions in the intestine. Trends Pharmacol. Sci. 2020, 41, 900–908. [Google Scholar] [CrossRef]
- Nieves, K.M.; Hirota, S.A.; Flannigan, K.L. Xenobiotic receptors and the regulation of intestinal homeostasis: Harnessing the chemical output of the intestinal microbiota. Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 322, G268–G281. [Google Scholar] [CrossRef]
- Schulte, K.W.; Green, E.; Wilz, A.; Platten, M.; Daumke, O. Structural basis for aryl hydrocarbon receptor-mediated gene activation. Structure 2017, 25, 1025–1033. [Google Scholar] [CrossRef]
- Sogawa, K.; Fujisawa-Sehara, A.; Yamane, M.; Fujii-Kuriyama, Y. Location of regulatory elements responsible for drug induction in the rat cytochrome P-450c gene. Proc. Natl. Acad. Sci. USA 1986, 83, 8044–8048. [Google Scholar] [CrossRef]
- Denison, M.S.; Fisher, J.M.; Whitlock, J.P., Jr. Inducible, receptor-dependent protein-DNA interactions at a dioxin-responsive transcriptional enhancer. Proc. Natl. Acad. Sci. USA 1988, 85, 2528–2532. [Google Scholar] [CrossRef]
- Lusska, A.; Shen, E.; Whitlock, J.P., Jr. Protein-DNA interactions at a dioxin-responsive enhancer. Analysis of six bona fide DNA-binding sites for the liganded Ah receptor. J. Biol. Chem. 1993, 268, 6575–6580. [Google Scholar] [CrossRef]
- Mimura, J.; Ema, M.; Sogawa, K.; Fujii-Kuriyama, Y. Identification of a novel mechanism of regulation of Ah (dioxin) receptor function. Genes Dev. 1999, 13, 20–25. [Google Scholar] [CrossRef]
- Bock, K.W.; Kohle, C. Ah receptor: Dioxin-mediated toxic responses as hints to deregulated physiologic functions. Biochem. Pharmacol. 2006, 72, 393–404. [Google Scholar] [CrossRef]
- Marlowe, J.L.; Puga, A. Aryl hydrocarbon receptor, cell cycle regulation, toxicity, and tumorigenesis. J. Cell. Biochem. 2005, 96, 1174–1184. [Google Scholar] [CrossRef]
- Murray, I.A.; Patterson, A.D.; Perdew, G.H. Aryl hydrocarbon receptor ligands in cancer: Friend and foe. Nat. Rev. Cancer 2014, 14, 801–814. [Google Scholar] [CrossRef]
- Yin, J.; Sheng, B.; Qiu, Y.; Yang, K.; Xiao, W.; Yang, H. Role of AhR in positive regulation of cell proliferation and survival. Cell Prolif. 2016, 49, 554–560. [Google Scholar] [CrossRef]
- Gutiérrez-Vázquez, C.; Quintana, F.J. Regulation of the immune response by the aryl hydrocarbon receptor. Immunity 2018, 48, 19–33. [Google Scholar] [CrossRef]
- Rothhammer, V.; Quintana, F.J. The aryl hydrocarbon receptor: An environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019, 19, 184–197. [Google Scholar] [CrossRef]
- Backlund, M.; Ingelman-Sundberg, M. Different structural requirements of the ligand binding domain of the aryl hydrocarbon receptor for high- and low-affinity ligand binding and receptor activation. Mol. Pharmacol. 2004, 65, 416–425. [Google Scholar] [CrossRef]
- Tagliabue, S.G.; Faber, S.C.; Motta, S.; Denison, M.S.; Bonati, L. Modeling the binding of diverse ligands within the Ah receptor ligand binding domain. Sci. Rep. 2019, 9, 10693. [Google Scholar] [CrossRef]
- Stepankova, M.; Bartonkova, I.; Jiskrova, E.; Vrzal, R.; Mani, S.; Kortagere, S.; Dvorak, Z. Methylindoles and methoxyindoles are agonists and antagonists of human aryl hydrocarbon receptor. Mol. Pharmacol. 2018, 93, 631–644. [Google Scholar] [CrossRef]
- Jin, U.H.; Cheng, Y.; Park, H.; Davidson, L.A.; Callaway, E.S.; Chapkin, R.S.; Jayaraman, A.; Asante, A.; Allred, C.; Weaver, E.A.; et al. Short chain fatty acids enhance aryl hydrocarbon (Ah) responsiveness in mouse colonocytes and caco-2 human colon cancer cells. Sci. Rep. 2017, 7, 10163. [Google Scholar] [CrossRef]
- Modoux, M.; Rolhion, N.; Lefevre, J.H.; Oeuvray, C.; Nádvorník, P.; Illes, P.; Emond, P.; Parc, Y.; Mani, S.; Dvorak, Z.; et al. Butyrate acts through HDAC inhibition to enhance aryl hydrocarbon receptor activation by gut microbiota-derived ligands. Gut. Microbes. 2022, 14, 2105637. [Google Scholar] [CrossRef]
- Darkoh, C.; Chappell, C.; Gonzales, C.; Okhuysen, P. A rapid and specific method for the detection of indole in complex biological samples. Appl. Environ. Microbiol. 2015, 81, 8093–8097. [Google Scholar] [CrossRef]
- Dong, F.; Hao, F.; Murray, I.A.; Smith, P.B.; Koo, I.; Tindall, A.M.; Kris-Etherton, P.M.; Gowda, K.; Amin, S.G.; Patterson, A.D.; et al. Intestinal microbiota-derived tryptophan metabolites are predictive of Ah receptor activity. Gut. Microbes. 2020, 12, 1788899. [Google Scholar] [CrossRef]
- Illés, P.; Krasulová, K.; Vyhlídalová, B.; Poulíková, K.; Marcalíková, A.; Pečinková, P.; Sirotová, N.; Vrzal, R.; Mani, S.; Dvořák, Z. Indole microbial intestinal metabolites expand the repertoire of ligands and agonists of the human pregnane X receptor. Toxicol. Lett. 2020, 334, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Tindall, A.M.; Petersen, K.S.; Skulas-Ray, A.C.; Richter, C.K.; Proctor, D.N.; Kris-Etherton, P.M. Replacing saturated fat with walnuts or vegetable oils improves central blood pressure and serum lipids in adults at risk for cardiovascular disease: A randomized controlled-feeding trial. J. Am. Heart Assoc. 2019, 8, e011512. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Illés, P.; Karunaratne, C.V.; Nordstrøm, L.U.; Luo, X.; Yang, A.; Qiu, Y.; Kurland, I.J.; Lukin, D.J.; Chen, W.; et al. Deciphering structural bases of intestinal and hepatic selectivity in targeting pregnane X receptor with indole-based microbial mimics. Bioorg. Chem. 2021, 109, 104661. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.K.; Balaguer, P.; Ekstrand, B.; Daujat-Chavanieu, M.; Gerbal-Chaloin, S. Skatole (3-Methylindole) is a partial aryl hydrocarbon receptor agonist and induces CYP1A1/2 and CYP1B1 expression in primary human hepatocytes. PLoS ONE 2016, 11, e0154629. [Google Scholar] [CrossRef]
- Rothhammer, V.; Mascanfroni, I.D.; Bunse, L.; Takenaka, M.C.; Kenison, J.E.; Mayo, L.; Chao, C.C.; Patel, B.; Yan, R.; Blain, M.; et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 2016, 22, 586–597. [Google Scholar] [CrossRef]
- Delfosse, V.; Dendele, B.; Huet, T.; Grimaldi, M.; Boulahtouf, A.; Gerbal-Chaloin, S.; Beucher, B.; Roecklin, D.; Muller, C.; Rahmani, R.; et al. Synergistic activation of human pregnane X receptor by binary cocktails of pharmaceutical and environmental compounds. Nat. Commun. 2015, 6, 8089. [Google Scholar] [CrossRef]
- Venkatesh, M.; Mukherjee, S.; Wang, H.; Li, H.; Sun, K.; Benechet, A.P.; Qiu, Z.; Maher, L.; Redinbo, M.R.; Phillips, R.S.; et al. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity 2014, 41, 296–310. [Google Scholar] [CrossRef]
- Gruszczyk, J.; Grandvuillemin, L.; Lai-Kee-Him, J.; Paloni, M.; Savva, C.G.; Germain, P.; Grimaldi, M.; Boulahtouf, A.; Kwong, H.-S.; Bous, J.; et al. Cryo-EM structure of the agonist-bound Hsp90-XAP2-AHR cytosolic complex. bioRxiv 2022. [Google Scholar] [CrossRef]
- Wincent, E.; Bengtsson, J.; Mohammadi Bardbori, A.; Alsberg, T.; Luecke, S.; Rannug, U.; Rannug, A. Inhibition of cytochrome P4501-dependent clearance of the endogenous agonist FICZ as a mechanism for activation of the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA 2012, 109, 4479–4484. [Google Scholar] [CrossRef]
- Vyhlidalova, B.; Poulikova, K.; Bartonkova, I.; Krasulova, K.; Vanco, J.; Travnicek, Z.; Mani, S.; Dvorak, Z. Mono-methylindoles induce CYP1A genes and inhibit CYP1A1 enzyme activity in human hepatocytes and HepaRG cells. Toxicol. Lett. 2019, 313, 66–76. [Google Scholar] [CrossRef]
- Novotna, A.; Pavek, P.; Dvorak, Z. Novel stably transfected gene reporter human hepatoma cell line for assessment of aryl hydrocarbon receptor transcriptional activity: Construction and characterization. Environ. Sci Technol. 2011, 45, 10133–10139. [Google Scholar] [CrossRef]
- Novotna, A.; Pavek, P.; Dvorak, Z. Construction and characterization of a reporter gene cell line for assessment of human glucocorticoid receptor activation. Eur. J. Pharm. Sci. 2012, 47, 842–847. [Google Scholar] [CrossRef]
- Bartonkova, I.; Grycova, A.; Dvorak, Z. Profiling of vitamin D metabolic intermediates toward VDR using novel stable gene reporter cell lines IZ-VDRE and IZ-CYP24. Chem. Res. Toxicol. 2016, 29, 1211–1222. [Google Scholar] [CrossRef]
- Bartonkova, I.; Novotna, A.; Dvorak, Z. Novel stably transfected human reporter cell line AIZ-AR as a tool for an assessment of human androgen receptor transcriptional activity. PLoS ONE 2015, 10, e0121316. [Google Scholar] [CrossRef]
- Illes, P.; Grycova, A.; Krasulova, K.; Dvorak, Z. Effects of flavored nonalcoholic beverages on transcriptional activities of nuclear and steroid hormone receptors: Proof of concept for novel reporter cell line PAZ-PPARg. J. Agric. Food Chem. 2018, 66, 12066–12078. [Google Scholar] [CrossRef]
- Chou, T.-C. Drug combination studies and their synergy quantification using the chou-talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vrzalová, A.; Pečinková, P.; Illés, P.; Gurská, S.; Džubák, P.; Szotkowski, M.; Hajdúch, M.; Mani, S.; Dvořák, Z. Mixture Effects of Tryptophan Intestinal Microbial Metabolites on Aryl Hydrocarbon Receptor Activity. Int. J. Mol. Sci. 2022, 23, 10825. https://doi.org/10.3390/ijms231810825
Vrzalová A, Pečinková P, Illés P, Gurská S, Džubák P, Szotkowski M, Hajdúch M, Mani S, Dvořák Z. Mixture Effects of Tryptophan Intestinal Microbial Metabolites on Aryl Hydrocarbon Receptor Activity. International Journal of Molecular Sciences. 2022; 23(18):10825. https://doi.org/10.3390/ijms231810825
Chicago/Turabian StyleVrzalová, Aneta, Petra Pečinková, Peter Illés, Soňa Gurská, Petr Džubák, Martin Szotkowski, Marián Hajdúch, Sridhar Mani, and Zdeněk Dvořák. 2022. "Mixture Effects of Tryptophan Intestinal Microbial Metabolites on Aryl Hydrocarbon Receptor Activity" International Journal of Molecular Sciences 23, no. 18: 10825. https://doi.org/10.3390/ijms231810825
APA StyleVrzalová, A., Pečinková, P., Illés, P., Gurská, S., Džubák, P., Szotkowski, M., Hajdúch, M., Mani, S., & Dvořák, Z. (2022). Mixture Effects of Tryptophan Intestinal Microbial Metabolites on Aryl Hydrocarbon Receptor Activity. International Journal of Molecular Sciences, 23(18), 10825. https://doi.org/10.3390/ijms231810825






