Abstract
In the human body, the intestine is the largest digestive and immune organ, where nutrients are digested and absorbed, and this organ plays a key role in host immunity. In recent years, intestinal health issues have gained attention and many studies have shown that oxidative stress, inflammation, intestinal barrier damage, and an imbalance of intestinal microbiota may cause a range of intestinal diseases, as well as other problems. Brown algae polysaccharides, mainly including alginate, fucoidan, and laminaran, are food-derived natural products that have received wide attention from scholars owing to their good biological activity and low toxic side effects. It has been found that brown algae polysaccharides can repair intestinal physical, chemical, immune and biological barrier damage. Principally, this review describes the protective effects and mechanisms of brown algae-derived polysaccharides on intestinal health, as indicated by the ability of polysaccharides to maintain intestinal barrier integrity, inhibit lipid peroxidation-associated damage, and suppress inflammatory cytokines. Furthermore, our review aims to provide new ideas on the prevention and treatment of intestinal diseases and act as a reference for the development of fucoidan as a functional product for intestinal protection.
1. Introduction
The intestine is the largest digestive and immune organ in the body, where nutrients are digested and absorbed. Immune cells in the gut can interact with microorganisms to maintain homeostasis in the intestinal environment, in addition to monitoring, recognizing, and differentiating food antigens from pathogens [1,2]. In recent years, intestinal health issues have gained attention and many studies have shown that oxidative stress, inflammation, malnutrition, medications, intestinal barrier damage, and imbalance of intestinal microbiota may cause a range of intestinal diseases, amid other disease problems. The intestinal barrier comprises physical, chemical, immune, and microbial barriers, which work to prevent pathogens and endotoxins from crossing into other tissues, organs, and blood. However, when the intestinal barrier is damaged via intestinal barrier injury (IBI), enterogenic infections, multi-organ failure, inflammatory bowel disease (IBD), sepsis, etc., may occur [3,4]. The intestinal tract is colonized by tens of trillions of microorganisms, referred to as intestinal microbiota, which are involved in digestion and the regulation of immunity, and act as biological barriers to protect the gastrointestinal tract from pathogens. There have been many studies which have shown that an imbalance of intestinal microbiota is associated with various immune-based, gastrointestinal, and metabolic diseases [5,6,7,8,9]. Therefore, maintaining the balance and health of intestinal microbiota, and protecting the integrity of the intestinal barrier are of great importance for the prevention and treatment of various diseases.
Seaweeds are the most abundant resources in the ocean; they can be classified into three main groups based on their pigmentation and chemical composition: brown, red, and green algae [10]. Brown algae are photosynthetic multicellular organisms that include the genera Laminaria (Kombu), Macrocystis, Kelp [11]. Brown algae-derived polysaccharides are one of the main active components of brown algae and mainly include alginate, fucoidan, and laminaran [12,13]. The polysaccharides have been reported to elicit protective effects on the intestinal tract by regulating intestinal microbiota, upregulating the expression of TJ proteins, inhibiting the expression of inflammatory factors, and suppressing oxidative stress, to repair intestinal barrier injury [14,15,16,17]. This paper reviews the protective effects and mechanisms of brown algae-derived polysaccharides in the intestine.
2. Brown Algae Polysaccharides
In addition to their food value, other important components found in brown algae include phenolic compounds, sulfated polysaccharides, quinones and several secondary metabolites, all of which have been studied for use against a variety of diseases [18]. Brown algae polysaccharides are collectively referred to as polysaccharides isolated and extracted from brown algae mainly include alginate, fucoidan, and laminaran. The main species and distribution of brown algae with protective effects on the intestine are shown in Table 1.

Table 1.
The main species and distribution of brown algae with protective effects on the intestine.
2.1. Classification of Brown Algae Polysaccharides
2.1.1. Alginate
Alginate, with the molecular formula C6H7NaO6, is the main polysaccharide component of both the cell wall and intercellular matrix of brown algae. It is a linear polysaccharide consisting of two conformational isomeric residues, β-D-mannuronate (M) and α-L-guluronate (G), connected by 1,4-glycosidic bonds [36]. The molecule can be arranged in three different conformations: MM, GG, and MG [37]. The structure of alginate is shown in Figure 1 [38,39]. The M/G ratio greatly affects the physicochemical properties, applications, and activities of alginate [40]; molecules with a high G content form firm gels for the food and cosmetic industries, while a high M content results in low viscosity appropriate for the production of nanoparticles, paper, for dyeing, or in the textile industry [37]. Alginate is readily fermented by intestinal bacteria to produce large amounts of short-chain fatty acids (SCFAs) to provide energy to intestinal epithelial and immune cells [41] and affects glucose and lipid metabolism, alleviating obesity, diabetes, insulinemia and other metabolic diseases [42]. Therefore, alginate has an important role in maintaining intestinal health and preventing the development of metabolic diseases. In addition, derivatives of alginate have a wide range of biological activities, and sulfated polymannuroguluronate (SPMG) is a sulfated form of sodium alginate (Figure 2) [43]. SPMG was shown to improve chemotherapy-induced leukopenia [44] and also effectively prevented the internalization of viral particles by interfering with the interaction between viral and host cell receptors [45,46]; SPMG significantly increases the abundance of Bifidobacterium and Lactobacillus in the intestine by altering the intestinal microbiota structure, prevents diet-induced obesity, and improves glucose tolerance and alleviates inflammation [47].
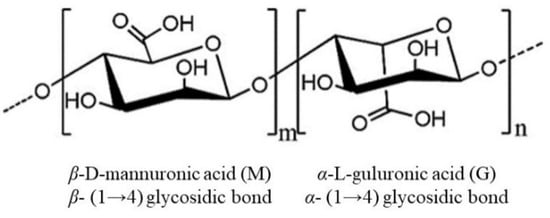
Figure 1.
The structure of the alginate.
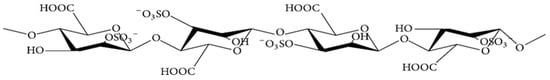
Figure 2.
Chemical structure of sulfated polymannuroguluronate (SPMG).
2.1.2. Fucoidan
Fucoidan, with the molecular formula (C6H10O7S)n, is a polysaccharide containing sulfate groups also known as sulfated polysaccharide. It is mainly present in the cell wall of brown algae where it maintains the stability of the cell membrane and protects its structure from dehydration [48]. There are remarkable differences in the structure and chemical compositions of the fucoidans found in different algal species, but they usually include (1→3)-linked α-L-amylopyranosyl skeletal structures and occasionally both (1→3)-linked and (1→4)-linked α-L-amylopyranosyl structures, where L-amylopyranose residues on C-2 or C-4 can be replaced by sulfate (SO3−) [49]. Fucoidan has a diverse chemical structure and composition, is usually sulfated and acetylated, and also contains glyoxalate [50,51,52], the structure of which is shown in Figure 3.
Fucoidan has a range of biological activities due to the presence of sulfate groups. Furthermore, it has been demonstrated that fucoidan can improve damage of the intestinal physical, chemical, immune, and biological barriers by upregulating intestinal epithelial cell TJ protein expression, inhibiting intestinal pro-inflammatory cytokine expression, and modulating intestinal microbiota abundance [15,53,54].
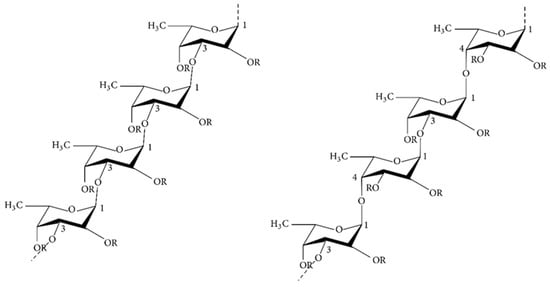
Figure 3.
Chemical structures of two different backbones for fucoidan [55].
2.1.3. Laminaran
Laminaran, with the molecular formula C18H32O16 and a molecular weight in the range of 1–10 KDa, is mainly found in the cytoplasm. The main chain consists of β-1,3-D-glucose and β-1,6 as a branched chain [56]. There are two types of chains: one linked by D-mannitol residue ends (M), and the other linked by D-glucose residue ends (G) (Figure 4) [57]. Laminaran has both water-soluble and water-insoluble forms; one form is characterized by complete solubility in cold water, while the other is soluble only in hot water. The presence of branched chains also affects solubility; the more branched chains, the higher the solubility in cold water [58]. Studies have shown that laminaran administration to rats affects the composition of intestinal mucus, increases the production of SCFAs, regulates intestinal metabolism, reshapes the structure of intestinal microbiota and reduces the incidence of obesity, NAFLD and diabetes [59,60].
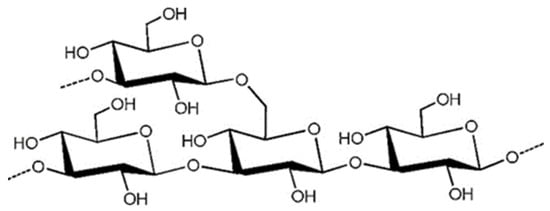
Figure 4.
The presumptive structure of the laminaran. (Reprinted with permission from Ref. [61]. Copyright 2020, Elsevier).
2.2. Extraction of Brown Algae Polysaccharides
Brown algae polysaccharide fractions are complex, and the extraction methods and conditions may affect the composition, yield, molecular weight and biological activity of polysaccharides [62]. Therefore, if we want to obtain the target components efficiently, we have to choose the corresponding extraction methods. The steps of polysaccharide extraction include: removal, extraction and purification [63]. According to the chemical properties of polysaccharides, different extraction methods can be chosen: water extraction, acid extraction, alkali extraction, auxiliary extraction, etc.; according to the chemical properties of polysaccharides, molecular weight size, different purification methods can be chosen: precipitation, column chromatography, etc. [64,65]. The extraction process of brown algae polysaccharide is shown in Figure 5 [66,67,68].
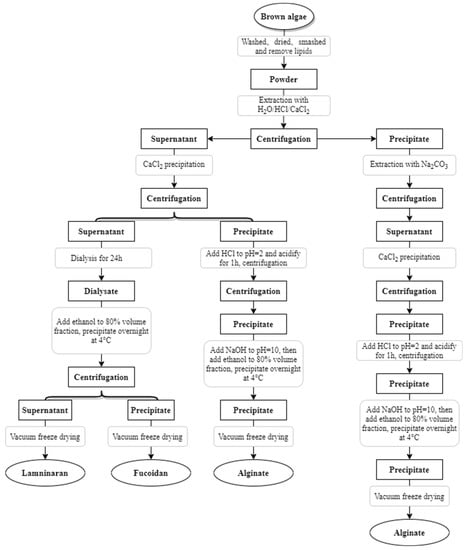
Figure 5.
Extraction and separation process of brown algae polysaccharide.
3. Protective Effect of Brown Algae-Derived Polysaccharides on Metabolic Diseases and Intestinal Barrier Injury
Many factors affect intestinal health, as shown in Figure 6; inflammation, drugs, lipid peroxidation, and damage to the intestinal barrier can all cause diseases related to intestinal inflammation. The activation of nuclear factor kappa-B (NF-κB) and mitogen-activated protein kinase (MAPK) pathways can promote the secretion of pro-inflammatory cytokines (TNF-α, NO, iNOS, COX-2, IL-1β, IL-6, etc.) by T cells, B cells and monocytes. Excessive accumulation of reactive oxygen species (ROS) can promote the production of inflammatory cytokines by immune cells, leading to oxidative stress and inflammation. This further activates signaling pathways such as MAPK, PKC, JNK and ERK, leading to the breakdown of the TJ complex between epithelial cells, and thus impairing intestinal epithelial barrier function [69]. Additionally, drug treatment can result in damage to the intestinal mechanical barriers [3,70].
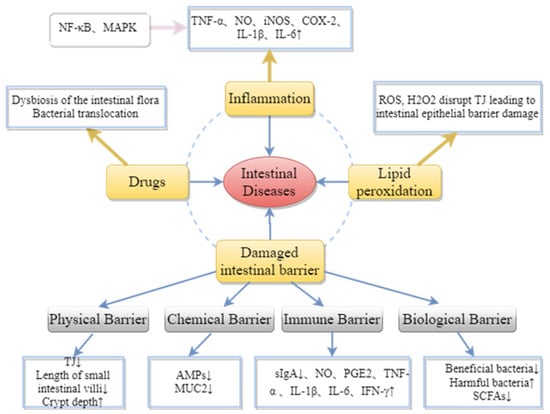
Figure 6.
Pathogenesis of intestinal diseases.
The protective effects of brown algae-derived polysaccharides on the intestine are shown in Table 2. These polysaccharides not only increase the synthesis of transmembrane proteins in the intestinal epithelial cells, but also regulate the intestinal microbiota, inhibit inflammatory responses, and play a protective and regulatory role against damage to the intestinal barrier and inflammation.

Table 2.
Protective effect and mechanism of brown algae-derived polysaccharides on intestinal health.
3.1. Brown Algae-Derived Polysaccharides Maintain Intestinal Barrier Integrity
The intestinal barriers include physical, chemical, immune, and biological elements; defects in barrier function may lead to chronic immune activation and so play a pathogenic role in many diseases such as celiac disease, colorectal cancer, inflammatory bowel disease (IBD), obesity, and diabetes [81]. Therefore, maintaining intestinal barrier integrity plays an important protective role in intestinal health and disease prevention.
3.1.1. Maintaining the Integrity of the Physical Barrier
The intestinal physical barrier consists of intestinal epithelial cells (IECs) and intercellular junctions: adherens junctions (AJs), bridging granules, and TJs [82]. The TJ is the most apical linker complex, consisting of transmembrane proteins, such as occludin, claudins, and junctional adhesion molecule (JAM), and intracellular plaque proteins, such as zonula occludens (ZO) [83]. These proteins are responsible for closing cellular gaps and regulating selective paracellular ion solute transport. TJs are regulated by several intracellular signaling pathways, such as myosin light chain kinase (MLCK), PKC, and MAPK [84]. In addition, the TJ is connected to the cytoskeleton supporting the epithelial cells; thus, forming a dynamic barrier system composed of IECs [85]. In fact, the TJ is the most important intercellular structure, and its disruption and increased paracellular permeability play a crucial role in the pathogenesis of IBD [3]. Furthermore, the length of small intestinal villi and crypt depth are important indicators of small intestinal digestion and absorption. The longer the length of the villi and the shallower the depth of the crypts, the better the digestion and absorption. Moreover, studies have shown that fucoidan can improve constipation, improve small intestinal tissue morphology, and repair tissue damage of IECs by promoting intestinal motility [17,86].
Matayoshi et al. [86] used a double-blind randomized clinical trial to study the efficacy of dried powder of Cladosiphon okamuranu in improving constipation. The results showed that C. okamuranu increase the frequency of defecation and regulate bowel function in patients with constipation. Xue et al. [17] showed that the consumption of fucoidan improved the structure of the small intestinal villi in mammary carcinoma rats, upregulated the levels of TJ proteins, ERK1/2, and p38 MAPK phosphorylation in rat jejunum tissues. It has also been demonstrated that fucoidan significantly reduced paracellular permeability and enhanced intestinal barrier function by upregulating claudin-1, Occludin, and ZO-1 expression [15,87]. Alginate oligosaccharides increased occludin abundance in TNF-α-treated IPEC-J2 cells, thereby ameliorating inflammatory damage in intestinal epithelial cells [88]. Thus, brown algae-derived polysaccharides can improve intestinal physical barrier damage by upregulating transmembrane protein expression, activating signaling pathways related to the regulation of TJs, and decreasing paracellular permeability.
3.1.2. Enhancement of Intestinal Chemical Barrier Function
The intestinal chemical barrier includes digestive juices, bile acids, antimicrobial peptides (AMPs), mucins, and other compounds secreted by IECs in the intestinal lumen to prevent bacterial adhesion [89]. Goblet cells can secrete mucins to form a dense gel covering the intestinal mucosa to prevent bacterial invasion [90]. MUC2 is the most abundant mucin in the small intestine and colon, and MUC2 deficiency makes the host susceptible to pathogenic bacteria invasions, alters mucus layers, defective AMPs, and can lead to bacterial translocation due to increased intestinal permeability, resulting in disease [91,92].
The addition of the brown algae-derived polysaccharides promoted intestinal mucin expression, and A.G. Smith found that the addition of laminarin to the diet had a significant effect on the expression of secreted MUC2 and the membrane-bound mucin MUC4 in the pig colon [93]. In addition, polysaccharides can bind bile acids, inhibit bile acid reabsorption, and promote cholesterol metabolism in the liver. Related studies have shown that the ability of polysaccharides to bind bile acids may be related to the sulfate groups in polysaccharides [94].
3.1.3. Improving Intestinal Immune Barrier Protection
The intestine is the largest immune system in the body, and the gut-associated lymphoid tissue (GALT) is the main component of the intestinal immune system, accounting for 70% of the systemic immune function. Intestinal immune cells include T cells, B cells, innate lymphocytes (ILC1, ILC2, ILC3), and the mononuclear phagocyte system (monocytes, dendritic cells (DC), and macrophages) [95,96]. These immune cells can secrete relevant pro- and anti-inflammatory cytokines and enzymes to regulate intestinal inflammation and immune responses [97]. Specific secretory immunoglobulins (sIgA) are mainly produced by lymphocytes and plasma cells, which are distributed on the surfaces of the intestinal mucosa. They are the most abundant immunoglobulins in intestinal secretions, functioning as the main factors stopping pathogen invasion, and so playing a key role in the intestinal immune system. Fucoidan has bi-directional immunomodulatory effects: immune-enhancing and immune-suppressing. The pattern recognition receptors (PRRs) that recognize polysaccharides in the intestine include Toll-like receptors (TLRs) and nucleotide oligomerization domain (NOD)-like receptors (NLRs). The specific recognition of polysaccharides by PRRs may trigger signaling pathways that regulate inflammation and immune cytokines, ultimately leading to upregulation of related gene expression and protein synthesis; thus, regulating the intestinal immunity to protect against compromised intestinal immune barriers [97].
Cancer is one of the most serious threats to human health, and CPA is commonly used to treat cancer, but its long-term use can suppress normal immune responses [98]. CPA significantly increases ROS levels and promotes apoptosis; it reduces the production of antioxidant enzymes (Catalase (CAT), Superoxide Dismutase (SOD), Glutathione peroxidase (GSH-Px)) in the spleen and thymus [99]. The fucoidan derived from Acaudina molpadioides can reduce intestinal inflammation, promote TJ protein expression, and increase the abundance of SCFAs-producing microorganisms (Coprococcus, Rikenella, and Butyricicoccus) by reducing the CPA-induced intestinal mucosal damage [54]. Additionally, it has been reported that Laminaria sodium alginate increased immune organ indices, decreased splenic T lymphocytes, and significantly increased serum immunoglobulin and cytokine secretion in immunosuppressed mice [73]. Fucoidan reversed CPA-induced immunosuppression, promoted intestinal immunity, and increased sIgA secretion [100]. Therefore, fucoidan can improve the damage to the intestinal immune barrier by promoting the secretion of intestinal sIgA and reducing intestinal inflammation-related cytokines; thus, protecting the intestine.
The inflammatory response is an excessive immune response; both LPS and dextran sodium sulfate (DSS) induce inflammatory responses. Jeong et al., [74] showed that fucoidan significantly inhibited LPS-induced secretion of pro-inflammatory mediators, including NO, PGE2, TNF-α, and IL-1β, without any remarkable cytotoxicity. Fucoidan also inhibited NF-κB translocation from the cytoplasm to the nucleus and attenuated LPS-induced intracellular ROS production in RAW 264.7 macrophage-like cells. The pro-inflammatory cytokine IL-6 is highly expressed in the serum of patients with Crohn’s disease (CD), and blocking IL-6 expression may provide benefit to patients with CD. It has been shown that IL-6 plays an important role in colitis in mice and accordingly, inhibition of IL-6 secretion plays a key role in the treatment of intestinal inflammation [101]. In a mouse model of chronic colitis, treatment with fucoidan (Cladosiphon okamuranus Tokida) inhibited the activation of NF-κB pathway, thereby suppressing IL-6 synthesis and down-regulating IFN-γ expression to improve chronic colitis in rats [102]. Fucoidan (20 mg kg−1) could inhibit IL-6, TNF expression, and IFN-γ to prevent gastric inflammation associated with aspirin-induced gastric ulcers [103]. It has been reported that low concentrations of fucoidan promote the secretion of NO, iNOS, ROS, IL-1β, IL-6, IL-12, and TNF-α by macrophages, while high concentrations of fucoidan inhibit the secretion of pro-inflammatory factors and thus act as an anti-inflammatory agent [100]. Alginate oligosaccharides inhibited apoptosis and decreased IL-6 and TNF-α concentrations in TNF-α-treated IPEC-J2 cells [88]. Bioactive molecules, such as NO and TNF-α, can indirectly induce cytotoxicity, but within a certain concentration range, pro-inflammatory factors can induce other immune cells to release cytokines and participate in immune response further activating their anti-tumor function. Therefore, further studies are needed to prove whether the promotion or inhibition of pro-inflammatory cytokine secretion in the bidirectional immunomodulatory effect of brown algae-derived polysaccharides can coordinate innate immunity and inflammatory response.
3.1.4. Maintenance of Intestinal Microbial Barrier by the Intestinal Microbiota Balance
The intestinal tract is colonized by tens of trillions of microorganisms—the intestinal microbiota—that are involved in digestion, modulate immunity, and act as a biological barrier to protect the gastrointestinal tract from pathogens. The intestinal microbiota can maintain host health by regulating metabolism, epithelial barrier integrity, and immune system development and function, intestinal microbiota can maintain host health by regulating metabolism, epithelial barrier integrity and immune system development and function, reducing the risk of obesity, hyperlipidemia and diabetes [104,105,106,107]. Polysaccharides typically promote the proliferation of SCFA-producing microorganisms by modulating immune cells to improve intestinal microbiota and the entry of undigested polysaccharides into the colon for fermentation.
Under normal circumstances, the immune system and intestinal microbiota interact to regulate the body’s immunity and metabolism to help maintain the host’s health, but once this mutual balance is disrupted, it will cause the intestinal microbiota and immune system to become dysregulated, making the body more susceptible to pathogenic infections and inducing various diseases [108]. In patients with IBD, the diversity of the intestinal microbiota is reduced, with fewer Bacteroides and an increase in Proteus (e.g., E. coli and Clostridium). The decrease in the number and impaired function of both Paneth and goblet cells leads to a decrease in the thickness of the mucus layer, which in turn reduces mucosal integrity, promotes dysbiosis of the intestinal microbiota, and finally leads to impaired physical barrier function of the intestine and thus bacterial translocation [109]. Dysbiosis of the intestinal microbiota increases bacterial translocation, which stimulates the activation of antigen-presenting cells (e.g., DCs and macrophages), which then induces alterations in T-cell subsets (increased Th1, Th2, and Th17 cells as well as decreased Treg cells), leading to pro-inflammatory responses and tissue damage [97]. Brown algae extracted laminaran and fucoidan were added to the diet of weaned pigs and fed to pigs, respectively, to increase the intestinal crypt depth ratio. A remarkable reduction in the abundance of Enterobacteriaceae in the intestine, and down-regulation of IL-6, IL-17A, and IL-1β mRNA expression in the colon was seen [110]. Acaudina molpadioides derived fucoidan increased the ratio of intestinal villi length to crypt depth and improved the IFN-γ/IL-4 ratio in an intestinal mucosa mouse model, allowing for Th1/Th2 immune homeostasis. Additionally, fucoidan promoted IgA expression to enhance intestinal adaptive immunity [111].
Another beneficial effect of the polysaccharides on intestinal microbiota is that they contribute to the proliferation of SCFAs-producing microorganisms. The anaerobic fermentation of polysaccharides into the intestine promotes anaerobic metabolism of bacteria to produce SCFAs, thereby lowering the pH of the intestine and inhibiting the proliferation of pathogenic gram-negative bacteria in the intestine [112,113]. Fucoidan exhibits prebiotic activity, which promotes the growth of beneficial intestinal bacteria and inhibits the growth of harmful bacteria. Liu et al. [114] showed that fucoidan from Undaria pinnatifida can increase the abundance of intestinal Bacteroidetes and Alloprevotella in BALB/c mice, decrease the abundance of Firmicutes, Staphylococcus, and Streptococcus, lower serum and liver cholesterol levels, and alleviate dyslipidemia in mice on a high-fat diet; fucoidan improved symptoms in diabetic mice associated with altered intestinal microbiota, reducing the relative abundance of diabetes-associated intestinal microbiota such as Oscillibacter, Ruminococcaceae, Peptostreptococcaceae, and Peptococcaceae [115]; fucoidan may also improve insulin resistance by reshaping the structure of the intestinal microbiota [25]. Alginate oligosaccharides decreased the relative abundance of bacteria of the phylum Bacteroidetes and increased the abundance of phyla Firmicutes and Actinobacteria in the intestine of mice with ulcerative colitis. These findings suggested that alginate oligosaccharides carried out the function of maintaining the mucosal barrier by modulating the intestinal ecosystem [116].
In addition, intestinal microbiota metabolites (SCFAs) can target the intestine, liver, pancreas and other organs to regulate gastrointestinal hormone secretion, control blood glucose, improve blood lipids, alleviate insulin resistance and inflammation, and have an impact on host physiology and immunity [117,118]. Acetate protects the host intestine from infection, whilst butyrate provides energy to colon cells, regulates stem cell proliferation, anti-inflammatory macrophage polarization with inhibition of histone deacetylase (HDAC), and activation of histone acetyltransferases, thus, affecting cellular transcriptional regulation [108]. SCFAs provide energy to intestinal cells, reduce the incidence of inflammatory diseases, and modulate the innate and adaptive immune systems [119,120]. Studies have shown that in vitro fecal fermentation of sodium alginate from Ascophyllum nodosum can significantly increase the concentrations of acetate and propionate [75]. Furthermore, it has been shown that fucoidan can significantly increase the concentrations of acetate and butyrate in the feces of mice with colitis to alleviate antimicrobial-induced colitis in mice [28]. In conclusion, the entry of indigestible fucoidan into the colon for fermentation can increase intestinal microbiota richness, promote the production of SCFAs, and lower intestinal pH to inhibit the proliferation of pathogenic microorganisms; thus, maintaining intestinal microbiota balance and enhancing intestinal immunity.
3.2. Inhibition of Lipid Peroxidation Damage by Brown Algae-Derived Polysaccharides
Oxidative stress is one of the factors that disrupt the intestinal barrier. Indeed, oxidative stress due to the accumulation of ROS is the pathological basis of many intestinal diseases. Oxidative stress can disrupt the intercellular TJ complex through multiple signaling pathways, namely, PKC, MAPK, JNK, and ERK; thereby, impairing intestinal epithelial barrier functions by redistributing TJs and AJs [69].
It has been shown that Fucus vesiculosus fucoidan (FVF) increased glucose consumption and alleviated sodium palmitate-induced insulin resistance through ROS-mediated JNK and Akt signaling pathways with decreased cellular levels of ROS [31]. Sargassum fusiforme alginate can reduce oxidative stress to some extent by increasing the activities of antioxidant enzymes (CAT, SOD) in the serum of high-fat diet-induced diabetic mice [6]. Thus, brown algae-derived polysaccharides may protect the intestine from oxidative stress-induced damage by decreasing ROS production, or by promoting an increase in the activities of antioxidant enzymes.
3.3. Inhibition of Inflammatory Cytokines by Brown Algae-Derived Polysaccharides
A moderate immune response protects the intestine from pathogens and eliminates them, whereas an excessive immune response makes the intestine more vulnerable to pathogens. Inflammation is a common excessive immune response in organisms [97]. Endotoxin LPS is capable of eliciting inflammatory responses, and many studies have used LPS to induce inflammatory cells and animal models; LPS is a major ligand for TLR4, and LPS binding to TLR4 can promote the expression of pro-inflammatory cytokines and enzymes, such as TNF-α, NO, iNOS, and COX-2. LPS can also increase pro-inflammatory cytokines and enzymes by activating expression of the MAPK pathway [121]. Therefore, the need to maintain pro-inflammatory and anti-inflammatory cytokines in a certain range is a useful approach for the treatment of inflammatory diseases.
Currently, brown algae-derived polysaccharides have been shown to possess inhibitory activities on the expression of pro-inflammatory cytokines. In LPS-treated RAW 264.7 cells, Sargassum horneri fucoidan could downregulate the protein expression levels of iNOS and COX-2, production of TNF-α and IL-1β, and inhibit the phosphorylation of ERK1/2 and p38 in a dose-dependent manner [122]. Fucoidan of Cladosiphon okamuranus could inhibit neutrophil extravasation into the peritoneal cavity, eliciting anti-inflammatory effects in a rat model of acute peritonitis [51]. In addition, the fucoidan derived from Sargassum hemiphyllum can inhibit LPS-induced inflammatory responses by inhibiting IL-1β and TNF-α, promoting IL-10 and IFN-γ, and significantly enhancing intestinal epithelial barrier and immune function [14]. Therefore, brown algae-derived polysaccharides can inhibit NF-κB and MAPK pathways, and the expression of related pro-inflammatory factors; thus, they have the potential to be developed into functional anti-inflammatory foods.
In summary, brown algae-derived polysaccharides can play both preventive and therapeutic roles in intestinal diseases by repairing intestinal barrier damage, inhibiting lipid peroxidation, and suppressing the expression of inflammatory factors. The protective mechanism of brown algae-derived polysaccharides against intestinal damage is shown in Figure 7. Using brown algae-derived polysaccharides as prebiotics can upregulate TJ protein expression to maintain the integrity of the intestinal physical barrier; thus, reducing bacterial translocation and pathogen invasion. The brown algae-derived polysaccharides regulated intestinal microbiota abundance, increased the abundance of beneficial intestinal bacteria, and promoted the production of SCFAs and AMP along with MUC2 by goblet cells. In addition, they enhanced intestinal chemical barrier function and stimulated DC, T, and B cells to secrete anti-inflammatory cytokines and antibodies, which improved intestinal immunity and strengthened the intestinal microbial barrier. Brown algae-derived polysaccharides can play an immune-enhancing role by activating NF-κB and MAPK signaling pathways, up-regulating inflammatory factor expression to alleviate the immunosuppressive effect induced by the chemotherapeutic drug CPA. The polysaccharides ameliorated production of reactive oxygen species by inhibiting the activation of the NF-κB pathway. This promoted the synthesis of antioxidant enzymes and inhibited the inflammatory response caused by LPS. Simply summarized, the polysaccharides prevented the activation of NF-κB and MAPK signaling pathways and resulted in anti-inflammatory effects through the inhibition of pro-inflammatory cytokines.
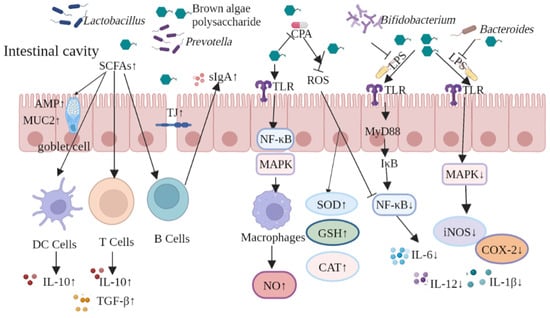
Figure 7.
Protective mechanisms of brown algae-derived polysaccharides against intestinal damage.
4. Conclusions and Future Perspectives
The biological activity of brown algae-derived polysaccharides is related to their molecular weight, size, monosaccharide composition, and the functional group content, location, conformation, and configuration [123]. Therefore, the classification of polysaccharide structures and the study of their structure-function relationships can help to reveal the biochemical basis of activity, and to lay the theoretical foundation for the screening of polysaccharides with protective effects on the intestine.
The development of intestinal diseases is often associated with damage to the intestinal barrier, so maintaining the integrity of the barrier plays an important role in protecting the intestine. Among them, the intestinal biological barrier plays a more important role in maintaining intestinal health. Intestinal microbiota, as well as intestinal microbiota metabolites, can affect the integrity of the epithelial barrier, chemical barrier, and immune barrier. Much of the current research on intestinal microbiota has focused on the following: the effects of targeting intestinal microbiota on disease, the effects of drugs and disease on the intestinal microbiota, and the effects of drugs and disease on microbiota diversity, along with the abundance of beneficial and harmful intestinal bacteria. Despite the rich diversity of intestinal microbiota, the effects of only a few microorganisms on disease and immunity have so far been demonstrated, and further studies on the effects of other microorganisms on intestinal disease and immunity are required to understand the relationship between intestinal microbiota and disease.
We have shown that fucoidan exhibits prebiotic activity and can protect and regulate the intestinal barrier. It functions by improving intestinal barrier damage, promoting the proliferation of beneficial intestinal microorganisms, inhibiting the proliferation of intestinal pathogenic bacteria, and promoting the growth of intestinal short-chain fatty acid-producing microorganisms. In summary, fucoidan possesses anti-inflammatory, anti-tumor, and immunomodulatory potentials. It also has the potential to be developed as a functional food for patients with inflammatory bowel disease and cancer.
Author Contributions
Conceptualization, L.S. and M.W.; investigation, M.L. and D.O.; writing—original draft preparation, Y.Y. and H.T.; writing—review and editing, Y.Y. and L.S.; supervision, L.S.; project administration, L.S.; funding acquisition, L.S. and M.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the National Natural Science Foundation of China (41876197), Agricultural national and industrial standards revision project (NYB-22270), Public Welfare Projects in Zhejiang Province (LGN20C200003), Key Scientific and Technological Innovation Project of Wenzhou (2022ZN0005 and ZD202003), and the Wenzhou Science and Technology Commissioner Project (X20210015).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, S.; Yu, M. Role of Goblet Cells in Intestinal Barrier and Mucosal Immunity. J. Inflamm. Res. 2021, 14, 3171–3183. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.C.; Schridde, A. Origin, Differentiation, and Function of Intestinal Macrophages. Front. Immunol. 2018, 9, 2733. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Wu, Z.; Sun, W.; Wang, Z.; Wu, J.; Huang, M.; Wang, B.; Sun, B. Protective Effects of Natural Polysaccharides on Intestinal Barrier Injury: A Review. J. Agric. Food Chem. 2022, 70, 711–735. [Google Scholar] [CrossRef] [PubMed]
- Sauruk da Silva, K.; Carla da Silveira, B.; Bueno, L.R.; Malaquias da Silva, L.C.; da Silva Fonseca, L.; Fernandes, E.S.; Maria-Ferreira, D. Beneficial Effects of Polysaccharides on the Epithelial Barrier Function in Intestinal Mucositis. Front. Physiol. 2021, 12, 714846. [Google Scholar] [CrossRef]
- Bao, Y.F.; Dong, C.; Ji, J.; Gu, Z.F. Dysregulation of gut microbiome is linked to disease activity of rheumatic diseases. Clin. Rheumatol. 2020, 39, 2523–2528. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, S.Y.; Cheng, Y.; Liu, Q.H.; Su, L.J.; Yang, Y.; Zhang, X.; Wu, M.J.; Choi, J.I.; Tong, H.B. Sargassum fusiforme Alginate Relieves Hyperglycemia and Modulates Intestinal Microbiota and Metabolites in Type 2 Diabetic Mice. Nutrients 2021, 13, 2887. [Google Scholar] [CrossRef]
- Ma, Q.T.; Li, Y.Q.; Li, P.F.; Wang, M.; Wang, J.K.; Tang, Z.Y.; Wang, T.; Luo, L.L.; Wang, C.G.; Zhao, B.S. Research progress in the relationship between type 2 diabetes mellitus and intestinal flora. Biomed. Pharm. 2019, 117, 109138. [Google Scholar] [CrossRef]
- Tang, Q.; Cao, L. Intestinal flora and neurological disorders. Sheng Wu Gong Cheng Xue Bao = Chin. J. Biotechnol. 2021, 37, 3757–3780. [Google Scholar] [CrossRef]
- Hills, R.D., Jr.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef]
- Xu, S.Y.; Huang, X.; Cheong, K.L. Recent Advances in Marine Algae Polysaccharides: Isolation, Structure, and Activities. Mar. Drugs 2017, 15, 388. [Google Scholar] [CrossRef]
- Li, Y.P.; Zheng, Y.T.; Zhang, Y.; Yang, Y.Y.; Wang, P.Y.; Imre, B.; Wong, A.C.Y.; Hsieh, Y.S.Y.; Wang, D.M. Brown Algae Carbohydrates: Structures, Pharmaceutical Properties, and Research Challenges. Mar. Drugs 2021, 19, 620. [Google Scholar] [CrossRef] [PubMed]
- Chi, S.; Liu, T.; Wang, X.; Wang, R.; Wang, S.; Wang, G.; Shan, G.; Liu, C. Functional genomics analysis reveals the biosynthesis pathways of important cellular components (alginate and fucoidan) of Saccharina. Curr. Genet. 2018, 64, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Dobrincic, A.; Balbino, S.; Zoric, Z.; Pedisic, S.; Bursac Kovacevic, D.; Elez Garofulic, I.; Dragovic-Uzelac, V. Advanced Technologies for the Extraction of Marine Brown Algal Polysaccharides. Mar. Drugs 2020, 18, 168. [Google Scholar] [CrossRef]
- Hwang, P.A.; Phan, N.N.; Lu, W.J.; Hieu, B.T.N.; Lin, Y.C. Low-molecular-weight fucoidan and high-stability fucoxanthin from brown seaweed exert prebiotics and anti-inflammatory activities in Caco-2 cells. Food Nutr. Res. 2016, 60, 32033. [Google Scholar] [CrossRef] [PubMed]
- Iraha, A.; Chinen, H.; Hokama, A.; Yonashiro, T.; Kinjo, T.; Kishimoto, K.; Nakamoto, M.; Hirata, T.; Kinjo, N.; Higa, F.; et al. Fucoidan enhances intestinal barrier function by upregulating the expression of claudin-1. World J. Gastroenterol. 2013, 19, 5500–5507. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Luthuli, S.; Yang, Y.; Cheng, Y.; Zhang, Y.; Wu, M.J.; Choi, J.I.; Tong, H.B. Therapeutic and nutraceutical potentials of a brown seaweed Sargassum Fusiforme. Food Sci. Nutr. 2020, 8, 5195–5205. [Google Scholar] [CrossRef]
- Xue, M.L.; Ji, X.Q.; Liang, H.; Liu, Y.; Wang, B.; Sun, L.L.; Li, W.W. The effect of fucoidan on intestinal flora and intestinal barrier function in rats with breast cancer. Food Funct. 2018, 9, 1214–1223. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Charoensiddhi, S.; Conlon, M.A.; Vuaran, M.S.; Franco, C.M.M.; Zhang, W. Impact of extraction processes on prebiotic potential of the brown seaweed Ecklonia radiata by in vitro human gut bacteria fermentation. J. Funct. Foods 2016, 24, 221–230. [Google Scholar] [CrossRef]
- Lee, S.H.; Ko, C.I.; Jee, Y.; Jeong, Y.; Kim, M.; Kim, J.S.; Jeon, Y.J. Anti-inflammatory effect of fucoidan extracted from Ecklonia cava in zebrafish model. Carbohydr. Polym. 2013, 92, 84–89. [Google Scholar] [CrossRef]
- Oh, S.; Shim, M.; Son, M.; Jang, J.T.; Son, K.H.; Byun, K. Attenuating Effects of Dieckol on Endothelial Cell Dysfunction via Modulation of Th17/Treg Balance in the Intestine and Aorta of Spontaneously Hypertensive Rats. Antioxidants 2021, 10, 298. [Google Scholar] [CrossRef]
- Khuituan, P.; Huipao, N.; Jeanmard, N.; Thantongsakul, S.; Promjun, W.; Chuthong, S.; Tipbunjong, C.; Peerakietkhajorn, S. Sargassum plagiophyllum Extract Enhances Colonic Functions and Modulates Gut Microbiota in Constipated Mice. Nutrients 2022, 14, 496. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Yan, J.; Bak, J.; Park, J.; Lee, H.; Kim, H. Sargassum thunbergii Extract Attenuates High-Fat Diet-Induced Obesity in Mice by Modulating AMPK Activation and the Gut Microbiota. Foods 2022, 11, 2529. [Google Scholar] [CrossRef]
- Wu, Q.; Wu, S.; Cheng, Y.; Zhang, Z.; Mao, G.; Li, S.; Yang, Y.; Zhang, X.; Wu, M.; Tong, H. Sargassum fusiforme fucoidan modifies gut microbiota and intestinal metabolites during alleviation of hyperglycemia in type 2 diabetic mice. Food Funct. 2021, 12, 3572–3585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zuo, J.; Yan, L.; Cheng, Y.; Li, Q.; Wu, S.; Chen, L.; Thring, R.W.; Yang, Y.; Gao, Y.; et al. Sargassum fusiforme Fucoidan Alleviates High-Fat Diet-Induced Obesity and Insulin Resistance Associated with the Improvement of Hepatic Oxidative Stress and Gut Microbiota Profile. J. Agric. Food Chem. 2020, 68, 10626–10638. [Google Scholar] [CrossRef]
- Fu, X.; Zhan, Y.; Li, N.; Yu, D.; Gao, W.; Gu, Z.; Zhu, L.; Li, R.; Zhu, C. Enzymatic Preparation of Low-Molecular-Weight Laminaria japonica Polysaccharides and Evaluation of Its Effect on Modulating Intestinal Microbiota in High-Fat-Diet-Fed Mice. Front. Bioeng. Biotechnol. 2021, 9, 820892. [Google Scholar] [CrossRef]
- Bolton, J.J. The biogeography of kelps (Laminariales, Phaeophyceae): A global analysis with new insights from recent advances in molecular phylogenetics. Helgol. Mar. Res. 2010, 64, 263–279. [Google Scholar] [CrossRef]
- Wang, L.; Ai, C.; Wen, C.; Qin, Y.; Liu, Z.; Wang, L.; Gong, Y.; Su, C.; Wang, Z.; Song, S. Fucoidan isolated from Ascophyllum nodosum alleviates gut microbiota dysbiosis and colonic inflammation in antibiotic-treated mice. Food Funct. 2020, 11, 5595–5606. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Morrison, L.; Shukla, P.S.; Critchley, A.T. A concise review of the brown macroalga Ascophyllum nodosum (Linnaeus) Le Jolis. J. Appl. Phycol. 2020, 32, 3561–3584. [Google Scholar] [CrossRef]
- Vodouhe, M.; Marois, J.; Guay, V.; Leblanc, N.; Weisnagel, S.J.; Bilodeau, J.F.; Jacques, H. Marginal Impact of Brown Seaweed Ascophyllum nodosum and Fucus vesiculosus Extract on Metabolic and Inflammatory Response in Overweight and Obese Prediabetic Subjects. Mar. Drugs 2022, 20, 174. [Google Scholar] [CrossRef]
- Wang, X.; Shan, X.; Dun, Y.; Cai, C.; Hao, J.; Li, G.; Cui, K.; Yu, G. Anti-Metabolic Syndrome Effects of Fucoidan from Fucus vesiculosus via Reactive Oxygen Species-Mediated Regulation of JNK, Akt, and AMPK Signaling. Molecules 2019, 24, 3319. [Google Scholar] [CrossRef]
- Jiang, P.; Zheng, W.; Sun, X.; Jiang, G.; Wu, S.; Xu, Y.; Song, S.; Ai, C. Sulfated polysaccharides from Undaria pinnatifida improved high fat diet-induced metabolic syndrome, gut microbiota dysbiosis and inflammation in BALB/c mice. Int. J. Biol. Macromol 2021, 167, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.P.; Jia, J.H.; Jiang, P.R.; Zheng, W.Y.; Li, X.F.; Song, S.; Ai, C.Q. Polysaccharides from edible brown seaweed Undaria pinnatifida are effective against high-fat diet-induced obesity in mice through the modulation of intestinal microecology. Food Funct. 2022, 13, 2581–2593. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.B.; Li, Z.R.; Lin, L.Z.; Luo, D.H.; Chen, C.; Zhao, M.M. The potential mechanisms of Macrocystis pyrifera polysaccharides mitigating type 2 diabetes in rats. Food Funct. 2022, 13, 7918–7929. [Google Scholar] [CrossRef] [PubMed]
- Purcell-Meyerink, D.; Packer, M.A.; Wheeler, T.T.; Hayes, M. Aquaculture Production of the Brown Seaweeds Laminaria digitata and Macrocystis pyrifera: Applications in Food and Pharmaceuticals. Molecules 2021, 26, 1306. [Google Scholar] [CrossRef]
- Brownlee, I.A.; Allen, A.; Pearson, J.P.; Dettmar, P.W.; Havler, M.E.; Atherton, M.R.; Onsoyen, E. Alginate as a source of dietary fiber. Crit. Rev. Food Sci. Nutr. 2005, 45, 497–510. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Gomaa, M.; Hifney, A.F.; Abdel-Gawad, K.M. Optimization of alginate alkaline extraction technology from Sargassum latifolium and its potential antioxidant and emulsifying properties. Carbohydr. Polym. 2017, 157, 1903–1912. [Google Scholar] [CrossRef]
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate hydrogels as biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef]
- Cong, Q.F.; Xiao, F.; Liao, W.F.; Dong, Q.; Ding, K. Structure and biological activities of an alginate from Sargassum fusiforme, and its sulfated derivative. Int. J. Biol. Macromol. 2014, 69, 252–259. [Google Scholar] [CrossRef]
- Sen, M. Effects of molecular weight and ratio of guluronic acid to mannuronic acid on the antioxidant properties of sodium alginate fractions prepared by radiation-induced degradation. Appl. Radiat. Isot. 2011, 69, 126–129. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Gupta, S.; Lokesh, J.; Abdelhafiz, Y.; Siriyappagouder, P.; Pierre, R.; Sorensen, M.; Fernandes, J.M.O.; Kiron, V. Macroalga-Derived Alginate Oligosaccharide Alters Intestinal Bacteria of Atlantic Salmon. Front. Microbiol. 2019, 10, 2037. [Google Scholar] [CrossRef] [PubMed]
- Miao, B.C.; Geng, M.Y.; Li, J.; Li, F.C.; Chen, H.X.; Guan, H.S.; Ding, J. Sulfated polymannuroguluronate, a novel anti-acquired immune deficiency syndrome (AIDS) drug candidate, targeting CD4 in lymphocytes. Biochem. Pharm. 2004, 68, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.Q.; Han, W.W.; Zhang, M.F.; Zang, R.C.; Du, K.X.; Li, L.; Ma, X.M.; Li, C.X.; Wang, S.X.; Qiu, P.J.; et al. Sulfated polymannuroguluronate TGC161 ameliorates leukopenia by inhibiting CD4(+) T cell apoptosis. Carbohydr. Polym. 2020, 247, 116728. [Google Scholar] [CrossRef] [PubMed]
- Salih, A.E.M.; Thissera, B.; Yaseen, M.; Hassane, A.S.I.; El-Seedi, H.R.; Sayed, A.M.; Rateb, M.E. Marine Sulfated Polysaccharides as Promising Antiviral Agents: A Comprehensive Report and Modeling Study Focusing on SARS CoV-2. Mar. Drugs 2021, 19, 406. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Aroca, A.; Ferrandis-Montesinos, M.; Wang, R.B. Antiviral Properties of Alginate-Based Biomaterials: Promising Antiviral Agents against SARS-CoV-2. ACS Appl. Bio Mater. 2021, 4, 5897–5907. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X.; Shi, H.J.; Wang, Y.M.; Xue, C.H.; Tang, Q.J. Polymannuronic acid ameliorated obesity and inflammation associated with a high-fat and high-sucrose diet by modulating the gut microbiome in a murine model. Brit. J. Nutr. 2017, 117, 1332–1342. [Google Scholar] [CrossRef]
- Vo, T.S.; Kim, S.K. Fucoidans as a natural bioactive ingredient for functional foods. J. Funct. Foods 2013, 5, 16–27. [Google Scholar] [CrossRef]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important Determinants for Fucoidan Bioactivity: A Critical Review of Structure-Function Relations and Extraction Methods for Fucose-Containing Sulfated Polysaccharides from Brown Seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef]
- Berteau, O.; Mulloy, B. Sulfated fucans, fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29R–40R. [Google Scholar] [CrossRef]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H.; Mourao, P.A.S. Structure, biology, evolution, and medical importance of sulfated fucans and galactans. Glycobiology 2008, 18, 1016–1027. [Google Scholar] [CrossRef]
- Luo, J.M.; Wang, Z.; Fan, B.; Wang, L.; Liu, M.Y.; An, Z.Z.; Zhao, X. A comparative study of the effects of different fucoidans on cefoperazone-induced gut microbiota disturbance and intestinal inflammation. Food Funct. 2021, 12, 9087–9097. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.J.; Chang, Y.G.; Gao, Y.; Wang, X.; Chen, X.; Wang, Y.M.; Xue, C.H.; Tang, Q.J. Dietary fucoidan of Acaudina molpadioides alters gut microbiota and mitigates intestinal mucosal injury induced by cyclophosphamide. Food Funct. 2017, 8, 3383–3393. [Google Scholar] [CrossRef]
- Ahmadi, A.; Moghadamtousi, S.Z.; Abubakar, S.; Zandi, K. Antiviral Potential of Algae Polysaccharides Isolated from Marine Sources: A Review. BioMed Res. Int. 2015, 2015, 825203. [Google Scholar] [CrossRef] [PubMed]
- Imbs, T.I.; Ermakova, S.P.; Malyarenko, O.S.; Isakov, V.V.; Zvyagintseva, T.N. Structural elucidation of polysaccharide fractions from the brown alga Coccophora langsdorfii and in vitro investigation of their anticancer activity. Carbohydr. Polym. 2016, 135, 162–168. [Google Scholar] [CrossRef]
- Rioux, L.E.; Turgeon, S.L.; Beaulieu, M. Structural characterization of laminaran and galactofucan extracted from the brown seaweed Saccharina longicruris. Phytochemistry 2010, 71, 1586–1595. [Google Scholar] [CrossRef]
- Pereira, M.S.; Mulloy, B.; Mourao, P.A.S. Structure and anticoagulant activity of sulfated fucans: Comparison between the regular, repetitive, and linear fucans from echinoderms with the more heterogeneous and branched polymers from brown algae. J. Biol. Chem. 1999, 274, 7656–7667. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.G.; Kim, J.; Guevarra, R.B.; Lee, J.H.; Kim, E.; Kim, S.I.; Unno, T. Laminarin favorably modulates gut microbiota in mice fed a high-fat diet. Food Funct. 2016, 7, 4193–4201. [Google Scholar] [CrossRef]
- An, C.; Kuda, T.; Yazaki, T.; Takahashi, H.; Kimura, B. FLX Pyrosequencing Analysis of the Effects of the Brown-Algal Fermentable Polysaccharides Alginate and Laminaran on Rat Cecal Microbiotas. Appl. Environ. Microb. 2013, 79, 860–866. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, X.X.; Tang, Y.X.; Mao, J.L. Composition, isolation, purification and biological activities of Sargassum fusiforme polysaccharides: A review. Carbohydr. Polym. 2020, 228, 115381. [Google Scholar] [CrossRef]
- Zhao, S.F.; He, Y.; Wang, C.G.; Assani, I.; Hou, P.L.; Feng, Y.; Yang, J.J.; Wang, Y.H.; Liao, Z.X.; Shen, S.D. Isolation, Characterization and Bioactive Properties of Alkali-Extracted Polysaccharides from Enteromorpha prolifera. Mar. Drugs 2020, 18, 552. [Google Scholar] [CrossRef] [PubMed]
- Hahn, T.; Lang, S.; Ulber, R.; Muffler, K. Novel procedures for the extraction of fucoidan from brown algae. Process. Biochem. 2012, 47, 1691–1698. [Google Scholar] [CrossRef]
- Jia, R.B.; Li, Z.R.; Wu, J.; Ou, Z.R.; Zhu, Q.Y.; Sun, B.G.; Lin, L.Z.; Zhao, M.M. Physicochemical properties of polysaccharide fractions from Sargassum fusiforme and their hypoglycemic and hypolipidemic activities in type 2 diabetic rats. Int. J. Biol. Macromol. 2020, 147, 428–438. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, S.Q.; Chakka, V.P.; Qian, W.W.; Wei, X.Y.; Zhu, Q.; Zhou, T. Purification and Biological Activities of Enzymatically Degraded Sargassum fusiforme Polysaccharides. Chem. Biodivers. 2021, 18, e2000930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, M.; Hu, C.; Liu, A.; Chen, J.; Gu, C.; Zhang, X.; You, C.; Tong, H.; Wu, M.; et al. Sargassum fusiforme Fucoidan SP2 Extends the Lifespan of Drosophila melanogaster by Upregulating the Nrf2-Mediated Antioxidant Signaling Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 8918914. [Google Scholar] [CrossRef] [PubMed]
- Abraham, R.E.; Su, P.; Puri, M.; Raston, C.L.; Zhang, W. Optimisation of biorefinery production of alginate, fucoidan and laminarin from brown seaweed Durvillaea potatorum. Algal Res. 2019, 38, 101389. [Google Scholar] [CrossRef]
- January, G.G.; Naidoo, R.K.; Kirby-McCullough, B.; Bauer, R. Assessing methodologies for fucoidan extraction from South African brown algae. Algal Res. 2019, 40, 101517, Corrigendum in Algal Res. 2019, 41, 101571. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Mall, J.P.G.; Fart, F.; Sabet, J.A.; Lindqvist, C.M.; Nestestog, R.; Hegge, F.T.; Keita, A.V.; Brummer, R.J.; Schoultz, I. Effects of Dietary Fibres on Acute Indomethacin-Induced Intestinal Hyperpermeability in the Elderly: A Randomised Placebo Controlled Parallel Clinical Trial. Nutrients 2020, 12, 1954. [Google Scholar] [CrossRef]
- Ikeda-Ohtsubo, W.; Nadal, A.L.; Zaccaria, E.; Iha, M.; Kitazawa, H.; Kleerebezem, M.; Brugman, S. Intestinal Microbiota and Immune Modulation in Zebrafish by Fucoidan From Okinawa Mozuku (Cladosiphon okamuranus). Front. Nutr. 2020, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.H.; Zhang, R.F.; You, L.J.; Ma, Y.X.; Liao, L.; Pedisic, S. In vitro fermentation characteristics of polysaccharide from Sargassum fusiforme and its modulation effects on gut microbiota. Food Chem. Toxicol. 2021, 151, 112145. [Google Scholar] [CrossRef]
- Huang, J.; Huang, J.L.; Li, Y.; Wang, Y.L.; Wang, F.H.; Qiu, X.; Liu, X.; Li, H.J. Sodium Alginate Modulates Immunity, Intestinal Mucosal Barrier Function, and Gut Microbiota in Cyclophosphamide-Induced Immunosuppressed BALB/c Mice. J. Agric Food Chem. 2021, 69, 7064–7073. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.W.; Hwang, S.J.; Han, M.H.; Lee, D.S.; Yoo, J.S.; Choi, I.W.; Cha, H.J.; Kim, S.; Kim, H.S.; Kim, G.Y.; et al. Fucoidan inhibits lipopolysaccharide-induced inflammatory responses in RAW 264.7 macrophages and zebrafish larvae. Mol. Cell Toxicol. 2017, 13, 405–417. [Google Scholar] [CrossRef]
- Ramnani, P.; Chitarrari, R.; Tuohy, K.; Grant, J.; Hotchkiss, S.; Philp, K.; Campbell, R.; Gill, C.; Rowland, I. In vitro fermentation and prebiotic potential of novel low molecular weight polysaccharides derived from agar and alginate seaweeds. Anaerobe 2012, 18, 1–6. [Google Scholar] [CrossRef]
- Ai, C.Q.; Jiang, P.R.; Liu, Y.L.; Duan, M.M.; Sun, X.N.; Luo, T.R.; Jiang, G.P.; Song, S. The specific use of alginate from Laminaria japonica by Bacteroides species determined its modulation of the Bacteroides community. Food Funct. 2019, 10, 4304–4314. [Google Scholar] [CrossRef] [PubMed]
- Nakata, T.; Kyoui, D.; Takahashi, H.; Kimura, B.; Kuda, T. Inhibitory effects of laminaran and alginate on production of putrefactive compounds from soy protein by intestinal microbiota in vitro and in rats. Carbohydr Polym. 2016, 143, 61–69. [Google Scholar] [CrossRef]
- Tang, Y.P.; Pu, Q.Y.; Zhao, Q.L.; Zhou, Y.F.; Jiang, X.X.; Han, T. Effects of Fucoidan Isolated From Laminaria japonica on Immune Response and Gut Microbiota in Cyclophosphamide-Treated Mice. Front. Immunol. 2022, 13, 916618. [Google Scholar] [CrossRef]
- Fu, X.; Cao, C.L.; Ren, B.B.; Zhang, B.; Huang, Q.; Li, C. Structural characterization and in vitro fermentation of a novel polysaccharide from Sargassum thunbergii and its impact on gut microbiota. Carbohydr. Polym. 2018, 183, 230–239. [Google Scholar] [CrossRef]
- Shang, Q.S.; Shan, X.D.; Cai, C.; Hao, J.J.; Li, G.Y.; Yu, G.L. Dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of Lactobacillus and Ruminococcaceae. Food Funct. 2016, 7, 3224–3232. [Google Scholar] [CrossRef]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroent 2017, 11, 821–834. [Google Scholar] [CrossRef]
- Branca, J.J.V.; Gulisano, M.; Nicoletti, C. Intestinal epithelial barrier functions in ageing. Ageing Res. Rev. 2019, 54, 100938. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Regulation of the intestinal barrier by nutrients: The role of tight junctions. Anim. Sci. J. 2020, 91, e13357. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.A.; Uppada, S.; Achkar, I.W.; Hashem, S.; Yadav, S.K.; Shanmugakonar, M.; Al-Naemi, H.A.; Haris, M.; Uddin, S. Tight Junction Proteins and Signaling Pathways in Cancer and Inflammation: A Functional Crosstalk. Front. Physiol. 2018, 9, 1942. [Google Scholar] [CrossRef] [PubMed]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Matayoshi, M.; Teruya, J.; Yasumoto-Hirose, M.; Teruya, R.; Miura, N.; Takeda, R. Improvement of defecation in healthy individuals with infrequent bowel movements through the ingestion of dried Mozuku powder: A randomized, double-blind, parallel-group study. Funct. Foods Health Dis. 2017, 7, 735–742. [Google Scholar] [CrossRef]
- Sun, T.; Liang, H.; Xue, M.L.; Liu, Y.; Gong, A.J.; Jiang, Y.S.; Qin, Y.M.; Yang, J.; Meng, D.Y. Protective effect and mechanism of fucoidan on intestinal mucosal barrier function in NOD mice. Food Agric. Immunol. 2020, 31, 922–936. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, J.; Yin, H.; Chen, D.; Yu, B.; He, J. Ameliorative effects of alginate oligosaccharide on tumour necrosis factor-alpha-induced intestinal epithelial cell injury. Int. Immunopharmacol. 2020, 89, 107084. [Google Scholar] [CrossRef]
- Ding, X.M.; Hu, X.Y.; Chen, Y.; Xie, J.H.; Ying, M.X.; Wang, Y.D.; Yu, Q. Differentiated Caco-2 cell models in food-intestine interaction study: Current applications and future trends. Trends Food Sci. Technol. 2021, 107, 455–465. [Google Scholar] [CrossRef]
- Zhang, M.M.; Wu, C.C. The relationship between intestinal goblet cells and the immune response. Biosci. Rep. 2020, 40, 10. [Google Scholar] [CrossRef]
- Sicard, J.F.; Le Bihan, G.; Vogeleer, P.; Jacques, M.; Harel, J. Interactions of Intestinal Bacteria with Components of the Intestinal Mucus. Front. Cell. Infect. Microbiol. 2017, 7, 387. [Google Scholar] [CrossRef] [PubMed]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.G.; Ryan, M.; O’Doherty, J.V.; Reilly, P.; Bahar, B.; Sweeney, T. Effects of dietary supplementation with laminarin derived from Laminaria hyperborea and Laminaria digitata on colonic mucin gene expression in pigs. Livest. Sci. 2010, 133, 204–206. [Google Scholar] [CrossRef]
- Gao, J.; Lin, L.Z.; Sun, B.G.; Zhao, M.M. Comparison Study on Polysaccharide Fractions from Laminaria japonica: Structural Characterization and Bile Acid Binding Capacity. J. Agric. Food Chem. 2017, 65, 9790–9798. [Google Scholar] [CrossRef] [PubMed]
- Ruder, B.; Becker, C. At the Forefront of the Mucosal Barrier: The Role of Macrophages in the Intestine. Cells 2020, 9, 2162. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, L.; Liu, F. Immunological Impact of Intestinal T Cells on Metabolic Diseases. Front. Immunol. 2021, 12, 639902. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Ding, R.X.; Sun, J.; Liu, J.; Kan, J.; Jin, C.H. The impacts of natural polysaccharides on intestinal microbiota and immune responses-a review. Food Funct. 2019, 10, 2290–2312. [Google Scholar] [CrossRef]
- Xu, X.F.; Zhang, X.W. Effects of cyclophosphamide on immune system and gut microbiota in mice. Microbiol. Res. 2015, 171, 97–106. [Google Scholar] [CrossRef]
- Li, W.J.; Li, L.; Zhen, W.Y.; Wang, L.F.; Pan, M.; Lv, J.Q.; Wang, F.; Yao, Y.F.; Nie, S.P.; Xie, M.Y. Ganoderma atrum polysaccharide ameliorates ROS generation and apoptosis in spleen and thymus of immunosuppressed mice. Food Chem. Toxicol. 2017, 99, 199–208. [Google Scholar] [CrossRef]
- Feng, G.; Su, L.J.; Chen, S.; Teng, W.; Zhou, D.J.; Yin, C.; Guo, S.D. In vitro and in vivo immunoregulatory activity of sulfated fucan from the sea cucumber A. leucoprocta. Int. J. Biol. Macromol. 2021, 187, 931–938. [Google Scholar] [CrossRef]
- Yamamoto, M.; Yoshizaki, K.; Kishimoto, T.; Ito, H. IL-6 is required for the development of Th1 cell-mediated murine colitis. J. Immunol. 2000, 164, 4878–4882. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Nagaoka, M.; Hara, T.; Kimura-Takagi, I.; Mistuyama, K.; Ueyama, S. Fucoidan derived from Cladosiphon okamuranus tokida ameliorates murine chronic colitis through the down-regulation of interleukin-6 production on colonic epithelial cells. Clin. Exp. Immunol. 2004, 136, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Raghavendran, H.R.B.; Srinivasan, P.; Rekha, S. Immunomodulatory activity of fucoidan against aspirin-induced gastric mucosal damage in rats. Int. Immunopharmacol. 2011, 11, 157–163. [Google Scholar] [CrossRef]
- Artis, D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat. Rev. Immunol. 2008, 8, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Wang, L.N.; Liu, B.; He, N.N. Unsaturated alginate oligosaccharides attenuated obesity-related metabolic abnormalities by modulating gut microbiota in high-fat-diet mice. Food Funct. 2020, 11, 4773–4784. [Google Scholar] [CrossRef]
- Luthuli, S.; Wu, S.; Cheng, Y.; Zheng, X.; Wu, M.; Tong, H. Therapeutic Effects of Fucoidan: A Review on Recent Studies. Mar. Drugs 2019, 17, 487. [Google Scholar] [CrossRef]
- Vandana, U.K.; Barlaskar, N.H.; Gulzar, A.B.M.; Laskar, I.H.; Kumar, D.; Paul, P.; Pandey, P.; Mazumder, P.B. Linking gut microbiota with the human diseases. Bioinformation 2020, 16, 196–208. [Google Scholar] [CrossRef]
- Spiljar, M.; Merkler, D.; Trajkovski, M. The Immune System Bridges the Gut Microbiota with Systemic Energy Homeostasis: Focus on TLRs, Mucosal Barrier, and SCFAs. Front. Immunol. 2017, 8, 1353. [Google Scholar] [CrossRef]
- Sommer, F.; Ruhlemann, M.C.; Bang, C.; Hoppner, M.; Rehman, A.; Kaleta, C.; Schmitt-Kopplin, P.; Dempfle, A.; Weidinger, S.; Ellinghaus, E.; et al. Microbiomarkers in inflammatory bowel diseases: Caveats come with caviar. Gut 2017, 66, 1734–1738. [Google Scholar] [CrossRef]
- Walsh, A.M.; Sweeney, T.; O’Shea, C.J.; Doyle, D.N.; O’Doherty, J.V. Effect of dietary laminarin and fucoidan on selected microbiota, intestinal morphology and immune status of the newly weaned pig. Brit. J. Nutr. 2013, 110, 1630–1638. [Google Scholar] [CrossRef]
- Zuo, T.; Li, X.M.; Chang, Y.G.; Duan, G.F.; Yu, L.; Zheng, R.; Xue, C.H.; Tang, Q.J. Dietary fucoidan of Acaudina molpadioides and its enzymatically degraded fragments could prevent intestinal mucositis induced by chemotherapy in mice. Food Funct. 2015, 6, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.Z.; O’Toole, P.W. Impact of diet on the human intestinal microbiota. Curr. Opin. Food Sci. 2015, 2, 71–77. [Google Scholar] [CrossRef]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ma, L.; Chen, Q.Z.; Zhang, P.Y.; Chen, C.; Jia, L.L.; Li, H.J. Fucoidan alleviates dyslipidemia and modulates gut microbiota in high-fat diet-induced mice. J. Funct. Foods 2018, 48, 220–227. [Google Scholar] [CrossRef]
- Cheng, Y.; Sibusiso, L.; Hou, L.F.; Jiang, H.J.; Chen, P.C.; Zhang, X.; Wu, M.J.; Tong, H.B. Sargassum fusiforme fucoidan modifies the gut microbiota during alleviation of streptozotocin-induced hyperglycemia in mice. Int. J. Biol. Macromol. 2019, 131, 1162–1170. [Google Scholar] [CrossRef]
- He, N.N.; Yang, Y.; Wang, H.Y.; Liu, N.A.; Yang, Z.Z.; Li, S.Y. Unsaturated alginate oligosaccharides (UAOS) protects against dextran sulfate sodium-induced colitis associated with regulation of gut microbiota. J. Funct. Foods 2021, 83, 104536. [Google Scholar] [CrossRef]
- Liu, J.; Tan, Y.Z.; Cheng, H.; Zhang, D.D.; Feng, W.W.; Peng, C. Functions of Gut Microbiota Metabolites, Current Status and Future Perspectives. Aging Dis. 2022, 13, 1106–1126. [Google Scholar] [CrossRef]
- Schoeler, M.; Caesar, R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottiere, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Phull, A.R.; Kim, S.J. Fucoidan as bio-functional molecule: Insights into the anti-inflammatory potential and associated molecular mechanisms. J. Funct. Foods 2017, 38, 415–426. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Fernando, I.P.S.; Kim, E.A.; Ahn, G.; Jee, Y.; Jeon, Y.J. Anti-inflammatory activity of a sulfated polysaccharide isolated from an enzymatic digest of brown seaweed Sargassum horneri in RAW 264.7 cells. Nutr. Res. Pract. 2017, 11, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Liu, H.J.; Zhang, R.F.; Dong, L.H.; Liu, L.; Ma, Y.X.; Jia, X.C.; Wang, G.J.; Zhang, M.W. Physicochemical properties and prebiotic activities of polysaccharides from longan pulp based on different extraction techniques. Carbohydr. Polym. 2019, 206, 344–351. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).