Dietary Risk Factors and Eating Behaviors in Peripheral Arterial Disease (PAD)
Abstract
1. Introduction
1.1. Materials, Methods and Study Design
1.2. Non-Dietary Risk Factors and Predictors of Peripheral Artery Disease
2. Interplay between Risk Factors and Predictors of PAD with Dietary Patterns/Eating Behaviors
2.1. Diets and Risk Factors/Predictors of Peripheral Artery Disease of Lower Limbs
2.1.1. The Mediterranean Diet
2.1.2. The Vegetarian, Vegan and Plant-Based Diet
2.1.3. The Low-Carbohydrate Ketogenic Diet
2.1.4. Intermittent Fasting Diet
2.2. Impact of Eating Behaviors on PAD Risk Factors and Predictors
2.2.1. Emotional Eating, Binge Eating, Bulimia Nervosa, Anorexia Nervosa
2.2.2. Night Eating and Sleep Disorders
2.2.3. Skipping Meals
2.2.4. Home Cooking, Fast Food Access, Ultra-Processed and Packaged Food Consumption
2.3. Smoking and Eating Behaviors
3. PAD of Lower Limbs and Nutrition
3.1. PAD and Nutritional Status: Obesity and Malnutrition
3.2. PAD of Lower Limbs and Nutrients
3.3. PAD of Lower Limbs and Diets
3.3.1. Mediterranean Diet
3.3.2. The Vegetarian, Vegan and Plant-Based Diets
3.3.3. Low Carbohydrate Ketogenic Diet
3.3.4. Intermittent Fasting Diet
3.4. PAD and Eating Behaviors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Downer, S.; Berkowitz, S.A.; Harlan, T.S.; Olstad, D.L.; Mozaffarian, D. Food is medicine: Actions to integrate food and nutrition into healthcare. BMJ 2020, 369, m2482. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G.D. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, H.; Itoh, H.; Kiriyama, H.; Kamon, T.; Fujiu, K.; Morita, K.; Michihata, N.; Jo, T.; Takeda, N.; Morita, H.; et al. Possible association between eating behaviors and cardiovascular disease in the general population: Analysis of a nationwide epidemiological database. Atherosclerosis 2021, 320, 79–85. [Google Scholar] [CrossRef]
- Viigimaa, M.; Sachinidis, A.; Toumpourleka, M.; Koutsampasopoulos, K.; Alliksoo, S.; Titma, T. Macrovascular Complications of Type 2 Diabetes Mellitus. Curr. Vasc. Pharmacol. 2020, 18, 110–116. [Google Scholar] [CrossRef]
- Asadollahi, S.; Rojulpote, C.; Bhattaru, A.; Patil, S.; Gonuguntla, K.; Karambelkar, P.; Borja, A.J.; Vuthaluru, K.; Seraj, S.M.; Zhang, V.; et al. Comparison of atherosclerotic burden in non-lower extremity arteries in patients with and without peripheral artery disease using. Am. J. Nucl. Med. Mol. Imaging 2020, 10, 272–278. [Google Scholar]
- Fowkes, F.G.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.; Williams, L.J.; Mensah, G.A.; et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013, 382, 1329–1340. [Google Scholar] [CrossRef]
- Agarwal, U.; Mishra, S.; Xu, J.; Levin, S.; Gonzales, J.; Barnard, N.D. A multicenter randomized controlled trial of a nutrition intervention program in a multiethnic adult population in the corporate setting reduces depression and anxiety and improves quality of life: The GEICO study. Am. J. Health Promot. 2015, 29, 245–254. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Laudisio, D.; Pugliese, G.; Salzano, C.; Savastano, S.; Colao, A. The management of very low-calorie ketogenic diet in obesity outpatient clinic: A practical guide. J. Transl. Med. 2019, 17, 356. [Google Scholar] [CrossRef]
- StatPearls. Available online: https://www.statpearls.com (accessed on 10 August 2022).
- Paoli, A. Ketogenic diet for obesity: Friend or foe? Int. J. Environ. Res. Public Health 2014, 11, 2092–2107. [Google Scholar] [CrossRef]
- Feinman, R.D.; Fine, E.J. Nonequilibrium thermodynamics and energy efficiency in weight loss diets. Theor. Biol. Med. Model. 2007, 4, 27. [Google Scholar] [CrossRef]
- Fine, E.J.; Feinman, R.D. Thermodynamics of weight loss diets. Nutr. Metab. 2004, 1, 15. [Google Scholar] [CrossRef]
- Veldhorst, M.A.; Westerterp-Plantenga, M.S.; Westerterp, K.R. Gluconeogenesis and energy expenditure after a high-protein, carbohydrate-free diet. Am. J. Clin. Nutr. 2009, 90, 519–526. [Google Scholar] [CrossRef]
- Cahill, G.F. Fuel metabolism in starvation. Annu. Rev. Nutr. 2006, 26, 1–22. [Google Scholar] [CrossRef]
- Tagliabue, A.; Bertoli, S.; Trentani, C.; Borrelli, P.; Veggiotti, P. Effects of the ketogenic diet on nutritional status, resting energy expenditure, and substrate oxidation in patients with medically refractory epilepsy: A 6-month prospective observational study. Clin. Nutr. 2012, 31, 246–249. [Google Scholar] [CrossRef]
- Westerterp-Plantenga, M.S.; Nieuwenhuizen, A.; Tomé, D.; Soenen, S.; Westerterp, K.R. Dietary protein, weight loss, and weight maintenance. Annu. Rev. Nutr. 2009, 29, 21–41. [Google Scholar] [CrossRef]
- Veldhorst, M.; Smeets, A.; Soenen, S.; Hochstenbach-Waelen, A.; Hursel, R.; Diepvens, K.; Lejeune, M.; Luscombe-Marsh, N.; Westerterp-Plantenga, M. Protein-induced satiety: Effects and mechanisms of different proteins. Physiol. Behav. 2008, 94, 300–307. [Google Scholar] [CrossRef]
- Sumithran, P.; Prendergast,, L.A.; Delbridge, E.; Purcell, K.; Shulkes, A.; Kriketos, A.; Proietto, J. Ketosis and appetite-mediating nutrients and hormones after weight loss. Eur. J. Clin. Nutr. 2013, 67, 759–764. [Google Scholar] [CrossRef]
- Johnstone, A.M.; Horgan, G.W.; Murison, S.D.; Bremner, D.M.; Lobley, G.E. Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Am. J. Clin. Nutr. 2008, 87, 44–55. [Google Scholar] [CrossRef]
- Westman, E.C.; Tondt, J.; Maguire, E.; Yancy, W.S. Implementing a low-carbohydrate, ketogenic diet to manage type 2 diabetes mellitus. Expert Rev. Endocrinol. Metab. 2018, 13, 263–272. [Google Scholar] [CrossRef]
- Dashti, H.M.; Mathew, T.C.; Al-Zaid, N.S. Efficacy of Low-Carbohydrate Ketogenic Diet in the Treatment of Type 2 Diabetes. Med. Princ. Pract. 2021, 30, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, J. Interest in the Ketogenic Diet Grows for Weight Loss and Type 2 Diabetes. JAMA 2018, 319, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Yancy, W.S.; Mitchell, N.S.; Westman, E.C. Ketogenic Diet for Obesity and Diabetes. JAMA Intern. Med. 2019, 179, 1734–1735. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S.; Phinney, S.D.; Forsythe, C.E.; Quann, E.E.; Wood, R.J.; Puglisi, M.J.; Kraemer, W.J.; Bibus, D.M.; Fernandez, M.L.; Feinman, R.D. Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet. Lipids 2009, 44, 297–309. [Google Scholar] [CrossRef]
- Saslow, L.R.; Mason, A.E.; Kim, S.; Goldman, V.; Ploutz-Snyder, R.; Bayandorian, H.; Daubenmier, J.; Hecht, F.M.; Moskowitz, J.T. An Online Intervention Comparing a Very Low-Carbohydrate Ketogenic Diet and Lifestyle Recommendations Versus a Plate Method Diet in Overweight Individuals with Type 2 Diabetes: A Randomized Controlled Trial. J. Med. Internet Res. 2017, 19, e36. [Google Scholar] [CrossRef]
- Kumar, S.; Behl, T.; Sachdeva, M.; Sehgal, A.; Kumari, S.; Kumar, A.; Kaur, G.; Yadav, H.N.; Bungau, S. Implicating the effect of ketogenic diet as a preventive measure to obesity and diabetes mellitus. Life Sci. 2021, 264, 118661. [Google Scholar] [CrossRef]
- Samaha, F.F.; Iqbal, N.; Seshadri, P.; Chicano, K.L.; Daily, D.A.; McGrory, J.; Williams, T.; Williams, M.; Gracely, E.J.; Stern, L. A low-carbohydrate as compared with a low-fat diet in severe obesity. N. Engl. J. Med. 2003, 348, 2074–2081. [Google Scholar] [CrossRef]
- You, Y.; Guo, Y.; Jia, P.; Zhuang, B.; Cheng, Y.; Deng, H.; Wang, X.; Zhang, C.; Luo, S.; Huang, B. Ketogenic diet aggravates cardiac remodeling in adult spontaneously hypertensive rats. Nutr. Metab. 2020, 17, 91. [Google Scholar] [CrossRef]
- Poole, R.K. The influence of growth substrate and capacity for oxidative phosphorylation on respiratory oscillations in synchronous cultures of Escherichia coli K12. J. Gen. Microbiol. 1977, 99, 369–377. [Google Scholar] [CrossRef]
- Praga, M. Synergy of low nephron number and obesity: A new focus on hyperfiltration nephropathy. Nephrol. Dial. Transplant. 2005, 20, 2594–2597. [Google Scholar] [CrossRef]
- Kosinski, C.; Jornayvaz, F.R. Effects of Ketogenic Diets on Cardiovascular Risk Factors: Evidence from Animal and Human Studies. Nutrients 2017, 9, 517. [Google Scholar] [CrossRef]
- Cicero, A.F.; Benelli, M.; Brancaleoni, M.; Dainelli, G.; Merlini, D.; Negri, R. Middle and Long-Term Impact of a Very Low-Carbohydrate Ketogenic Diet on Cardiometabolic Factors: A Multi-Center, Cross-Sectional, Clinical Study. High. Blood Press. Cardiovasc.Prev. 2015, 22, 389–394. [Google Scholar] [CrossRef]
- Castellana, M.; Conte, E.; Cignarelli, A.; Perrini, S.; Giustina, A.; Giovanella, L.; Giorgino, F.; Trimboli, P. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2020, 21, 5–16. [Google Scholar] [CrossRef]
- Walton, C.M.; Perry, K.; Hart, R.H.; Berry, S.L.; Bikman, B.T. Improvement in Glycemic and Lipid Profiles in Type 2 Diabetics with a 90-Day Ketogenic Diet. J. Diabetes Res. 2019, 2019, 8681959. [Google Scholar] [CrossRef]
- Paoli, A.; Rubini, A.; Volek, J.S.; Grimaldi, K.A. Beyond weight loss: A review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur. J. Clin. Nutr. 2013, 67, 789–796. [Google Scholar] [CrossRef]
- Bueno, N.B.; de Melo, I.S.; de Oliveira, S.L.; Ataide, T.d. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2013, 110, 1178–1187. [Google Scholar] [CrossRef]
- Sharman, M.J.; Kraemer, W.J.; Love, D.M.; Avery, N.G.; Goómez, A.L.; Scheett, T.P.; Volek, J.S. A ketogenic diet favorably affects serum biomarkers for cardiovascular disease in normal-weight men. J. Nutr. 2002, 132, 1879–1885. [Google Scholar] [CrossRef]
- Rosenbaum, M.; Hall, K.D.; Guo, J.; Ravussin, E.; Mayer, L.S.; Reitman, M.L.; Smith, S.R.; Walsh, B.T.; Leibel, R.L. Glucose and Lipid Homeostasis and Inflammation in Humans Following an Isocaloric Ketogenic Diet. Obesity 2019, 27, 971–981. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, J.; Yang, S.; Gao, M.; Cao, L.; Li, X.; Hong, D.; Tian, S.; Sun, C. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: A systematic review and meta-analysis. Nutr. Diabetes 2020, 10, 38. [Google Scholar] [CrossRef]
- Choi, Y.J.; Jeon, S.M.; Shin, S. Impact of a Ketogenic Diet on Metabolic Parameters in Patients with Obesity or Overweight and with or without Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2020, 12, 2005. [Google Scholar] [CrossRef]
- O’Neill, B.J. Effect of low-carbohydrate diets on cardiometabolic risk, insulin resistance, and metabolic syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 301–307. [Google Scholar] [CrossRef]
- Draaisma, J.M.T.; Hampsink, B.M.; Janssen, M.; van Houdt, N.B.M.; Linders, E.T.A.M.; Willemsen, M.A. The Ketogenic Diet and Its Effect on Bone Mineral Density: A Retrospective Observational Cohort Study. Neuropediatrics 2019, 50, 353–358. [Google Scholar] [CrossRef]
- Bruci, A.; Tuccinardi, D.; Tozzi, R.; Balena, A.; Santucci, S.; Frontani, R.; Mariani, S.; Basciani, S.; Spera, G.; Gnessi, L.; et al. Very Low-Calorie Ketogenic Diet: A Safe and Effective Tool for Weight Loss in Patients with Obesity and Mild Kidney Failure. Nutrients 2020, 12, 333. [Google Scholar] [CrossRef]
- Carriazo, S.; Perez-Gomez, M.V.; Cordido, A.; García-González, M.A.; Sanz, A.B.; Ortiz, A.; Sanchez-Niño, M.D. Dietary Care for ADPKD Patients: Current Status and Future Directions. Nutrients 2019, 11, 1576. [Google Scholar] [CrossRef]
- Torres, J.A.; Kruger, S.L.; Broderick, C.; Amarlkhagva, T.; Agrawal, S.; Dodam, J.R.; Mrug, M.; Lyons, L.A.; Weimbs, T. Ketosis Ameliorates Renal Cyst Growth in Polycystic Kidney Disease. Cell Metab. 2019, 30, 1007–1023.e5. [Google Scholar] [CrossRef]
- Koh, S.; Dupuis, N.; Auvin, S. Ketogenic diet and Neuroinflammation. Epilepsy Res. 2020, 167, 106454. [Google Scholar] [CrossRef]
- Monda, V.; Polito, R.; Lovino, A.; Finaldi, A.; Valenzano, A.; Nigro, E.; Corso, G.; Sessa, F.; Asmundo, A.; Di Nunno, N.; et al. Short-Term Physiological Effects of a Very Low-Calorie Ketogenic Diet: Effects on Adiponectin Levels and Inflammatory States. Int. J. Mol. Sci. 2020, 21, 3228. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.; Bonucci, A.; Maggi, E.; Corsi, M.; Businaro, R. Anti-Oxidant and Anti-Inflammatory Activity of Ketogenic Diet: New Perspectives for Neuroprotection in Alzheimer’s Disease. Antioxidants 2018, 7, 63. [Google Scholar] [CrossRef]
- Ang, Q.Y.; Alexander, M.; Newman, J.C.; Tian, Y.; Cai, J.; Upadhyay, V.; Turnbaugh, J.A.; Verdin, E.; Hall, K.D.; Leibel, R.L.; et al. Ketogenic Diets Alter the Gut Microbiome Resulting in Decreased Intestinal Th17 Cells. Cell 2020, 181, 1263–1275.e16. [Google Scholar] [CrossRef]
- Johnstone, A. Fasting for weight loss: An effective strategy or latest dieting trend? Int. J. Obes 2015, 39, 727–733. [Google Scholar] [CrossRef]
- Jane, L.; Atkinson, G.; Jaime, V.; Hamilton, S.; Waller, G.; Harrison, S. Intermittent fasting interventions for the treatment of overweight and obesity in adults aged 18 years and over: A systematic review protocol. JBI Database Syst. Rev. Implement. Rep. 2015, 13, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Zubrzycki, A.; Cierpka-Kmiec, K.; Kmiec, Z.; Wronska, A. The role of low-calorie diets and intermittent fasting in the treatment of obesity and type-2 diabetes. J. Physiol. Pharmacol. 2018, 69, 663–683.e4. [Google Scholar] [CrossRef]
- Li, G.; Xie, C.; Lu, S.; Nichols, R.G.; Tian, Y.; Li, L.; Patel, D.; Ma, Y.; Brocker, C.N.; Yan, T.; et al. Intermittent Fasting Promotes White Adipose Browning and Decreases Obesity by Shaping the Gut Microbiota. Cell Metab. 2017, 26, 672–685. [Google Scholar] [CrossRef] [PubMed]
- Welton, S.; Minty, R.; O’Driscoll, T.; Willms, H.; Poirier, D.; Madden, S.; Kelly, L. Intermittent fasting and weight loss: Systematic review. Can. Fam. Physician 2020, 66, 117–125. [Google Scholar]
- Stockman, M.C.; Thomas, D.; Burke, J.; Apovian, C.M. Intermittent Fasting: Is the Wait Worth the Weight? Curr. Obes. Rep. 2018, 7, 172–185. [Google Scholar] [CrossRef]
- Harris, L.; Hamilton, S.; Azevedo, L.B.; Olajide, J.; De Brún, C.; Waller, G.; Whittaker, V.; Sharp, T.; Lean, M.; Hankey, C.; et al. Intermittent fasting interventions for treatment of overweight and obesity in adults: A systematic review and meta-analysis. JBI Database Syst. Rev. Implement. Rep. 2018, 16, 507–547. [Google Scholar] [CrossRef]
- Dong, T.A.; Sandesara, P.B.; Dhindsa, D.S.; Mehta, A.; Arneson, L.C.; Dollar, A.L.; Taub, P.R.; Sperling, L.S. Intermittent Fasting: A Heart Healthy Dietary Pattern? Am. J. Med. 2020, 133, 901–907. [Google Scholar] [CrossRef]
- Corley, B.T.; Carroll, R.W.; Hall, R.M.; Weatherall, M.; Parry-Strong, A.; Krebs, J.D. Intermittent fasting in Type 2 diabetes mellitus and the risk of hypoglycaemia: A randomized controlled trial. Diabet. Med. 2018, 35, 588–594. [Google Scholar] [CrossRef]
- Grajower, M.M.; Horne, B.D. Clinical Management of Intermittent Fasting in Patients with Diabetes Mellitus. Nutrients 2019, 11, 873. [Google Scholar] [CrossRef]
- Ganesan, K.; Habboush, Y.; Sultan, S. Intermittent Fasting: The Choice for a Healthier Lifestyle. Cureus 2018, 10, e2947. [Google Scholar] [CrossRef]
- Rothberg, A.E.; McEwen, L.N.; Kraftson, A.T.; Ajluni, N.; Fowler, C.E.; Nay, C.K.; Miller, N.M.; Burant, C.F.; Herman, W.H. Impact of weight loss on waist circumference and the components of the metabolic syndrome. BMJ Open Diabetes Res. Care 2017, 5, e000341. [Google Scholar] [CrossRef]
- Stote, K.S.; Baer, D.J.; Spears, K.; Paul, D.R.; Harris, G.K.; Rumpler, W.V.; Strycula, P.; Najjar, S.S.; Ferrucci, L.; Ingram, D.K.; et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am. J. Clin. Nutr. 2007, 85, 981–988. [Google Scholar] [CrossRef]
- Li, C.; Sadraie, B.; Steckhan, N.; Kessler, C.; Stange, R.; Jeitler, M.; Michalsen, A. Effects of A One-week Fasting Therapy in Patients with Type-2 Diabetes Mellitus and Metabolic Syndrome—A Randomized Controlled Explorative Study. Exp. Clin. Endocrinol. Diabetes 2017, 125, 618–624. [Google Scholar] [CrossRef]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221.e3. [Google Scholar] [CrossRef]
- Crupi, A.N.; Haase, J.; Brandhorst, S.; Longo, V.D. Periodic and Intermittent Fasting in Diabetes and Cardiovascular Disease. Curr. Diabetes Rep. 2020, 20, 83. [Google Scholar] [CrossRef]
- Erdem, Y.; Ozkan, G.; Ulusoy, S.; Arici, M.; Derici, U.; Sengul, S.; Sindel, S.; Erturk, S.; Turkish Society of Hypertension and Renal Diseases. The effect of intermittent fasting on blood pressure variability in patients with newly diagnosed hypertension or prehypertension. J. Am. Soc. Hypertens. 2018, 12, 42–49. [Google Scholar] [CrossRef]
- Wei, M.; Brandhorst, S.; Shelehchi, M.; Mirzaei, H.; Cheng, C.W.; Budniak, J.; Groshen, S.; Mack, W.J.; Guen, E.; Di Biase, S.; et al. Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci. Transl. Med. 2017, 9, eaai8700. [Google Scholar] [CrossRef]
- Sundfør, T.M.; Svendsen, M.; Tonstad, S. Effect of intermittent versus continuous energy restriction on weight loss, maintenance and cardiometabolic risk: A randomized 1-year trial. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 698–706. [Google Scholar] [CrossRef]
- Sanford, M.A.; Sanford, T.S.; Campbell, K.F.; Davis, D.; Tandberg, T.; Road, L.N.E. Do Adults Utilizing Intermittent Fasting Improve Lipids More Than Those Following a Restricted-Calorie Diet? A Clin-IQ. J. Patient Cent. Res. Rev. 2020, 7, 282–285. [Google Scholar] [CrossRef]
- Ahmed, N.; Farooq, J.; Siddiqi, H.S.; Meo, S.A.; Kulsoom, B.; Laghari, A.H.; Jamshed, H.; Pasha, F. Impact of Intermittent Fasting on Lipid Profile-A Quasi-Randomized Clinical Trial. Front. Nutr. 2020, 7, 596787. [Google Scholar] [CrossRef]
- Das, S.; McCreary, J.; Shamim, S.; Kalayjian, T. Reversal of severe hypertriglyceridemia with intermittent fasting and a very-low-carbohydrate ketogenic diet: A case series. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 308–311. [Google Scholar] [CrossRef]
- D’Souza, M.S.; Dong, T.A.; Ragazzo, G.; Dhindsa, D.S.; Mehta, A.; Sandesara, P.B.; Freeman, A.M.; Taub, P.; Sperling, L.S. From Fad to Fact: Evaluating the Impact of Emerging Diets on the Prevention of Cardiovascular Disease. Am. J. Med. 2020, 133, 1126–1134. [Google Scholar] [CrossRef]
- Kunduraci, Y.E.; Ozbek, H. Does the Energy Restriction Intermittent Fasting Diet Alleviate Metabolic Syndrome Biomarkers? A Randomized Controlled Trial. Nutrients 2020, 12, 3213. [Google Scholar] [CrossRef]
- Cho, A.R.; Moon, J.-Y.; Kim, S.; An, K.-Y.; Oh, M.; Jeon, J.Y.; Jung, D.-H.; Choi, M.H.; Lee, J.-W. Effects of alternate day fasting and exercise on cholesterol metabolism in overweight or obese adults: A pilot randomized controlled trial. Metabolism 2019, 93, 52–60. [Google Scholar] [CrossRef]
- Bhutani, S.; Klempel, M.C.; Kroeger, C.M.; Trepanowski, J.F.; Varady, K.A. Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity 2013, 21, 1370–1379. [Google Scholar] [CrossRef]
- Malinowski, B.; Zalewska, K.; Węsierska, A.; Sokołowska, M.M.; Socha, M.; Liczner, G.; Pawlak-Osińska, K.; Wiciński, M. Intermittent Fasting in Cardiovascular Disorders-An Overview. Nutrients 2019, 11, 673. [Google Scholar] [CrossRef]
- Wilson, R.A.; Deasy, W.; Stathis, C.G.; Hayes, A.; Cooke, M.B. Intermittent Fasting with or without Exercise Prevents Weight Gain and Improves Lipids in Diet-Induced Obese Mice. Nutrients 2018, 10, 346. [Google Scholar] [CrossRef]
- Dedual, M.A.; Wueest, S.; Borsigova, M.; Konrad, D. Intermittent fasting improves metabolic flexibility in short-term high-fat diet-fed mice. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E773–E782. [Google Scholar] [CrossRef]
- Eldeeb, A.A.; Mahmoud, M.A.; Ibrahim, A.B.; Yousef, E.A.; Sabry, A.A. Effect of Ramadan fasting on arterial stiffness parameters among Egyptian hypertensive patients with and without chronic kidney disease. Saudi J. Kidney Dis. Transpl. 2020, 31, 582–588. [Google Scholar] [CrossRef]
- Bernieh, B.; al Hakim, M.R.; Boobes, Y.; Zidan, F.M.A. Fasting Ramadan in chronic kidney disease patients: Clinical and biochemical effects. Saudi J. Kidney Dis. Transpl. 2010, 21, 898–902. [Google Scholar]
- Ahmad, S.; Chowdhury, T.A. Fasting during Ramadan in people with chronic kidney disease: A review of the literature. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018819889019. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.; Khan, H.; Lasker, S.S.; Chowdhury, T.A. Fasting outcomes in people with diabetes and chronic kidney disease in East London during Ramadan 2018: The East London diabetes in Ramadan survey. Diabetes Res. Clin. Pract. 2019, 152, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Alawadi, F.; Rashid, F.; Bashier, A.; Abdelgadir, E.; Al Saeed, M.; Abuelkheir, S.; Khalifa, A.; Al Sayyah, F.; Bachet, F.; Elsayed, M.; et al. The use of Free Style Libre Continues Glucose Monitoring (FSL-CGM) to monitor the impact of Ramadan fasting on glycemic changes and kidney function in high-risk patients with diabetes and chronic kidney disease stage 3 under optimal diabetes care. Diabetes Res. Clin. Pract. 2019, 151, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Bragazzi, N.L. Ramadan fasting and chronic kidney disease: Does estimated glomerular filtration rate change after and before Ramadan? Insights from a mini meta-analysis. Int. J. Nephrol. Renovasc. Dis. 2015, 8, 53–57. [Google Scholar] [CrossRef]
- Chandrasekar, B.; Nelson, J.F.; Colston, J.T.; Freeman, G.L. Calorie restriction attenuates inflammatory responses to myocardial ischemia-reperfusion injury. Am. J. Physiol Heart Circ. Physio 2001, 280, H2094–H2102. [Google Scholar] [CrossRef]
- Aksungar, F.B.; Topkaya, A.E.; Akyildiz, M. Interleukin-6, C-reactive protein and biochemical parameters during prolonged intermittent fasting. Ann. Nutr. Metab. 2007, 51, 88–95. [Google Scholar] [CrossRef]
- Moro, T.; Tinsley, G.; Bianco, A.; Marcolin, G.; Pacelli, Q.F.; Battaglia, G.; Palma, A.; Gentil, P.; Neri, M.; Paoli, A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med. 2016, 14, 290. [Google Scholar] [CrossRef]
- Bhutani, S.; Klempel, M.C.; Berger, R.A.; Varady, K.A. Improvements in coronary heart disease risk indicators by alternate-day fasting involve adipose tissue modulations. Obesity 2010, 18, 2152–2159. [Google Scholar] [CrossRef]
- Ouchi, N.; Kihara, S.; Arita, Y.; Maeda, K.; Kuriyama, H.; Okamoto, Y.; Hotta, K.; Nishida, M.; Takahashi, M.; Nakamura, T.; et al. Novel modulator for endothelial adhesion molecules: Adipocyte-derived plasma protein adiponectin. Circulation 1999, 100, 2473–2476. [Google Scholar] [CrossRef]
- Ouchi, N.; Kihara, S.; Arita, Y.; Okamoto, Y.; Maeda, K.; Kuriyama, H.; Hotta, K.; Nishida, M.; Takahashi, M.; Muraguchi, M.; et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation 2000, 102, 1296–1301. [Google Scholar] [CrossRef]
- Matsuda, M.; Shimomura, I.; Sata, M.; Arita, Y.; Nishida, M.; Maeda, N.; Kumada, M.; Okamoto, Y.; Nagaretani, H.; Nishizawa, H.; et al. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J. Biol. Chem. 2002, 277, 37487–37491. [Google Scholar] [CrossRef]
- Bowman, J.D.; Bowman, C.D.; Bush, J.E.; Delheij, P.P.J.; Frankle, C.M.; Gould, C.R.; Haase, D.G.; Knudson, J.; Mitchell, G.E.; Penttila, S.; et al. Parity nonconservation for neutron resonances in 238U. Phys. Rev. Lett. 1990, 65, 1192–1195. [Google Scholar] [CrossRef]
- Burnett, M.S.; Lee, C.W.; Kinnaird, T.D.; Stabile, E.; Durrani, S.; Dullum, M.K.; Devaney, J.M.; Fishman, C.; Stamou, S.; Canos, D.; et al. The potential role of resistin in atherogenesis. Atherosclerosis 2005, 182, 241–248. [Google Scholar] [CrossRef]
- Jiang, S.; Park, D.W.; Tadie, J.-M.; Gregoire, M.; Deshane, J.; Pittet, J.F.; Abraham, E.; Zmijewski, J.W. Human resistin promotes neutrophil proinflammatory activation and neutrophil extracellular trap formation and increases severity of acute lung injury. J. Immunol. 2014, 192, 4795–4803. [Google Scholar] [CrossRef]
- Marinho, T.S.; Ornellas, F.; Barbosa-da-Silva, S.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Beneficial effects of intermittent fasting on steatosis and inflammation of the liver in mice fed a high-fat or a high-fructose diet. Nutrition 2019, 65, 103–112. [Google Scholar] [CrossRef]
- Zhang, X.; Zou, Q.; Zhao, B.; Zhang, J.; Zhao, W.; Li, Y.; Liu, R.; Liu, X.; Liu, Z. Effects of alternate-day fasting, time-restricted fasting and intermittent energy restriction DSS-induced on colitis and behavioral disorders. Redox Biol. 2020, 32, 101535. [Google Scholar] [CrossRef]
- Rangan, P.; Choi, I.; Wei, M.; Navarrete, G.; Guen, E.; Brandhorst, S.; Enyati, N.; Pasia, G.; Maesincee, D.; Ocon, V.; et al. Fasting-Mimicking Diet Modulates Microbiota and Promotes Intestinal Regeneration to Reduce Inflammatory Bowel Disease Pathology. Cell Rep. 2019, 26, 2704–2719.e6. [Google Scholar] [CrossRef]
- Le, L.T.; Sabaté, J. Beyond meatless, the health effects of vegan diets: Findings from the Adventist cohorts. Nutrients 2014, 6, 2131–2147. [Google Scholar] [CrossRef]
- Sabaté, J.; Wien, M. Vegetarian diets and childhood obesity prevention. Am. J. Clin. Nutr. 2010, 91, 1525S–1529S. [Google Scholar] [CrossRef]
- Najjar, R.S.; Feresin, R.G. Plant-Based Diets in the Reduction of Body Fat: Physiological Effects and Biochemical Insights. Nutrients 2019, 11, 2712. [Google Scholar] [CrossRef]
- Kahleova, H.; Fleeman, R.; Hlozkova, A.; Holubkov, R.; Barnard, N.D. A plant-based diet in overweight individuals in a 16-week randomized clinical trial: Metabolic benefits of plant protein. Nutr. Diabetes 2018, 8, 58. [Google Scholar] [CrossRef]
- Rosell, M.; Appleby, P.; Spencer, E.; Key, T. Weight gain over 5 years in 21,966 meat-eating, fish-eating, vegetarian, and vegan men and women in EPIC-Oxford. Int. J. Obes. 2006, 30, 1389–1396. [Google Scholar] [CrossRef]
- Wright, N.; Wilson, L.; Smith, M.; Duncan, B.; McHugh, P. The BROAD study: A randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr. Diabetes 2017, 7, e256. [Google Scholar] [CrossRef]
- Medawar, E.; Huhn, S.; Villringer, A.; Witte, A.V. The effects of plant-based diets on the body and the brain: A systematic review. Transl. Psychiatry 2019, 9, 226. [Google Scholar] [CrossRef]
- Magkos, F.; Tetens, I.; Bügel, S.G.; Felby, C.; Schacht, S.R.; Hill, J.O.; Ravussin, E.; Astrup, A. A Perspective on the Transition to Plant-Based Diets: A Diet Change May Attenuate Climate Change, but Can It Also Attenuate Obesity and Chronic Disease Risk? Adv. Nutr. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Grant, J.D. Time for change: Benefits of a plant-based diet. Can. Fam. Physician 2017, 63, 744–746. [Google Scholar] [PubMed]
- Turner-McGrievy, G.; Mandes, T.; Crimarco, A. A plant-based diet for overweight and obesity prevention and treatment. J. Geriatr. Cardiol. 2017, 14, 369–374. [Google Scholar] [CrossRef]
- Szabó, Z.; Erdélyi, A.; Kisbenedek, A.G.; Ungár, T.; Polyák, L.; Szabó, S.S.; Kovács, R.E.; Raposa, L.B.; Figler, M. Plant-based diets: A review. Orv. Hetil. 2016, 157, 1859–1865. (In Hungarian) [Google Scholar] [CrossRef]
- Kahleova, H.; Levin, S.; Barnard, N. Cardio-Metabolic Benefits of Plant-Based Diets. Nutrients 2017, 9, 848. [Google Scholar] [CrossRef]
- Craig, W.J.; Mangels, A.R.; Association, A.D. Position of the American Dietetic Association: Vegetarian diets. J. Am. Diet. Assoc. 2009, 109, 1266–1282. [Google Scholar] [CrossRef]
- Tonstad, S.; Stewart, K.; Oda, K.; Batech, M.; Herring, R.P.; Fraser, G.E. Vegetarian diets and incidence of diabetes in the Adventist Health Study-2. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Ward, K. High-carbohydrate, high-fiber diets for insulin-treated men with diabetes mellitus. Am. J. Clin. Nutr. 1979, 32, 2312–2321. [Google Scholar] [CrossRef] [PubMed]
- Barnard, R.J.; Jung, T.; Inkeles, S.B. Diet and exercise in the treatment of NIDDM. The need for early emphasis. Diabetes Care 1994, 17, 1469–1472. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Barnard, N.D.; Levin, S.M.; Watanabe, M. Vegetarian diets and glycemic control in diabetes: A systematic review and meta-analysis. Cardiovasc. Diagn. Ther. 2014, 4, 373–382. [Google Scholar] [CrossRef]
- Kahleova, H.; Matoulek, M.; Malinska, H.; Oliyarnik, O.; Kazdova, L.; Neskudla, T.; Skoch, A.; Hajek, M.; Hill, M.; Kahle, M.; et al. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with Type 2 diabetes. Diabet. Med. 2011, 28, 549–559. [Google Scholar] [CrossRef]
- Barnard, N.D.; Cohen, J.; Jenkins, D.J.; Turner-McGrievy, G.; Gloede, L.; Jaster, B.; Seidl, K.; Green, A.A.; Talpers, S. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care 2006, 29, 1777–1783. [Google Scholar] [CrossRef]
- Nicholson, A.S.; Sklar, M.; Barnard, N.D.; Gore, S.; Sullivan, R.; Browning, S. Toward improved management of NIDDM: A randomized, controlled, pilot intervention using a lowfat, vegetarian diet. Prev. Med. 1999, 29, 87–91. [Google Scholar] [CrossRef]
- Kahleova, H.; Tura, A.; Klementova, M.; Thieme, L.; Haluzik, M.; Pavlovicova, R.; Hill, M.; Pelikanova, T. A Plant-Based Meal Stimulates Incretin and Insulin Secretion More Than an Energy- and Macronutrient-Matched Standard Meal in Type 2 Diabetes: A Randomized Crossover Study. Nutrients 2019, 11, 486. [Google Scholar] [CrossRef]
- Belinova, L.; Kahleova, H.; Malinska, H.; Topolcan, O.; Vrzalova, J.; Oliyarnyk, O.; Kazdova, L.; Hill, M.; Pelikanova, T. Differential acute postprandial effects of processed meat and isocaloric vegan meals on the gastrointestinal hormone response in subjects suffering from type 2 diabetes and healthy controls: A randomized crossover study. PLoS ONE 2014, 9, e107561. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Becerra-Tomás, N.; Papandreou, C.; Bulló, M. Dietary Patterns Emphasizing the Consumption of Plant Foods in the Management of Type 2 Diabetes: A Narrative Review. Adv. Nutr. 2019, 10 (Suppl. S4), S320–S331. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; González-Lucán, M.; Fernández-Fernández, C.; Carneiro-Freire, N.; Seco-Filgueira, M.; Pedre-Piñeiro, A.M. Effect of diet composition on insulin sensitivity in humans. Clin. Nutr. ESPEN 2019, 33, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Johannesen, C.O.; Dale, H.F.; Jensen, C.; Lied, G.A. Effects of Plant-Based Diets on Outcomes Related to Glucose Metabolism: A Systematic Review. Diabetes Metab. Syndr. Obes. 2020, 13, 2811–2822. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Nishimura, K.; Barnard, N.D.; Takegami, M.; Watanabe, M.; Sekikawa, A.; Okamura, T.; Miyamoto, Y. Vegetarian diets and blood pressure: A meta-analysis. JAMA Intern. Med. 2014, 174, 577–587. [Google Scholar] [CrossRef]
- Rouse, I.L.; Beilin, L.J.; Armstrong, B.K.; Vandongen, R. Blood-pressure-lowering effect of a vegetarian diet: Controlled trial in normotensive subjects. Lancet 1983, 1, 5–10. [Google Scholar] [CrossRef]
- Lee, K.W.; Loh, H.C.; Ching, S.M.; Devaraj, N.K.; Hoo, F.K. Effects of Vegetarian Diets on Blood Pressure Lowering: A Systematic Review with Meta-Analysis and Trial Sequential Analysis. Nutrients 2020, 12, 1604. [Google Scholar] [CrossRef]
- Bradbury, K.E.; Crowe, F.L.; Appleby, P.N.; Schmidt, J.A.; Travis, R.C.; Key, T.J. Serum concentrations of cholesterol, apolipoprotein A-I and apolipoprotein B in a total of 1694 meat-eaters, fish-eaters, vegetarians and vegans. Eur. J. Clin. Nutr. 2014, 68, 178–183. [Google Scholar] [CrossRef]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.Y.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary Fats and Cardiovascular Disease: A Presidential Advisory from the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zheng, J.; Yang, B.; Jiang, J.; Fu, Y.; Li, D. Effects of Vegetarian Diets on Blood Lipids: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2015, 4, e002408. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Wong, J.M.W.; Kendall, C.W.C.; Esfahani, A.; Ng, V.W.Y.; Leong, T.C.K.; Faulkner, D.A.; Vidgen, E.; Paul, G.; Mukherjea, R.; et al. Effect of a 6-month vegan low-carbohydrate (‘Eco-Atkins’) diet on cardiovascular risk factors and body weight in hyperlipidaemic adults: A randomised controlled trial. BMJ Open 2014, 4, e003505. [Google Scholar] [CrossRef]
- Ornish, D.; Brown, S.; Billings, J.; Scherwitz, L.; Armstrong, W.; Ports, T.; McLanahan, S.; Kirkeeide, R.; Gould, K.; Brand, R. Can lifestyle changes reverse coronary heart disease? The Lifestyle Heart Trial. Lancet 1990, 336, 129–133. [Google Scholar] [CrossRef]
- Kahleova, H.; Petersen, K.F.; Shulman, G.I.; Alwarith, J.; Rembert, E.; Tura, A.; Hill, M.; Holubkov, R.; Barnard, N.D. Effect of a Low-Fat Vegan Diet on Body Weight, Insulin Sensitivity, Postprandial Metabolism, and Intramyocellular and Hepatocellular Lipid Levels in Overweight Adults: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2025454. [Google Scholar] [CrossRef] [PubMed]
- Adair, K.E.; Bowden, R.G. Ameliorating Chronic Kidney Disease Using a Whole Food Plant-Based Diet. Nutrients 2020, 12, 1007. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Joshi, S.; Schlueter, R.; Cooke, J.; Brown-Tortorici, A.; Donnelly, M.; Schulman, S.; Lau, W.-L.; Rhee, C.M.; Streja, E.; et al. Plant-Dominant Low-Protein Diet for Conservative Management of Chronic Kidney Disease. Nutrients 2020, 12, 1931. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; McMacken, M.; Kalantar-Zadeh, K. Plant-Based Diets for Kidney Disease: A Guide for Clinicians. Am. J. Kidney Dis. 2021, 77, 287–296. [Google Scholar] [CrossRef]
- Carrero, J.J.; González-Ortiz, A.; Avesani, C.M.; Bakker, S.J.L.; Bellizzi, V.; Chauveau, P.; Clase, C.M.; Cupisti, A.; Espinosa-Cuevas, A.; Molina, P.; et al. Plant-based diets to manage the risks and complications of chronic kidney disease. Nat. Rev. Nephrol. 2020, 16, 525–542. [Google Scholar] [CrossRef]
- Gluba-Brzózka, A.; Franczyk, B.; Rysz, J. Vegetarian Diet in Chronic Kidney Disease-A Friend or Foe. Nutrients 2017, 9, 374. [Google Scholar] [CrossRef]
- Eichelmann, F.; Schwingshackl, L.; Fedirko, V.; Aleksandrova, K. Effect of plant-based diets on obesity-related inflammatory profiles: A systematic review and meta-analysis of intervention trials. Obes. Rev. 2016, 17, 1067–1079. [Google Scholar] [CrossRef]
- Craddock, J.C.; Neale, E.P.; Peoples, G.E.; Probst, Y.C. Vegetarian-Based Dietary Patterns and their Relation with Inflammatory and Immune Biomarkers: A Systematic Review and Meta-Analysis. Adv. Nutr. 2019, 10, 433–451. [Google Scholar] [CrossRef]
- Barnard, N.D.; Goldman, D.M.; Loomis, J.F.; Kahleova, H.; Levin, S.M.; Neabore, S.; Batts, T.C. Plant-Based Diets for Cardiovascular Safety and Performance in Endurance Sports. Nutrients 2019, 11, 130. [Google Scholar] [CrossRef]
- Bolori, P.; Setaysh, L.; Rasaei, N.; Jarrahi, F.; Yekaninejad, M.S.; Mirzaei, K. Adherence to a healthy plant diet may reduce inflammatory factors in obese and overweight women-a cross-sectional study. Diabetes Metab. Syndr. 2019, 13, 2795–2802. [Google Scholar] [CrossRef]
- Nakou, E.S.; Liberopoulos, E.N.; Milionis, H.J.; Elisaf, M.S. The role of C-reactive protein in atherosclerotic cardiovascular disease: An overview. Curr. Vasc. Pharmacol. 2008, 6, 258–270. [Google Scholar] [CrossRef]
- Menzel, J.; Jabakhanji, A.; Biemann, R.; Mai, K.; Abraham, K.; Weikert, C. Systematic review and meta-analysis of the associations of vegan and vegetarian diets with inflammatory biomarkers. Sci. Rep. 2020, 10, 21736. [Google Scholar] [CrossRef]
- Najjar, R.S.; Moore, C.E.; Montgomery, B.D. Consumption of a defined, plant-based diet reduces lipoprotein(a), inflammation, and other atherogenic lipoproteins and particles within 4 weeks. Clin. Cardiol. 2018, 41, 1062–1068. [Google Scholar] [CrossRef]
- Grosse, C.S.J.; Christophersen, C.T.; Devine, A.; Lawrance, I.C. The role of a plant-based diet in the pathogenesis, etiology and management of the inflammatory bowel diseases. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 137–145. [Google Scholar] [CrossRef]
- Strowig, T.; Henao-Mejia, J.; Elinav, E.; Flavell, R. Inflammasomes in health and disease. Nature 2012, 481, 278–286. [Google Scholar] [CrossRef]
- Franco-de-Moraes, A.C.; de Almeida-Pititto, B.; da Fernandes, G.; Gomes, E.P.; da Pereira, A.; Ferreira, S.R.G. Worse inflammatory profile in omnivores than in vegetarians associates with the gut microbiota composition. Diabetol. Metab. Syndr. 2017, 9, 62. [Google Scholar] [CrossRef]
- D’Innocenzo, S.; Biagi, C.; Lanari, M. Obesity and the Mediterranean Diet: A Review of Evidence of the Role and Sustainability of the Mediterranean Diet. Nutrients 2019, 11, 1306. [Google Scholar] [CrossRef]
- Oggioni, C.; Lara, J.; Wells, J.C.; Soroka, K.; Siervo, M. Shifts in population dietary patterns and physical inactivity as determinants of global trends in the prevalence of diabetes: An ecological analysis. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1105–1111. [Google Scholar] [CrossRef]
- Thow, A.M. Trade liberalisation and the nutrition transition: Mapping the pathways for public health nutritionists. Public Health Nutr. 2009, 11, 2150–2158. [Google Scholar] [CrossRef]
- Ogden, C.L.; Kuczmarski, R.J.; Flegal, K.M.; Mei, Z.; Guo, S.; Wei, R.; Grummer-Strawn, L.M.; Curtin, L.R.; Roche, A.F.; Johnson, C.L. Centers for Disease Control and Prevention 2000 growth charts for the United States: Improvements to the 1977 National Center for Health Statistics version. Pediatrics 2002, 109, 45–60. [Google Scholar] [CrossRef]
- Lobstein, T.; Baur, L.; Uauy, R.; TaskForce, I.I.O. Obesity in children and young people: A crisis in public health. Obes. Rev. 2004, 5 (Suppl. S1), 4–104. [Google Scholar] [CrossRef] [PubMed]
- Magarey, A.M.; Daniels, L.A.; Boulton, T.J. Prevalence of overweight and obesity in Australian children and adolescents: Reassessment of 1985 and 1995 data against new standard international definitions. Med. J. Aust. 2001, 174, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Kearney, J. Food consumption trends and drivers. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 2793–2807. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Pugliese, G.; Laudisio, D.; Colao, A.; Savastano, S.; Muscogiuri, G. Mediterranean diet as medical prescription in menopausal women with obesity: A practical guide for nutritionists. Crit. Rev. Food Sci. Nutr. 2021, 61, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Castro-Barquero, S.; Lamuela-Raventós, R.M.; Doménech, M.; Estruch, R. Relationship between Mediterranean Dietary Polyphenol Intake and Obesity. Nutrients 2018, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S. The glycemic index: Physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 2002, 287, 2414–2423. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Simonetta, I.; Daidone, M.; Mogavero, A.; Ortello, A.; Pinto, A. Metabolic and Vascular Effect of the Mediterranean Diet. Int. J. Mol. Sci. 2019, 20, 4716. [Google Scholar] [CrossRef]
- Lovejoy, J.C. Dietary fatty acids and insulin resistance. Curr. Atheroscler. Rep. 1999, 1, 215–220. [Google Scholar] [CrossRef]
- Brown-Borg, H.M.; Buffenstein, R. Cutting back on the essentials: Can manipulating intake of specific amino acids modulate health and lifespan? Ageing Res. Rev. 2017, 39, 87–95. [Google Scholar] [CrossRef]
- Vessby, B. Dietary fat and insulin action in humans. Br. J. Nutr. 2000, 83 (Suppl. S1), S91–S96. [Google Scholar] [CrossRef]
- Åkesson, A. Go nuts and go extra virgin olive oil! Mediterranean diets reduce blood pressure. Hypertension 2014, 64, 26–27. [Google Scholar] [CrossRef]
- Estruch, R.; Martinez-Gonzalez, M.A.; Corella, D.; Salas-Salvadó, J.; Ruiz-Gutiérrez, V.; Covas, M.I.; Fiol, M.; Gómez-Gracia, E.; López-Sabater, M.C.; Vinyoles, E.; et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 1–11. [Google Scholar] [CrossRef]
- Toledo, E.; Hu, F.B.; Estruch, R.; Buil-Cosiales, P.; Corella, D.; Salas-Salvadó, J.; Covas, M.I.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. Effect of the Mediterranean diet on blood pressure in the PREDIMED trial: Results from a randomized controlled trial. BMC Med. 2013, 11, 207. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef]
- Razquin, C.; Martinez-Gonzalez, M.A. A Traditional Mediterranean Diet Effectively Reduces Inflammation and Improves Cardiovascular Health. Nutrients 2019, 11, 1842. [Google Scholar] [CrossRef]
- Jennings, A.; Berendsen, A.M.; De Groot, L.C.P.G.M.; Feskens, E.J.M.; Brzozowska, A.; Sicińska, E.; Pietruszka, B.; Meunier, N.; Caumon, E.; Malpuech-Brugère, C.; et al. Mediterranean-Style Diet Improves Systolic Blood Pressure and Arterial Stiffness in Older Adults. Hypertension 2019, 73, 578–586. [Google Scholar] [CrossRef]
- Storniolo, C.E.; Casillas, R.; Bulló, M.; Castañer, O.; Ros, E.; Sáez, G.; Toledo, E.; Estruch, R.; Ruiz-Gutiérrez, V.; Fitó, M.; et al. A Mediterranean diet supplemented with extra virgin olive oil or nuts improves endothelial markers involved in blood pressure control in hypertensive women. Eur. J. Nutr. 2017, 56, 89–97. [Google Scholar] [CrossRef]
- Konstantinidou, V.; Covas, M.; Muñoz-Aguayo, D.; Khymenets, O.; de la Torre, R.; Saez, G.; Tormos, M.D.C.; Toledo, E.; Marti, A.; Ruiz-Gutiérrez, V.; et al. In vivo nutrigenomic effects of virgin olive oil polyphenols within the frame of the Mediterranean diet: A randomized controlled trial. FASEB J. 2010, 24, 2546–2557. [Google Scholar] [CrossRef]
- Fleming, I. Molecular mechanisms underlying the activation of eNOS. Pflugers Arch. 2010, 459, 793–806. [Google Scholar] [CrossRef]
- Davis, C.R.; Hodgson, J.M.; Woodman, R.; Bryan, J.; Wilson, C.; Murphy, K.J. A Mediterranean diet lowers blood pressure and improves endothelial function: Results from the MedLey randomized intervention trial. Am. J. Clin. Nutr. 2017, 105, 1305–1313. [Google Scholar] [CrossRef]
- Camargo, A.; Ruano, J.; Fernandez, J.M.; Parnell, L.D.; Jimenez, A.; Santos-Gonzalez, M.; Marin, C.; Perez-Martinez, P.; Uceda, M.; Lopez-Miranda, J.; et al. Gene expression changes in mononuclear cells in patients with metabolic syndrome after acute intake of phenol-rich virgin olive oil. BMC Genom. 2010, 11, 253. [Google Scholar] [CrossRef]
- Yang, Q.; Alemany, R.; Casas, J.; Kitajka, K.; Lanier, S.M.; Escribá, P.V. Influence of the membrane lipid structure on signal processing via G protein-coupled receptors. Mol. Pharmacol. 2005, 68, 210–217. [Google Scholar] [CrossRef]
- Platania, A.; Zappala, G.; Mirabella, M.U.; Gullo, C.; Mellini, G.; Beneventano, G.; Maugeri, G.; Marranzano, M. Association between Mediterranean diet adherence and dyslipidaemia in a cohort of adults living in the Mediterranean area. Int. J. Food Sci. Nutr. 2018, 69, 608–618. [Google Scholar] [CrossRef]
- Roldan, C.C.; Marcos, M.L.T.; Marcos, F.M.; Albero, J.S.; Rios, R.S.; Rodriguez, A.C.; Royo, J.M.P.; López, P.J.T. Adhesion to the Mediterranean diet in diabetic patients with poor control. Clin. Investig. Arterioscler. 2019, 31, 210–217, (In English and Spanish). [Google Scholar] [CrossRef]
- Antoniazzi, L.; Arroyo-Olivares, R.; Bittencourt, M.S.; Tada, M.T.; Lima, I.; Jannes, C.E.; Krieger, J.E.; Pereira, A.C.; Quintana-Navarro, G.; Muñiz-Grijalvo, O.; et al. Adherence to a Mediterranean diet, dyslipidemia and inflammation in familial hypercholesterolemia. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2014–2022. [Google Scholar] [CrossRef]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean diet, its components, and cardiovascular disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef]
- Neuenschwander, M.; Hoffmann, G.; Schwingshackl, L.; Schlesinger, S. Impact of different dietary approaches on blood lipid control in patients with type 2 diabetes mellitus: A systematic review and network meta-analysis. Eur. J. Epidemiol. 2019, 34, 837–852. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Clarke, R. Quantitative effects on cardiovascular risk factors and coronary heart disease risk of replacing partially hydrogenated vegetable oils with other fats and oils. Eur. J. Clin. Nutr. 2009, 63 (Suppl. S2), S22–S33. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Farrés, X.; Luque, X.; Narejos, S.; Borrell, M.; Basora, J.; Anguera, A.; Torres, F.; Bulló, M.; Balanza, R.; et al. Effect of two doses of a mixture of soluble fibres on body weight and metabolic variables in overweight or obese patients: A randomised trial. Br. J. Nutr. 2008, 99, 1380–1387. [Google Scholar] [CrossRef]
- Theuwissen, E.; Mensink, R.P. Water-soluble dietary fibers and cardiovascular disease. Physiol. Behav. 2008, 94, 285–292. [Google Scholar] [CrossRef]
- Trautwein, E.A.; McKay, S. The Role of Specific Components of a Plant-Based Diet in Management of Dyslipidemia and the Impact on Cardiovascular Risk. Nutrients 2020, 12, 2671. [Google Scholar] [CrossRef] [PubMed]
- Abumweis, S.S.; Barake, R.; Jones, P.J. Plant sterols/stanols as cholesterol lowering agents: A meta-analysis of randomized controlled trials. Food Nutr. Res. 2008, 52, 1811. [Google Scholar] [CrossRef] [PubMed]
- Bédard, A.; Corneau, L.; Vohl, M.C.; Dodin, S.; Lemieux, S. Effect of the Mediterranean diet on the lipid-lipoprotein profile: Is it influenced by the family history of dyslipidemia? J. Nutrigenet. Nutrigenom. 2014, 7, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Lampropoulou, M.; Chaini, M.; Rigopoulos, N.; Evangeliou, A.; Papadopoulou-Legbelou, K.; Koutelidakis, A.E. Association between Serum Lipid Levels in Greek Children with Dyslipidemia and Mediterranean Diet Adherence, Dietary Habits, Lifestyle and Family Socioeconomic Factors. Nutrients 2020, 12, 1600. [Google Scholar] [CrossRef]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. A Biol. Sci.Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef]
- Gomez-Marin, B.; Gomez-Delgado, F.; Lopez-Moreno, J.; Alcala-Diaz, J.F.; Jimenez-Lucena, R.; Torres-Peña, J.D.; Garcia-Rios, A.; Ortiz-Morales, A.M.; Yubero-Serrano, E.M.; Malagon, M.D.M.; et al. Long-term consumption of a Mediterranean diet improves postprandial lipemia in patients with type 2 diabetes: The Cordioprev randomized trial. Am. J. Clin. Nutr. 2018, 108, 963–970. [Google Scholar] [CrossRef]
- Georgia-Eirini, D.; Athina, S.; Wim, V.B.; Christos, K.; Theodoros, C. Natural Products from Mediterranean Diet: From Anti-hyperlipidemic Agents to Dietary Epigenetic Modulators. Curr. Pharm. Biotechnol. 2019, 20, 825–844. [Google Scholar] [CrossRef]
- Pignanelli, M.; Just, C.; Bogiatzi, C.; Dinculescu, V.; Gloor, G.B.; Allen-Vercoe, E.; Reid, G.; Urquhart, B.L.; Ruetz, K.N.; Velenosi, T.J.; et al. Mediterranean Diet Score: Associations with Metabolic Products of the Intestinal Microbiome, Carotid Plaque Burden, and Renal Function. Nutrients 2018, 10, 779. [Google Scholar] [CrossRef]
- De Lorenzo, A.; Noce, A.; Bigioni, M.; Calabrese, V.; Della Rocca, D.G.; Di Daniele, N.; Tozzo, C.; Di Renzo, L. The effects of Italian Mediterranean organic diet (IMOD) on health status. Curr. Pharm. Des. 2010, 16, 814–824. [Google Scholar] [CrossRef]
- Hansrivijit, P.; Oli, S.; Khanal, R.; Ghahramani, N.; Thongprayoon, C.; Cheungpasitporn, W. Mediterranean diet and the risk of chronic kidney disease: A systematic review and meta-analysis. Nephrology 2020, 25, 913–918. [Google Scholar] [CrossRef]
- Asghari, G.; Farhadnejad, H.; Mirmiran, P.; Dizavi, A.; Yuzbashian, E.; Azizi, F. Adherence to the Mediterranean diet is associated with reduced risk of incident chronic kidney diseases among Tehranian adults. Hypertens. Res. 2017, 40, 96–102. [Google Scholar] [CrossRef]
- Hu, E.A.; Steffen, L.M.; Grams, M.E.; Crews, D.C.; Coresh, J.; Appel, L.J.; Rebholz, C.M. Dietary patterns and risk of incident chronic kidney disease: The Atherosclerosis Risk in Communities study. Am. J. Clin. Nutr. 2019, 110, 713–721. [Google Scholar] [CrossRef]
- Geng, T.T.; Jafar, T.H.; Neelakantan, N.; Yuan, J.M.; van Dam, R.M.; Koh, W.P. Healthful dietary patterns and risk of end-stage kidney disease: The Singapore Chinese Health Study. Am. J. Clin. Nutr. 2021, 113, 675–683. [Google Scholar] [CrossRef]
- Picard, K.; Senior, P.A.; Perez, S.A.; Jindal, K.; Richard, C.; Mager, D.R. Low Mediterranean Diet scores are associated with reduced kidney function and health related quality of life but not other markers of cardiovascular risk in adults with diabetes and chronic kidney disease. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1445–1453. [Google Scholar] [CrossRef]
- Losappio, V.; Infante, B.; Leo, S.; Troise, D.; Calvaruso, M.; Vitale, P.; Renzi, S.; Stallone, G.; Castellano, G. Nutrition-Based Management of Inflammaging in CKD and Renal Replacement Therapies. Nutrients 2021, 13, 267. [Google Scholar] [CrossRef]
- Chauveau, P.; Aparicio, M.; Bellizzi, V.; Campbell, K.; Hong, X.; Johansson, L.; Kolko, A.; Molina, P.; Sezer, S.; Wanner, C.; et al. Mediterranean diet as the diet of choice for patients with chronic kidney disease. Nephrol. Dial. Transplant. 2018, 33, 725–735. [Google Scholar] [CrossRef]
- Mekki, K.; Bouzidi-bekada, N.; Kaddous, A.; Bouchenak, M. Mediterranean diet improves dyslipidemia and biomarkers in chronic renal failure patients. Food Funct. 2010, 1, 110–115. [Google Scholar] [CrossRef]
- Gomes-Neto, A.W.; Osté, M.C.J.; Sotomayor, C.G.; van den Berg, E.; Geleijnse, J.M.; Berger, S.P.; Gans, R.O.B.; Bakker, S.J.L.; Navis, G.J. Mediterranean Style Diet and Kidney Function Loss in Kidney Transplant Recipients. Clin. J. Am. Soc. Nephrol. 2020, 15, 238–246. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Bargagli, M. Dietetic and lifestyle recommendations for stone formers. Arch. Esp. Urol. 2021, 74, 112–122, (In English and Spanish). [Google Scholar]
- Martínez-Pineda, M.; Yagüe-Ruiz, C.; Caverni-Muñoz, A.; Vercet-Tormo, A. Cooking Legumes: A Way for Their Inclusion in the Renal Patient Diet. J. Ren. Nutr. 2019, 29, 118–125. [Google Scholar] [CrossRef]
- Kammoun, K.; Chaker, H.; Mahfoudh, H.; Makhlouf, N.; Jarraya, F.; Hachicha, J. Diet in chronic kidney disease in a Mediterranean African country. BMC Nephrol. 2017, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Daneshzad, E.; Najafabadi, M.M.; Bellissimo, N.; Suitor, K.; Azadbakht, L. Association between adherence to the Mediterranean diet and renal function biomarkers and cardiovascular risk factors among diabetic patients with nephropathy. Clin. Nutr. ESPEN 2020, 40, 156–163. [Google Scholar] [CrossRef]
- Palmer, S.C.; Maggo, J.K.; Campbell, K.L.; Craig, J.C.; Johnson, D.W.; Sutanto, B.; Ruospo, M.; Tong, A.; Strippoli, G.F. Dietary interventions for adults with chronic kidney disease. Cochrane Database Syst. Rev. 2017, 4, CD011998. [Google Scholar] [CrossRef]
- Saglimbene, V.M.; Wong, G.; Craig, J.; Ruospo, M.; Palmer, S.C.; Campbell, K.; Larsen, V.G.; Natale, P.; Teixeira-Pinto, A.; Carrero, J.-J.; et al. The Association of Mediterranean and DASH Diets with Mortality in Adults on Hemodialysis: The DIET-HD Multinational Cohort Study. J. Am. Soc. Nephrol. 2018, 29, 1741–1751. [Google Scholar] [CrossRef]
- Martucci, M.; Ostan, R.; Biondi, F.; Bellavista, E.; Fabbri, C.; Bertarelli, C.; Salvioli, S.; Capri, M.; Franceschi, C.; Santoro, A. Mediterranean diet and inflammaging within the hormesis paradigm. Nutr Rev. 2017, 75, 442–455. [Google Scholar] [CrossRef]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jönsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011, 106 (Suppl. S3), S5–S78. [Google Scholar] [CrossRef]
- Pounis, G.; Bonaccio, M.; Di Castelnuovo, A.; Costanzo, S.; De Curtis, A.; Persichillo, M.; Sieri, S.; Donati, M.B.; Cerletti, C.; de Gaetano, G.; et al. Polyphenol intake is associated with low-grade inflammation, using a novel data analysis from the Moli-sani study. Thromb. Haemost. 2016, 115, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Temple, N.J.; Guercio, V.; Tavani, A. The Mediterranean Diet and Cardiovascular Disease: Gaps in the Evidence and Research Challenges. Cardiol. Rev. 2019, 27, 127–130. [Google Scholar] [CrossRef]
- Arouca, A.B.; Meirhaeghe, A.; Dallongeville, J.; Moreno, L.A.; Lourenço, G.J.; Marcos, A.; Huybrechts, I.; Manios, Y.; Lambrinou, C.-P.; Gottrand, F.; et al. Interplay between the Mediterranean diet and C-reactive protein genetic polymorphisms towards inflammation in adolescents. Clin. Nutr. 2020, 39, 1919–1926. [Google Scholar] [CrossRef]
- Richard, C.; Couture, P.; Desroches, S.; Lamarche, B. Effect of the Mediterranean diet with and without weight loss on markers of inflammation in men with metabolic syndrome. Obesity 2013, 21, 51–57. [Google Scholar] [CrossRef]
- Bendall, C.L.; Mayr, H.L.; Opie, R.S.; Bes-Rastrollo, M.; Itsiopoulos, C.; Thomas, C.J. Central obesity and the Mediterranean diet: A systematic review of intervention trials. Crit. Rev. Food Sci. Nutr. 2018, 58, 3070–3084. [Google Scholar] [CrossRef] [PubMed]
- Bonaccio, M.; Cerletti, C.; Iacoviello, L.; de Gaetano, G. Mediterranean diet and low-grade subclinical inflammation: The Moli-sani study. Endocr. Metab. Immune Disord. Drug Targets 2015, 15, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Luisi, M.L.E.; Lucarini, L.; Biffi, B.; Rafanelli, E.; Pietramellara, G.; Durante, M.; Vidali, S.; Provensi, G.; Madiai, S.; Gheri, C.F.; et al. Effect of Mediterranean Diet Enriched in High Quality Extra Virgin Olive Oil on Oxidative Stress, Inflammation and Gut Microbiota in Obese and Normal Weight Adult Subjects. Front. Pharmacol. 2019, 10, 1366. [Google Scholar] [CrossRef]
- Casas, R.; Sacanella, E.; Urpi, M.; Chiva-Blanch, G.; Ros, E.; Martinez-Gonzalez, M.A.; Covas, M.-I.; Salas-Salvadó, J.; Fiol, M.; Arós, F.; et al. The effects of the mediterranean diet on biomarkers of vascular wall inflammation and plaque vulnerability in subjects with high risk for cardiovascular disease. A randomized trial. PLoS ONE 2014, 9, e100084. [Google Scholar] [CrossRef]
- Urpi-Sarda, M.; Casas, R.; Chiva-Blanch, G.; Romero-Mamani, E.S.; Valderas-Martínez, P.; Salas-Salvadó, J.; Covas, M.I.; Toledo, E.; Andres-Lacueva, C.; Llorach, R.; et al. The Mediterranean diet pattern and its main components are associated with lower plasma concentrations of tumor necrosis factor receptor 60 in patients at high risk for cardiovascular disease. J. Nutr. 2012, 142, 1019–1025. [Google Scholar] [CrossRef]
- Marcelino, G.; Hiane, P.A.; Freitas, K.D.C.; Santana, L.F.; Pott, A.; Donadon, J.R.; Guimarães, R.D.C.A. Effects of Olive Oil and Its Minor Components on Cardiovascular Diseases, Inflammation, and Gut Microbiota. Nutrients 2019, 11, 1826. [Google Scholar] [CrossRef]
- Ramos, I.R.; Rangel-Zuñiga, O.A.; Lopez-Moreno, J.; Alcala-Diaz, J.F.; Perez-Martinez, P.; Jimenez-Lucena, R.; Castaño, J.P.; Roche, H.; Delgado-Lista, J.; Ordovas, J.M.; et al. Mediterranean Diet, Glucose Homeostasis, and Inflammasome Genetic Variants: The CORDIOPREV Study. Mol. Nutr. Food Res. 2018, 62, e1700960. [Google Scholar] [CrossRef]
- Reddavide, R.; Rotolo, O.; Caruso, M.G.; Stasi, E.; Notarnicola, M.; Miraglia, C.; Nouvenne, A.; Meschi, T.; Angelis, G.L.D.; Di Mario, F.; et al. The role of diet in the prevention and treatment of Inflammatory Bowel Diseases. Acta Biomed. 2018, 89, 60–75. [Google Scholar] [CrossRef]
- Weber, A.T.; Shah, N.D.; Sauk, J.; Limketkai, B.N. Popular Diet Trends for Inflammatory Bowel Diseases: Claims and Evidence. Curr. Treat. Options Gastroenterol. 2019, 17, 564–576. [Google Scholar] [CrossRef]
- Mazzocchi, A.; Leone, L.; Agostoni, C.; Pali-Schöll, I. The Secrets of the Mediterranean Diet. Does [Only] Olive Oil Matter? Nutrients 2019, 11, 2941. [Google Scholar] [CrossRef]
- Cerletti, C.; Gianfagna, F.; Tamburrelli, C.; De Curtis, A.; D’Imperio, M.; Coletta, W.; Giordano, L.; Lorenzet, R.; Rapisarda, P.; Recupero, G.R.; et al. Orange juice intake during a fatty meal consumption reduces the postprandial low-grade inflammatory response in healthy subjects. Thromb. Res. 2015, 135, 255–259. [Google Scholar] [CrossRef]
- Spazzafumo, L.; Olivieri, F.; Abbatecola, A.M.; Castellani, G.; Monti, D.; Lisa, R.; Galeazzi, R.; Sirolla, C.; Testa, R.; Ostan, R.; et al. Remodelling of biological parameters during human ageing: Evidence for complex regulation in longevity and in type 2 diabetes. Age 2013, 35, 419–429. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. S1), S4–S9. [Google Scholar] [CrossRef]
- Kennedy, B.K.; Berger, S.L.; Brunet, A.; Campisi, J.; Cuervo, A.M.; Epel, E.S.; Franceschi, C.; Lithgow, G.J.; Morimoto, R.I.; Pessin, J.E.; et al. Geroscience: Linking aging to chronic disease. Cell 2014, 159, 709–713. [Google Scholar] [CrossRef]
- Tsigalou, C.; Konstantinidis, T.; Paraschaki, A.; Stavropoulou, E.; Voidarou, C.; Bezirtzoglou, E. Mediterranean Diet as a Tool to Combat Inflammation and Chronic Diseases. An Overview. Biomedicines 2020, 8, 201. [Google Scholar] [CrossRef]
- Val-Laillet, D.; Aarts, E.; Weber, B.; Ferrari, M.; Quaresima, V.; Stoeckel, L.; Alonso-Alonso, M.; Audette, M.; Malbert, C.; Stice, E. Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. Neuroimage Clin. 2015, 8, 1–31. [Google Scholar] [CrossRef]
- Klatzkin, R.R.; Gaffney, S.; Cyrus, K.; Bigus, E.; Brownley, K.A. Stress-induced eating in women with binge-eating disorder and obesity. Biol. Psychol. 2018, 131, 96–106. [Google Scholar] [CrossRef]
- Van Strien, T. Causes of Emotional Eating and Matched Treatment of Obesity. Curr. Diabetes Rep. 2018, 18, 35. [Google Scholar] [CrossRef]
- Kałużna-Czaplińska, J.; Gątarek, P.; Chirumbolo, S.; Chartrand, M.S.; Bjørklund, G. How important is tryptophan in human health? Crit. Rev. Food Sci. Nutr. 2019, 59, 72–88. [Google Scholar] [CrossRef]
- Yau, Y.H.; Potenza, M.N. Stress and eating behaviors. Minerva Endocrinol. 2013, 38, 255–267. [Google Scholar] [PubMed]
- O’Reilly, G.A.; Cook, L.; Spruijt-Metz, D.; Black, D.S. Mindfulness-based interventions for obesity-related eating behaviours: A literature review. Obes. Rev. 2014, 15, 453–461. [Google Scholar] [CrossRef]
- Candela, C.G. Consensus document about the nutritional evaluation and management of eating disorders: Bulimia nervosa, binge eating disorder, and others. Nutr. Hosp. 2018, 35, 49–97. (In Spanish) [Google Scholar] [CrossRef]
- Morse, S.S. Mouse thymic necrosis virus: A novel murine lymphotropic agent. Lab. Anim. Sci. 1987, 37, 717–725. [Google Scholar] [PubMed]
- Puhl, R.; Suh, Y. Stigma and eating and weight disorders. Curr. Psychiatry Rep. 2015, 17, 552. [Google Scholar] [CrossRef] [PubMed]
- Winston, A.P. Eating Disorders and Diabetes. Curr. Diabetes Rep. 2020, 20, 32. [Google Scholar] [CrossRef] [PubMed]
- Henríquez-Tejo, R.; Cartes-Velásquez, R. Psychosocial impact of type 1 diabetes mellitus in children, adolescents and their families. Literature review. Rev. Chil. Pediatr. 2018, 89, 391–398. (In Spanish) [Google Scholar] [CrossRef]
- Pursey, K.M.; Hay, P.; Bussey, K.; Trompeter, N.; Lonergan, A.; Pike, K.M.; Mond, J.; Mitchison, D. Diabetes and disordered eating behaviours in a community-based sample of Australian adolescents. J. Eat. Disord. 2020, 8, 5. [Google Scholar] [CrossRef]
- Lorettu, L.; Pes, G.M.; Dore, M.P.; Milia, P.; Nivoli, A. Eating disorders and diabetes: Behavioural patterns and psychopathology. Two case reports. Riv. Psichiatr. 2020, 55, 240–244. [Google Scholar] [CrossRef]
- Moskovich, A.A.; Dmitrieva, N.O.; Babyak, M.A.; Smith, P.J.; Honeycutt, L.K.; Mooney, J.; Merwin, R.M. Real-time predictors and consequences of binge eating among adults with type 1 diabetes. J. Eat. Disord. 2019, 7, 7. [Google Scholar] [CrossRef]
- Harris, S.R.; Carrillo, M.; Fujioka, K. Binge-Eating Disorder and Type 2 Diabetes: A Review. Endocr. Pract. 2021, 27, 158–164. [Google Scholar] [CrossRef]
- Papelbaum, M.; Moreira, R.D.O.; Coutinho, W.F.; Kupfer, R.; Freitas, S.; Luz, R.R.; Appolinario, J. Does binge-eating matter for glycemic control in type 2 diabetes patients? J. Eat. Disord. 2019, 7, 30. [Google Scholar] [CrossRef]
- Van Bloemendaal, L.; Veltman, D.J.; Kulve, J.S.T.; Drent, M.L.; Barkhof, F.; Diamant, M.; Ijzerman, R.G. Emotional eating is associated with increased brain responses to food-cues and reduced sensitivity to GLP-1 receptor activation. Obesity 2015, 23, 2075–2082. [Google Scholar] [CrossRef]
- Chevinsky, J.D.; Wadden, T.A.; Chao, A.M. Binge Eating Disorder in Patients with Type 2 Diabetes: Diagnostic and Management Challenges. Diabetes Metab. Syndr. Obes. 2020, 13, 1117–1131. [Google Scholar] [CrossRef]
- Abbott, S.; Dindol, N.; Tahrani, A.A.; Piya, M.K. Binge eating disorder and night eating syndrome in adults with type 2 diabetes: A systematic review. J. Eat. Disord. 2018, 6, 36. [Google Scholar] [CrossRef]
- Hotz, M.M.; Gnoinski, W.M. Effects of early maxillary orthopaedics in coordination with delayed surgery for cleft lip and palate. J. Maxillofac. Surg. 1979, 7, 201–210. [Google Scholar] [CrossRef]
- Blom, T.J.; Guerdjikova, A.I.; Mori, N.; Casuto, L.S.; McElroy, S.L. Placebo cessation in binge eating disorder: Effect on anthropometric, cardiovascular, and metabolic variables. Eur. Eat. Disord. Rev. 2015, 23, 86–88. [Google Scholar] [CrossRef]
- Shank, L.; Tanofsky-Kraff, M.; Kelly, N.R.; Schvey, N.A.; Marwitz, S.E.; Mehari, R.D.; Brady, S.M.; Demidowich, A.P.; Broadney, M.M.; Galescu, O.A.; et al. Pediatric Loss of Control Eating and High-Sensitivity C-Reactive Protein Concentrations. Child. Obes. 2017, 13, 1–8. [Google Scholar] [CrossRef]
- Radin, R.; Tanofsky-Kraff, M.; Shomaker, L.B.; Kelly, N.R.; Pickworth, C.K.; Shank, L.; Altschul, A.M.; Brady, S.M.; Demidowich, A.P.; Yanovski, S.Z.; et al. Metabolic characteristics of youth with loss of control eating. Eat. Behav. 2015, 19, 86–89. [Google Scholar] [CrossRef]
- Olguin, P.; Fuentes, M.; Gabler, G.; Guerdjikova, A.I.; Keck, P.E.; McElroy, S.L. Medical comorbidity of binge eating disorder. Eat. Weight Disord. 2017, 22, 13–26. [Google Scholar] [CrossRef]
- Stein, D.J.; Aguilar-Gaxiola, S.; Alonso, J.; Bruffaerts, R.; de Jonge, P.; Liu, Z.; Caldas-De-Almeida, J.M.; O’Neill, S.; Viana, M.C.; Al-Hamzawi, A.O.; et al. Associations between mental disorders and subsequent onset of hypertension. Gen. Hosp. Psychiatry 2014, 36, 142–149. [Google Scholar] [CrossRef]
- Mensorio, M.S.; Cebolla, A.; Lisón, J.F.; Rodilla, E.; Palomar, G.; Miragall, M.; Baños, R.M. Emotional eating as a mediator between anxiety and cholesterol in population with overweight and hypertension. Psychol. Health Med. 2017, 22, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Noma, S.; Fukusima, M.; Taniguchi, A.; Teramukai, S. Serum Lipid Levels in Patients with Eating Disorders. Intern. Med. 2016, 55, 1853–1857. [Google Scholar] [CrossRef] [PubMed]
- Succurro, E.; Segura-Garcia, C.; Ruffo, M.; Caroleo, M.; Rania, M.; Aloi, M.; De Fazio, P.; Sesti, G.; Arturi, F. Obese Patients with a Binge Eating Disorder Have an Unfavorable Metabolic and Inflammatory Profile. Medicine 2015, 94, e2098. [Google Scholar] [CrossRef] [PubMed]
- Solmi, F.; Bulik, C.M.; de Stavola, B.L.; Dalman, C.; Khandaker, G.M.; Lewis, G. Longitudinal associations between circulating interleukin-6 and C-reactive protein in childhood, and eating disorders and disordered eating in adolescence. Brain Behav. Immun. 2020, 89, 491–500. [Google Scholar] [CrossRef]
- Caroleo, M.; Carbone, E.A.; Greco, M.; Corigliano, D.M.; Arcidiacono, B.; Fazia, G.; Rania, M.; Aloi, M.; Gallelli, L.; Segura-Garcia, C.; et al. Brain-Behavior-Immune Interaction: Serum Cytokines and Growth Factors in Patients with Eating Disorders at Extremes of the Body Mass Index (BMI) Spectrum. Nutrients 2019, 11, 1995. [Google Scholar] [CrossRef]
- Moran, G.W.; Thapaliya, G. The Gut-Brain Axis and Its Role in Controlling Eating Behavior in Intestinal Inflammation. Nutrients 2021, 13, 981. [Google Scholar] [CrossRef]
- Dalton, B.; Bartholdy, S.; Robinson, L.; Solmi, M.; Ibrahim, M.A.; Breen, G.; Schmidt, U.; Himmerich, H. A meta-analysis of cytokine concentrations in eating disorders. J. Psychiatr. Res. 2018, 103, 252–264. [Google Scholar] [CrossRef]
- Butler, M.J.; Perrini, A.A.; Eckel, L.A. The Role of the Gut Microbiome, Immunity, and Neuroinflammation in the Pathophysiology of Eating Disorders. Nutrients 2021, 13, 500. [Google Scholar] [CrossRef]
- Yu, Y.; Fernandez, I.D.; Meng, Y.; Zhao, W.; Groth, S.W. Gut hormones, adipokines, and pro- and anti-inflammatory cytokines/markers in loss of control eating: A scoping review. Appetite 2021, 166, 105442. [Google Scholar] [CrossRef]
- Belegri, E.; Eggels, L.; Unmehopa, U.A.; Mul, J.D.; Boelen, A.; la Fleur, S.E. The effects of overnight nutrient intake on hypothalamic inflammation in a free-choice diet-induced obesity rat model. Appetite 2018, 120, 527–535. [Google Scholar] [CrossRef]
- Bruzas, M.B.; Allison, K.C. A Review of the Relationship between Night Eating Syndrome and Body Mass Index. Curr. Obes. Rep. 2019, 8, 145–155. [Google Scholar] [CrossRef]
- Kara, Y.; Tuzun, S.; Oner, C.; Simsek, E.E. Night Eating Syndrome According to Obesity Groups and the Related Factors. J. Coll Physicians Surg. Pak. 2020, 30, 833–838. [Google Scholar] [CrossRef]
- McCuen-Wurst, C.; Ruggieri, M.; Allison, K.C. Disordered eating and obesity: Associations between binge-eating disorder, night-eating syndrome, and weight-related comorbidities. Ann. N. Y. Acad. Sci. 2018, 1411, 96–105. [Google Scholar] [CrossRef]
- Gallant, A.R.; Lundgren, J.; Drapeau, V. The night-eating syndrome and obesity. Obes. Rev. 2012, 13, 528–536. [Google Scholar] [CrossRef]
- Pinto, T.F.; Silva, F.G.; Bruin, V.M.; Bruin, P.F. Night eating syndrome: How to treat it? Rev. Assoc. Med. Bras. 2016, 62, 701–707. [Google Scholar] [CrossRef]
- Cleator, J.; Abbott, J.; Judd, P.; Sutton, C.; Wilding, J.P. Night eating syndrome: Implications for severe obesity. Nutr. Diabetes 2012, 2, e44. [Google Scholar] [CrossRef]
- Yahia, N.; Brown, C.; Potter, S.; Szymanski, H.; Smith, K.; Pringle, L.; Herman, C.; Uribe, M.; Fu, Z.; Chung, M.; et al. Night eating syndrome and its association with weight status, physical activity, eating habits, smoking status, and sleep patterns among college students. Eat. Weight Disord. 2017, 22, 421–433. [Google Scholar] [CrossRef]
- Sutcu, C.; Pamuk, G.; Ongel, K. Evaluation of night eating syndrome in individuals with and without obesity. Endokrynol. Pol. 2021, 72, 539–544. [Google Scholar] [CrossRef]
- Wal, J.S.V. Night eating syndrome: A critical review of the literature. Clin. Psychol. Rev. 2012, 32, 49–59. [Google Scholar] [CrossRef]
- Chaput, J.P. Sleep patterns, diet quality and energy balance. Physiol. Behav. 2014, 134, 86–91. [Google Scholar] [CrossRef]
- Ogilvie, R.P.; Patel, S.R. The Epidemiology of Sleep and Diabetes. Curr. Diabetes Rep. 2018, 18, 82. [Google Scholar] [CrossRef]
- Anothaisintawee, T.; Reutrakul, S.; van Cauter, E.; Thakkinstian, A. Sleep disturbances compared to traditional risk factors for diabetes development: Systematic review and meta-analysis. Sleep Med. Rev. 2016, 30, 11–24. [Google Scholar] [CrossRef]
- Reutrakul, S.; Mokhlesi, B. Obstructive Sleep Apnea and Diabetes: A State of the Art Review. Chest 2017, 152, 1070–1086. [Google Scholar] [CrossRef]
- Ryan, S. Sleep and diabetes. Curr. Opin. Pulm. Med. 2018, 24, 555–560. [Google Scholar] [CrossRef]
- Larcher, S.; Benhamou, P.Y.; Pépin, J.L.; Borel, A.L. Sleep habits and diabetes. Diabetes Metab. 2015, 41, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.H.; Ng, K.Y.; Chin, W.K. The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: A systematic review and meta-analysis. Sleep Med. Rev. 2017, 31, 91–101. [Google Scholar] [CrossRef]
- Muraki, I.; Wada, H.; Tanigawa, T. Sleep apnea and type 2 diabetes. J. Diabetes Investig. 2018, 9, 991–997. [Google Scholar] [CrossRef]
- Grandner, M.A.; Seixas, A.; Shetty, S.; Shenoy, S. Sleep Duration and Diabetes Risk: Population Trends and Potential Mechanisms. Curr. Diabetes Rep. 2016, 16, 106. [Google Scholar] [CrossRef]
- Reutrakul, S.; van Cauter, E. Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metabolism 2018, 84, 56–66. [Google Scholar] [CrossRef]
- Sakai, R.; Hashimoto, Y.; Ushigome, E.; Miki, A.; Okamura, T.; Matsugasumi, M.; Fukuda, T.; Majima, S.; Matsumoto, S.; Senmaru, T.; et al. Late-night-dinner is associated with poor glycemic control in people with type 2 diabetes: The KAMOGAWA-DM cohort study. Endocr. J. 2018, 65, 395–402. [Google Scholar] [CrossRef]
- Brouwer, A.; van Raalte, D.H.; Rutters, F.; Elders, P.J.; Snoek, F.J.; Beekman, A.T.; Bremmer, M.A. Sleep and HbA. Diabetes Care 2020, 43, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Majewski, C.; Xie, M.; Yan, P.; Guo, Y.; Bao, W.; Rong, Y.; Jackson, C.L.; Hu, F.B.; Liu, L. Sleep duration and risk of type 2 diabetes: A meta-analysis of prospective studies. Diabetes Care 2015, 38, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Dutil, C.; Chaput, J.P. Inadequate sleep as a contributor to type 2 diabetes in children and adolescents. Nutr. Diabetes 2017, 7, e266. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, A.R.; Nightingale, C.M.; Donin, A.S.; Sattar, N.; Cook, D.G.; Whincup, P.H.; Owen, C.G. Sleep Duration and Risk of Type 2 Diabetes. Pediatrics 2017, 140, e20170338. [Google Scholar] [CrossRef]
- Griggs, S.; Redeker, N.S.; Grey, M. Sleep characteristics in young adults with type 1 diabetes. Diabetes Res. Clin. Pract. 2019, 150, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Perez, K.M.; Hamburger, E.R.; Lyttle, M.; Williams, R.; Bergner, E.; Kahanda, S.; Cobry, E.; Jaser, S.S. Sleep in Type 1 Diabetes: Implications for Glycemic Control and Diabetes Management. Curr. Diabetes Rep. 2018, 18, 5. [Google Scholar] [CrossRef]
- Calhoun, D.A.; Harding, S.M. Sleep and hypertension. Chest 2010, 138, 434–443. [Google Scholar] [CrossRef]
- Chen, Y.; Fang, Z.; Zhu, L.; He, L.; Liu, H.; Zhou, C. The association of eating behaviors with blood pressure levels in college students: A cross-sectional study. Ann. Transl. Med. 2021, 9, 155. [Google Scholar] [CrossRef]
- Barikani, A.; Javadi, M.; Rafiei, S. Sleep Quality and Blood Lipid Composition Among Patients with Diabetes. Int. J. Endocrinol. Metab. 2019, 17, e81062. [Google Scholar] [CrossRef]
- Lemke, M.K.; Apostolopoulos, Y.; Hege, A.; Wideman, L.; Sönmez, S. Work, sleep, and cholesterol levels of U.S. long-haul truck drivers. Ind. Health 2017, 55, 149–161. [Google Scholar] [CrossRef]
- Smiley, A.; King, D.; Harezlak, J.; Dinh, P.; Bidulescu, A. The association between sleep duration and lipid profiles: The NHANES 2013–2014. J. Diabetes Metab. Disord. 2019, 18, 315–322. [Google Scholar] [CrossRef]
- Kim, C.E.; Shin, S.; Lee, H.-W.; Lim, J.; Lee, J.-K.; Shin, A.; Kang, D. Association between sleep duration and metabolic syndrome: A cross-sectional study. BMC Public Health 2018, 18, 720. [Google Scholar] [CrossRef]
- Korostovtseva, L.; Alieva, A.; Rotar, O.; Bochkarev, M.; Boyarinova, M.; Sviryaev, Y.; Konradi, A.; Shlyakhto, E. Sleep Duration, Lipid Profile and Insulin Resistance: Potential Role of Lipoprotein(a). Int. J. Mol. Sci. 2020, 21, 4680. [Google Scholar] [CrossRef]
- Smiley, A.; King, D.; Bidulescu, A. The Association between Sleep Duration and Metabolic Syndrome: The NHANES 2013/2014. Nutrients 2019, 11, 2582. [Google Scholar] [CrossRef]
- Xing, C.; Huang, X.; Zhang, Y.; Zhang, C.; Wang, W.; Wu, L.; Ding, M.; Zhang, M.; Song, L. Sleep Disturbance Induces Increased Cholesterol Level by NR1D1 Mediated CYP7A1 Inhibition. Front. Genet. 2020, 11, 610496. [Google Scholar] [CrossRef]
- Landry, S.A.; Joosten, S.A. Obstructive sleep apnoea and cholesterol: Independence in context. Respirology 2018, 23, 1092–1093. [Google Scholar] [CrossRef]
- Chopra, S.; Rathore, A.; Younas, H.; Pham, L.V.; Gu, C.; Beselman, A.; Kim, I.-Y.; Wolfe, R.R.; Perin, J.; Polotsky, V.Y.; et al. Obstructive Sleep Apnea Dynamically Increases Nocturnal Plasma Free Fatty Acids, Glucose, and Cortisol During Sleep. J. Clin. Endocrinol. Metab. 2017, 102, 3172–3181. [Google Scholar] [CrossRef]
- Barros, D.; García-Río, F. Obstructive sleep apnea and dyslipidemia: From animal models to clinical evidence. Sleep 2019, 42, zsy236. [Google Scholar] [CrossRef]
- Yoshida, J.; Eguchi, E.; Nagaoka, K.; Ito, T.; Ogino, K. Association of night eating habits with metabolic syndrome and its components: A longitudinal study. BMC Public Health 2018, 18, 1366. [Google Scholar] [CrossRef]
- Bonham, M.P.; Kaias, E.; Zimberg, I.; Leung, G.K.W.; Davis, R.; Sletten, T.L.; Windsor-Aubrey, H.; Huggins, C.E. Effect of Night Time Eating on Postprandial Triglyceride Metabolism in Healthy Adults: A Systematic Literature Review. J. Biol. Rhythms 2019, 34, 119–130. [Google Scholar] [CrossRef]
- Grant, L.K.; Czeisler, C.A.; Lockley, S.W.; Rahman, S.A. Time-of-day and Meal Size Effects on Clinical Lipid Markers. J. Clin. Endocrinol. Metab. 2021, 106, e1373–e1379. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chen, Q.; Pu, Y.; Guo, M.; Jiang, Z.; Huang, W.; Long, Y.; Xu, Y. Skipping breakfast is associated with overweight and obesity: A systematic review and meta-analysis. Obes. Res. Clin. Pract. 2020, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Monzani, A.; Ricotti, R.; Caputo, M.; Solito, A.; Archero, F.; Bellone, S.; Prodam, F. A Systematic Review of the Association of Skipping Breakfast with Weight and Cardiometabolic Risk Factors in Children and Adolescents. What Should We Better Investigate in the Future? Nutrients 2019, 11, 387. [Google Scholar] [CrossRef] [PubMed]
- Ofori-Asenso, R.; Owen, A.J.; Liew, D. Skipping Breakfast and the Risk of Cardiovascular Disease and Death: A Systematic Review of Prospective Cohort Studies in Primary Prevention Settings. J. Cardiovasc. Dev. Dis. 2019, 6, 30. [Google Scholar] [CrossRef]
- Carew, A.S.; Mekary, R.A.; Kirkland, S.; Theou, O.; Siddiqi, F.; Urquhart, R.; Blanchard, C.; Parkash, R.; Bennett, M.; Ivey, K.L.; et al. Prospective Study of Skipping Meals to Lose Weight as a Predictor of Incident Type 2 Diabetes with Potential Modification by Cardiometabolic Risk Factors: The Canadian 1995 Nova Scotia Health Survey. Can. J. Diabetes 2021, 45, 306–312. [Google Scholar] [CrossRef]
- Ahola, A.J.; Mutter, S.; Forsblom, C.; Harjutsalo, V.; Groop, P.H. Meal timing, meal frequency, and breakfast skipping in adult individuals with type 1 diabetes—Associations with glycaemic control. Sci. Rep. 2019, 9, 20063. [Google Scholar] [CrossRef]
- Nas, A.; Mirza, N.; Hägele, F.; Kahlhöfer, J.; Keller, J.; Rising, R.; Kufer, T.A.; Bosy-Westphal, A. Impact of breakfast skipping compared with dinner skipping on regulation of energy balance and metabolic risk. Am. J. Clin. Nutr. 2017, 105, 1351–1361. [Google Scholar] [CrossRef]
- Uzhova, I.; Mullally, D.; Peñalvo, J.L.; Gibney, E.R. Regularity of Breakfast Consumption and Diet: Insights from National Adult Nutrition Survey. Nutrients 2018, 10, 1578. [Google Scholar] [CrossRef]
- Ballon, A.; Neuenschwander, M.; Schlesinger, S. Breakfast Skipping Is Associated with Increased Risk of Type 2 Diabetes among Adults: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. J. Nutr. 2019, 149, 106–113. [Google Scholar] [CrossRef]
- Mekary, R.A. Breakfast Skipping and Type 2 Diabetes: Where Do We Stand? J. Nutr. 2019, 149, 1–3. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Kaji, A.; Sakai, R.; Osaka, T.; Ushigome, E.; Hamaguchi, M.; Yamazaki, M.; Fukui, M. Skipping breakfast is associated with glycemic variability in patients with type 2 diabetes. Nutrition 2020, 71, 110639. [Google Scholar] [CrossRef]
- Mita, T.; Osonoi, Y.; Osonoi, T.; Saito, M.; Nakayama, S.; Someya, Y.; Ishida, H.; Gosho, M.; Watada, H. Breakfast skipping is associated with persistently increased arterial stiffness in patients with type 2 diabetes. BMJ Open Diabetes Res. Care 2020, 8. [Google Scholar] [CrossRef]
- Dong, J.-Y.; Ikehara, S.; Kimura, T.; Cui, M.; Kawanishi, Y.; Ueda, K.; Iso, H.; the Japan Environment and Children’s Study Group. Skipping breakfast before and during early pregnancy and incidence of gestational diabetes mellitus: The Japan Environment and Children’s Study. Am. J. Clin. Nutr. 2020, 111, 829–834. [Google Scholar] [CrossRef]
- Ogata, H.; Hatamoto, Y.; Goto, Y.; Tajiri, E.; Yoshimura, E.; Kiyono, K.; Uehara, Y.; Kawanaka, K.; Omi, N.; Tanaka, H. Association between breakfast skipping and postprandial hyperglycaemia after lunch in healthy young individuals. Br. J. Nutr. 2019, 122, 431–440. [Google Scholar] [CrossRef]
- Azami, Y.; Funakoshi, M.; Matsumoto, H.; Ikota, A.; Ito, K.; Okimoto, H.; Shimizu, N.; Tsujimura, F.; Fukuda, H.; Miyagi, C.; et al. Long working hours and skipping breakfast concomitant with late evening meals are associated with suboptimal glycemic control among young male Japanese patients with type 2 diabetes. J. Diabetes Investig. 2019, 10, 73–83. [Google Scholar] [CrossRef]
- Bonnet, J.P.; Cardel, M.I.; Cellini, J.; Hu, F.B.; Guasch-Ferré, M. Breakfast Skipping, Body Composition, and Cardiometabolic Risk: A Systematic Review and Meta-Analysis of Randomized Trials. Obesity 2020, 28, 1098–1109. [Google Scholar] [CrossRef]
- Lavelle, F.; McGowan, L.; Hollywood, L.; Surgenor, D.; McCloat, A.; Mooney, E.; Caraher, M.; Raats, M.; Dean, M. The development and validation of measures to assess cooking skills and food skills. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 118. [Google Scholar] [CrossRef]
- Rees, J.; Christophersen, C.C.; Lewis, J.R.; Lo, J.; Sambell, R.; Costello, L.; Walker, C.; Byrne, M.F.; Boyce, M.C.; Newton, R.U.; et al. The study protocol for a pseudo-randomised pre-post designed controlled intervention trial to study the effects of a 7-week cooking program on self-efficacy and biomarkers of health: The ECU lifestyle and biomarkers get connected study (ECULABJMOF) including the Jamie’s Ministry of Food WA participant experience. BMC Public Health 2020, 20, 1037. [Google Scholar] [CrossRef]
- Asigbee, F.M.; Davis, J.N.; Markowitz, A.K.; Landry, M.J.; Vandyousefi, S.; Ghaddar, R.; Ranjit, N.; Warren, J.; Berg, A.V.D. The Association between Child Cooking Involvement in Food Preparation and Fruit and Vegetable Intake in a Hispanic Youth Population. Curr. Dev. Nutr. 2020, 4, nzaa028. [Google Scholar] [CrossRef]
- Woumbo, C.Y.; Kuate, D.; Womeni, H.M. Cooking methods affect phytochemical composition and anti-obesity potential of soybean. Heliyon 2017, 3, e00382. [Google Scholar] [CrossRef]
- Gupta, L.; Khandelwal, D. Pragmatic selection of cooking oils. J. Pak. Med. Assoc. 2017, 67, 957–958. [Google Scholar] [PubMed]
- Gatto, N.M.; Martinez, L.C.; Spruijt-Metz, D.; Davis, J.N. LA sprouts randomized controlled nutrition, cooking and gardening programme reduces obesity and metabolic risk in Hispanic/Latino youth. Pediatr. Obes. 2017, 12, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Taillie, L.S.; Poti, J.M. Associations of Cooking with Dietary Intake and Obesity Among Supplemental Nutrition Assistance Program Participants. Am. J. Prev. Med. 2017, 52, S151–S160. [Google Scholar] [CrossRef]
- Jayawardena, R.; Ranasinghe, P.; Wijayabandara, M.; Hills, A.P.; Misra, A. Nutrition Transition and Obesity among Teenagers and Young Adults in South Asia. Curr. Diabetes Rev. 2017, 13, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Gadiraju, T.V.; Patel, Y.; Gaziano, J.M.; Djoussé, L. Fried Food Consumption and Cardiovascular Health: A Review of Current Evidence. Nutrient 2015, 7, 8424–8430. [Google Scholar] [CrossRef] [PubMed]
- Farmer, N.; Lee, L.J.; Powell-Wiley, T.M.; Wallen, G.R. Cooking Frequency and Perception of Diet among US Adults Are Associated with US Healthy and Healthy Mediterranean-Style Dietary Related Classes: A Latent Class Profile Analysis. Nutrients 2020, 12, 3268. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Misra, A. Abdominal obesity and type 2 diabetes in Asian Indians: Dietary strategies including edible oils, cooking practices and sugar intake. Eur. J. Clin. Nutr. 2017, 71, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Vergeer, L.; Vanderlee, L.; Ahmed, M.; Franco-Arellano, B.; Mulligan, C.; Dickinson, K.; L’Abbé, M.R. A comparison of the nutritional quality of products offered by the top packaged food and beverage companies in Canada. BMC Public Health 2020, 20, 650. [Google Scholar] [CrossRef] [PubMed]
- Heindel, J.J.; Blumberg, B. Environmental Obesogens: Mechanisms and Controversies. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 89–106. [Google Scholar] [CrossRef]
- Heras-González, L.; Latorre, J.A.; Martinez-Bebia, M.; Espino, D.; Olea-Serrano, F.; Mariscal-Arcas, M. The relationship of obesity with lifestyle and dietary exposure to endocrine-disrupting chemicals. Food Chem. Toxicol. 2020, 136, 110983. [Google Scholar] [CrossRef]
- Oostenbach, L.H.; Slits, E.; Robinson, E.; Sacks, G. Systematic review of the impact of nutrition claims related to fat, sugar and energy content on food choices and energy intake. BMC Public Health 2019, 19, 1296. [Google Scholar] [CrossRef]
- Daepp, M.I.G.; Gortmaker, S.L.; Wang, Y.C.; Long, M.W.; Kenney, E.L. WIC Food Package Changes: Trends in Childhood Obesity Prevalence. Pediatrics 2019, 143, e20182841. [Google Scholar] [CrossRef]
- Jandorf, S.; Siersma, V.; Køster-Rasmussen, R.; Olivarius, N.d.; Waldorff, F.B. The impact of patients’ involvement in cooking on their mortality and morbidity: A 19-year follow-up of patients diagnosed with type 2 diabetes mellitus. Scand. J. Prim. Health Care 2015, 33, 33–39. [Google Scholar] [CrossRef]
- Polak, R.; Tirosh, A.; Livingston, B.; Pober, D.; Eubanks, J.E.; Silver, J.K.; Minezaki, K.; Loten, R.; Phillips, E.M. Preventing Type 2 Diabetes with Home Cooking: Current Evidence and Future Potential. Curr. Diabetes Rep. 2018, 18, 99. [Google Scholar] [CrossRef]
- Liu, G.; Zong, G.; Hu, F.B.; Willett, W.C.; Eisenberg, D.M.; Sun, Q. Cooking Methods for Red Meats and Risk of Type 2 Diabetes: A Prospective Study of U.S. Women. Diabetes Care 2017, 40, 1041–1049. [Google Scholar] [CrossRef]
- Liu, G.; Zong, G.; Wu, K.; Hu, Y.; Li, Y.; Willett, W.C.; Eisenberg, D.M.; Hu, F.B.; Sun, Q. Meat Cooking Methods and Risk of Type 2 Diabetes: Results From Three Prospective Cohort Studies. Diabetes Care 2018, 41, 1049–1060. [Google Scholar] [CrossRef]
- Zhuang, P.; Mao, L.; Wu, F.; Wang, J.; Jiao, J.; Zhang, Y. Cooking Oil Consumption Is Positively Associated with Risk of Type 2 Diabetes in a Chinese Nationwide Cohort Study. J. Nutr. 2020, 150, 1799–1807. [Google Scholar] [CrossRef]
- Moore, L.V.; Roux, A.V.D.; Nettleton, J.A.; Jacobs, D.R.; Franco, M. Fast-food consumption, diet quality, and neighborhood exposure to fast food: The multi-ethnic study of atherosclerosis. Am. J. Epidemiol. 2009, 170, 29–36. [Google Scholar] [CrossRef]
- Schröder, H.; Fïto, M.; Covas, M.I. Association of fast food consumption with energy intake, diet quality, body mass index and the risk of obesity in a representative Mediterranean population. Br. J. Nutr. 2007, 98, 1274–1280. [Google Scholar] [CrossRef]
- Duffey, K.J.; Gordon-Larsen, P.; Steffen, L.M.; Jacobs, D.R.; Popkin, B.M. Regular consumption from fast food establishments relative to other restaurants is differentially associated with metabolic outcomes in young adults. J. Nutr. 2009, 139, 2113–3118. [Google Scholar] [CrossRef]
- Stender, S.; Dyerberg, J.; Astrup, A. Fast food: Unfriendly and unhealthy. Int. J. Obes. 2007, 31, 887–890. [Google Scholar] [CrossRef]
- Larson, N.I.; Neumark-Sztainer, D.R.; Story, M.T.; Wall, M.M.; Harnack, L.J.; Eisenberg, M.E. Fast food intake: Longitudinal trends during the transition to young adulthood and correlates of intake. J. Adolesc. Health 2008, 43, 79–86. [Google Scholar] [CrossRef]
- Halton, T.L.; Willett, W.C.; Liu, S.; Manson, J.E.; Stampfer, M.J.; Hu, F.B. Potato and french fry consumption and risk of type 2 diabetes in women. Am. J. Clin. Nutr. 2006, 83, 284–290. [Google Scholar] [CrossRef]
- Mazidi, M.; Speakman, J.R. Higher densities of fast-food and full-service restaurants are not associated with obesity prevalence. Am. J. Clin. Nutr. 2017, 106, 603–613. [Google Scholar] [CrossRef]
- Babio, N.; Sorlí, M.; Bulló, M.; Basora, J.; Ibarrola-Jurado, N.; Fernández-Ballart, J.; Martínez-González, M.; Serra-Majem, L.; González-Pérez, R.; Salas-Salvadó, J. Association between red meat consumption and metabolic syndrome in a Mediterranean population at high cardiovascular risk: Cross-sectional and 1-year follow-up assessment. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 200–207. [Google Scholar] [CrossRef]
- Lopez, S.; Bermudez, B.; Ortega-Gomez, A.; Varela, L.M.; Pacheco, Y.M.; Villar, J.; Abia, R.; Muriana, F.J. Effects of meals rich in either monounsaturated or saturated fat on lipid concentrations and on insulin secretion and action in subjects with high fasting triglyceride concentrations. Am. J. Clin. Nutr. 2011, 93, 494–499. [Google Scholar] [CrossRef]
- Sharma, A.; Baldi, A.; Sharma, D.K. Impact of physical activity and cooking oil amongst diabetes with coexisting hypertension patients on economic cost and length of stay: A 1914 patient’s observational study. Int. J. Clin. Pract. 2021, 75, e14163. [Google Scholar] [CrossRef]
- Adavi, M.; Salehi, M.; Roudbari, M. Artificial neural networks versus bivariate logistic regression in prediction diagnosis of patients with hypertension and diabetes. Med. J. Islam. Repub. Iran 2016, 30, 312. [Google Scholar]
- Cahill, L.E.; Pan, A.; Chiuve, S.E.; Sun, Q.; Willett, W.C.; Hu, F.B.; Rimm, E.B. Fried-food consumption and risk of type 2 diabetes and coronary artery disease: A prospective study in 2 cohorts of US women and men. Am. J. Clin. Nutr. 2014, 100, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.; Ribeiro, I.; Jurema-Santos, G.; Nobre, I.; Santos, R.; Rodrigues, C.; Oliveira, K.; Henrique, R.; Ferreira-E-Silva, W.; Araújo, A. Can the Consumption of Ultra-Processed Food Be Associated with Anthropometric Indicators of Obesity and Blood Pressure in Children 7 to 10 Years Old? Foods 2020, 9, 1567. [Google Scholar] [CrossRef]
- Mendonça, R.D.; Lopes, A.C.; Pimenta, A.M.; Gea, A.; Martinez-Gonzalez, M.A.; Bes-Rastrollo, M. Ultra-Processed Food Consumption and the Incidence of Hypertension in a Mediterranean Cohort: The Seguimiento Universidad de Navarra Project. Am. J. Hypertens. 2017, 30, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Alsabieh, M.; Alqahtani, M.; Altamimi, A.; Albasha, A.; Alsulaiman, A.; Alkhamshi, A.; Habib, S.S.; Bashir, S. Fast food consumption and its associations with heart rate, blood pressure, cognitive function and quality of life. Pilot study. Heliyon 2019, 5, e01566. [Google Scholar] [CrossRef] [PubMed]
- Roddy, G.W.; Rosa, R.H.; Viker, K.B.; Holman, B.H.; Hann, C.R.; Krishnan, A.; Gores, G.J.; Bakri, S.J.; Fautsch, M.P. Diet Mimicking Fast Food Causes Structural Changes to the Retina Relevant to Age-Related Macular Degeneration. Curr. Eye Res. 2020, 45, 726–732. [Google Scholar] [CrossRef]
- Hojat, M.; Jahromi, M.K.; Koshkaki, S.R.; Rahmanian, M. Comparison of risk factors of cardiovascular diseases in male and female nurses. J. Educ. Health Promot. 2019, 8, 19. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.; Xue, H.; Wang, H.; Wang, Y. Fast food consumption and its associations with obesity and hypertension among children: Results from the baseline data of the Childhood Obesity Study in China Mega-cities. BMC Public Health 2017, 17, 933. [Google Scholar] [CrossRef]
- Santos, F.S.D.; Dias, M.D.S.; Mintem, G.C.; Oliveira, I.O.; Gigante, D.P. Food processing and cardiometabolic risk factors: A systematic review. Rev. Saude Publ. 2020, 54, 70. [Google Scholar] [CrossRef]
- Poti, J.M.; Braga, B.; Qin, B. Ultra-processed Food Intake and Obesity: What Really Matters for Health-Processing or Nutrient Content? Curr. Obes. Rep. 2017, 6, 420–431. [Google Scholar] [CrossRef]
- Yoon, S.R.; Fogleman, S.K.; Kim, H.; Lee, K.E.; Kim, O.Y. Breakfast Intake Effect on the Association between Fast-Food Consumption and the Risk of Obesity and Dyslipidemia in Korean Adults Aged 20–39 Years Based on the Korea National Health and Nutrition Examination Survey IV 2013–2014. Clin. Nutr. Res. 2020, 9, 107–121. [Google Scholar] [CrossRef]
- Asghari, G.; Yuzbashian, E.; Mirmiran, P.; Mahmoodi, B.; Azizi, F. Fast Food Intake Increases the Incidence of Metabolic Syndrome in Children and Adolescents: Tehran Lipid and Glucose Study. PLoS ONE 2015, 10, e0139641. [Google Scholar] [CrossRef]
- Al-Duais, M.A.; Al-Awthan, Y.S. Prevalence of dyslipidemia among students of a Yemeni University. J. Taibah Univ. Med. Sci. 2019, 14, 163–171. [Google Scholar] [CrossRef]
- Lima, L.R.; Nascimento, L.M.; Gomes, K.R.O.; Martins, M.D.C.C.; Rodrigues, M.T.P.; Frota, K.M.G. Association between ultra-processed food consumption and lipid parameters among adolescents. Cien. Saude Colet. 2020, 25, 4055–4064. (In Portuguese) [Google Scholar] [CrossRef] [PubMed]
- Cupisti, A.; Kovesdy, C.P.; D’Alessandro, C.; Kalantar-Zadeh, K. Dietary Approach to Recurrent or Chronic Hyperkalaemia in Patients with Decreased Kidney Function. Nutrients 2018, 10, 261. [Google Scholar] [CrossRef]
- Arentz, L. Kidney-Friendly Frozen Meals Update: Quick and Convenient Options for Chronic Kidney Disease Patients. J. Ren. Nutr. 2016, 26, e15–e17. [Google Scholar] [CrossRef]
- Madrigal, J.M.; Cedillo-Couvert, E.; Ricardo, A.; Appel, L.J.; Anderson, C.A.; Deo, R.; Hamm, L.L.; Cornish-Zirker, D.; Tan, T.C.; Sha, D.; et al. Neighborhood Food Outlet Access and Dietary Intake among Adults with Chronic Kidney Disease: Results from the Chronic Renal Insufficiency Cohort Study. J. Acad. Nutr. Diet. 2020, 120, 1151–1162.e3. [Google Scholar] [CrossRef]
- Edlich, R.F.; Rodeheaver, G.T.; Thacker, J.G.; Winn, H.R.; Edgerton, M.T. Management of soft tissue injury. Clin. Plast. Surg. 1977, 4, 191–198. [Google Scholar] [CrossRef]
- Martínez-Pineda, M.; Yagüe-Ruiz, C.; Caverni-Muñoz, A.; Vercet-Tormo, A. Reduction of potassium content of green bean pods and chard by culinary processing. Tools for chronic kidney disease. Nefrologia 2016, 36, 427–432. [Google Scholar] [CrossRef]
- Gutiérrez, O.M. Contextual poverty, nutrition, and chronic kidney disease. Adv. Chronic Kidney Dis. 2015, 22, 31–38. [Google Scholar] [CrossRef]
- Wong, K.K.; Velasquez, A.; Powe, N.R.; Tuot, D.S. Association between health literacy and self-care behaviors among patients with chronic kidney disease. BMC Nephrol. 2018, 19, 196. [Google Scholar] [CrossRef]
- Watanabe, M.T.; Araujo, R.M.; Vogt, B.P.; Barretti, P.; Caramori, J.C.T. Most consumed processed foods by patients on hemodialysis: Alert for phosphate-containing additives and the phosphate-to-protein ratio. Clin. Nutr. ESPEN 2016, 14, 37–41. [Google Scholar] [CrossRef]
- Rizzo, G. The Antioxidant Role of Soy and Soy Foods in Human Health. Antioxidants 2020, 9, 635. [Google Scholar] [CrossRef]
- Salehi-Abargouei, A.; Saraf-Bank, S.; Bellissimo, N.; Azadbakht, L. Effects of non-soy legume consumption on C-reactive protein: A systematic review and meta-analysis. Nutrition 2015, 31, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Hematdar, Z.; Ghasemifard, N.; Phishdad, G.; Faghih, S. Substitution of red meat with soybean but not non- soy legumes improves inflammation in patients with type 2 diabetes; a randomized clinical trial. J. Diabetes Metab. Disord. 2018, 17, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpour-Niazi, S.; Mirmiran, P.; Fallah-Ghohroudi, A.; Azizi, F. Non-soya legume-based therapeutic lifestyle change diet reduces inflammatory status in diabetic patients: A randomised cross-over clinical trial. Br. J. Nutr. 2015, 114, 213–219. [Google Scholar] [CrossRef]
- Lopes, A.E.D.S.; Araújo, L.F.; Levy, R.B.; Barreto, S.M.; Giatti, L. Association between consumption of ultra-processed foods and serum C-reactive protein levels: Cross-sectional results from the ELSA-Brasil study. Sao Paulo Med. J. 2019, 137, 169–176. [Google Scholar] [CrossRef]
- Ahmad, M.I.; Ijaz, M.U.; Haq, I.U.; Li, C. The Role of Meat Protein in Generation of Oxidative Stress and Pathophysiology of Metabolic Syndromes. Food Sci. Anim. Resour. 2020, 40, 1–10. [Google Scholar] [CrossRef]
- Pelletier, J.E.; Lytle, L.A.; Laska, M.N. Stress, Health Risk Behaviors, and Weight Status Among Community College Students. Health Educ. Behav. 2016, 43, 139–144. [Google Scholar] [CrossRef]
- Jones, J.; Kauffman, B.Y.; Rosenfield, D.; Smits, J.A.J.; Zvolensky, M.J. Emotion dysregulation and body mass index: The explanatory role of emotional eating among adult smokers. Eat. Behav. 2019, 33, 97–101. [Google Scholar] [CrossRef]
- Rodríguez-Muñoz, P.M.; Carmona-Torres, J.M.; Rodríguez-Borrego, M.A. Influence of tobacco, alcohol consumption, eating habits and physical activity in nursing students. Rev. Lat. Am. Enferm. 2020, 28, e3230. [Google Scholar] [CrossRef]
- Papadopoulou, S.; Hassapidou, M.N.; Katsiki, N.; Fachantidis, P.; Fachantidou, A.I.; Daskalou, E.; Deligiannis, A.P. Relationships between Alcohol Consumption, Smoking Status and Food Habits in Greek Adolescents. Vascular Implications for the Future. Curr. Vasc. Pharmacol. 2017, 15, 167–173. [Google Scholar] [CrossRef]
- Lee, B.; Yi, Y. Smoking, Physical Activity, and Eating Habits among Adolescents. West. J. Nurs. Res. 2016, 38, 27–42. [Google Scholar] [CrossRef]
- Meule, A.; Reichenberger, J.; Blechert, J. Smoking, Stress Eating, and Body Weight: The Moderating Role of Perceived Stress. Subst. Use Misuse 2018, 53, 2152–2156. [Google Scholar] [CrossRef] [PubMed]
- Farris, S.G.; DiBello, A.M.; Bloom, E.L.; Abrantes, A.M. A Confirmatory Factor Analysis of the Smoking and Weight Eating Episodes Test (SWEET). Int. J. Behav. Med. 2018, 25, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Rathod, K.S.; Velmurugan, S.; Ahluwalia, A. A ‘green’ diet-based approach to cardiovascular health? Is inorganic nitrate the answer? Mol. Nutr. Food Res. 2016, 60, 185–202. [Google Scholar] [CrossRef]
- Nasser, S.; Vialichka, V.; Biesiekierska, M.; Balcerczyk, A.; Pirola, L. Effects of ketogenic diet and ketone bodies on the cardiovascular system: Concentration matters. World J. Diabetes 2020, 11, 584–595. [Google Scholar] [CrossRef]
- Tinsley, G.M.; Horne, B.D. Intermittent fasting and cardiovascular disease: Current evidence and unresolved questions. Future Cardiol. 2018, 14, 47–54. [Google Scholar] [CrossRef]
- Mattson, M.P.; Allison, D.B.; Fontana, L.; Harvie, M.; Longo, V.D.; Malaisse, W.J.; Mosley, M.; Notterpek, L.; Ravussin, E.; Scheer, F.A.J.L.; et al. Meal frequency and timing in health and disease. Proc. Natl. Acad. Sci. USA 2014, 111, 16647–16653. [Google Scholar] [CrossRef]
- Planavila, A.; Redondo, I.; Hondares, E.; Vinciguerra, M.; Munts, C.; Iglesias, R.; Gabrielli, L.A.; Sitges, M.; Giralt, M.; Van Bilsen, M.; et al. Fibroblast growth factor 21 protects against cardiac hypertrophy in mice. Nat. Commun. 2013, 4, 2019. [Google Scholar] [CrossRef]
- Chen, Y.; Su, J.; Yan, Y.; Zhao, Q.; Ma, J.; Zhu, M.; He, X.; Zhang, B.; Xu, H.; Yang, X.; et al. Intermittent Fasting Inhibits High-Fat Diet-Induced Atherosclerosis by Ameliorating Hypercholesterolemia and Reducing Monocyte Chemoattraction. Front. Pharmacol. 2021, 12, 719750. [Google Scholar] [CrossRef]
- Dardano, A.; Penno, G.; del Prato, S.; Miccoli, R. Optimal therapy of type 2 diabetes: A controversial challenge. Aging 2014, 6, 187–206. [Google Scholar] [CrossRef]
- Dalgaard, F.; Bondonno, N.P.; Murray, K.; Bondonno, C.P.; Lewis, J.R.; Croft, K.; Kyrø, C.; Gislason, G.; Scalbert, A.; Cassidy, A.; et al. Associations between habitual flavonoid intake and hospital admissions for atherosclerotic cardiovascular disease: A prospective cohort study. Lancet Planet. Health 2019, 3, e450–e459. [Google Scholar] [CrossRef]
- Ferdowsian, H.R.; Barnard, N.D. Effects of plant-based diets on plasma lipids. Am. J. Cardiol. 2009, 104, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Bork, C.S.; Venø, S.K.; Lasota, A.N.; Lundbye-Christensen, S.; Schmidt, E.B. Marine and plant-based. Proc. Nutr. Soc. 2020, 79, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Delaney, C.L.; Smale, M.K.; Miller, M.D. Nutritional Considerations for Peripheral Arterial Disease: A Narrative Review. Nutrients 2019, 11, 1219. [Google Scholar] [CrossRef]
- Pedro-Botet, J.; Pintó, X. LDL-cholesterol: The lower the better. Clin. Investig. Arterioscler. 2019, 31 (Suppl. S2), 16–27, (In English and Spanish). [Google Scholar] [CrossRef]
- du Mont, L.S.; Leclerc, B.; Morgant, M.-C.; Besch, G.; Laubriet, A.; Steinmetz, E.; Rinckenbach, S. Impact of Nutritional State on Critical Limb Ischemia Early Outcomes (DENUCRITICC Study). Ann. Vasc Surg. 2017, 45, 10–15. [Google Scholar] [CrossRef]
- Tuso, P.; Stoll, S.R.; Li, W.W. A plant-based diet, atherogenesis, and coronary artery disease prevention. Perm. J. 2015, 19, 62–67. [Google Scholar] [CrossRef]
- Ruiz-Canela, M.; Estruch, R.; Corella, D.; Salas-Salvadó, J.; Martínez-González, M.A. Association of Mediterranean diet with peripheral artery disease: The PREDIMED randomized trial. JAMA 2014, 311, 415–417. [Google Scholar] [CrossRef]
- Wischmeyer, P.E.; Carli, F.; Evans, D.; Guilbert, S.; Kozar, R.; Pryor, A.; Thiele, R.H.; Everett, S.; Grocott, M.; Gan, T.J.; et al. American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on Nutrition Screening and Therapy within a Surgical Enhanced Recovery Pathway. Anesth. Analg. 2018, 126, 1883–1895. [Google Scholar] [CrossRef]
- Roncal, C.; Martínez-Aguilar, E.; Orbe, J.; Ravassa, S.; Fernandez-Montero, A.; Saenz-Pipaon, G.; Ugarte, A.; de Mendoza, A.E.-H.; Rodriguez, J.A.; Fernández-Alonso, S.; et al. Trimethylamine-N-Oxide (TMAO) Predicts Cardiovascular Mortality in Peripheral Artery Disease. Sci. Rep. 2019, 9, 15580. [Google Scholar] [CrossRef]
- Senthong, V.; Wang, Z.; Fan, Y.; Wu, Y.; Hazen, S.L.; Tang, W.H. Trimethylamine N-Oxide and Mortality Risk in Patients with Peripheral Artery Disease. J. Am. Heart Assoc. 2016, 5, e004237. [Google Scholar] [CrossRef]
- Sagris, M.; Kokkinidis, D.G.; Lempesis, I.G.; Giannopoulos, S.; Rallidis, L.; Mena-Hurtado, C.; Bakoyiannis, C. Nutrition, dietary habits, and weight management to prevent and treat patients with peripheral artery disease. Rev. Cardiovasc. Med. 2020, 21, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Levitan, E.B.; Ahmed, A.; Arnett, D.K.; Polak, J.F.; Hundley, W.G.; Bluemke, D.; Heckbert, S.R.; Jacobs, D.R.; Nettleton, J.A. Mediterranean diet score and left ventricular structure and function: The Multi-Ethnic Study of Atherosclerosis. Am. J. Clin. Nutr. 2016, 104, 595–602. [Google Scholar] [CrossRef]
- Ciccarone, E.; Di Castelnuovo, A.; Salcuni, M.; Siani, A.; Giacco, A.; Donati, M.B.; De Gaetano, G.; Capani, F.; Iacoviello, L.; on behalf of the Gendiabe Investigators. A high-score Mediterranean dietary pattern is associated with a reduced risk of peripheral arterial disease in Italian patients with Type 2 diabetes. J. Thromb. Haemost. 2003, 1, 1744–1752. [Google Scholar] [CrossRef]
- Ruiz-Canela, M.; Martínez-González, M.A. Lifestyle and dietary risk factors for peripheral artery disease. Circ. J. 2014, 78, 553–599. [Google Scholar] [CrossRef]
- Tith, R.M.; Paradis, G.; Potter, B.J.; Low, N.; Healy-Profitós, J.; He, S.; Auger, N. Association of Bulimia Nervosa with Long-term Risk of Cardiovascular Disease and Mortality among Women. JAMA Psychiatry 2020, 77, 44–51. [Google Scholar] [CrossRef]
- Casiero, D.; Frishman, W.H. Cardiovascular complications of eating disorders. Cardiol. Rev. 2006, 14, 227–231. [Google Scholar] [CrossRef]
- Monteleone, P.; Fabrazzo, M.; Martiadis, V.; Fuschino, A.; Serritella, C.; Milici, N.; Maj, M. Opposite changes in circulating adiponectin in women with bulimia nervosa or binge eating disorder. J. Clin. Endocrinol. Metab. 2003, 88, 5387–5391. [Google Scholar] [CrossRef][Green Version]
- Biscetti, F.; Nardella, E.; Cecchini, A.L.; Flex, A.; Landolfi, R. Biomarkers of vascular disease in diabetes: The adipose-immune system cross talk. Intern. Emerg. Med. 2020, 15, 381–393. [Google Scholar] [CrossRef]
- Biscetti, F.; Nardella, E.; Bonadia, N.; Angelini, F.; Pitocco, D.; Santoliquido, A.; Filipponi, M.; Landolfi, R.; Flex, A. Association between plasma omentin-1 levels in type 2 diabetic patients and peripheral artery disease. Cardiovasc. Diabetol. 2019, 18, 74. [Google Scholar] [CrossRef]
- Biscetti, F.; Bonadia, N.; Santini, F.; Angelini, F.; Nardella, E.; Pitocco, D.; Santoliquido, A.; Filipponi, M.; Landolfi, R.; Flex, A. Sortilin levels are associated with peripheral arterial disease in type 2 diabetic subjects. Cardiovasc. Diabetol. 2019, 18, 5. [Google Scholar] [CrossRef]
- Biscetti, F.; Nardella, E.; Rando, M.M.; Cecchini, A.L.; Angelini, F.; Cina, A.; Iezzi, R.; Filipponi, M.; Santoliquido, A.; Pitocco, D.; et al. Association between omentin-1 and major cardiovascular events after lower extremity endovascular revascularization in diabetic patients: A prospective cohort study. Cardiovasc. Diabetol. 2020, 19, 170. [Google Scholar] [CrossRef] [PubMed]
- Biscetti, F.; Nardella, E.; Rando, M.M.; Cecchini, A.L.; Bonadia, N.; Bruno, P.; Angelini, F.; Di Stasi, C.; Contegiacomo, A.; Santoliquido, A.; et al. Sortilin levels correlate with major cardiovascular events of diabetic patients with peripheral artery disease following revascularization: A prospective study. Cardiovasc. Diabetol. 2020, 19, 147. [Google Scholar] [CrossRef] [PubMed]
- Pizzimenti, M.; Meyer, A.; Charles, A.L.; Giannini, M.; Chakfé, N.; Lejay, A.; Geny, B. Sarcopenia and peripheral arterial disease: A systematic review. J. Cachexia Sarcopenia Muscle 2020, 11, 866–886. [Google Scholar] [CrossRef]
- Aziz, M.; Ali, S.S.; Das, S.; Younus, A.; Malik, R.; Latif, M.A.; Humayun, C.; Anugula, D.; Abbas, G.; Salami, J.; et al. Association of Subjective and Objective Sleep Duration as well as Sleep Quality with Non-Invasive Markers of Sub-Clinical Cardiovascular Disease (CVD): A Systematic Review. J. Atheroscler. Thromb. 2017, 24, 208–226. [Google Scholar] [CrossRef]
- Pizarro, C.; Schaefer, C.; Kimeu, I.; Pingel, S.; Horlbeck, F.; Tuleta, I.; Nickenig, G.; Skowasch, D. Underdiagnosis of Obstructive Sleep Apnoea in Peripheral Arterial Disease. Respiration 2015, 89, 214–220. [Google Scholar] [CrossRef]
- Xia, Y.; You, K.; Xiong, Y.; Jiang, H. Sleep-Disordered Breathing and Peripheral Arterial Disease: Current Evidence. Ear Nose Throat J. 2021, 100, 185–191. [Google Scholar] [CrossRef]
- Szymanski, F.M.; Gorko, D.; Platek, A.E.; Ostrowski, T.; Celejewski, K.; Chudzinski, W.; Szymanska, A.; Stepkowski, K.; Rys-Czaporowska, A.; Semczuk-Kaczmarek, K.; et al. Prevalence of obstructive sleep apnea in patients with peripheral arterial diseases. Sleep Breath 2020, 24, 1035–1041. [Google Scholar] [CrossRef]
- Huang, S.; Sun, H.; Yu, J.; Shi, H.; Ren, L.; He, Y.; Zhang, M.; Peng, H.; Guo, H. The Interaction between Self-Reported Sleep Duration and Physical Activity on Peripheral Artery Disease in Chinese Adults: A Cross-Sectional Analysis in the Tianning Cohort Study. Risk Manag Healthc Policy 2021, 14, 4063–4072. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Zhao, L.; Wu, G. Association between sleep-disordered breathing and lower extremity artery disease: A meta-analysis. Sleep Breath 2021, 25, 227–236. [Google Scholar] [CrossRef]
- Rich, K. The connection between obstructive sleep apnea and peripheral artery disease. J. Vasc. Nurs. 2020, 38, 195–197. [Google Scholar] [CrossRef]
- Szymański, F.M.; Gałązka, Z.; Platek, A.E.; Górko, D.; Ostrowski, T.; Adamkiewicz, K.; Łęgosz, P.; Ryś, A.; Semczuk-Kaczmarek, K.; Celejewski, K.; et al. Peripheral ARtery Atherosclerotic DIsease and SlEep disordered breathing (PARADISE) trial—Protocol for an observational cohort study. Kardiol. Pol. 2017, 75, 1332–1338. [Google Scholar] [CrossRef] [PubMed]
- Schahab, N.; Sudan, S.; Schaefer, C.; Tiyerili, V.; Steinmetz, M.; Nickenig, G.; Skowasch, D.; Pizarro, C. Sleep apnoea is common in severe peripheral arterial disease. PLoS ONE 2017, 12, e018173. [Google Scholar] [CrossRef] [PubMed]
- Utriainen, K.T.; Airaksinen, J.K.; Polo, O.; Laitio, R.; Pietilä, M.J.; Scheinin, H.; Vahlberg, T.; Leino, K.A.; Kentala, E.S.; Jalonen, J.R.; et al. Sleep apnoea is associated with major cardiac events in peripheral arterial disease. Eur. Respir. J. 2014, 43, 1652–1660. [Google Scholar] [CrossRef]
- Jelani, Q.-U.; Mena-Hurtado, C.; Gosch, K.; Mohammed, M.; Labrosciano, C.; Regan, C.; Scierka, L.E.; Spertus, J.A.; Nagpal, S.; Smolderen, K.G. Association of sleep apnea with outcomes in peripheral artery disease: Insights from the PORTRAIT study. PLoS ONE 2021, 16, e0256933. [Google Scholar] [CrossRef]
- Le, N.A. Lipoprotein-associated oxidative stress: A new twist to the postprandial hypothesis. Int. J. Mol. Sci. 2014, 16, 401–419. [Google Scholar] [CrossRef]
- Lupattelli, G.; Pasqualini, L.; Siepi, D.; Marchesi, S.; Pirro, M.; Vaudo, G.; Ciuffetti, G.; Mannarino, E. Increased postprandial lipemia in patients with normolipemic peripheral arterial disease. Am. Heart J. 2002, 143, 733–738. [Google Scholar] [CrossRef]
- Valdivielso, P.; Hidalgo, A.; Rioja, J.; Aguilar, I.; Ariza, M.J.; González-Alegre, T.; González-Santos, P. Smoking and postprandial triglycerides are associated with vascular disease in patients with type 2 diabetes. Atherosclerosis 2007, 194, 391–396. [Google Scholar] [CrossRef]
- Jakubowicz, D.; Wainstein, J.; Landau, Z.; Raz, I.; Ahren, B.; Chapnik, N.; Ganz, T.; Menaged, M.; Barnea, M.; Bar-Dayan, Y.; et al. Influences of Breakfast on Clock Gene Expression and Postprandial Glycemia in Healthy Individuals and Individuals with Diabetes: A Randomized Clinical Trial. Diabetes Care 2017, 40, 1573–1579. [Google Scholar] [CrossRef]
- Gordin, D.; Saraheimo, M.; Tuomikangas, J.; Soro-Paavonen, A.; Forsblom, C.; Paavonen, K.; Steckel-Hamann, B.; Vandenhende, F.; Nicolaou, L.; Pavo, I.; et al. Influence of Postprandial Hyperglycemic Conditions on Arterial Stiffness in Patients with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 1134–1143. [Google Scholar] [CrossRef]
- Huang, D.; Refaat, M.; Mohammedi, K.; Jayyousi, A.; al Suwaidi, J.; Khalil, C.A. Macrovascular Complications in Patients with Diabetes and Prediabetes. Biomed. Res. Int. 2017, 2017, 7839101. [Google Scholar] [CrossRef]
- Yosefy, C. Hyperglycaemia and its relation to cardiovascular morbidity and mortality: Has it been resolved? Acta Diabetol. 2003, 40 (Suppl. S2), S380–S388. [Google Scholar] [CrossRef]
- Yamagishi, S.I.; Nakamura, K.; Matsui, T.; Ueda, S.I.; Imaizumi, T. Role of postprandial hyperglycaemia in cardiovascular disease in diabetes. Int. J. Clin. Pract. 2007, 61, 83–87. [Google Scholar] [CrossRef]
- Smiljanec, K.; Mbakwe, A.U.; Ramos-Gonzalez, M.; Mesbah, C.; Lennon, S.L. Associations of Ultra-Processed and Unprocessed/Minimally Processed Food Consumption with Peripheral and Central Hemodynamics, and Arterial Stiffness in Young Healthy Adults. Nutrients 2020, 12, 3229. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.; Felício, M.B.; Caldas, A.P.S.; Hermsdorff, H.H.; Torreglosa, C.R.; Bersch-Ferreira, C.; Weber, B.; Marcadenti, A.; Bressan, J. Ultra-processed foods consumption is associated with cardiovascular disease and cardiometabolic risk factors in Brazilians with established cardiovascular events. Int. J. Food Sci. Nutr. 2021, 72, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Hicks, C.W.; Yang, C.; Ndumele, C.E.; Folsom, A.R.; Heiss, G.; Black, J.H.; Selvin, E.; Matsushita, K. Associations of Obesity with Incident Hospitalization Related to Peripheral Artery Disease and Critical Limb Ischemia in the ARIC Study. J. Am. Heart Assoc. 2018, 7, e008644. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.; Hobohm, L.; Geyer, M.; Münzel, T.; Lavie, C.J.; Ostad, M.A.; Espinola-Klein, C. Obesity paradox in peripheral artery disease. Clin. Nutr. 2019, 38, 2269–2276. [Google Scholar] [CrossRef] [PubMed]
- Selçuk, N.; Albeyoğlu, Ş.; Bastopcu, M.; Selçuk, İ.; Barutca, H.; Şahan, H. Sarcopenia is a risk factor for major adverse cardiac events after surgical revascularization for critical limb ischemia. Vascular 2022, 17085381211059383. [Google Scholar] [CrossRef]
- Morisaki, K.; Furuyama, T.; Matsubara, Y.; Inoue, K.; Kurose, S.; Yoshino, S.; Nakayama, K.; Yamashita, S.; Yoshiya, K.; Mori, M. Thigh sarcopenia and hypoalbuminemia predict impaired overall survival after infrainguinal revascularization in patients with critical limb ischemia. Vascular 2020, 28, 542–547. [Google Scholar] [CrossRef]
- Sivaharan, A.; Boylan, L.; Witham, M.D.; Nandhra, S.; Centre, N.V. Sarcopenia in patients undergoing lower limb bypass surgery is associated with higher mortality and major amputation rates. Ann. Vasc. Surg. 2021, 75, 227–236. [Google Scholar] [CrossRef]
- Matsubara, Y.; Matsumoto, T.; Inoue, K.; Matsuda, D.; Yoshiga, R.; Yoshiya, K.; Furuyama, T.; Maehara, Y. Sarcopenia is a risk factor for cardiovascular events experienced by patients with critical limb ischemia. J. Vasc. Surg. 2017, 65, 1390–1397. [Google Scholar] [CrossRef]
- Matsubara, Y.; Matsumoto, T.; Aoyagi, Y.; Tanaka, S.; Okadome, J.; Morisaki, K.; Shirabe, K.; Maehara, Y. Sarcopenia is a prognostic factor for overall survival in patients with critical limb ischemia. J. Vasc. Surg. 2015, 61, 945–950. [Google Scholar] [CrossRef]
- Earl, E.; Wong, R.; Payne, M.W. Low vitamin B12 in patients on admission to an amputation rehabilitation unit: A retrospective study. Nutr. Clin. Pract. 2015, 30, 122–127. [Google Scholar] [CrossRef]
- Chua, G.T.; Chan, Y.C.; Cheng, S.W. Vitamin D status and peripheral arterial disease: Evidence so far. Vasc. Health Risk. Manag. 2011, 7, 671–675. [Google Scholar] [CrossRef]
- Chao, J.Y.; Li, C.Y.; Wang, M.C.; Yang, Y.H.K. The use of activated vitamin D and risks of hospitalization for infection and amputation in incident hemodialysis patients in Taiwan: A nationwide population-based cohort study. BMC Nephrol. 2020, 21, 331. [Google Scholar] [CrossRef]
- Gunton, J.E.; Girgis, C.M.; Lau, T.; Vicaretti, M.; Begg, L.; Flood, V. Vitamin C improves healing of foot ulcers: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2021, 126, 1451–1458. [Google Scholar] [CrossRef]
- Brookes, J.D.L.; Jaya, J.S.; Tran, H.; Vaska, A.; Werner-Gibbings, K.; D’Mello, A.C.; Wong, J.; Lemoh, C.N.; Saunder, A.C.; Yii, M.K. Broad-Ranging Nutritional Deficiencies Predict Amputation in Diabetic Foot Ulcers. Int. J. Low Extrem. Wounds 2020, 19, 27–33. [Google Scholar] [CrossRef]
- Moore, Z.E.; Corcoran, M.A.; Patton, D. Nutritional interventions for treating foot ulcers in people with diabetes. Cochrane Database Syst. Rev. 2020, 7, CD011378. [Google Scholar] [CrossRef]
- Ito, N.; Hishikari, K.; Abe, F.; Kanno, Y.; Iiya, M.; Murai, T.; Hikita, H.; Takahashi, A.; Yonetsu, T.; Sasano, T. Eicosapentaenoic acid levels predict prognosis of peripheral artery disease caused by aortoiliac artery lesions. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 263–268. [Google Scholar] [CrossRef]
- Hishikari, K.; Kimura, S.; Yamakami, Y.; Kojima, K.; Sagawa, Y.; Otani, H.; Sugiyama, T.; Kuwahara, T.; Hikita, H.; Takahashi, A.; et al. The prognostic value of the serum eicosapentaenoic acid to arachidonic acid ratio in relation to clinical outcomes after endovascular therapy in patients with peripheral artery disease caused by femoropopliteal artery lesions. Atherosclerosis 2015, 239, 583–588. [Google Scholar] [CrossRef]
- Czupryniak, L.; Joshi, S.R.; Gogtay, J.A.; Lopez, M. Effect of micronized fenofibrate on microvascular complications of type 2 diabetes: A systematic review. Expert. Opin. Pharmacother. 2016, 17, 1463–1473. [Google Scholar] [CrossRef]
- Rajamani, K.; Colman, P.G.; Li, L.P.; Best, J.D.; Voysey, M.; D’Emden, M.C.; Laakso, M.; Baker, J.R.; Keech, A.C. Effect of fenofibrate on amputation events in people with type 2 diabetes mellitus (FIELD study): A prespecified analysis of a randomised controlled trial. Lancet 2009, 373, 1780–1788. [Google Scholar] [CrossRef]
- Ansquer, J.C.; Foucher, C.; Aubonnet, P.; le Malicot, K. Fibrates and microvascular complications in diabetes—Insight from the FIELD study. Curr. Pharm. Des. 2009, 15, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P. Preventing diabetic complications: A primary care perspective. Diabetes Res. Clin. Pract. 2009, 84, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M. After the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study: Implications for fenofibrate. Am. J. Cardiol. 2008, 102, 34L–40L. [Google Scholar] [CrossRef]
- Fanaroff, A.C.; Yang, L.; Nathan, A.S.; Khatana, S.A.M.; Julien, H.; Wang, T.Y.; Armstrong, E.J.; Treat-Jacobson, D.; Glaser, J.D.; Wang, G.; et al. Geographic and Socioeconomic Disparities in Major Lower Extremity Amputation Rates in Metropolitan Areas. J. Am. Heart Assoc. 2021, 10, e021456. [Google Scholar] [CrossRef]
- Hughes, K.; Mota, L.; Nunez, M.; Sehgal, N.; Ortega, G. The effect of income and insurance on the likelihood of major leg amputation. J. Vasc. Surg. 2019, 70, 580–587. [Google Scholar] [CrossRef]
- Belon, H.P.; Vigoda, D.F. Emotional adaptation to limb loss. Phys. Med. Rehabil. Clin. N. Am. 2014, 25, 53–74. [Google Scholar] [CrossRef]
- Andrews, K.L.; Dib, M.; Shives, T.C.; Hoskin, T.L.; Liedl, D.A.; Boon, A.J. The effect of obstructive sleep apnea on amputation site healing. J. Vasc. Nurs. 2012, 30, 61–63. [Google Scholar] [CrossRef]
- Holla, J.F.M.; Akker, L.E.V.D.; Dadema, T.; de Groot, S.; Tieland, M.; Weijs, P.J.M.; Deutekom, M.; on behalf of the WHEELS-study group. Determinants of dietary behaviour in wheelchair users with spinal cord injury or lower limb amputation: Perspectives of rehabilitation professionals and wheelchair users. PLoS ONE 2020, 15, e0228465. [Google Scholar] [CrossRef]
- Bauersachs, R.; Zeymer, U.; Brière, J.B.; Marre, C.; Bowrin, K.; Huelsebeck, M. Burden of Coronary Artery Disease and Peripheral Artery Disease: A Literature Review. Cardiovasc. Ther. 2019, 2019, 8295054. [Google Scholar] [CrossRef]
- Unkart, J.T.; Allison, M.A.; Araneta, M.R.G.; Ix, J.H.; Matsushita, K.; Criqui, M.H. Burden of Peripheral Artery Disease on Mortality and Incident Cardiovascular Events. Am. J. Epidemiol. 2020, 189, 951–962. [Google Scholar] [CrossRef]
- Golledge, J.; Leicht, A.S.; Yip, L.; Rowbotham, S.E.; Pinchbeck, J.; Jenkins, J.S.; Clapperton, R.; Dally-Watkins, M.; Singh, M.A.F.; Mavros, Y.; et al. Relationship between Disease Specific Quality of Life Measures, Physical Performance, and Activity in People with Intermittent Claudication Caused by Peripheral Artery Disease. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 957–964. [Google Scholar] [CrossRef]
- Agnelli, G.; Belch, J.J.F.; Baumgartner, I.; Giovas, P.; Hoffmann, U. Morbidity and mortality associated with atherosclerotic peripheral artery disease: A systematic review. Atherosclerosis 2020, 293, 94–100. [Google Scholar] [CrossRef]
- Biscetti, F.; Nardella, E.; Rando, M.; Cecchini, A.; Gasbarrini, A.; Massetti, M.; Flex, A. Outcomes of Lower Extremity Endovascular Revascularization: Potential Predictors and Prevention Strategies. Int. J. Mol. Sci. 2021, 22, 2002. [Google Scholar] [CrossRef]
- Heffron, S.P.; Dwivedi, A.; Rockman, C.B.; Xia, Y.; Guo, Y.; Zhong, J.; Berger, J.S. Body mass index and peripheral artery disease. Atherosclerosis 2020, 292, 31–36. [Google Scholar] [CrossRef]
- Umuerri, E.M.; Obasohan, A.O. Obesity Indices and Peripheral Artery Disease Measured by Ankle Brachial Index in Nigerian Out-Patients. West Afr. J. Med. 2018, 35, 3–8, (In English and French). [Google Scholar]
- Huang, Y.; Xu, M.; Xie, L.; Wang, T.; Huang, X.; Lv, X.; Chen, Y.; Ding, L.; Lin, L.; Wang, W.; et al. Obesity and peripheral arterial disease: A Mendelian Randomization analysis. Atherosclerosis 2016, 247, 218–224. [Google Scholar] [CrossRef]
- Swenty, C.F.; Hall, M. Peripheral Vascular Disease. Home Healthc. Now 2020, 38, 294–301. [Google Scholar] [CrossRef]
- Moussa, O.; Ardissino, M.; Muttoni, S.; Faraj, A.; Tang, A.; Khan, O.; Collins, P.; Jaffer, U.; Purkayastha, S. Long-term incidence and outcomes of obesity-related peripheral vascular disease after bariatric surgery. Langenbecks Arch. Surg. 2021, 406, 1029–1036. [Google Scholar] [CrossRef]
- Kanegusuku, H.; Cucato, G.G.; Domiciano, R.M.; Longano, P.; Puech-Leao, P.; Wolosker, N.; Ritti-Dias, R.M.; Correia, M.A. Impact of obesity on walking capacity and cardiovascular parameters in patients with peripheral artery disease: A cross-sectional study. J. Vasc. Nurs. 2020, 38, 66–71. [Google Scholar] [CrossRef]
- Taylor, B.V.; Oudit, G.Y.; Kalman, P.G.; Liu, P. Clinical and pathophysiological effects of active and passive smoking on the cardiovascular system. Can. J. Cardiol. 1998, 14, 1129–1139. [Google Scholar] [PubMed]
- Willigendael, E.M.; Teijink, J.A.; Bartelink, M.-L.; Kuiken, B.W.; Boiten, J.; Moll, F.L.; Büller, H.R.; Prins, M.H. Influence of smoking on incidence and prevalence of peripheral arterial disease. J. Vasc. Surg. 2004, 40, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S. The association of active and passive smoking with peripheral arterial disease: Results from NHANES 1999–2004. Angiology 2009, 60, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Mackay, D.F.; Pell, J.P. Association between level of exposure to secondhand smoke and peripheral arterial disease: Cross-sectional study of 5686 never smokers. Atherosclerosis 2013, 229, 273–376. [Google Scholar] [CrossRef]
- Ngu, N.L.; McEvoy, M. Environmental tobacco smoke and peripheral arterial disease: A review. Atherosclerosis 2017, 266, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Pirri, D.; Fragiadaki, M.; Evans, P.C. Diabetic atherosclerosis: Is there a role for the hypoxia-inducible factors? Biosci. Rep. 2020, 40, BSR20200026. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Ohman, E.M.; Hirsch, A.T.; Ikeda, Y.; Mas, J.-L.; Goto, S.; Liau, C.-S.; Richard, A.J.; Röther, J.; et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA 2006, 295, 180–189. [Google Scholar] [CrossRef]
- Fowkes, F.G.; Aboyans, V.; Fowkes, F.J.; McDermott, M.M.; Sampson, U.K.; Criqui, M.H. Peripheral artery disease: Epidemiology and global perspectives. Nat. Rev. Cardiol. 2017, 14, 156–170. [Google Scholar] [CrossRef]
- Meijer, W.T.; Grobbee, D.E.; Hunink, M.G.; Hofman, A.; Hoes, A.W. Determinants of peripheral arterial disease in the elderly: The Rotterdam study. Arch. Intern. Med. 2000, 160, 2934–2938. [Google Scholar] [CrossRef]
- Lin, T.Y.; Chou, H.H.; Huang, H.L.; Hung, S.C. Indoxyl Sulfate and Incident Peripheral Artery Disease in Hemodialysis Patients. Toxins 2020, 12, 696. [Google Scholar] [CrossRef]
- Firnhaber, J.M.; Powell, C.S. Lower Extremity Peripheral Artery Disease: Diagnosis and Treatment. Am. Fam. Physician 2019, 99, 362–369. [Google Scholar]
- Matsushita, K.; Ballew, S.; Coresh, J.; Arima, H.; Ärnlöv, J.; Cirillo, M.; Ebert, N.; Hiramoto, J.S.; Kimm, H.; Shlipak, M.G.; et al. Measures of chronic kidney disease and risk of incident peripheral artery disease: A collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2017, 5, 718–728. [Google Scholar] [CrossRef]
- Bourrier, M.; Ferguson, T.W.; Embil, J.M.; Rigatto, C.; Komenda, P.; Tangri, N. Peripheral Artery Disease: Its Adverse Consequences With and Without CKD. Am. J. Kidney Dis. 2020, 75, 705–712. [Google Scholar] [CrossRef]
- Smilowitz, N.R.; Bhandari, N.; Berger, J.S. Chronic kidney disease and outcomes of lower extremity revascularization for peripheral artery disease. Atherosclerosis 2020, 297, 149–156. [Google Scholar] [CrossRef]
- Bosevski, M. Peripheral Arterial Disease and Chronic Kidney Disease. Pril 2017, 38, 29–33. [Google Scholar] [CrossRef]
- Wattanakit, K.; Folsom, A.R.; Selvin, E.; Coresh, J.; Hirsch, A.T.; Weatherley, B.D. Kidney function and risk of peripheral arterial disease: Results from the Atherosclerosis Risk in Communities (ARIC) Study. J. Am. Soc. Nephrol. 2007, 18, 629–636. [Google Scholar] [CrossRef]
- Arinze, N.V.; Gregory, A.; Francis, J.M.; Farber, A.; Chitalia, V.C. Unique aspects of peripheral artery disease in patients with chronic kidney disease. Vasc. Med. 2019, 24, 251–260. [Google Scholar] [CrossRef]
- Lu, Y.; Ballew, S.H.; Tanaka, H.; Szklo, M.; Heiss, G.; Coresh, J.; Matsushita, K. 2017 ACC/AHA blood pressure classification and incident peripheral artery disease: The Atherosclerosis Risk in Communities (ARIC) Study. Eur. J. Prev. Cardiol. 2020, 27, 51–59. [Google Scholar] [CrossRef]
- Yousefian, M.; Shakour, N.; Hosseinzadeh, H.; Hayes, A.W.; Hadizadeh, F.; Karimi, G. The natural phenolic compounds as modulators of NADPH oxidases in hypertension. Phytomedicine 2019, 55, 200–213. [Google Scholar] [CrossRef]
- Manapurathe, D.T.; Moxon, J.V.; Krishna, S.; Rowbotham, S.; Quigley, F.; Jenkins, J.; Bourke, M.; Bourke, B.; Jones, R.E.; Golledge, J. Cohort Study Examining the Association between Blood Pressure and Cardiovascular Events in Patients with Peripheral Artery Disease. J. Am. Heart Assoc. 2019, 8, e010748. [Google Scholar] [CrossRef]
- Ya’qoub, L.; Peri-Okonny, P.; Wang, J.; Patel, K.K.; Stone, N.; Smolderen, K. Blood pressure management in patients with symptomatic peripheral artery disease: Insights from the PORTRAIT registry. Eur. Heart J. Qual Care Clin. Outcomes 2019, 5, 79–81. [Google Scholar] [CrossRef]
- Ridker, P.M.; Stampfer, M.J.; Rifai, N. Novel risk factors for systemic atherosclerosis: A comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA 2001, 285, 2481–2485. [Google Scholar] [CrossRef]
- Steinberg, D. In celebration of the 100th anniversary of the lipid hypothesis of atherosclerosis. J. Lipid Res. 2013, 54, 2946–2949. [Google Scholar] [CrossRef]
- Lewington, S.; Whitlock, G.; Clarke, R.; Sherliker, P.; Emberson, J.; Halsey, J.; Qizilbash, N.; Peto, R.; Collins, R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: A meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 2007, 370, 1829–1839. [Google Scholar] [CrossRef]
- di Angelantonio, E.; Sarwar, N.; Perry, P.; Kaptoge, S.; Ray, K.K.; Thompson, A.; Wood, A.M.; Lewington, S.; Sattar, N.; Packard, C.J.; et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009, 302, 1993–2000. [Google Scholar] [CrossRef]
- Bellussi, L.; Bernocchi, D.; Ciferri, G.; Manini, G.; Passali, D. Sobrerol in the treatment of secretory otitis media in childhood. J. Int. Med. Res. 1989, 17, 277–286. [Google Scholar] [CrossRef]
- Barani, J.; Nilsson, J.A.; Mattiasson, I.; Lindblad, B.; Gottsäter, A. Inflammatory mediators are associated with 1-year mortality in critical limb ischemia. J. Vasc. Surg. 2005, 42, 75–80. [Google Scholar] [CrossRef]
- Biscetti, F.; Ferraro, P.M.; Hiatt, W.R.; Angelini, F.; Nardella, E.; Cecchini, A.L.; Santoliquido, A.; Pitocco, D.; Landolfi, R.; Flex, A. Inflammatory Cytokines Associated with Failure of Lower-Extremity Endovascular Revascularization (LER): A Prospective Study of a Population with Diabetes. Diabetes Care 2019, 42, 1939–1945. [Google Scholar] [CrossRef]
- Stone, P.A.; Schlarb, H.; Campbell, J.E.; Williams, D.; Thompson, S.N.; John, M.; Campbell, J.R.; AbuRahma, A.F. C-reactive protein and brain natriuretic peptide as predictors of adverse events after lower extremity endovascular revascularization. J. Vasc. Surg. 2014, 60, 652–660. [Google Scholar] [CrossRef]
- Shameer, K.; Dow, G.; Glicksberg, B.S.; Johnson, K.W.; Ze, Y.; Tomlinson, M.S.; Readhead, B.; Dudley, J.T.; Kullo, I.J. A Network-Biology Informed Computational Drug Repositioning Strategy to Target Disease Risk Trajectories and Comorbidities of Peripheral Artery Disease. AMIA Jt. Summits Transl. Sci. Proc. 2018, 2017, 108–117. [Google Scholar]
- Maddatu, J.; Anderson-Baucum, E.; Evans-Molina, C. Smoking and the risk of type 2 diabetes. Transl. Res. 2017, 184, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.J.; Raggi, P.; Wolf, M.; Gold, A.M.; Chertow, G.M.; Roe, M.T. Targeting Vascular Calcification in Chronic Kidney Disease. JACC Basic Transl. Sci. 2020, 5, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Biscetti, F.; Giovannini, S.; Straface, G.; Bertucci, F.; Angelini, F.; Porreca, C.; Landolfi, R.; Flex, A. RANK/RANKL/OPG pathway: Genetic association with history of ischemic stroke in Italian population. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4574–4580. [Google Scholar] [PubMed]
- Conte, M.S.; Bradbury, A.W.; Kolh, P.; White, J.V.; Dick, F.; Fitridge, R.; Mills, J.L.; Ricco, J.-B.; Suresh, K.R.; Murad, M.H.; et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J. Vasc. Surg. 2019, 69, 3S–125S.e40. [Google Scholar] [CrossRef]
- Kithcart, A.P.; Beckman, J.A. ACC/AHA Versus ESC Guidelines for Diagnosis and Management of Peripheral Artery Disease: JACC Guideline Comparison. J. Am. Coll Cardiol. 2018, 72, 2789–2801. [Google Scholar] [CrossRef]
- Parvar, S.L.; Fitridge, R.; Dawson, J.; Nicholls, S.J. Medical and lifestyle management of peripheral arterial disease. J. Vasc. Surg. 2018, 68, 1595–1606. [Google Scholar] [CrossRef]
- Bevan, G.H.; Solaru, K.T.W. Evidence-Based Medical Management of Peripheral Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 541–553. [Google Scholar] [CrossRef]
- Ajala, O.N.; Everett, B.M. Targeting Inflammation to Reduce Residual Cardiovascular Risk. Curr. Atheroscler. Rep. 2020, 22, 66. [Google Scholar] [CrossRef]
- Signorelli, S.S.; Katsiki, N. Oxidative Stress and Inflammation: Their Role in the Pathogenesis of Peripheral Artery Disease with or Without Type 2 Diabetes Mellitus. Curr. Vasc. Pharmacol. 2018, 6, 547–554. [Google Scholar] [CrossRef]
- Trinquart, L.; Johns, D.M.; Galea, S. Why do we think we know what we know? A metaknowledge analysis of the salt controversy. Int. J. Epidemiol. 2016, 45, 251–260. [Google Scholar] [CrossRef]
- GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef]
- Afshin, A.; Penalvo, J.; Del Gobbo, L.; Kashaf, M.; Micha, R.; Morrish, K.; Pearson-Stuttard, J.; Rehm, C.; Shangguan, S.; Smith, J.D.; et al. CVD Prevention Through Policy: A Review of Mass Media, Food/Menu Labeling, Taxation/Subsidies, Built Environment, School Procurement, Worksite Wellness, and Marketing Standards to Improve Diet. Curr. Cardiol. Rep. 2015, 17, 98. [Google Scholar] [CrossRef]
- Cobiac, L.J.; Veerman, L.; Vos, T. The role of cost-effectiveness analysis in developing nutrition policy. Annu. Rev. Nutr. 2013, 33, 373–393. [Google Scholar] [CrossRef]
- Bibbins-Domingo, K.; Chertow, G.M.; Coxson, P.G.; Moran, A.; Lightwood, J.M.; Pletcher, M.J.; Goldman, L. Projected effect of dietary salt reductions on future cardiovascular disease. N. Engl. J. Med. 2010, 362, 590–599. [Google Scholar] [CrossRef]
- Smith-Spangler, C.M.; Juusola, J.L.; Enns, E.A.; Owens, D.K.; Garber, A.M. Population strategies to decrease sodium intake and the burden of cardiovascular disease: A cost-effectiveness analysis. Ann. Intern. Med. 2010, 152, 481–487. [Google Scholar] [CrossRef]
- Owen, L.; Morgan, A.; Fischer, A.; Ellis, S.; Hoy, A.; Kelly, M.P. The cost-effectiveness of public health interventions. J. Public Health 2012, 34, 37–45. [Google Scholar] [CrossRef]
- AAnand, S.S.; Hawkes, C.; de Souza, R.J.; Mente, A.; Dehghan, M.; Nugent, R.; Zulyniak, M.A.; Weis, T.; Bernstein, A.M.; Krauss, R.M. Food Consumption and its Impact on Cardiovascular Disease: Importance of Solutions Focused on the Globalized Food System: A Report from the Workshop Convened by the World Heart Federation. J. Am. Coll. Cardiol. 2015, 66, 1590–1614. [Google Scholar] [CrossRef]
- Micha, R.; Shulkin, M.L.; Penalvo, J.L.; Khatibzadeh, S.; Singh, G.M.; Rao, M.; Fahimi, S.; Powles, J.; Mozaffarian, D. Etiologic effects and optimal intakes of foods and nutrients for risk of cardiovascular diseases and diabetes: Systematic reviews and meta-analyses from the Nutrition and Chronic Diseases Expert Group (NutriCoDE). PLoS ONE 2017, 12, e0175149. [Google Scholar] [CrossRef]
- Micha, R.; Kalantarian, S.; Wirojratana, P.; Byers, T.; Danaei, G.; Elmadfa, I.; Ding, E.; Giovannucci, E.; Powles, J.; Smith-Warner, S.; et al. Estimating the global and regional burden of suboptimal nutrition on chronic disease: Methods and inputs to the analysis. Eur. J. Clin. Nutr. 2012, 66, 119–129. [Google Scholar] [CrossRef]
- Colditz, G.A. Overview of the epidemiology methods and applications: Strengths and limitations of observational study designs. Crit. Rev. Food Sci. Nutr. 2010, 50 (Suppl. S1), 10–12. [Google Scholar] [CrossRef]
- Nishida, C.; Uauy, R.; Kumanyika, S.; Shetty, P. The joint WHO/FAO expert consultation on diet, nutrition and the prevention of chronic diseases: Process, product and policy implications. Public Health Nutr. 2004, 7, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 2010, 121, 586–613. [Google Scholar] [CrossRef]
- McGuire, S.U.S. Department of Agriculture and U.S. Department of Health and Human Services, Dietary Guidelines for Americans, 2010. 7th Edition, Washington, DC: U.S. Government Printing Office, January 2011. Adv. Nutr. 2011, 2, 293–294. [Google Scholar] [CrossRef]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; AlMazroa, M.A.; Amann, M.; Anderson, H.R.; Andrews, K.G.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef]
- GBD 2013 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 2287–2323. [Google Scholar] [CrossRef]
- Reedy, J.; Krebs-Smith, S.M.; Miller, P.E.; Liese, A.D.; Kahle, L.L.; Park, Y.; Subar, A.F. Higher diet quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. J. Nutr. 2014, 144, 881–889. [Google Scholar] [CrossRef]
- Medina-Remón, A.; Kirwan, R.; Lamuela-Raventós, R.M.; Estruch, R. Dietary patterns and the risk of obesity, type 2 diabetes mellitus, cardiovascular diseases, asthma, and neurodegenerative diseases. Crit. Rev. Food Sci. Nutr. 2018, 58, 262–296. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Becerra-Tomás, N.; García-Gavilán, J.F.; Bulló, M.; Barrubés, L. Mediterranean Diet and Cardiovascular Disease Prevention: What Do We Know? Prog. Cardiovasc. Dis. 2018, 61, 62–67. [Google Scholar] [CrossRef]
- Shively, C.A.; Appt, S.E.; Vitolins, M.Z.; Uberseder, B.; Michalson, K.T.; Silverstein-Metzler, M.G.; Register, T.C. Mediterranean versus Western Diet Effects on Caloric Intake, Obesity, Metabolism, and Hepatosteatosis in Nonhuman Primates. Obesity 2019, 27, 777–784. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Lagiou, P. Healthy traditional Mediterranean diet: An expression of culture, history, and lifestyle. Nutr. Rev. 1997, 55 Pt 1, 383–389. [Google Scholar] [CrossRef]
- Rees, K.; Takeda, A.; Martin, N.; Ellis, L.; Wijesekara, D.; Vepa, A.; Das, A.; Hartley, L.; Stranges, S. Mediterranean-style diet for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2019, 3, CD009825. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Mediterranean dietary pattern, inflammation and endothelial function: A systematic review and meta-analysis of intervention trials. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef]
- Beauchamp, G.K.; Keast, R.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.-H.; Smith, A.B.; Breslin, P.A.S. Phytochemistry: Ibuprofen-like activity in extra-virgin olive oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Aprano, S.; Framondi, L.; Di Matteo, R.; Laudisio, D.; Pugliese, G.; Savastano, S.; Colao, A. Sleep Quality in Obesity: Does Adherence to the Mediterranean Diet Matter? Nutrients 2020, 12, 1364. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, M.A.; Bes-Rastrollo, M.; Serra-Majem, L.; Lairon, D.; Estruch, R.; Trichopoulou, A. Mediterranean food pattern and the primary prevention of chronic disease: Recent developments. Nutr. Rev. 2009, 67 (Suppl. S1), S111–S116. [Google Scholar] [CrossRef]
- Cavaliere, A.; de Marchi, E.; Banterle, A. Exploring the Adherence to the Mediterranean Diet and Its Relationship with Individual Lifestyle: The Role of Healthy Behaviors, Pro-Environmental Behaviors, Income, and Education. Nutrients 2018, 10, 141. [Google Scholar] [CrossRef]
- Mamalaki, E.; Anastasiou, C.A.; Ntanasi, E.; Tsapanou, A.; Kosmidis, M.H.; Dardiotis, E.; Hadjigeorgiou, G.M.; Sakka, P.; Scarmeas, N.; Yannakoulia, M. Associations between the mediterranean diet and sleep in older adults: Results from the hellenic longitudinal investigation of aging and diet study. Geriatr. Gerontol. Int. 2018, 18, 1543–1548. [Google Scholar] [CrossRef]
- Ruggiero, E.; Di Castelnuovo, A.; Costanzo, S.; Persichillo, M.; Bracone, F.; Cerletti, C.; Donati, M.B.; De Gaetano, G.; Iacoviello, L.; Bonaccio, M.; et al. Socioeconomic and psychosocial determinants of adherence to the Mediterranean diet in a general adult Italian population. Eur. J. Public Health 2019, 29, 328–335. [Google Scholar] [CrossRef]
- Zurita-Ortega, F.; Román-Mata, S.S.; Chacón-Cuberos, R.; Castro-Sánchez, M.; Muros, J.J. Adherence to the Mediterranean Diet Is Associated with Physical Activity, Self-Concept and Sociodemographic Factors in University Student. Nutrients 2018, 10, 966. [Google Scholar] [CrossRef]
- Bonaccio, M.; Di Castelnuovo, A.; Costanzo, S.; Persichillo, M.; De Curtis, A.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L. Interaction between Mediterranean diet and statins on mortality risk in patients with cardiovascular disease: Findings from the Moli-sani Study. Int. J. Cardiol. 2019, 276, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Alvarez, I.; de Rojas, J.P.; Fernandez-Montero, A.; Zazpe, I.; Ruiz-Canela, M.; Hidalgo-Santamaría, M.; Bes-Rastrollo, M.; Martínez-González, M. Strong inverse associations of Mediterranean diet, physical activity and their combination with cardiovascular disease: The Seguimiento Universidad de Navarra (SUN) cohort. Eur. J. Prev. Cardiol. 2018, 25, 1186–1197. [Google Scholar] [CrossRef] [PubMed]
- Bonaccio, M.; Gianfagna, F.; de Gaetano, G.; Iacoviello, L. Too many individuals are unaware of their blood lipid levels, but might still get health benefit from the Mediterranean diet through lipid-independent mechanisms. Eur. J. Prev. Cardiol. 2019, 26, 1953–1956. [Google Scholar] [CrossRef] [PubMed]
- Bonaccio, M.; Pounis, G.; Cerletti, C.; Donati, M.B.; Iacoviello, L.; de Gaetano, G.; on behalf of the MOLI-SANI Study Investigators. Mediterranean diet, dietary polyphenols and low grade inflammation: Results from the MOLI-SANI study. Br. J. Clin. Pharmacol. 2017, 83, 107–113. [Google Scholar] [CrossRef]
- Biscetti, F.; Porreca, C.F.; Bertucci, F.; Straface, G.; Santoliquido, A.; Tondi, P.; Angelini, F.; Pitocco, D.; Santoro, L.; Gasbarrini, A.; et al. TNFRSF11B gene polymorphisms increased risk of peripheral arterial occlusive disease and critical limb ischemia in patients with type 2 diabetes. Acta Diabetol. 2014, 51, 1025–1032. [Google Scholar] [CrossRef]
- Biscetti, F.; Straface, G.; Giovannini, S.; Santoliquido, A.; Angelini, F.; Santoro, L.; Porreca, C.F.; Pecorini, G.; Ghirlanda, G.; Flex, A. Association between TNFRSF11B gene polymorphisms and history of ischemic stroke in Italian diabetic patients. Hum. Genet. 2013, 132, 49–55. [Google Scholar] [CrossRef]
- Papa, A.; Danese, S.; Urgesi, R.; Grillo, A.; Guglielmo, S.; Roberto, I.; Semeraro, S.; Scaldaferri, F.; Pola, R.; Flex, A.; et al. Intercellular adhesion molecule 1 gene polymorphisms in inflammatory bowel disease. Eur. Rev. Med. Pharmacol. Sci. 2004, 8, 187–191. [Google Scholar]
- Biscetti, F.; Gaetani, E.; Flex, A.; Straface, G.; Pecorini, G.; Angelini, F.; Stigliano, E.; Aprahamian, T.; Smith, R.C.; Castellot, J.J.; et al. Peroxisome proliferator-activated receptor alpha is crucial for iloprost-induced in vivo angiogenesis and vascular endothelial growth factor upregulation. J. Vasc. Res. 2009, 46, 103–108. [Google Scholar] [CrossRef]
- Janiszewska, J.; Ostrowska, J.; Szostak-Węgierek, D. The Influence of Nutrition on Adiponectin—A Narrative Review. Nutrients 2021, 13, 1394. [Google Scholar] [CrossRef]
- Chen, J.H.; Song, J.; Chen, Y.; Ding, Q.; Peng, A.; Mao, L. The Effect of Vegan Protein-Based Diets on Metabolic Parameters, Expressions of Adiponectin and Its Receptors in Wistar Rats. Nutrients 2016, 8, 643. [Google Scholar] [CrossRef]
- Gogga, P.; Śliwińska, A.; Aleksandrowicz-Wrona, E.; Małgorzewicz, S. Association between different types of plant-based diets and leptin levels in healthy volunteers. Acta Biochim. Pol. 2019, 66, 77–82. [Google Scholar] [CrossRef]
- Pola, R.; Flex, A.; Gaetani, E.; Pola, P.; Bernabei, R. The -174 G/C polymorphism of the interleukin-6 gene promoter and essential hypertension in an elderly Italian population. J. Hum. Hypertens. 2002, 16, 637–640. [Google Scholar] [CrossRef][Green Version]
- Fukao, T.; Lopaschuk, G.D.; Mitchell, G.A. Pathways and control of ketone body metabolism: On the fringe of lipid biochemistry. Prostaglandins Leukot. Essent. Fatty Acids 2004, 70, 243–251. [Google Scholar] [CrossRef]
- Bolla, A.M.; Caretto, A.; Laurenzi, A.; Scavini, M.; Piemonti, L. Low-Carb and Ketogenic Diets in Type 1 and Type 2 Diabetes. Nutrients 2019, 11, 962. [Google Scholar] [CrossRef]
- Joshi, S.; Ostfeld, R.J.; McMacken, M. The Ketogenic Diet for Obesity and Diabetes-Enthusiasm Outpaces Evidence. JAMA Intern. Med. 2019, 179, 1163–1164. [Google Scholar] [CrossRef]
- Ludwig, D.S. The Ketogenic Diet: Evidence for Optimism but High-Quality Research Needed. J. Nutr. 2020, 150, 1354–1359. [Google Scholar] [CrossRef]
- Tangnijkul, Y.; Adams, L.E.; Herman, J.H. Serum ribonucleotide binding activity in osteoarthritis. Clin. Exp. Rheumatol. 1988, 6, 233–237. [Google Scholar]
- Mohorko, N.; Černelič-Bizjak, M.; Poklar-Vatovec, T.; Grom, G.; Kenig, S.; Petelin, A.; Jenko-Pražnikar, Z. Weight loss, improved physical performance, cognitive function, eating behavior, and metabolic profile in a 12-week ketogenic diet in obese adults. Nutr. Res. 2019, 62, 64–77. [Google Scholar] [CrossRef]
- Volek, J.S.; Sharman, M.J.; Forsythe, C.E. Modification of lipoproteins by very low-carbohydrate diets. J. Nutr. 2005, 135, 1339–1342. [Google Scholar] [CrossRef]
- Noain, J.S.; Minupuri, A.; Kulkarni, A.; Zheng, S. Significant Impact of the Ketogenic Diet on Low-Density Lipoprotein Cholesterol Levels. Cureus 2020, 12, e9418. [Google Scholar] [CrossRef]
- Cenci, L.; Paoli, A.; Omar, H.R.; Dalvi, P.; Camporesi, E.M.; Mangar, D.; Quartesan, S.; Fiorito, A.; Bosco, G. Internist, anesthesiologist and surgeon use of ketogenic diet. Minerva Gastroenterol. Dietol. 2018, 64, 84–93. [Google Scholar] [CrossRef]
- Ko, G.J.; Rhee, C.M.; Kalantar-Zadeh, K.; Joshi, S. The Effects of High-Protein Diets on Kidney Health and Longevity. J. Am. Soc. Nephrol. 2020, 31, 1667–1679. [Google Scholar] [CrossRef]
- Flore, R.; Ponziani, F.R.; Di Rienzo, T.A.; Zocco, M.A.; Flex, A.; Gerardino, L.; Lupascu, A.; Santoro, L.; Santoliquido, A.; Di Stasio, E.; et al. Something more to say about calcium homeostasis: The role of vitamin K2 in vascular calcification and osteoporosis. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2433–2440. [Google Scholar]
- Paoli, A.; Cenci, L.; Grimaldi, K.A. Effect of ketogenic Mediterranean diet with phytoextracts and low carbohydrates/high-protein meals on weight, cardiovascular risk factors, body composition and diet compliance in Italian council employees. Nutr. J. 2011, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Grimaldi, K.; D’Agostino, D.; Cenci, L.; Moro, T.; Bianco, A.; Palma, A. Ketogenic diet does not affect strength performance in elite artistic gymnasts. J. Int. Soc. Sports Nutr. 2012, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Youm, Y.-H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.-D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Mayor, A.; Tschopp, J. The inflammasomes: Guardians of the body. Annu. Rev. Immunol. 2009, 27, 229–265. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and functions of inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef]
- Varady, K.A.; Bhutani, S.; Klempel, M.C.; Kroeger, C.M.; Trepanowski, J.F.; Haus, J.M.; Hoddy, K.K.; Calvo, Y. Alternate day fasting for weight loss in normal weight and overweight subjects: A randomized controlled trial. Nutr. J. 2013, 12, 146. [Google Scholar] [CrossRef]
- Catenacci, V.A.; Pan, Z.; Ostendorf, D.; Brannon, S.; Gozansky, W.S.; Mattson, M.P.; Martin, B.; MacLean, P.S.; Melanson, E.L.; Donahoo, W.T. A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity 2016, 24, 1874–1883. [Google Scholar] [CrossRef]
- Carter, S.; Clifton, P.M.; Keogh, J.B. The effects of intermittent compared to continuous energy restriction on glycaemic control in type 2 diabetes; a pragmatic pilot trial. Diabetes Res. Clin. Pract. 2016, 122, 106–112. [Google Scholar] [CrossRef]
- Aksungar, F.B.; Sarıkaya, M.; Coskun, A.; Serteser, M.; Unsal, I. Comparison of Intermittent Fasting versus Caloric Restriction in Obese Subjects: A Two Year Follow-Up. J. Nutr. Health Aging 2017, 21, 681–685. [Google Scholar] [CrossRef]
- Furmli, S.; Elmasry, R.; Ramos, M.; Fung, J. Therapeutic use of intermittent fasting for people with type 2 diabetes as an alternative to insulin. BMJ Case Rep. 2018, 2018, bcr2017221854. [Google Scholar] [CrossRef]
- Biscetti, F.; Pitocco, D.; Straface, G.; Zaccardi, F.; De Cristofaro, R.; Rizzo, P.; Lancellotti, S.; Arena, V.; Stigliano, E.; Musella, T.; et al. Glycaemic variability affects ischaemia-induced angiogenesis in diabetic mice. Clin. Sci. 2011, 121, 555–564. [Google Scholar] [CrossRef]
- Liu, H.; Javaheri, A.; Godar, R.J.; Murphy, J.; Ma, X.; Rohatgi, N.; Mahadevan, J.; Hyrc, K.; Saftig, P.; Marshall, C.; et al. Intermittent fasting preserves beta-cell mass in obesity-induced diabetes via the autophagy-lysosome pathway. Autophagy 2017, 13, 1952–1968. [Google Scholar] [CrossRef]
- Turner, N.; Cooney, G.J.; Kraegen, E.W.; Bruce, C.R. Fatty acid metabolism, energy expenditure and insulin resistance in muscle. J. Endocrinol. 2014, 220, T61–T69. [Google Scholar] [CrossRef]
- Barnosky, A.R.; Hoddy, K.K.; Unterman, T.G.; Varady, K.A. Intermittent fasting vs. daily calorie restriction for type 2 diabetes prevention: A review of human findings. Transl. Res. 2014, 164, 302–311. [Google Scholar] [CrossRef]
- Hallak, M.H.; Nomani, M.Z. Body weight loss and changes in blood lipid levels in normal men on hypocaloric diets during Ramadan fasting. Am. J. Clin. Nutr. 1988, 48, 1197–1210. [Google Scholar] [CrossRef]
- Afrasiabi, A.; Hassanzadeh, S.; Sattarivand, R.; Mahboob, S. Effects of Ramadan fasting on serum lipid profiles on 2 hyperlipidemic groups with or without diet pattern. Saudi Med. J. 2003, 24, 23–26. [Google Scholar]
- Santos, H.O.; Macedo, R.C.O. Impact of intermittent fasting on the lipid profile: Assessment associated with diet and weight loss. Clin. Nutr. ESPEN 2018, 24, 14–21. [Google Scholar] [CrossRef]
- Mirmiran, P.; Bahadoran, Z.; Gaeini, Z.; Moslehi, N.; Azizi, F. Effects of Ramadan intermittent fasting on lipid and lipoprotein parameters: An updated meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Emami-Naini, A.; Roomizadeh, P.; Baradaran, A.; Abedini, A.; Abtahi, M. Ramadan fasting and patients with renal diseases: A mini review of the literature. J. Res. Med. Sci. 2013, 18, 711–716. [Google Scholar] [PubMed]
- Baloglu, I.; Turkmen, K.; Kocyigit, I.; Altunoren, O.; Demirtas, L.; Zararsız, G.; Eroglu, E. The effect of Ramadan fasting on kidney function in patients with chronic kidney disease. Int. Urol. Nephrol. 2020, 52, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Alsughayer, L.Y.; Altamimi, L.A.; Tarakji, A.R. Ramadan fasting among advanced chronic kidney disease patients. Nephrologists’ perspectives in Saudi Arabia. Saudi Med. J. 2020, 41, 1070–1075. [Google Scholar] [CrossRef]
- Bragazzi, N.L. Ramadan fasting and chronic kidney disease: A systematic review. J. Res. Med. Sci. 2014, 19, 665–676. [Google Scholar]
- Pola, R.; Gaetani, E.; Flex, A.; Gerardino, L.; Aloi, F.; Flore, R.; Serricchio, M.; Pola, P.; Bernabei, R. Lack of association between Alzheimer’s disease and Gln-Arg 192 Q/R polymorphism of the PON-1 gene in an Italian population. Dement. Geriatr. Cogn. Disord. 2003, 15, 88–91. [Google Scholar] [CrossRef]
- Pola, R.; Flex, A.; Gaetani, E.; Santoliquido, A.; Serricchio, M.; Pola, P.; Bernabei, R. Intercellular adhesion molecule-1 K469E gene polymorphism and Alzheimer’s disease. Neurobiol. Aging 2003, 24, 385–387. [Google Scholar] [CrossRef]
- Flex, A.; Gaetani, E.; Proia, A.S.; Pecorini, G.; Straface, G.; Biscetti, F.; Fioroni, G.; Sabusco, A.; Flore, R.; Tondi, P.; et al. Analysis of functional polymorphisms of metalloproteinase genes in persons with vascular dementia and Alzheimer’s disease. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1065–1069. [Google Scholar] [CrossRef][Green Version]
- Pola, R.; Flex, A.; Ciaburri, M.; Rovella, E.; Valiani, A.; Reali, G.; Silveri, M.C.; Bernabei, R. Responsiveness to cholinesterase inhibitors in Alzheimer’s disease: A possible role for the 192 Q/R polymorphism of the PON-1 gene. Neurosci. Lett. 2005, 382, 338–341. [Google Scholar] [CrossRef]
- Liu, B.; Hutchison, A.T.; Thompson, C.H.; Lange, K.; Heilbronn, L.K. Markers of adipose tissue inflammation are transiently elevated during intermittent fasting in women who are overweight or obese. Obes. Res. Clin. Pract. 2019, 13, 408–415. [Google Scholar] [CrossRef]
- Trepanowski, J.F.; Kroeger, C.M.; Barnosky, A.; Klempel, M.C.; Bhutani, S.; Hoddy, K.K.; Gabel, K.; Freels, S.; Rigdon, J.; Rood, J.; et al. Effect of Alternate-Day Fasting on Weight Loss, Weight Maintenance, and Cardioprotection among Metabolically Healthy Obese Adults: A Randomized Clinical Trial. JAMA Intern. Med. 2017, 177, 930–938. [Google Scholar] [CrossRef]
- Tinsley, G.M.; la Bounty, P.M. Effects of intermittent fasting on body composition and clinical health markers in humans. Nutr. Rev. 2015, 73, 661–674. [Google Scholar] [CrossRef]
- Vist, G.E.; Reinar, L.M.; Straumann, G.H.; Wisting, L. Treatment of Persons Who Suffer from Both an Eating Disorder and Diabetes; Knowledge Centre for the Health Services at The Norwegian Institute of Public Health (NIPH): Oslo, Norway, 2015. [Google Scholar]
- Zhu, B.; Martyn-Nemeth, P.; Ruggiero, L.; Park, C.G.; Zhang, Y.; Fritschi, C. Associations between fatigue, sleep disturbance and eating style in adults with type 2 diabetes: A correlational study. J. Clin. Nurs. 2019, 28, 3200–3209. [Google Scholar] [CrossRef]
- Petroni, M.L.; Barbanti, F.A.; Bonadonna, R.; Bruno, G.; Caletti, M.T.; Croci, M.; D’Eusebio, C.; Cas, A.D.; Invitti, C.; Merlo, F.; et al. Dysfunctional eating in type 2 diabetes mellitus: A multicenter Italian study of socio-demographic and clinical associations. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 983–990. [Google Scholar] [CrossRef]
- Racicka, E.; Bryńska, A. Eating Disorders in children and adolescents with Type 1 and Type 2 Diabetes: Prevalence, risk factors, warning signs. Psychiatr. Pol. 2015, 49, 1017–1024, (In English and Polish). [Google Scholar] [CrossRef]
- Raevuori, A.; Suokas, J.; Haukka, J.; Gissler, M.; Linna, M.; Grainger, M.; Suvisaari, J. Highly increased risk of type 2 diabetes in patients with binge eating disorder and bulimia nervosa. Int. J. Eat. Disord. 2015, 48, 555–562. [Google Scholar] [CrossRef]
- Yahya, A.S.; Khawaja, S.; Chukwuma, J.; Chukwuma, C. Early Diagnosis and Management of Bulimia Nervosa in Type 1 Diabetes. Prim. Care Companion CNS Disord. 2020, 22, 20nr02707. [Google Scholar] [CrossRef]
- Nieto-Martínez, R.; González-Rivas, J.P.; Medina-Inojosa, J.R.; Florez, H. Are Eating Disorders Risk Factors for Type 2 Diabetes? A Systematic Review and Meta-analysis. Curr. Diabetes Rep. 2017, 17, 138. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Na, M.; Lichtenstein, A.H.; Xing, A.; Chen, S.; Wu, S.; Gao, X. Habitual Night Eating Was Positively Associated with Progress of Arterial Stiffness in Chinese Adults. J. Am. Heart Assoc. 2020, 9, e016455. [Google Scholar] [CrossRef]
- Davis, R.; Murgia, C.; Dordevic, A.L.; Bonham, M.P.; Huggins, C.E. Diurnal variation in gene expression of human peripheral blood mononuclear cells after eating a standard meal compared with a high protein meal: A cross-over study. Clin. Nutr. 2021, 40, 4349–4359. [Google Scholar] [CrossRef]
- Dashti, H.S.; Scheer, F.A.; Jacques, P.F.; Lamon-Fava, S.; Ordovás, J.M. Short sleep duration and dietary intake: Epidemiologic evidence, mechanisms, and health implications. Adv. Nutr. 2015, 6, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Balieiro, L.C.; Rossato, L.T.; Waterhouse, J.; Paim, S.L.; Mota, M.C.; Crispim, C.A. Nutritional status and eating habits of bus drivers during the day and night. Chronobiol. Int. 2014, 31, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Okada, C.; Tabuchi, T.; Iso, H. Association between skipping breakfast in parents and children and childhood overweight/obesity among children: A nationwide 10.5-year prospective study in Japan. Int. J. Obes. 2018, 42, 1724–1732. [Google Scholar] [CrossRef] [PubMed]
- Traub, M.; Lauer, R.; Kesztyüs, T.; Wartha, O.; Steinacker, J.M.; Kesztyüs, D. Skipping breakfast, overconsumption of soft drinks and screen media: Longitudinal analysis of the combined influence on weight development in primary schoolchildren. BMC Public Health 2018, 18, 363. [Google Scholar] [CrossRef] [PubMed]
- Wadolowska, L.; Hamulka, J.; Kowalkowska, J.; Ulewicz, N.; Gornicka, M.; Jeruszka-Bielak, M.; Kostecka, M.; Wawrzyniak, A. Skipping Breakfast and a Meal at School: Its Correlates in Adiposity Context. Report from the ABC of Healthy Eating Study of Polish Teenagers. Nutrients 2019, 11, 1563. [Google Scholar] [CrossRef]
- Kudo, A.; Asahi, K.; Satoh, H.; Iseki, K.; Moriyama, T.; Yamagata, K.; Tsuruya, K.; Fujimoto, S.; Narita, I.; Konta, T.; et al. Fast eating is a strong risk factor for new-onset diabetes among the Japanese general population. Sci. Rep. 2019, 9, 8210. [Google Scholar] [CrossRef]
- Luigetti, M.; Iorio, R.; Bentivoglio, A.R.; Tricoli, L.; Riso, V.; Marotta, J.; Piano, C.; Primiano, G.; Del Verme, L.Z.; Monaco, M.R.L.; et al. Assessment of neurological manifestations in hospitalized patients with COVID-19. Eur. J. Neurol. 2020, 27, 2322–2328. [Google Scholar] [CrossRef]
- Crimarco, A.; Turner-McGrievy, G.M.; Wirth, M.D. The effects of meal-timing on self-rated hunger and dietary inflammatory potential among a sample of college students. J. Am. Coll. Health 2019, 67, 328–337. [Google Scholar] [CrossRef]
- Kiguli, J.; Alvesson, H.M.; Mayega, R.W.; Kasujja, F.X.; Muyingo, A.; Kirunda, B.; Kiracho, E.E.; Nalwadda, C.K.; Naggayi, G.; Peterson, S.; et al. Dietary patterns and practices in rural eastern Uganda: Implications for prevention and management of type 2 diabetes. Appetite 2019, 143, 104409. [Google Scholar] [CrossRef]
- Sapkota, S.; Brien, J.E.; Gwynn, J.; Flood, V.; Aslani, P. Perceived impact of Nepalese food and food culture in diabetes. Appetite 2017, 113, 376–386. [Google Scholar] [CrossRef]
- Mazidi, M.; Speakman, J.R. Association of Fast-Food and Full-Service Restaurant Densities with Mortality From Cardiovascular Disease and Stroke, and the Prevalence of Diabetes Mellitus. J. Am. Heart Assoc. 2018, 7, e007651. [Google Scholar] [CrossRef]
- Raza, L.; Raza, A.; Ali, A.; Hasnain, A. Influence of dietary practices on Blood pressure: Comparison between house wives and employed women. J. Pak. Med. Assoc. 2019, 69, 857–863. [Google Scholar]
- Ahmad, M.I.; Zou, X.; Ijaz, M.U.; Hussain, M.; Liu, C.; Xu, X.; Zhou, G.; Li, C. Processed Meat Protein Promoted Inflammation and Hepatic Lipogenesis by Upregulating Nrf2/Keap1 Signaling Pathway in Glrx-Deficient Mice. J. Agric. Food Chem. 2019, 67, 8794–8809. [Google Scholar] [CrossRef]
- Macho-González, A.; Garcimartín, A.; López-Oliva, M.E.; Bastida, S.; Benedí, J.; Ros, G.; Nieto, G.; Sánchez-Muniz, F.J. Can Meat and Meat-Products Induce Oxidative Stress? Antioxidants 2020, 9, 638. [Google Scholar] [CrossRef]
- Park, K.H.; Ye, Z.W.; Zhang, J.; Kim, S.H. Palmitic Acid-Enriched Diet Induces Hepatic Steatosis and Injury in Adult Zebrafish. Zebrafish 2019, 16, 497–504. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Fast Food Pattern and Cardiometabolic Disorders: A Review of Current Studies. Health Promot. Perspect. 2015, 5, 231–240. [Google Scholar] [CrossRef]
- Castelao-Naval, O.; Blanco-Fernández, A.; Meseguer-Barros, C.M.; Thuissard-Vasallo, I.J.; Cerdá, B.; Larrosa, M. Life style and risk of atypical eating disorders in university students: Reality versus perception. Enferm. Clin. 2019, 2019, 280–290. [Google Scholar] [CrossRef]
- Salk, R.H.; Germeroth, L.J.; Emery, R.L.; Conlon, R.P.K.; Wang, Z.; Cheng, Y.; Marcus, M.D.; Perkins, K.A.; Levine, M.D. Predictive utility of subtyping women smokers on depression, eating, and weight-related symptoms. Health Psychol. 2019, 38, 248–258. [Google Scholar] [CrossRef]
- Kaluza, J.; Håkansson, N.; Harris, H.R.; Orsini, N.; Michaëlsson, K.; Wolk, A. Influence of anti-inflammatory diet and smoking on mortality and survival in men and women: Two prospective cohort studies. J. Intern. Med. 2019, 285, 75–91. [Google Scholar] [CrossRef]
- Mahmood, S.S.; Levy, D.; Vasan, R.S.; Wang, T.J. The Framingham Heart Study and the epidemiology of cardiovascular disease: A historical perspective. Lancet 2014, 383, 999–1008. [Google Scholar] [CrossRef]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Economic Burden of Cardiovascular Disease in Type 2 Diabetes: A Systematic Review. Value Health 2018, 21, 881–890. [Google Scholar] [CrossRef]
- Suárez, C.; Zeymer, U.; Limbourg, T.; Baumgartner, I.; Cacoub, P.; Poldermans, D.; Röther, J.; Bhatt, D.L.; Steg, P.G. Influence of polyvascular disease on cardiovascular event rates. Insights from the REACH Registry. Vasc. Med. 2010, 15, 259–265. [Google Scholar] [CrossRef]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Gardner, A.W.; Montgomery, P.S.; Wang, M.; Shen, B.; Casanegra, A.I.; Silva-Palacios, F.; Knehans, A.W. Diet is associated with ankle-brachial index, inflammation, and ambulation in patients with intermittent claudication. J. Vasc. Surg. 2020, 72, 1375–1384. [Google Scholar] [CrossRef]
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Bautista, L.; Franzosi, M.G.; Commerford, P.; Lang, C.C.; Rumboldt, Z.; Onen, C.L.; Lisheng, L.; et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: A case-control study. Lancet 2005, 366, 1640–1649. [Google Scholar] [CrossRef]
- Polonsky, T.S.; Tian, L.; Zhang, D.; Bazzano, L.A.; Criqui, M.H.; Ferrucci, L.; Guralnik, J.M.; Kibbe, M.R.; Leeuwenburgh, C.; Sufit, R.L.; et al. Associations of Weight Change with Changes in Calf Muscle Characteristics and Functional Decline in Peripheral Artery Disease. J. Am. Heart Assoc. 2019, 8, e010890. [Google Scholar] [CrossRef]
- Arinze, N.; Farber, A.; Levin, S.R.; Cheng, T.W.; Jones, D.W.; Patel, V.I.; Rybin, D.; Doros, G.; Siracuse, J.J. Perioperative outcomes after lower extremity bypass and peripheral vascular interventions in patients with morbid obesity and superobesity. J. Vasc. Surg. 2020, 71, 567–574.e4. [Google Scholar] [CrossRef]
- Pitan, O.; Williams, M.; Obirieze, A.; Tran, D.; Rose, D.; Fullum, T.; Cornwell, E.; Hughes, K. Lower extremity arterial reconstruction in obese patients. Am. J. Surg. 2015, 209, 640–644. [Google Scholar] [CrossRef]
- Kokkinidis, D.G.; Armstrong, E.J.; Giri, J. Balancing Weight Loss and Sarcopenia in Elderly Patients with Peripheral Artery Disease. J. Am. Heart Assoc. 2019, 8, e013200. [Google Scholar] [CrossRef]
- Tzoulaki, I.; Murray, G.D.; Lee, A.J.; Rumley, A.; Lowe, G.D.; Fowkes, F.G. C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh Artery Study. Circulation 2005, 112, 976–983. [Google Scholar] [CrossRef]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Thomas, J.; Delaney, C.; Penna, D.; Puckridge, P.; Spark, J.I.; Liang, L. A 3-year follow-up study of inpatients with lower limb ulcers: Evidence of an obesity paradox? J. Multidiscip. Healthc. 2012, 5, 181–186. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Palaiodimos, L.; Kokkinidis, D.G.; Li, W.; Karamanis, D.; Ognibene, J.; Arora, S.; Southern, W.N.; Mantzoros, C.S. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism 2020, 108, 154262. [Google Scholar] [CrossRef] [PubMed]
- Kokkinidis, D.G.; Strobel, A.; Jawaid, O.; Haider, M.N.; Alvandi, B.; Singh, G.D.; Laird, J.R.; Waldo, S.W.; Armstrong, E.J. Development and validation of a predictive score for anterograde crossing of infrapopliteal chronic total occlusions: (The Infrapop-CTO Score). Catheter. Cardiovasc. Interv. 2020, 95, 748–755. [Google Scholar] [CrossRef]
- Nosova, E.V.; Conte, M.S.; Grenon, S.M. Advancing beyond the heart-healthy diet for peripheral arterial disease. J. Vasc. Surg. 2015, 61, 265–274. [Google Scholar] [CrossRef]
- Casas, R.; Castro-Barquero, S.; Estruch, R.; Sacanella, E. Nutrition and Cardiovascular Health. Int. J. Mol. Sci. 2018, 19, 3988. [Google Scholar] [CrossRef]
- Sabeva, N.S.; McPhaul, C.M.; Li, X.; Cory, T.J.; Feola, D.J.; Graf, G.A. Phytosterols differentially influence ABC transporter expression, cholesterol efflux and inflammatory cytokine secretion in macrophage foam cells. J. Nutr. Biochem. 2011, 22, 777–783. [Google Scholar] [CrossRef]
- Andersson, S.W.; Skinner, J.; Ellegård, L.; Welch, A.A.; Bingham, S.; Mulligan, A.; Andersson, H.; Shaw, K.-T. Intake of dietary plant sterols is inversely related to serum cholesterol concentration in men and women in the EPIC Norfolk population: A cross-sectional study. Eur. J. Clin. Nutr. 2004, 58, 1378–1385. [Google Scholar] [CrossRef]
- Katan, M.B.; Grundy, S.M.; Jones, P.; Law, M.; Miettinen, T.; Paoletti, R. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin. Proc. 2003, 78, 965–978. [Google Scholar] [CrossRef]
- Ferreyra, M.L.F.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant. Sci. 2012, 3, 222. [Google Scholar] [CrossRef]
- Biscetti, F.; Ghirlanda, G.; Flex, A. Therapeutic potential of high mobility group box-1 in ischemic injury and tissue regeneration. Curr. Vasc. Pharmacol. 2011, 9, 677–681. [Google Scholar] [CrossRef]
- Fontana, L. Interventions to promote cardiometabolic health and slow cardiovascular ageing. Nat. Rev. Cardiol. 2018, 15, 566–577. [Google Scholar] [CrossRef]
- Biscetti, F.; Straface, G.; Bertoletti, G.; Vincenzoni, C.; Snider, F.; Arena, V.; Landolfi, R.; Flex, A. Identification of a potential proinflammatory genetic profile influencing carotid plaque vulnerability. J. Vasc. Surg. 2015, 61, 374–381. [Google Scholar] [CrossRef]
- Straface, G.; Biscetti, F.; Pitocco, D.; Bertoletti, G.; Misuraca, M.; Vincenzoni, C.; Snider, F.; Arena, V.; Stigliano, E.; Angelini, F.; et al. Assessment of the genetic effects of polymorphisms in the osteoprotegerin gene, TNFRSF11B, on serum osteoprotegerin levels and carotid plaque vulnerability. Stroke 2011, 42, 3022–3028. [Google Scholar] [CrossRef]
- Biscetti, F.; Tinelli, G.; Rando, M.M.; Nardella, E.; Cecchini, A.L.; Angelini, F.; Straface, G.; Filipponi, M.; Arena, V.; Pitocco, D.; et al. Association between carotid plaque vulnerability and high mobility group box-1 serum levels in a diabetic population. Cardiovasc. Diabetol. 2021, 20, 114. [Google Scholar] [CrossRef]
- Sunkara, A.; Raizner, A. Supplemental Vitamins and Minerals for Cardiovascular Disease Prevention and Treatment. Methodist. Debakey Cardiovasc. J. 2019, 15, 179–184. [Google Scholar] [CrossRef]
- Villacorta, L.; Graça-Souza, A.V.; Ricciarelli, R.; Zingg, J.M.; Azzi, A. Alpha-tocopherol induces expression of connective tissue growth factor and antagonizes tumor necrosis factor-alpha-mediated downregulation in human smooth muscle cells. Circ. Res. 2003, 92, 104–110. [Google Scholar] [CrossRef]
- Biscetti, F.; Rando, M.M.; Nardella, E.; Cecchini, A.L.; Pecorini, G.; Landolfi, R.; Flex, A. High Mobility Group Box-1 and Diabetes Mellitus Complications: State of the Art and Future Perspectives. Int. J. Mol. Sci. 2019, 20, 6258. [Google Scholar] [CrossRef]
- Giovannini, S.; Tinelli, G.; Biscetti, F.; Straface, G.; Angelini, F.; Pitocco, D.; Mucci, L.; Landolfi, R.; Flex, A. Serum high mobility group box-1 and osteoprotegerin levels are associated with peripheral arterial disease and critical limb ischemia in type 2 diabetic subjects. Cardiovasc. Diabetol. 2017, 16, 99. [Google Scholar] [CrossRef]
- Hustad, S.; Ueland, P.M.; Vollset, S.E.; Zhang, Y.; Bjørke-Monsen, A.L.; Schneede, J. Riboflavin as a determinant of plasma total homocysteine: Effect modification by the methylenetetrahydrofolate reductase C677T polymorphism. Clin. Chem. 2000, 46, 1065–1071. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Lee, W.-R.; Su, Y.-F.; Hsu, S.-P.; Lin, H.-C.; Ho, P.-Y.; Hou, T.-C.; Chou, Y.-P.; Kuo, C.-T.; Lee, W.-S. Folic acid inhibits endothelial cell proliferation through activating the cSrc/ERK 2/NF-κB/p53 pathway mediated by folic acid receptor. Angiogenesis 2012, 15, 671–683. [Google Scholar] [CrossRef]
- Fenton, R.; Brook-Barclay, L.; Delaney, C.L.; Spark, J.I.; Miller, M.D. Do Medications Commonly Prescribed to Patients with Peripheral Arterial Disease Have an Effect on Nutritional Status? A Review of the Literature. Ann. Vasc. Surg. 2016, 32, 145–175. [Google Scholar] [CrossRef]
- Blom, H.J.; Smulders, Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J. Inherit. Metab. Dis. 2011, 34, 75–81. [Google Scholar] [CrossRef]
- Violi, F.; Micheletta, F.; Iuliano, L. Vitamin E, atherosclerosis and thrombosis. Thromb. Haemost. 2001, 85, 766–770. [Google Scholar]
- Dionisio, N.; Jardín, I.; Salido, G.M.; Rosado, J.A. Homocysteine, intracellular signaling and thrombotic disorders. Curr. Med. Chem. 2010, 17, 3109–3119. [Google Scholar] [CrossRef]
- Aguilar, E.; Leonel, A.; Teixeira, L.; Silva, A.; Silva, J.; Pelaez, J.; Capettini, L.; Lemos, V.; Santos, R.; Alvarez-Leite, J. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFκB activation. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 606–613. [Google Scholar] [CrossRef]
- Menzel, T.; Lührs, H.; Zirlik, S.; Schauber, J.; Kudlich, T.; Gerke, T.; Gostner, A.; Neumann, M.; Melcher, R.; Scheppach, W. Butyrate inhibits leukocyte adhesion to endothelial cells via modulation of VCAM-1. Inflamm. Bowel Dis. 2004, 10, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Kulezic, A.; Bergwall, S.; Fatemi, S.; Sonestedt, E.; Zarrouk, M.; Gottsäter, A.; Acosta, S. Healthy diet and fiber intake are associated with decreased risk of incident symptomatic peripheral artery disease—A prospective cohort study. Vasc. Med. 2019, 24, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Hartley, L.; May, M.D.; Loveman, E.; Colquitt, J.L.; Rees, K. Dietary fibre for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2016, CD011472. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Je, Y. Dietary fibre intake and mortality from cardiovascular disease and all cancers: A meta-analysis of prospective cohort studies. Arch. Cardiovasc. Dis. 2016, 109, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Zhang, W.; Zhang, R.; Jiao, J.; Fu, C.; Tong, X.; Zhang, W.; Qin, L. Cereal fiber improves blood cholesterol profiles and modulates intestinal cholesterol metabolism in C57BL/6 mice fed a high-fat, high-cholesterol diet. Food Nutr. Res. 2019, 63. [Google Scholar] [CrossRef]
- Keech, A.C.; Simes, R.J.; Barter, P.J.; Best, J.D.; Scott, R.A.P.; Taskinen, M.R.; Forder, P.M.; Pillai, A.; Davis, T.M.; Glasziou, P.; et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): Randomised controlled trial. Lancet 2005, 366, 1849–1861. [Google Scholar] [CrossRef]
- Ismail-Beigi, F.; Craven, T.; Banerji, M.A.; Basile, J.; Calles, J.; Cohen, R.M.; Cuddihy, R.; Cushman, W.C.; Genuth, S.; Grimm, R.H.; et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: An analysis of the ACCORD randomised trial. Lancet 2010, 376, 419–430. [Google Scholar] [CrossRef]
- Chew, E.Y.; Ambrosius, W.T.; Davis, M.D.; Danis, R.P.; Gangaputra, S.; Greven, C.M.; Hubbard, L.; Esser, B.A.; Lovato, J.F.; Perdue, L.H.; et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N. Engl. J. Med. 2010, 363, 233–244. [Google Scholar] [CrossRef]
- Hermans, M.P. Non-invited review: Prevention of microvascular diabetic complications by fenofibrate: Lessons from FIELD and ACCORD. Diabetes Vasc. Dis. Res. 2011, 8, 180–189. [Google Scholar] [CrossRef]
- Li, P.; Shibata, R.; Maruyama, S.; Kondo, M.; Ohashi, K.; Ouchi, N.; Murohara, T. Fenofibrate promotes ischemia-induced revascularization through the adiponectin-dependent pathway. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E560–E566. [Google Scholar] [CrossRef][Green Version]
- Zayed, M.A.; Jin, X.; Yang, C.; Belaygorod, L.; Engel, C.; Desai, K.; Harroun, N.; Saffaf, O.; Patterson, B.W.; Hsu, F.-F.; et al. CEPT1-Mediated Phospholipogenesis Regulates Endothelial Cell Function and Ischemia-Induced Angiogenesis Through PPARα. Diabetes 2021, 70, 549–561. [Google Scholar] [CrossRef]
- Liu, P.-H.; Cao, Y.; Keeley, B.R.; Tam, I.; Wu, K.; Strate, L.L.; Giovannucci, E.L.; Chan, A.T. Adherence to a Healthy Lifestyle is Associated with a Lower Risk of Diverticulitis among Men. Am. J. Gastroenterol. 2017, 112, 1868–1876. [Google Scholar] [CrossRef]
- Wan, D.; Li, V.; Banfield, L.; Azab, S.; de Souza, R.J.; Anand, S.S. Diet and Nutrition in Peripheral Artery Disease: A Systematic Review. Can. J. Cardiol. 2022, 38, 672–680. [Google Scholar] [CrossRef]
- Chen, M.; Creger, T.; Howard, V.; Judd, S.E.; Harrington, K.F.; Fontaine, K.R. Geospatial analysis of Mediterranean diet adherence in the United States. Public Health Nutr. 2021, 24, 2920–2928. [Google Scholar] [CrossRef]
- Bonaccio, M.; Bonanni, A.E.; Di Castelnuovo, A.; De Lucia, F.; Donati, M.B.; de Gaetano, G.; Iacoviello, L.; on behalf of the Moli-sani Project Investigators. Low income is associated with poor adherence to a Mediterranean diet and a higher prevalence of obesity: Cross-sectional results from the Moli-sani study. BMJ Open 2012, 2, e001685. [Google Scholar] [CrossRef] [PubMed]
- Regmi, A.; Ballenger, N.; Putnam, J. Globalisation and income growth promote the Mediterranean diet. Public Health Nutr. 2004, 7, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Veech, R.L. The therapeutic implications of ketone bodies: The effects of ketone bodies in pathological conditions: Ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot. Essent. Fatty Acids 2004, 70, 309–319. [Google Scholar] [CrossRef]
- Tateishi, K.; Saito, Y.; Kitahara, H.; Kobayashi, Y. Impact of glycemic variability on coronary and peripheral endothelial dysfunction in patients with coronary artery disease. J. Cardiol. 2021, 79, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Juul, F.; Vaidean, G.; Parekh, N. Ultra-processed Foods and Cardiovascular Diseases: Potential Mechanisms of Action. Adv. Nutr. 2021, 12, 1673–1680. [Google Scholar] [CrossRef]
- Crimarco, A.; Landry, M.J.; Gardner, C.D. Ultra-processed Foods, Weight Gain, and Co-morbidity Risk. Curr. Obes. Rep. 2021, 11, 80–92. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Flex, A.; Biscetti, F.; Iachininoto, M.G.; Nuzzolo, E.R.; Orlando, N.; Capodimonti, S.; Angelini, F.; Valentini, C.G.; Bianchi, M.; Larocca, L.M.; et al. Human cord blood endothelial progenitors promote post-ischemic angiogenesis in immunocompetent mouse model. Thromb. Res. 2016, 141, 106–111. [Google Scholar] [CrossRef]
- Boswell, N.; Byrne, R.; Davies, P.S.W. Aetiology of eating behaviours: A possible mechanism to understand obesity development in early childhood. Neurosci. Biobehav. Rev. 2018, 95, 438–448. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.A.; Zheng, D.; Elinav, E. Diet-microbiota interactions and personalized nutrition. Nat. Rev. Microbiol. 2019, 17, 742–753. [Google Scholar] [CrossRef]
- Campia, U.; Gerhard-Herman, M.; Piazza, G.; Goldhaber, S.Z. Peripheral Artery Disease: Past, Present, and Future. Am. J. Med. 2019, 132, 1133–1141. [Google Scholar] [CrossRef]
- Warren, J.M.; Smith, N.; Ashwell, M. A structured literature review on the role of mindfulness, mindful eating and intuitive eating in changing eating behaviours: Effectiveness and associated potential mechanisms. Nutr. Res. Rev. 2017, 30, 272–283. [Google Scholar] [CrossRef]
- Wei, T.; Liu, J.; Zhang, D.; Wang, X.; Li, G.; Ma, R.; Chen, G.; Lin, X.; Guo, X. The Relationship between Nutrition and Atherosclerosis. Front. Bioeng. Biotechnol. 2021, 9, 635504. [Google Scholar] [CrossRef]
- Kahan, S.; Manson, J.E. Nutrition Counseling in Clinical Practice: How Clinicians Can Do Better. JAMA 2017, 318, 1101–1102. [Google Scholar] [CrossRef]
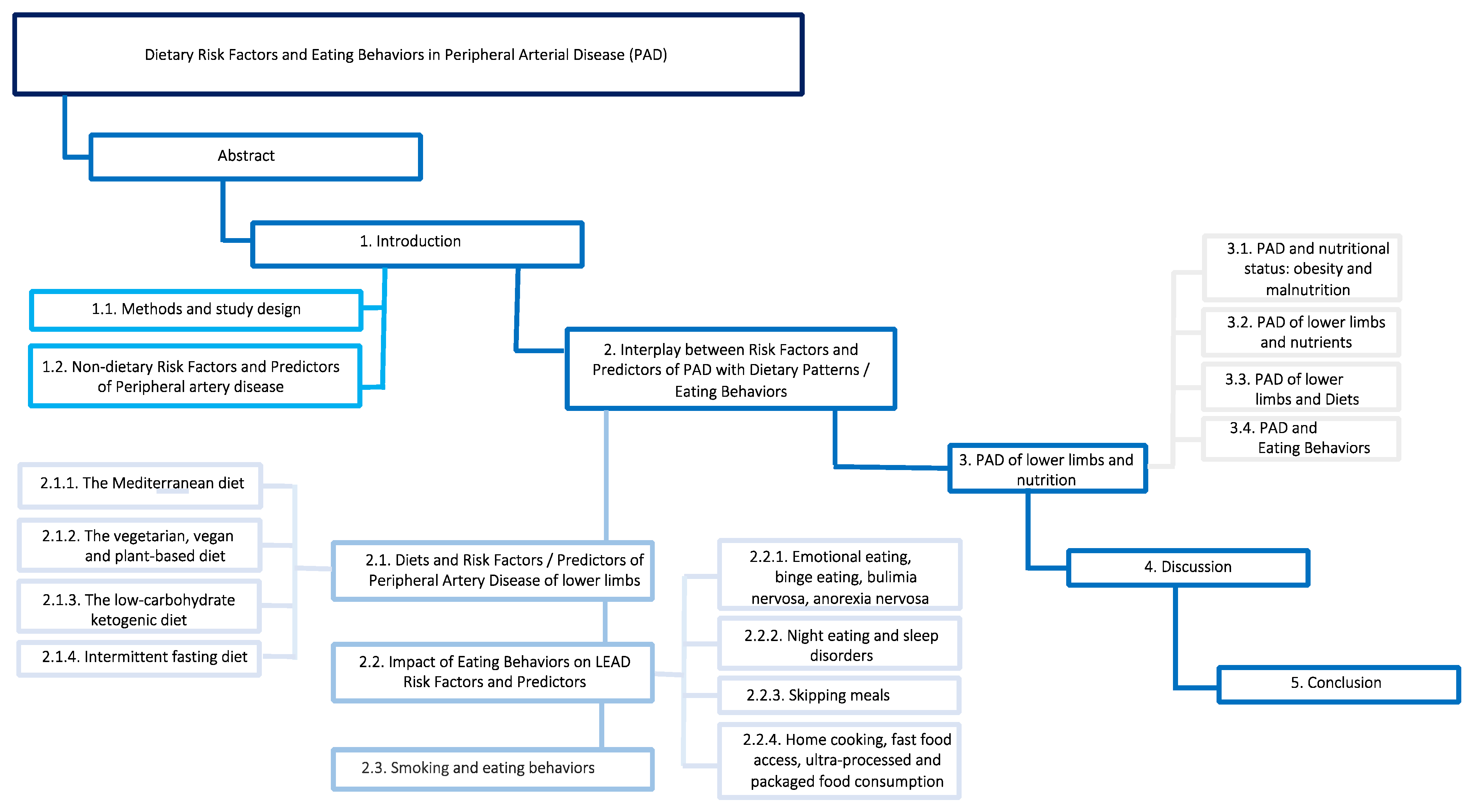
| Low Carbohydrate/Ketogenic Diet | ||
|---|---|---|
| PAD Risk Factors/Predictors | Evidence | Reference |
| Obesity | Efficient weight loss | [9,10,11,12,13,14,15,16,17,18,19,20] |
| Diabetes mellitus | Reduction in total insulin requirements due to reduced insulin resistance. Need for further safety studies | [21,22,23,24,25,26,27] |
| Hypertension | Controversial results with risk of worsening of blood pressure control in particular in CKD | [11,28,29,30,31,32,33,34,35] |
| Dyslipidemia | Reduction in the size of LDL-c molecules | [11,25,35,36,37,38,39,40,41,42] |
| Chronic kidney disease | Risk of calcium–phosphate homeostasis disruption, risk of progression of CKD due to acidosis and protein intake. Promotion of cyst regression in polycystic kidney disease | [43,44,45,46] |
| Inflammation | Modulation of the inflammasome with a net reduction in oxidative stress | [42,47,48,49,50] |
| Intermittent fasting diet | ||
| PAD Risk Factors/Predictors | Evidence | Reference |
| Obesity | Promotion of weight loss and change in body composition by consuming fat stores. May cause fatigue and dizziness | [51,52,53,54,55,56,57] |
| Diabetes mellitus | Reduction in serum glucose values regardless of weight loss but increased risk of hypoglycemia | [53,58,59,60] |
| Hypertension | Promising results by modulating cortisol levels and circadian rhythm. However, further confirmation for safe use in clinical practice is needed | [58,61,62,63,64,65,66,67,68] |
| Dyslipidemia | Evidence from observational studies on Ramadan. The amelioration of lipid profile results in an overall reduction in cardiovascular risk | [53,69,70,71,72,73,74,75,76,77,78,79] |
| Chronic kidney disease | Apparently safe in mild/moderate CKD when closely monitored | [80,81,82,83,84,85] |
| Inflammation | Regression of the systemic inflammatory state by promoting anti-inflammatory molecules | [56,58,77,86,87,88,89,90,91,92,93,94,95,96,97,98] |
| Vegetarian and Vegan diet | ||
| PAD Risk Factors/Predictors | Evidence | Reference |
| Obesity | Prevention and management of overweight but supplementation of vitamin B12 in the vegan diet is recommended | [99,100,101,102,103,104,105,106,107,108,109,110] |
| Diabetes mellitus | Improvement of pancreatic beta cell function, and increase in the production of gastrointestinal incretins resulting in a lower total insulin requirement | [99,111,112,113,114,115,116,117,118,119,120,121,122,123] |
| Hypertension | Additional improvement of the pressure profile if adequate adherence | [124,125,126] |
| Dyslipidemia | Efficacy comparable to statin therapy | [110,127,128,129,130,131,132] |
| Chronic kidney disease | Delayed need for dialysis but need to monitor serum potassium and risk of possible malnutrition | [133,134,135,136,137] |
| Inflammation | Restoration of intestinal microbiota homeostasis (Firmicutes/Bacteroidetes ratio) and reduction in local and systemic inflammation | [138,139,140,141,142,143,144,145,146,147] |
| Mediterranean diet | ||
| PAD Risk Factors/Predictors | Evidence | Reference |
| Obesity | Multifactorial properties that lead to significant weight loss | [148,149,150,151,152,153,154,155,156] |
| Diabetes mellitus | Lower intake of high glycemic index foods with a positive impact on glucose management | [148,149,150,151,152,153,154,157,158,159,160,161] |
| Hypertension | Regression of arterial degenerative processes exerting benefits on endothelial function and stiffness that result in a more effective blood pressure control | [162,163,164,165,166,167,168,169,170,171,172,173] |
| Dyslipidemia | Reduction in intestinal absorption and endogenous production of cholesterol along with epigenetic control on lipid metabolism | [174,175,176,177,178,179,180,181,182,183,184,185,186,187,188] |
| Chronic kidney disease | Not effective in preventing the accumulation of GDUTS but promising results in kidney transplantation and in the prevention of kidney stones | [189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205] |
| Inflammation | “Hormetic therapy” with multilevel regulation of the inflammatory process and homeostasis of the immune system | [158,166,186,197,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226] |
| Emotional Eating, Binge Eating, Bulimia Nervosa, Anorexia Nervosa | ||
|---|---|---|
| PAD Risk Factors/Predictors | Evidence | Reference |
| Obesity | Maladaptive response to distress and discomfort lead to recurrent episodes of overeating resulting in a net weight gain | [227,228,229,230,231,232,233,234,235] |
| Diabetes mellitus | Harmful compensatory behaviors due to emotional involvement in relation to food that lead to low compliance with therapy, high risk of hypoglycemia and worsening of glucose plasma level control | [236,237,238,239,240,241,242,243,244,245,246] |
| Hypertension | The emotional substrate directly regulates blood pressure values hindering the achievement of blood pressure targets | [247,248,249,250,251,252] |
| Dyslipidemia | Stress decompression through overeating increases cholesterol levels | [249,250,252,253] |
| Chronic kidney disease | - | - |
| Inflammation | Each eating disorder exhibits a typical inflammatory pattern that requires further evaluation | [248,254,255,256,257,258,259,260,261] |
| Night eating and sleep disorders | ||
| PAD Risk Factors/Predictors | Evidence | Reference |
| Obesity | Impairment of food regulation patterns leading to hyperphagia due to hedonic eating | [262,263,264,265,266,267,268,269,270,271] |
| Diabetes mellitus | Hypoxia activates neuro-hormonal triggers resulting in insulin resistance | [272,273,274,275,276,277,278,279,280,281,282,283,284,285,286,287] |
| Hypertension | Frequent awakenings during sleep alter the night-time blood pressure profile | [288,289] |
| Dyslipidemia | U-shaped association between sleep habits and serum lipid concentrations. Hypoxic episodes trigger neuro-adrenocortical hyper-activation that increases cortisol plasma levels | [290,291,292,293,294,295,296,297,298,299,300,301,302] |
| Chronic kidney disease | - | - |
| Inflammation | - | - |
| Skipping meals | ||
| PAD Risk Factors/Predictors | Evidence | Reference |
| Obesity | The uneven distribution of calories throughout the day leads to obesity | [303,304,305] |
| Diabetes mellitus | Skipping meals randomly, alters glucose sensitivity and worsens HbA1c values | [305,306,307,308,309,310,311,312,313,314,315,316] |
| Hypertension | Progression of the neuro-hormonal mechanisms underlying hypertension | [305] |
| Dyslipidemia | Unsafe and ineffective long-term strategy for serum lipid control. Skipping meals worsens post-prandial lipid profile | [304,305,308,317] |
| Chronic kidney disease | - | - |
| Inflammation | Dysregulation of intestinal microbiota homeostasis with worrying consequences on systemic atherosclerosis | [308] |
| Home cooking, fast food access, ultra-processed and packaged food consumption | ||
| PAD Risk Factors/Predictors | Evidence | Reference |
| Obesity | Weight control is supported by a wide selection of foods | [318,319,320,321,322,323,324,325,326,327,328,329,330,331,332,333] |
| Diabetes mellitus | Promotion of a greater perspective on the impact of food on health, especially in low-income countries, that helps prevent chronic diseases such as DM | [334,335,336,337,338,339,340,341,342,343,344,345,346,347] |
| Hypertension | Patients with hypertension should be trained with educational cooking programs and avoid foods that worsen blood pressure control (e.g., salt consumption) | [348,349,350,351,352,353,354,355,356] |
| Dyslipidemia | The spread of low-quality foods has anticipated the incidence of atherosclerosis and related complications with a parallel increase in plasma lipid levels | [321,357,358,359,360,361,362] |
| Chronic kidney disease | Proper cooking methods could reduce the excessive intake of minerals (e.g., potassium, phosphorus) and proteins frequently contained in fast food, ultra-processed and packaged food | [363,364,365,366,367,368,369,370] |
| Inflammation | The low-quality of ultra-processed and packaged food was observed to increase oxidative stress and decrease in the reducing power of cells | [371,372,373,374,375,376] |
| Smoking and eating behaviors | ||
| Evidence | Reference | |
| High prevalence of eating disorders, particularly after quitting smoking due to withdrawal symptoms | [268,377,378,379,380,381,382,383] | |
| LEAD and Diets | ||
|---|---|---|
| Diet | Evidence | Reference |
| Low carbohydrate/ketogenic diet | Improvement of the metabolic syndrome and beneficial effect on all common comorbidities/risk factors in the PAD population. This dietary model can be adopted for short periods alternating with the MD | [44,384,385] |
| Intermittent fasting | Correction of dysbiosis with a reduction in inflammation; prevention of atherosclerotic plaque vulnerability by modulating local inflammation and change in the lipid core of the plaque of peripheral arteries; improvement of liver function and glucose/lipid metabolism with consequent secondary prevention of CV risk; concerns about osteoporosis and hypoglycemia in diabetic patients with PAD | [77,386,387,388,389,390] |
| Vegetarian and Vegan diet | Primary prevention by counteracting the progression of risk factors and comorbidities underlying LEAD development; effect similar to a reversal of the atherosclerotic process underlying the deterioration of the peripheral arteries; antioxidant protection on the inflammatory load of PAD; reduction in the incidence of MACE | [391,392,393,394,395,396,397,398,399,400,401] |
| Mediterranean diet | Probably the most effective and safe dietary model to adopt in order to prevent the incidence of PAD through the improvement of all coexisting risk factors; additional benefit on secondary prevention along with pharmacological therapies | [398,402,403,404,405] |
| PAD and Eating disorders | ||
| LEAD Risk Factors/Predictors | Evidence | Reference |
| Emotional eating, binge eating, bulimia nervosa, anorexia nervosa | Patients with PAD and psychiatric disorders involving the emotional sphere have the highest mortality rate due to the progression of the atherosclerotic process underlying PAD; eating disorders including over-eating and malnutrition are prevalent in advanced stages of PAD | [247,406,407,408,409,410,411,412,413,414] |
| Night eating and sleep disorders | Progression of the clinical stages of PAD; however, eating disorders involving sleep quality are rarely investigated | [415,416,417,418,419,420,421,422,423,424,425] |
| Skipping meals | Increased morbidity and mortality of patients with PAD by deteriorating the metabolic homeostasis | [308,315,426,427,428,429,430,431,432,433] |
| Home cooking, fast food access, ultra-processed and packaged food consumption | Dedicated educational cooking programs should be encouraged to prevent and even correct the deleterious dietary patterns often adopted by LEAD patients. The physical and functional impairment experienced by patients in the advanced stages of the disease hinders the proper provision/cooking of healthy foods | [434,435] |
| CTLI Progression, Nutrition, Dietary Models and Eating Behaviors/Disorders | ||
|---|---|---|
| Nutritional status and PAD | Evidence | Reference |
| Obesity | Obese patients with LEAD experience an overall approximately 1.5-fold increase in the development of CTLI regardless of other confounding factors | [436] |
| Malnutrition and sarcopenia | Malnutrition and sarcopenia have a devastating effect on patient outcomes with a low success rate in foot wound healing, a higher incidence of MACE and major amputations even with appropriate drug treatment. Malnutrition and sarcopenia have a strong prognostic role in limb salvage | [394,437,438,439,440,441,442] |
| Nutrients and PAD | Evidence | Reference |
| Vitamins | Vitamin deficiency (especially vitamin B12, C and D) appears to accelerate progression to CTLI, increase the rate of infections (particularly in PAD patients on dialysis), worsen post-amputation outcomes and increase the incidence of MACE and MALE | [443,444,445,446,447,448] |
| Micronutrients (zinc, magnesium) | Supplements to reduce the risk of progression to CTLI, incidence of revascularization failure or amputation are not supported by evidence | [448] |
| PUFAs | Eicosapentaenoic acid deficiency might be correlated with a higher incidence of MALE and MACE | [449,450] |
| Synthetic Fibrate | Fenofibrate in patients with diabetes mellitus further reduces the residual risk of microvascular complications including lower extremity amputation (LEA) | [451,452,453,454,455] |
| Diet and PAD | Evidence | Reference |
| Low carbohydrate/ketogenic diet | - | |
| Intermittent fasting | - | |
| Vegetarian and Vegan diet | This dietary model is rich in nutrients (especially PUFAs) that reduce the morbidity and mortality of patients with PAD and slow the progression to the advanced stages of the disease | [392,401] |
| Mediterranean diet | The MD may offer significant benefits in terms of lower mortality and morbidity with protective effects on MACE and MALE. However, the population with PAD often has socioeconomic limitations that reduce adherence to MD resulting in a higher amputation rate in these subcategories of low-income patients. | [398,456,457] |
| Eating behaviors/disorders and PAD | Evidence | Reference |
| Emotional eating, binge eating, bulimia nervosa, anorexia nervosa | The emotional burden resulting from progressive physical and functional limitation is often associated with eating disorders (emotional and binge eating), which further worsen survival and limb outcomes. Serum adipokines could interact with this complex interaction between emotional substrate, eating disorders and MALE incidence | [408,409,410,411,412,413,458] |
| Night eating and sleep disorders | Treatment of sleep and eating disorders is a preventative strategy for MACE and MALE | [424,425,459] |
| Skipping meals | - | |
| Home cooking, fast food access, ultra-processed and packaged food consumption | Physical and functional barriers to healthy food supplying and preparation resulting from the disabling complications of PAD further increase the risk of progression into CTLI | [434,435,460] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cecchini, A.L.; Biscetti, F.; Rando, M.M.; Nardella, E.; Pecorini, G.; Eraso, L.H.; Dimuzio, P.J.; Gasbarrini, A.; Massetti, M.; Flex, A. Dietary Risk Factors and Eating Behaviors in Peripheral Arterial Disease (PAD). Int. J. Mol. Sci. 2022, 23, 10814. https://doi.org/10.3390/ijms231810814
Cecchini AL, Biscetti F, Rando MM, Nardella E, Pecorini G, Eraso LH, Dimuzio PJ, Gasbarrini A, Massetti M, Flex A. Dietary Risk Factors and Eating Behaviors in Peripheral Arterial Disease (PAD). International Journal of Molecular Sciences. 2022; 23(18):10814. https://doi.org/10.3390/ijms231810814
Chicago/Turabian StyleCecchini, Andrea Leonardo, Federico Biscetti, Maria Margherita Rando, Elisabetta Nardella, Giovanni Pecorini, Luis H. Eraso, Paul J. Dimuzio, Antonio Gasbarrini, Massimo Massetti, and Andrea Flex. 2022. "Dietary Risk Factors and Eating Behaviors in Peripheral Arterial Disease (PAD)" International Journal of Molecular Sciences 23, no. 18: 10814. https://doi.org/10.3390/ijms231810814
APA StyleCecchini, A. L., Biscetti, F., Rando, M. M., Nardella, E., Pecorini, G., Eraso, L. H., Dimuzio, P. J., Gasbarrini, A., Massetti, M., & Flex, A. (2022). Dietary Risk Factors and Eating Behaviors in Peripheral Arterial Disease (PAD). International Journal of Molecular Sciences, 23(18), 10814. https://doi.org/10.3390/ijms231810814









