Tumor-Suppressive and Oncogenic Roles of microRNA-149-5p in Human Cancers
Abstract
1. Introduction
2. Physiological Roles of miRNA-149-5p
2.1. miRNA-149-5p and Adipogenesis
2.2. miR-149-5p and Vascular Smooth Muscle Cells
2.3. miR-149-5p and Biological Stem Cells
3. miR-149-5p in Different Human Cancers
3.1. Cancers of the Endocrine System
Thyroid Cancer
3.2. Hematologic Malignancies
3.3. Cancers of the Central Nervous System (CNS)
3.3.1. Glioma
3.3.2. Pituitary Cancers
3.4. Bone Cancers
3.5. Cancers of the Digestive System
3.5.1. Hepatocellular Carcinoma
3.5.2. Gastric Cancer
3.5.3. Colorectal Cancer
3.5.4. Oral Cancer
3.5.5. Esophageal Cancer
3.6. Cancers of the Respiratory System
3.6.1. Lung Cancer
3.6.2. Nasopharyngeal Cancer
3.7. Cancers of the Reproductive System
3.7.1. Breast Cancer
3.7.2. Cervical Cancer
3.7.3. Ovarian Cancer
3.7.4. Endometrial Cancer
3.7.5. Prostate Cancer
3.8. Cancers of the Urological System
3.8.1. Kidney Cancer
3.8.2. Bladder Cancer
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ozsolak, F.; Poling, L.L.; Wang, Z.; Liu, H.; Liu, X.S.; Roeder, R.G.; Zhang, X.; Song, J.S.; Fisher, D.E. Chromatin structure analyses identify miRNA promoters. Genes Dev. 2008, 22, 3172–3183. [Google Scholar] [CrossRef] [PubMed]
- Kabekkodu, S.P.; Shukla, V.; Varghese, V.K.; D’Souza, J.; Chakrabarty, S.; Satyamoorthy, K. Clustered miRNAs and their role in biological functions and diseases. Biol. Rev. Camb. Philos. Soc. 2018, 93, 1955–1986. [Google Scholar] [CrossRef] [PubMed]
- Correia de Sousa, M.; Gjorgjieva, M.; Dolicka, D.; Sobolewski, C.; Foti, M. Deciphering miRNAs’ Action through miRNA Editing. Int. J. Mol. Sci. 2019, 20, 6249. [Google Scholar] [CrossRef] [PubMed]
- Dakhlallah, D.; Batte, K.; Wang, Y.; Cantemir-Stone, C.Z.; Yan, P.; Nuovo, G.; Mikhail, A.; Hitchcock, C.L.; Wright, V.P.; Nana-Sinkam, S.P.; et al. Epigenetic regulation of miR-17~92 contributes to the pathogenesis of pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2013, 187, 397–405. [Google Scholar] [CrossRef]
- Lujambio, A.; Calin, G.A.; Villanueva, A.; Ropero, S.; Sánchez-Céspedes, M.; Blanco, D.; Montuenga, L.M.; Rossi, S.; Nicoloso, M.S.; Faller, W.J.; et al. A microRNA DNA methylation signature for human cancer metastasis. Proc. Natl. Acad. Sci. USA 2008, 105, 13556–13561. [Google Scholar] [CrossRef] [PubMed]
- Dai, E.; Yu, X.; Zhang, Y.; Meng, F.; Wang, S.; Liu, X.; Liu, D.; Wang, J.; Li, X.; Jiang, W. EpimiR: A database of curated mutual regulation between miRNAs and epigenetic modifications. Database 2014, 2014, bau023. [Google Scholar] [CrossRef]
- Torsin, L.I.; Petrescu, G.E.D.; Sabo, A.A.; Chen, B.; Brehar, F.M.; Dragomir, M.P.; Calin, G.A. Editing and Chemical Modifications on Non-Coding RNAs in Cancer: A New Tale with Clinical Significance. Int. J. Mol. Sci. 2021, 22, 581. [Google Scholar] [CrossRef]
- He, B.; Zhao, Z.; Cai, Q.; Zhang, Y.; Zhang, P.; Shi, S.; Xie, H.; Peng, X.; Yin, W.; Tao, Y.; et al. miRNA-based biomarkers, therapies, and resistance in Cancer. Int. J. Biol. Sci. 2020, 16, 2628–2647. [Google Scholar] [CrossRef]
- Hussen, B.M.; Hidayat, H.J.; Salihi, A.; Sabir, D.K.; Taheri, M.; Ghafouri-Fard, S. MicroRNA: A signature for cancer progression. Biomed. Pharmacother. 2021, 138, 111528. [Google Scholar] [CrossRef]
- Lu, Y.; Deng, M.; Wang, K.; Peng, Y.; Ouyang, M. The Regulatory Effects of MicroRNAs on Tumor Immunity. BioMed Res. Int. 2022, 2022, 2121993. [Google Scholar] [CrossRef]
- Di, C.; Zhang, Q.; Wang, Y.; Wang, F.; Chen, Y.; Gan, L.; Zhou, R.; Sun, C.; Li, H.; Zhang, X.; et al. Exosomes as drug carriers for clinical application. Artif. Cells Nanomed. Biotechnol. 2018, 46, S564–S570. [Google Scholar] [CrossRef]
- Liu, J.; Meng, T.; Yuan, M.; Wen, L.; Cheng, B.; Liu, N.; Huang, X.; Hong, Y.; Yuan, H.; Hu, F. MicroRNA-200c delivered by solid lipid nanoparticles enhances the effect of paclitaxel on breast cancer stem cell. Int. J. Nanomed. 2016, 11, 6713–6725. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cui, J.; Gong, Y.; Wei, S.; Wei, Y.; Yi, L. MicroRNA: A novel implication for damage and protection against ionizing radiation. Environ. Sci. Pollut. Res. 2021, 28, 15584–15596. [Google Scholar] [CrossRef] [PubMed]
- Mireștean, C.C.; Iancu, R.I.; Iancu, D.P.T. Micro-RNAs, the Cornerstones of the Future of Radiobiology in Head and Neck Cancers? Curr. Oncol. 2022, 29, 816–833. [Google Scholar] [CrossRef]
- Ren, F.-J.; Yao, Y.; Cai, X.-Y.; Cai, Y.-T.; Su, Q.; Fang, G.-Y. MiR-149-5p: An Important miRNA Regulated by Competing Endogenous RNAs in Diverse Human Cancers. Front. Oncol. 2021, 11, 743077. [Google Scholar] [CrossRef]
- Guo, H.; Khan, R.; Abbas Raza, S.H.; Suhail, S.M.; Khan, H.; Khan, S.B.; Abd El-Aziz, A.H.; Zan, L. RNA-Seq Reveals Function of Bta-miR-149-5p in the Regulation of Bovine Adipocyte Differentiation. Animals 2021, 11, 1207. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hong, B.S.; Ryu, H.S.; Lee, H.B.; Lee, M.; Park, I.A.; Kim, J.; Han, W.; Noh, D.Y.; Moon, H.G. Transition into inflammatory cancer-associated adipocytes in breast cancer microenvironment requires microRNA regulatory mechanism. PLoS ONE 2017, 12, e0174126. [Google Scholar] [CrossRef]
- Rajarajan, D.; Selvarajan, S.; Charan Raja, M.R.; Kar Mahapatra, S.; Kasiappan, R. Genome-wide analysis reveals miR-3184-5p and miR-181c-3p as a critical regulator for adipocytes-associated breast cancer. J. Cell. Physiol. 2019, 234, 17959–17974. [Google Scholar] [CrossRef]
- Wang, G.; Jacquet, L.; Karamariti, E.; Xu, Q. Origin and differentiation of vascular smooth muscle cells. J. Physiol. 2015, 593, 3013–3030. [Google Scholar] [CrossRef]
- Zhang, B.; Dong, Y.; Liu, M.; Yang, L.; Zhao, Z. miR-149-5p Inhibits Vascular Smooth Muscle Cells Proliferation, Invasion, and Migration by Targeting Histone Deacetylase 4 (HDAC4). Med. Sci. Monit. 2019, 25, 7581–7590. [Google Scholar] [CrossRef]
- Peng, W.; Li, T.; Pi, S.; Huang, L.; Liu, Y. Suppression of circular RNA circDHCR24 alleviates aortic smooth muscle cell proliferation and migration by targeting miR-149-5p/MMP9 axis. Biochem. Biophys. Res. Commun. 2020, 529, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, C.; Cai, T.; Zhang, A.; Cao, J.; Xin, H. Circ_CHFR Promotes Platelet-Derived Growth Factor-BB–Induced Proliferation, Invasion, and Migration in Vascular Smooth Muscle Cells via the miR-149-5p/NRP2 Axis. J. Cardiovasc. Pharmacol. 2022, 79, e94–e102. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qu, J.; Li, Y.; Feng, Y.; Ma, J.; Zhang, L.; Chu, C.; Hu, H.; Wang, Y.; Ji, D. miR-149-5p Regulates Goat Hair Follicle Stem Cell Proliferation and Apoptosis by Targeting the CMTM3/AR Axis During Superior-Quality Brush Hair Formation. Front. Genet. 2020, 11, 529757. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Fan, D.; Yuan, W. CMTM3 suppresses bone formation and osteogenic differentiation of mesenchymal stem cells through inhibiting Erk1/2 and RUNX2 pathways. Genes Dis. 2021, 8, 882–890. [Google Scholar] [CrossRef]
- Prete, A.; Borges de Souza, P.; Censi, S.; Muzza, M.; Nucci, N.; Sponziello, M. Update on Fundamental Mechanisms of Thyroid Cancer. Front. Endocrinol. 2020, 11, 102. [Google Scholar] [CrossRef]
- Wei, W.-J.; Lu, Z.-W.; Li, D.-S.; Wang, Y.; Zhu, Y.-X.; Wang, Z.-Y.; Wu, Y.; Wang, Y.-L.; Ji, Q.-H. Association of the miR-149 Rs2292832 Polymorphism with Papillary Thyroid Cancer Risk and Clinicopathologic Characteristics in a Chinese Population. Int. J. Mol. Sci. 2014, 15, 20968–20981. [Google Scholar] [CrossRef]
- Ouyang, X.; Feng, L.; Yao, L.; Xiao, Y.; Hu, X.; Zhang, G.; Liu, G.; Wang, Z. Testicular orphan receptor 4 (TR4) promotes papillary thyroid cancer invasion via activating circ-FNLA/miR-149-5p/MMP9 signaling. Mol. Ther. Nucleic Acids 2021, 24, 755–767. [Google Scholar] [CrossRef]
- Sun, C.; Wang, P.; Gao, T.; Chi, J. CCL18 Knockdown Suppresses Cell Growth and Migration in Thyroid Cancer. J. Healthc. Eng. 2022, 2022, 1548155. [Google Scholar] [CrossRef]
- Chen, H.; Li, Q.; Yi, R.; Li, B.; Xiong, D.; Peng, H. CircRNA casein kinase 1 gamma 1 (circ-CSNK1G1) plays carcinogenic effects in thyroid cancer by acting as miR-149-5p sponge and relieving the suppression of miR-149-5p on mitogen-activated protein kinase 1 (MAPK1). J. Clin. Lab. Anal. 2022, 36, e24188. [Google Scholar] [CrossRef]
- Ye, X.; Chen, X. miR-149-5p inhibits cell proliferation and invasion through targeting GIT1 in medullary thyroid carcinoma. Oncol. Lett. 2019, 17, 372–378. [Google Scholar] [CrossRef]
- Tian, P.; Yan, L. Inhibition of MicroRNA-149-5p Induces Apoptosis of Acute Myeloid Leukemia Cell Line THP-1 by Targeting Fas Ligand (FASLG). Med. Sci. Monit. 2016, 22, 5116–5123. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, F.; Fang, E.; Xiao, W.; Mei, H.; Li, H.; Li, D.; Song, H.; Wang, J.; Hong, M.; et al. Circular RNA circAGO2 drives cancer progression through facilitating HuR-repressed functions of AGO2-miRNA complexes. Cell Death Differ. 2019, 26, 1346–1364. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ma, X.; Zhang, H.; Wu, Y.; Kang, M.; Fang, Y.; Xue, Y. Mechanism of circADD2 as ceRNA in Childhood Acute Lymphoblastic Leukemia. Front. Cell Dev. Biol. 2021, 9, 639910. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Luo, X.; Ning, X.; Luo, J.; Guo, J.; Liu, Q.; Ling, G. miR-149 rs2292832 C allele enhances the cytotoxic effect of temozolomide against glioma cells. NeuroReport 2020, 31, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Kasuki, L.; Raverot, G. Definition and diagnosis of aggressive pituitary tumors. Rev. Endocr. Metab. Disord. 2020, 21, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Cheng, J.; Li, B.; Nie, D.; Li, C.; Gui, S.; Wang, H.; Zhang, Y. Up-regulation of the expressions of MiR-149-5p and MiR-99a-3p in exosome inhibits the progress of pituitary adenomas. Cell Biol. Toxicol. 2021, 37, 633–651. [Google Scholar] [CrossRef]
- MacDonald, I.J.; Lin, C.-Y.; Kuo, S.-J.; Su, C.-M.; Tang, C.-H. An update on current and future treatment options for chondrosarcoma. Expert Rev. Anticancer Ther. 2019, 19, 773–786. [Google Scholar] [CrossRef]
- Tzeng, H.-E.; Lin, S.-L.; Thadevoos, L.A.; Lien, M.-Y.; Yang, W.-H.; Ko, C.-Y.; Lin, C.-Y.; Huang, Y.-W.; Liu, J.-F.; Fong, Y.-C.; et al. Nerve growth factor promotes lysyl oxidase-dependent chondrosarcoma cell metastasis by suppressing miR-149-5p synthesis. Cell Death Dis. 2021, 12, 1101. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Dong, J.; Teng, F.; Guo, W.; Yang, J.; Ding, G.; Fu, Z. lncRNA SNHG8 Promotes the Tumorigenesis and Metastasis by Sponging miR-149-5p and Predicts Tumor Recurrence in Hepatocellular Carcinoma. Cell. Physiol. Biochem. 2018, 51, 2262–2274. [Google Scholar] [CrossRef]
- Ji, D.; Wang, Y.; Li, H.; Sun, B.; Luo, X. Long non-coding RNA LINC00461/miR-149-5p/LRIG2 axis regulates hepatocellular carcinoma progression. Biochem. Biophys. Res. Commun. 2019, 512, 176–181. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, P.; Tu, M.; Huang, Y.; Xiong, F.; Wu, Y. Long Non-Coding RNA PART1 Promotes Proliferation, Migration and Invasion of Hepatocellular Carcinoma Cells via miR-149-5p/MAP2K1 Axis. Cancer Manag. Res. 2020, 12, 3771–3782. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Ji, Q.; Wang, C.; Yu, L.; Yu, R. Aloin decelerates the progression of hepatocellular carcinoma through circ_0011385/miR-149-5p/WT1 axis. Cell Cycle 2021, 20, 2476–2493. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yin, L.; Ouyang, X.; Zeng, K.; Xiao, Y.; Li, Y. M2 Macrophages Promote HCC Cells Invasion and Migration via miR-149-5p/MMP9 Signaling. J. Cancer 2020, 11, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Keating, G.M. Sorafenib: A Review in Hepatocellular Carcinoma. Target. Oncol. 2017, 12, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Tang, G.; Wu, X.; Wu, C. LncRNA NEAT1 modulates sorafenib resistance in hepatocellular carcinoma through regulating the miR-149-5p/AKT1 axis. Saudi J. Gastroenterol. 2020, 26, 194–203. [Google Scholar]
- Wang, S.; Zhang, J.H.; Wang, H.; Yang, L.; Hong, S.; Yu, B.; Guo, J.C.; Liu, J.; Zhu, Y.B. A novel multidimensional signature predicts prognosis in hepatocellular carcinoma patients. J. Cell. Physiol. 2019, 234, 11610–11619. [Google Scholar] [CrossRef]
- Fang, S.S.; Guo, J.C.; Zhang, J.H.; Liu, J.N.; Hong, S.; Yu, B.; Gao, Y.; Hu, S.P.; Liu, H.Z.; Sun, L.; et al. A P53-related microRNA model for predicting the prognosis of hepatocellular carcinoma patients. J. Cell. Physiol. 2020, 235, 3569–3578. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, G.; Xu, H.; Wang, H.; Bai, D. Identification of a Novel Metastasis-Related miRNAs-Based Signature for Predicting the Prognosis of Hepatocellular Carcinoma. J. Oncol. 2021, 2021, 6629633. [Google Scholar] [CrossRef]
- Chen, E.B.; Zhou, Z.J.; Xiao, K.; Zhu, G.Q.; Yang, Y.; Wang, B.; Zhou, S.L.; Chen, Q.; Yin, D.; Wang, Z.; et al. The miR-561-5p/CX(3)CL1 Signaling Axis Regulates Pulmonary Metastasis in Hepatocellular Carcinoma Involving CX(3)CR1(+) Natural Killer Cells Infiltration. Theranostics 2019, 9, 4779–4794. [Google Scholar] [CrossRef]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Wang, H.; Cao, J.; Huang, X.; Chen, Z.; Xu, P.; Sun, G.; Xu, J.; Lv, J.; et al. Circular RNA circNRIP1 acts as a microRNA-149-5p sponge to promote gastric cancer progression via the AKT1/mTOR pathway. Mol. Cancer 2019, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.; Tian, L.; He, X. Circular RNA circNHSL1 Contributes to Gastric Cancer Progression Through the miR-149-5p/YWHAZ Axis. Cancer Manag. Res. 2020, 12, 7117–7130. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cai, B.; Shi, X.; Duan, C.; Tong, T.; Yu, C. circ_0044516 functions in the progression of gastric cancer by modulating MicroRNA-149-5p/HuR axis. Mol. Cell. Biochem. 2021, 477, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Li, F.; Liu, H. Circ-DONSON Facilitates the Malignant Progression of Gastric Cancer Depending on the Regulation of miR-149-5p/LDHA Axis. Biochem. Genet. 2022, 60, 640–655. [Google Scholar] [CrossRef]
- Jin, D.; Huang, K.; Peng, L.; Xu, P.; Dang, Y.; Yang, J.; Chen, M.; Zhu, X.; Wei, S.; Yan, J.; et al. Circular RNA circDNA2 upregulates CCDC6 expression to promote the progression of gastric cancer via miR-149-5p suppression. Mol. Ther. Nucleic Acids 2021, 26, 360–373. [Google Scholar] [CrossRef]
- Cui, H.; Ruan, M.; Xu, H.; Qi, J.; Ruan, L.; Gao, X.; Sun, X.; Zhang, S.; Zuo, R.; Yin, Y. LINC01420 Serves as a Novel Prognostic Biomarker and Promotes Cell Proliferation, Migration, and Invasion by Suppressing miR-149-5p in Gastric Cancer. Crit. Rev. Eukaryot. Gene Expr. 2021, 31, 49–58. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, D.; Wang, H.; Xie, J.; Peng, L.; Peng, Y.; Zheng, X.; Jiang, J. SLCO4A1-AS1 Facilitates the Malignant Phenotype via miR-149-5p/STAT3 Axis in Gastric Cancer Cells. J. Oncol. 2021, 2021, 1698771. [Google Scholar] [CrossRef]
- Qin, H.; Li, X.; Zhang, W.; Ding, Z. LncRNA OGFRP1 promotes cell proliferation and suppresses cell radiosensitivity in gastric cancer by targeting the miR-149-5p/MAP3K3 axis. J. Mol. Histol. 2022, 53, 257–271. [Google Scholar] [CrossRef]
- Liu, H.; Dai, W. Circular RNA 0000654 facilitates the growth of gastric cancer cells through absorbing microRNA-149-5p to up-regulate inhibin-beta A. Bioengineered 2022, 13, 469–480. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Peng, X.; Zhang, C.; Liu, K.; Huang, G.; Lai, Y. Use of a Four-miRNA Panel as a Biomarker for the Diagnosis of Stomach Adenocarcinoma. Dis. Markers 2020, 2020, 8880937. [Google Scholar] [CrossRef]
- Liu, X.; Pu, K.; Wang, Y.; Chen, Y.; Zhou, Y. Gastric cancer-associated microRNA expression signatures: Integrated bioinformatics analysis, validation, and clinical significance. Ann. Transl. Med. 2021, 9, 797. [Google Scholar] [CrossRef]
- Kurata, J.S.; Lin, R.J. MicroRNA-focused CRISPR-Cas9 library screen reveals fitness-associated miRNAs. RNA 2018, 24, 966–981. [Google Scholar] [CrossRef]
- Lian, Y.; Yan, C.; Xu, H.; Yang, J.; Yu, Y.; Zhou, J.; Shi, Y.; Ren, J.; Ji, G.; Wang, K. A Novel lncRNA, LINC00460, Affects Cell Proliferation and Apoptosis by Regulating KLF2 and CUL4A Expression in Colorectal Cancer. Mol. Ther. Nucleic Acids 2018, 12, 684–697. [Google Scholar] [CrossRef]
- Wang, A.H.; Fan, W.J.; Fu, L.; Wang, X.T. LncRNA PCAT-1 regulated cell proliferation, invasion, migration and apoptosis in colorectal cancer through targeting miR-149-5. Eur. Rev. Med. Pharm. Sci. 2019, 23, 8310–8320. [Google Scholar]
- Ma, Z.; Han, C.; Xia, W.; Wang, S.; Li, X.; Fang, P.; Yin, R.; Xu, L.; Yang, L. circ5615 functions as a ceRNA to promote colorectal cancer progression by upregulating TNKS. Cell Death Dis. 2020, 11, 356. [Google Scholar] [CrossRef]
- Chen, P.; Yao, Y.; Yang, N.; Gong, L.; Kong, Y.; Wu, A. Circular RNA circCTNNA1 promotes colorectal cancer progression by sponging miR-149-5p and regulating FOXM1 expression. Cell Death Dis. 2020, 11, 557. [Google Scholar] [CrossRef]
- Ruan, T.; Lu, S.; Xu, J.; Zhou, J.-Y. lncRNA LINC00460 Functions as a Competing Endogenous RNA and Regulates Expression of BGN by Sponging miR-149-5p in Colorectal Cancer. Technol. Cancer Res. Treat. 2021, 20, 1533033820964238. [Google Scholar] [CrossRef]
- Qu, L.; Chen, Y.; Zhang, F.; He, L. The lncRNA DLGAP1-AS1/miR-149-5p/TGFB2 axis contributes to colorectal cancer progression and 5-FU resistance by regulating smad2 pathway. Mol. Ther. Oncolytics 2021, 20, 607–624. [Google Scholar] [CrossRef]
- Yang, A.D.; Fan, F.; Camp, E.R.; van Buren, G.; Liu, W.; Somcio, R.; Gray, M.J.; Cheng, H.; Hoff, P.M.; Ellis, L.M. Chronic oxaliplatin resistance induces epithelial-to-mesenchymal transition in colorectal cancer cell lines. Clin. Cancer Res. 2006, 12, 4147–4153. [Google Scholar] [CrossRef]
- Meng, X.; Sun, W.; Yu, J.; Zhou, Y.; Gu, Y.; Han, J.; Zhou, L.; Jiang, X.; Wang, C. LINC00460-miR-149-5p/miR-150-5p-Mutant p53 Feedback Loop Promotes Oxaliplatin Resistance in Colorectal Cancer. Mol. Ther. Nucleic Acids 2020, 22, 1004–1015. [Google Scholar] [CrossRef]
- Qi, F.F.; Yang, Y.; Zhang, H.; Chen, H. Long non-coding RNAs: Key regulators in oxaliplatin resistance of colorectal cancer. Biomed. Pharm. 2020, 128, 110329. [Google Scholar] [CrossRef]
- Milanesi, E.; Dobre, M.; Bucuroiu, A.I.; Herlea, V.; Manuc, T.E.; Salvi, A.; De Petro, G.; Manuc, M.; Becheanu, G. miRNAs-Based Molecular Signature for KRAS Mutated and Wild Type Colorectal Cancer: An Explorative Study. J. Immunol. Res. 2020, 2020, 4927120. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.; Li, W. MicroRNA-149 targets specificity protein 1 to suppress human tongue squamous cell carcinoma cell proliferation and motility. Oncol. Lett. 2017, 13, 851–856. [Google Scholar] [CrossRef]
- Lai, H.; Xu, G.; Meng, H.; Zhu, H. Association of SP1 rs1353058818 and STAT3 rs1053004 gene polymorphisms with human tongue squamous cell carcinoma. Biosci. Rep. 2019, 39, BSR20190955. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, S.; Gao, L.; Zhi, K.; Ren, W. The Molecular Basis and Therapeutic Aspects of Cisplatin Resistance in Oral Squamous Cell Carcinoma. Front. Oncol. 2021, 11, 761379. [Google Scholar] [CrossRef]
- Luo, K.; He, J.; Yu, D.; Açil, Y. MiR-149-5p regulates cisplatin chemosensitivity, cell growth, and metastasis of oral squamous cell carcinoma cells by targeting TGFβ2. Int. J. Clin. Exp. Pathol. 2019, 12, 3728–3739. [Google Scholar]
- Qiu, L.; Zheng, L.; Gan, C.; Deng, W.; Sun, Y.; Wang, T. circBICD2 targets miR-149-5p/IGF2BP1 axis to regulate oral squamous cell carcinoma progression. J. Oral Pathol. Med. 2021, 50, 668–680. [Google Scholar] [CrossRef]
- Lv, T.; Liu, H.; Wu, Y.; Huang, W. Knockdown of lncRNA DLEU1 inhibits the tumorigenesis of oral squamous cell carcinoma via regulation of miR-149-5p/CDK6 axis. Mol. Med. Rep. 2021, 23, 447. [Google Scholar] [CrossRef]
- Xu, Z.; Tie, X.; Li, N.; Yi, Z.; Shen, F.; Zhang, Y. Circular RNA hsa_circ_0000654 promotes esophageal squamous cell carcinoma progression by regulating the miR-149-5p/IL-6/STAT3 pathway. IUBMB Life 2020, 72, 426–439. [Google Scholar] [CrossRef]
- Li, F.; Zhou, X.; Chen, M.; Fan, W. Regulatory effect of LncRNA DRAIC/miR-149-5p/NFIB molecular network on autophagy of esophageal cancer cells and its biological behavior. Exp. Mol. Pathol. 2020, 116, 104491. [Google Scholar] [CrossRef]
- The, L. Lung cancer: Some progress, but still a lot more to do. Lancet 2019, 394, 1880. [Google Scholar]
- Li, J.; Li, Y.; Wang, B.; Ma, Y.; Chen, P. LncRNA-PCAT-1 promotes non-small cell lung cancer progression by regulating miR-149-5p/LRIG2 axis. J. Cell Biochem. 2018, 120, 7725–7733. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Y.; Li, Q.; Duan, P. lncRNA HNF1A-AS1 modulates non-small cell lung cancer progression by targeting miR-149-5p/Cdk6. J. Cell Biochem. 2019, 120, 18736–18750. [Google Scholar] [CrossRef]
- Chen, Q.; Luo, J.; Wu, C.; Lu, H.; Cai, S.; Bao, C.; Liu, D.; Kong, J. The miRNA-149-5p/MyD88 axis is responsible for ursolic acid-mediated attenuation of the stemness and chemoresistance of non-small cell lung cancer cells. Environ. Toxicol. 2020, 35, 561–569. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, S.; Xiong, Y. Long noncoding RNA MIAT promotes non-small cell lung cancer progression by sponging miR-149-5p and regulating FOXM1 expression. Cancer Cell Int. 2020, 20, 348. [Google Scholar] [CrossRef]
- Li, H.; Cui, Z.; Lv, X.; Li, J.; Gao, M.; Yang, Z.; Bi, Y.; Zhang, Z.; Wang, S.; Li, S.; et al. Long Non-coding RNA HOTAIR Function as a Competing Endogenous RNA for miR-149-5p to Promote the Cell Growth, Migration, and Invasion in Non-small Cell Lung Cancer. Front. Oncol. 2020, 10, 528520. [Google Scholar] [CrossRef]
- Wei, H.; Li, L.; Zhang, H.; Xu, F.; Chen, L.; Che, G.; Wang, Y. Circ-FOXM1 knockdown suppresses non-small cell lung cancer development by regulating the miR-149-5p/ATG5 axis. Cell Cycle 2021, 20, 166–178. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, T.; Xian, L.; Liu, W.; Liu, J.; Zhou, H. B3GNT3, a Direct Target of miR-149-5p, Promotes Lung Cancer Development and Indicates Poor Prognosis of Lung Cancer. Cancer Manag. Res. 2020, 12, 2381–2391. [Google Scholar] [CrossRef]
- Zhan, Y.; Abuduwaili, K.; Wang, X.; Shen, Y.; Nuerlan, S.; Liu, C. Knockdown of Long Non-Coding RNA HOTAIR Suppresses Cisplatin Resistance, Cell Proliferation, Migration and Invasion of DDP-Resistant NSCLC Cells by Targeting miR-149-5p/Doublecortin-Like Kinase 1 Axis. Cancer Manag. Res. 2020, 12, 7725–7737. [Google Scholar] [CrossRef]
- Nakano, Y.; Isobe, K.; Kobayashi, H.; Kaburaki, K.; Isshiki, T.; Sakamoto, S.; Takai, Y.; Tochigi, N.; Mikami, T.; Iyoda, A.; et al. Clinical importance of long non-coding RNA LINC00460 expression in EGFR-mutant lung adenocarcinoma. Int. J. Oncol. 2020, 56, 243–257. [Google Scholar] [CrossRef]
- Konoshenko, M.; Lansukhay, Y.; Krasilnikov, S.; Laktionov, P. MicroRNAs as Predictors of Lung-Cancer Resistance and Sensitivity to Cisplatin. Int. J. Mol. Sci. 2022, 23, 7594. [Google Scholar] [CrossRef]
- Tian, W.; Yang, H.; Zhou, B. Integrative analysis of exosomal microRNA-149-5p in lung adenocarcinoma. Aging 2021, 13, 7382–7396. [Google Scholar] [CrossRef]
- Kong, Y.G.; Cui, M.; Chen, S.M.; Xu, Y.; Xu, Y.; Tao, Z.Z. LncRNA-LINC00460 facilitates nasopharyngeal carcinoma tumorigenesis through sponging miR-149-5p to up-regulate IL6. Gene 2018, 639, 77–84. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Xiang, F.; Fan, Y.; Ni, Z.; Liu, Q.; Zhu, Z.; Chen, Z.; Hao, W.; Yue, H.; Wu, R.; Kang, X. Ursolic Acid Reverses the Chemoresistance of Breast Cancer Cells to Paclitaxel by Targeting MiRNA-149-5p/MyD88. Front. Oncol. 2019, 9, 501. [Google Scholar] [CrossRef]
- Barok, M.; Joensuu, H.; Isola, J. Trastuzumab emtansine: Mechanisms of action and drug resistance. Breast Cancer Res. 2014, 16, 209. [Google Scholar] [CrossRef]
- Tian, D.; Tian, M.; Ma, Z.M.; Zhang, L.L.; Cui, Y.F.; Li, J.L. Anesthetic propofol epigenetically regulates breast cancer trastuzumab resistance through IL-6/miR-149-5p axis. Sci. Rep. 2020, 10, 8858. [Google Scholar] [CrossRef]
- Wang, J.; Yang, K.; Cao, J.; Li, L. Knockdown of circular RNA septin 9 inhibits the malignant progression of breast cancer by reducing the expression of solute carrier family 1 member 5 in a microRNA-149-5p-dependent manner. Bioengineered 2021, 12, 10624–10637. [Google Scholar] [CrossRef]
- Qi, C.; Qin, X.; Zhou, Z.; Wang, Y.; Yang, Q.; Liao, T. Circ_0072995 Promotes Cell Carcinogenesis via Up-Regulating miR-149-5p-Mediated SHMT2 in Breast Cancer. Cancer Manag. Res. 2020, 12, 11169–11181. [Google Scholar] [CrossRef]
- Li, H.; Yin, H.; Yan, Y. Circ_0041732 regulates tumor properties of triple-negative breast cancer cells by the miR-149-5p/FGF5 pathway. Int. J. Biol. Markers 2022, 37, 178–190. [Google Scholar] [CrossRef]
- Maimaiti, Y.; Zhang, N.; Zhang, Y.; Zhou, J.; Song, H.; Wang, S. CircFAM64A enhances cellular processes in triple-negative breast cancer by targeting the miR-149-5p/CDT1 axis. Environ. Toxicol. 2022, 37, 1081–1092. [Google Scholar] [CrossRef]
- Yuan, Y.; Shi, X.; Li, B.; Peng, M.; Zhu, T.; Lv, G.; Liu, L.; Jin, H.; Li, L.; Qin, D. Integrated analysis of key microRNAs/TFs/mRNAs/in HPV-positive cervical cancer based on microRNA sequencing and bioinformatics analysis. Pathol. Res. Pr. 2020, 216, 152952. [Google Scholar] [CrossRef]
- Shao, S.; Wang, C.; Wang, S.; Zhang, H.; Zhang, Y. Hsa_circ_0075341 is up-regulated and exerts oncogenic properties by sponging miR-149-5p in cervical cancer. Biomed. Pharm. 2020, 121, 109582. [Google Scholar] [CrossRef]
- Xu, A.L.; Wang, W.S.; Zhao, M.Y.; Sun, J.N.; Chen, X.R.; Hou, J.Q. Circular RNA circ_0011385 promotes cervical cancer progression through competitively binding to miR-149-5p and up-regulating SOX4 expression. Kaohsiung J. Med. Sci. 2021, 37, 1058–1068. [Google Scholar] [CrossRef]
- Hu, L.; Zhou, M.; Xue, L.; Zhang, J. Circular RNA hsa_circ_0011385 contributes to cervical cancer progression through sequestering miR-149-5p and increasing PRDX6 expression. Reprod. Biol. 2022, 22, 100619. [Google Scholar] [CrossRef]
- Stage, T.B.; Bergmann, T.K.; Kroetz, D.L. Clinical Pharmacokinetics of Paclitaxel Monotherapy: An Updated Literature Review. Clin. Pharm. 2018, 57, 7–19. [Google Scholar] [CrossRef]
- Vasey, P.A. Resistance to chemotherapy in advanced ovarian cancer: Mechanisms and current strategies. Br. J. Cancer 2003, 89, S23–S28. [Google Scholar] [CrossRef]
- Wei, S.; Qi, L.; Wang, L. Overexpression of circ_CELSR1 facilitates paclitaxel resistance of ovarian cancer by regulating miR-149-5p/SIK2 axis. Anticancer Drugs 2021, 32, 496–507. [Google Scholar] [CrossRef]
- Sun, L.; Zhai, R.; Zhang, L.; Zhao, S. MicroRNA-149 suppresses the proliferation and increases the sensitivity of ovarian cancer cells to cisplatin by targeting X-linked inhibitor of apoptosis. Oncol. Lett. 2018, 15, 7328–7334. [Google Scholar] [CrossRef]
- Xu, M.; Xiao, J.; Chen, M.; Yuan, L.; Li, J.; Shen, H.; Yao, S. miR-149-5p promotes chemotherapeutic resistance in ovarian cancer via the inactivation of the Hippo signaling pathway. Int. J. Oncol. 2018, 52, 815–827. [Google Scholar] [CrossRef]
- Liu, Y.; Chang, Y.; Cai, Y. Hsa_circ_0061140 promotes endometrial carcinoma progression via regulating miR-149-5p/STAT3. Gene 2020, 745, 144625. [Google Scholar] [CrossRef]
- Fu, W.; Hong, Z.; You, X.; Din, J.; Chen, B.; Zhao, B.; Yuan, G.; Li, Q. Enhancement of anticancer activity of docetaxel by combination with Fuzheng Yiliu decoction in a mouse model of castration-resistant prostate cancer. Biomed. Pharm. 2019, 118, 109374. [Google Scholar] [CrossRef]
- Ma, J.; Wei, H.; Li, X.; Qu, X. Hsa-miR-149-5p Suppresses Prostate Carcinoma Malignancy by Suppressing RGS17. Cancer Manag. Res. 2021, 13, 2773–2783. [Google Scholar] [CrossRef]
- Temiz, E.; Koyuncu, İ.; Sahin, E. CCT3 suppression prompts apoptotic machinery through oxidative stress and energy deprivation in breast and prostate cancers. Free Radic. Biol. Med. 2021, 165, 88–99. [Google Scholar] [CrossRef]
- Jonasch, E.; Gao, J.; Rathmell, W.K. Renal cell carcinoma. BMJ 2014, 349, g4797. [Google Scholar] [CrossRef]
- Patard, J.J.; Leray, E.; Rioux-Leclercq, N.; Cindolo, L.; Ficarra, V.; Zisman, A.; De La Taille, A.; Tostain, J.; Artibani, W.; Abbou, C.C.; et al. Prognostic value of histologic subtypes in renal cell carcinoma: A multicenter experience. J. Clin. Oncol. 2005, 23, 2763–2771. [Google Scholar] [CrossRef]
- Jin, L.; Li, Y.; Liu, J.; Yang, S.; Gui, Y.; Mao, X.; Nie, G.; Lai, Y. Tumor suppressor miR-149-5p is associated with cellular migration, proliferation and apoptosis in renal cell carcinoma. Mol. Med. Rep. 2016, 13, 5386–5392. [Google Scholar] [CrossRef]
- Gremel, G.; Djureinovic, D.; Niinivirta, M.; Laird, A.; Ljungqvist, O.; Johannesson, H.; Bergman, J.; Edqvist, P.H.; Navani, S.; Khan, N.; et al. A systematic search strategy identifies cubilin as independent prognostic marker for renal cell carcinoma. BMC Cancer 2017, 17, 9. [Google Scholar] [CrossRef]
- Okato, A.; Arai, T.; Yamada, Y.; Sugawara, S.; Koshizuka, K.; Fujimura, L.; Kurozumi, A.; Kato, M.; Kojima, S.; Naya, Y.; et al. Dual Strands of Pre-miR-149 Inhibit Cancer Cell Migration and Invasion through Targeting FOXM1 in Renal Cell Carcinoma. Int. J. Mol. Sci. 2017, 18, 1969. [Google Scholar] [CrossRef]
- Xie, M.; Lv, Y.; Liu, Z.; Zhang, J.; Liang, C.; Liao, X.; Liang, R.; Lin, Y.; Li, Y. Identification and validation of a four-miRNA (miRNA-21-5p, miRNA-9-5p, miR-149-5p, and miRNA-30b-5p) prognosis signature in clear cell renal cell carcinoma. Cancer Manag. Res. 2018, 10, 5759–5766. [Google Scholar] [CrossRef] [PubMed]
- Mathes, J.; Rausch, S.; Todenhöfer, T.; Stenzl, A. Trimodal therapy for muscle-invasive bladder cancer. Expert Rev. Anticancer Ther. 2018, 18, 1219–1229. [Google Scholar] [CrossRef]
- Pham, A.; Ballas, L.K. Trimodality therapy for bladder cancer: Modern management and future directions. Curr. Opin. Urol. 2019, 29, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Niu, X.; Mao, F.; Liu, X.; Zhong, B.; Jiang, H.; Fu, G. Hsa_circRNA_100146 Acts as a Sponge of miR-149-5p in Promoting Bladder Cancer Progression via Regulating RNF2. Onco Targets Ther. 2020, 13, 11007. [Google Scholar] [CrossRef]
- Koguchi, D.; Matsumoto, K.; Shiba, I.; Harano, T.; Okuda, S.; Mori, K.; Hirano, S.; Kitajima, K.; Ikeda, M.; Iwamura, M. Diagnostic Potential of Circulating Tumor Cells, Urinary MicroRNA, and Urinary Cell-Free DNA for Bladder Cancer: A Review. Int. J. Mol. Sci. 2022, 23, 9148. [Google Scholar] [CrossRef]
- Afzalinia, A.; Mirzaee, M. Ultrasensitive Fluorescent miRNA Biosensor Based on a “Sandwich” Oligonucleotide Hybridization and Fluorescence Resonance Energy Transfer Process Using an Ln(III)-MOF and Ag Nanoparticles for Early Cancer Diagnosis: Application of Central Composite Design. ACS Appl. Mater. Interfaces 2020, 12, 16076–16087. [Google Scholar] [CrossRef] [PubMed]
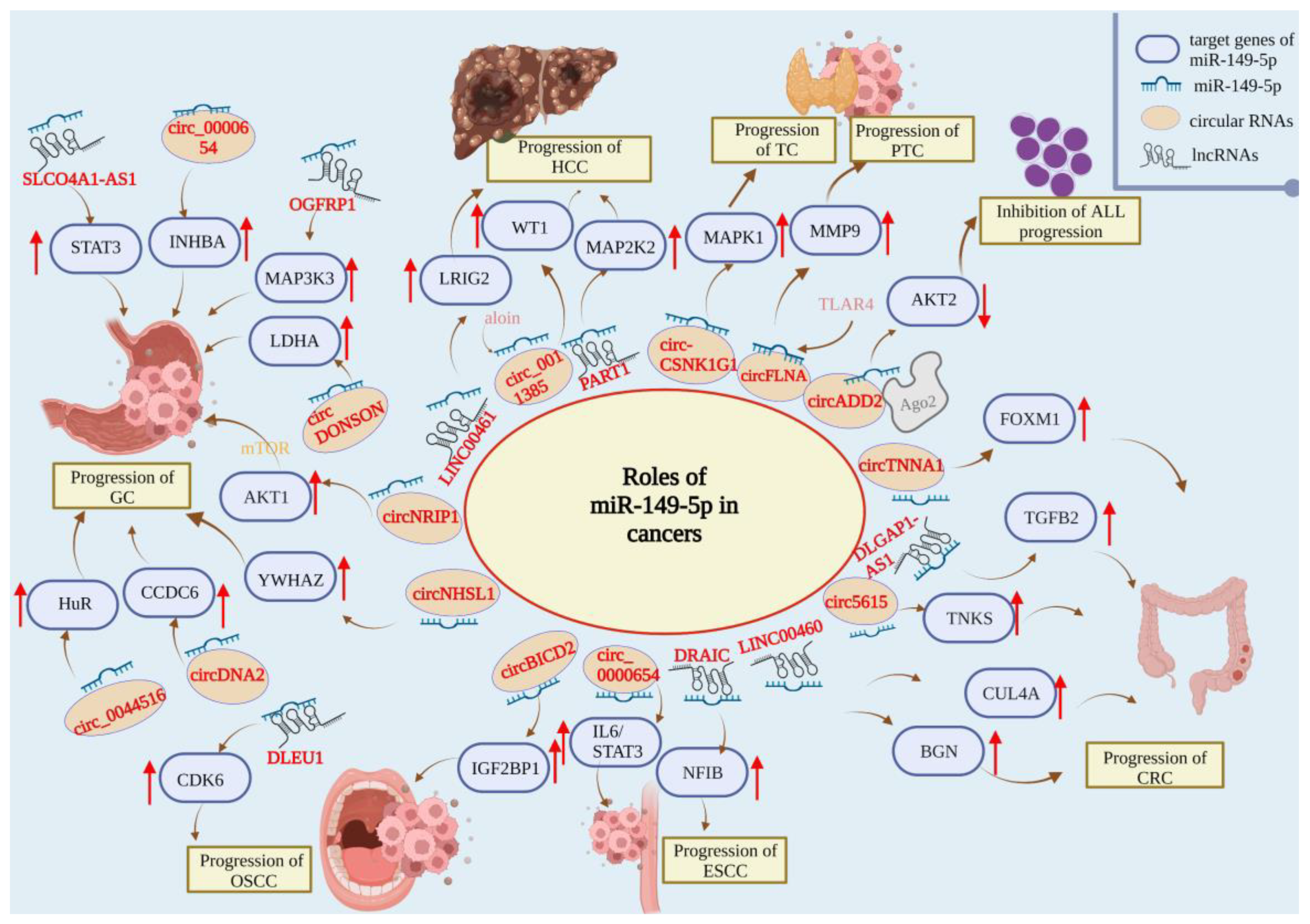
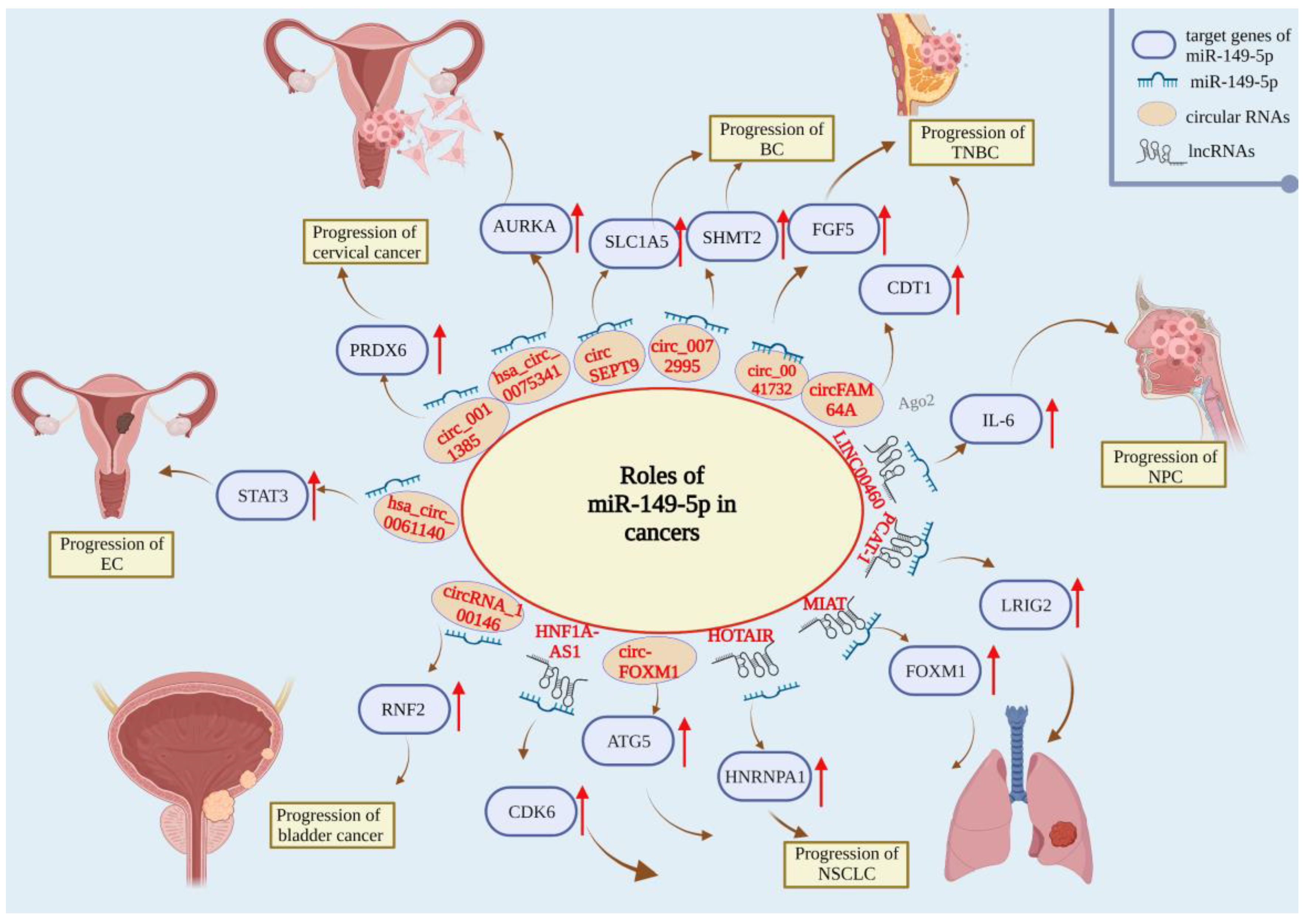
| Human System | Tumor Type | Upstream Regulator | Target Gene | Mechanism | Role | References |
|---|---|---|---|---|---|---|
| Endocrine system | TC | rs2292832 | NA | Inhibits the local progression of PTC | Tumor suppressor | [26] |
| ciecFLNA | MMP9 | Promotes the invasion and migration of PTC cells | [27] | |||
| NA | CCL18 | Attenuates the lymph-node metastasis of TC cells | [28] | |||
| circ-CSNK1G1 | MAPK1 | Inhibits cell proliferation and invasion | [29] | |||
| NA | GIT1 | Inhibits the proliferation and invasion of MTC cells | [30] | |||
| Hematological system | Leukemia | NA | FASLG | Activates FADD and caspase and promotes apoptosis | Tumor suppressor | [31] |
| hsa_circ_0120872 | AKT2 | Inhibits cell proliferation and induces cell apoptosis | [32,33] | |||
| Skeletal system | Chondrosarcoma | NGF | PI3K/Akt/mTOR | Inhibits the occurrence and metastasis of chondrosarcoma | Tumor suppressor | [38] |
| Digestive system | HCC | SNHG8 | NA | Inhibits HCC proliferation, invasion, and lung metastasis | Tumor suppressor | [40] |
| LINC00461 | LRIG2 | Inhibits the proliferation, migration, and invasion of HCC cells | [41] | |||
| NEAT1 | AKT1 | Reverses the resistance of HCC cells to sorafenib | [46] | |||
| PART1 | MAP2K1 | Inhibits the proliferation, migration, and invasion of HCC cells | [42] | |||
| circ_0011385 | WT1 | Inhibits cell proliferation, invasion, and tumor growth and promotes the apoptosis and autophagy of HCC | [43] | |||
| M2Macrophages | MMP9 | Inhibits HCC progression | [44] | |||
| GC | circNRIP1 | AKT1 | Inhibits GC proliferation, migration, and invasion | Tumor suppressor | [52] | |
| circNHSL1 | YWHAZ | Inhibits GC migration, invasion, and glutaminolysis | [53] | |||
| circ_0044516 | HuR | Inhibits GC-cell proliferation, migration, and invasion | [54] | |||
| circ-DONSON | LDHA | Inhibits GC-cell proliferation, metastasis, and angiogenesis | [55] | |||
| circDNA2 | CCDC6 | Inhibits GC growth and lymphatic metastasis | [56] | |||
| LINC01420 | NA | Inhibits GC-cell proliferation, migration, and invasion | [57] | |||
| SLCO4A1-AS1 | STAT3 | Inhibits GC-cell migration and invasion | [58] | |||
| OGFRP1 | MAP3K3 | Inhibits GC-cell proliferation and enhances apoptosis and radiosensitivity | [59] | |||
| circ_0000654 | INHBA | Inhibits the growth of GC cells | [60] | |||
| CRC | LINC00460 | CUL4A | Inhibits CRC tumorigenesis and progression | Tumor suppressor | [64] | |
| PCAT-1 | NA | Inhibits the proliferation, migration, and invasion of CRC cells and promotes the apoptosis of CRC cells | [65] | |||
| circ5615 | TNKS | Inhibits CRC tumorigenesis and progression | [66] | |||
| circTNNA1 | FOXM1 | Inhibits the proliferation and invasion of CRC cells | [67] | |||
| LINC00460 | BGN | Inhibits CRC tumor metastasis | [68] | |||
| DLGAP1-AS1 | TGFB2 | Inhibits the proliferation and invasion of CRC cells and inhibits apoptosis | [69] | |||
| LINC00460 | p53 | Leads to oxaliplatin resistance | [71] | |||
| Oral cancer | NA | SP1 | Inhibits TSCC-cell proliferation, migration, and invasion | Tumor suppressor | [74] | |
| NA | TGF-β | Promotes the chemical sensitivity of cisplatin and reduces cell proliferation, apoptosis, migration, and invasion | [77] | |||
| circBICD2 | IGF2BP1 | Promotes OSCC-cell proliferation, migration and invasion; glutamine dissolution; and apoptosis | [78] | |||
| DLEU1 | CDK6 | Inhibits OSCC-cell proliferation, migration, and invasion and induces OSCC-cell apoptosis and G1 stagnation | [79] | |||
| Esophageal cancer | circ_0000654 | IL-6/STAT3 | Inhibits the proliferation, migration, invasion, and apoptosis of ESCC cells | Tumor suppressor | [80] | |
| DRAIC | NFIB | Inhibits EC-cell proliferation and invasion and promotes esophageal-cancer-cell apoptosis and autophagy | [81] | |||
| Respiratory system | Lung cancer | PCAT-1 | LRIG2 | Inhibits the growth of NSCLC cells | Controversial | [83] |
| HNF1A-AS1 | CDK6 | Inhibits the proliferation, migration, and invasion of NSCLC cells | [84] | |||
| Ursolic acid | MyD88 | Promotes NSCLC-cell stemness | [85] | |||
| MIAT | FOXM1 | Inhibits proliferation, invasion, and migration of NSCLC cells | [86] | |||
| HOTAIR | HNRNPA1 | Inhibits the growth, migration, and invasion of NSCLC cells | [87] | |||
| circ-FOXM1 | ATG5 | Inhibits cell migration and autophagy in NSCLC | [88] | |||
| HOTAIR | DCLK1 | Promotes cisplatin resistance in NSCLC | [90] | |||
| LINC00460 | IL-6 | Induces EMT and promotes EGFR-TKI resistance | [91] | |||
| NA | B3GNT3 | Inhibits the proliferation and invasion of lung cancer cells | [89] | |||
| NPC | LINC00460 | IL-6 | Inhibits the malignant progression of NPC | Tumor suppressor | [94] | |
| Reproductive system | BC | NA | My88 | Reverses PXT resistance | Tumor suppressor | [96] |
| NA | IL-6 | Reverses trastuzumab resistance | [98] | |||
| circSEPT9 | SLC1A5 | Inhibits glutamine uptake and inhibits cell proliferation | [99] | |||
| circ_0072995 | SHMT2 | Inhibits malignant phenotype and anaerobic glycolysis in breast cancer cells | [100] | |||
| circ_0041732 | FGF5 | Inhibits cell proliferation, migration, invasion, and vascular formation and increased cell apoptosis | [101] | |||
| circFAM64A | CDT1 | Inhibits TNBC proliferation, cell-cycle migration, and invasion | [102] | |||
| Cervical cancer | has_circ_0075341 | AURKA | Reduces the proliferation and invasion of cervical cancer cells | Tumor suppressor | [104] | |
| circ_0011385 | SOX4 | Inhibits the proliferation, migration, and invasion of cervical cancer cells and inhibits apoptosis | [105] | |||
| hsa_circ_0011385 | PRDX6 | Reverses the angiogenesis and malignant biological behavior of cervical cancer cells | [106] | |||
| Ovarian cancer | circ_CELSR1 | SIK2 | Inhibits paclitaxel resistance and inhibits malignant cell progression | Controversial | [109] | |
| NA | MST1 and SAV1 | Promotes chemotherapy resistance of ovarian cancer cells to cisplatin | [111] | |||
| NA | XIAP | Inhibits ovarian-cancer-cell growth and promotes apoptosis and cisplatin sensitivity | [110] | |||
| EC | hsa_circ_0061140 | STAT3 | Inhibits EC malignant progression | Tumor suppressor | [112] | |
| PCa | NA | PI3K-Akt | Enhances the therapeutic effect of Fuzheng Yiliu decoction | Tumor suppressor | [113] | |
| NA | RGS17 | Inhibits the active proliferation and migration of PCa cells | [114] | |||
| NA | CCT3 | Destroys ROS homeostasis and promotes tumor-cell apoptosis | [115] | |||
| Urinary system | RCC | NA | FOXM1 | Inhibits the proliferation, migration, and invasion of renal carcinoma cells | Tumor suppressor | [120] |
| Bladder cancer | circ_100146 | RNF2 | Inhibits the proliferation, migration, and invasion of cysts | Tumor suppressor | [124] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Zhao, N.; Zhao, N.; Hu, X.; He, X.; Xu, Y.; Chen, J.; Chen, W.; Liu, X.; Zhou, Z.; et al. Tumor-Suppressive and Oncogenic Roles of microRNA-149-5p in Human Cancers. Int. J. Mol. Sci. 2022, 23, 10823. https://doi.org/10.3390/ijms231810823
Shen Y, Zhao N, Zhao N, Hu X, He X, Xu Y, Chen J, Chen W, Liu X, Zhou Z, et al. Tumor-Suppressive and Oncogenic Roles of microRNA-149-5p in Human Cancers. International Journal of Molecular Sciences. 2022; 23(18):10823. https://doi.org/10.3390/ijms231810823
Chicago/Turabian StyleShen, Yang, Nan Zhao, Nan Zhao, Xinyao Hu, Xiaoqin He, Yangtao Xu, Jiayu Chen, Wenliang Chen, Xin Liu, Zhuolin Zhou, and et al. 2022. "Tumor-Suppressive and Oncogenic Roles of microRNA-149-5p in Human Cancers" International Journal of Molecular Sciences 23, no. 18: 10823. https://doi.org/10.3390/ijms231810823
APA StyleShen, Y., Zhao, N., Zhao, N., Hu, X., He, X., Xu, Y., Chen, J., Chen, W., Liu, X., Zhou, Z., Cao, D., & Xu, X. (2022). Tumor-Suppressive and Oncogenic Roles of microRNA-149-5p in Human Cancers. International Journal of Molecular Sciences, 23(18), 10823. https://doi.org/10.3390/ijms231810823






