Cholinergic Modulation of Locomotor Circuits in Vertebrates
Abstract
1. Introduction
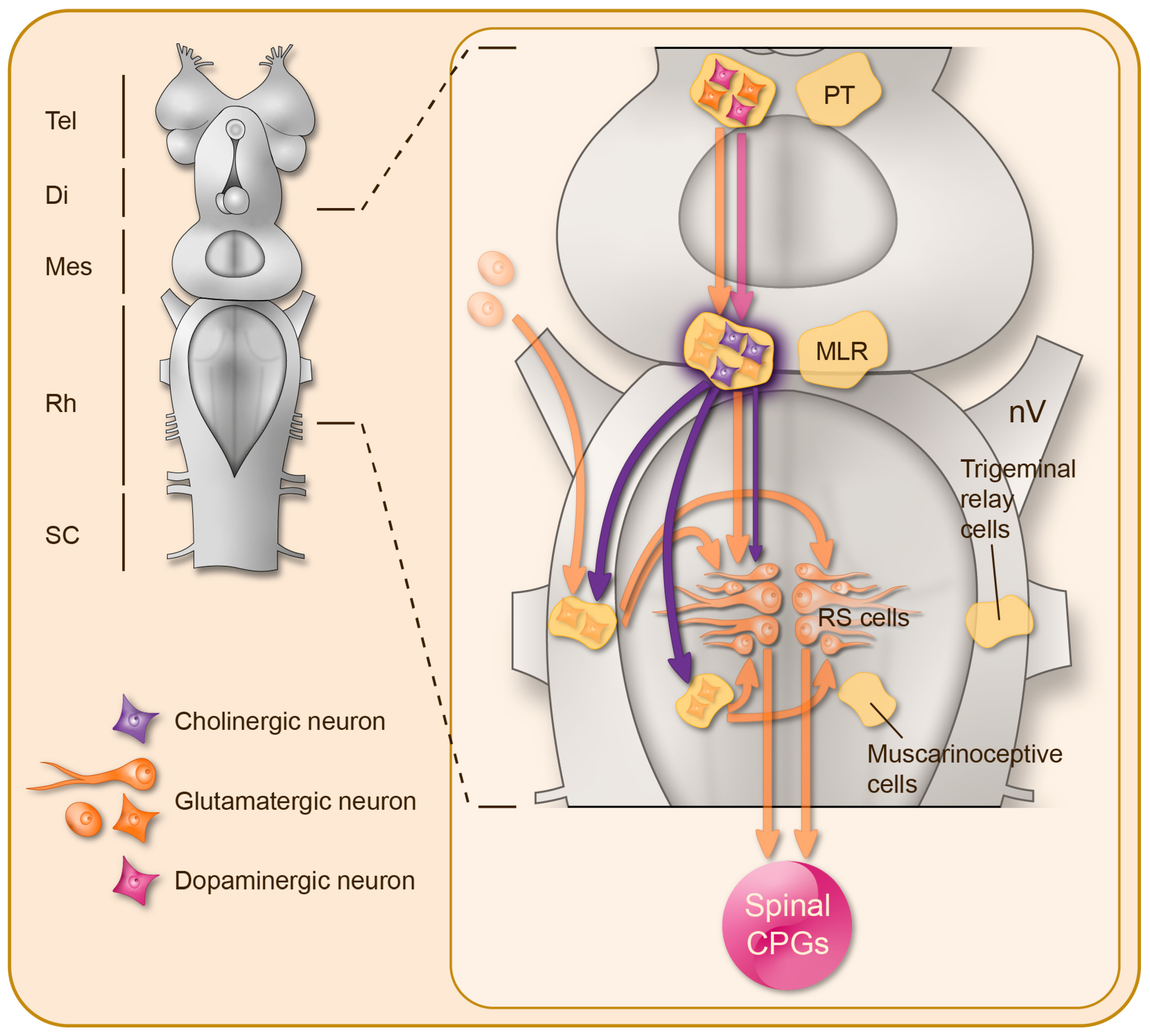
2. Brainstem Cholinergic Mechanisms Controlling Motor Activity
2.1. The MLR Contains ACh Neurons
2.2. MLR Implications in Locomotor Control: Targets, Pathways, and Pharmacology
2.3. Cholinergic Effects on Reticulospinal Neurons
2.4. A Parallel Muscarinic Hyperdrive to Boost the Locomotor Output
2.5. Muscarinic Control of Brainstem Sensory Inputs
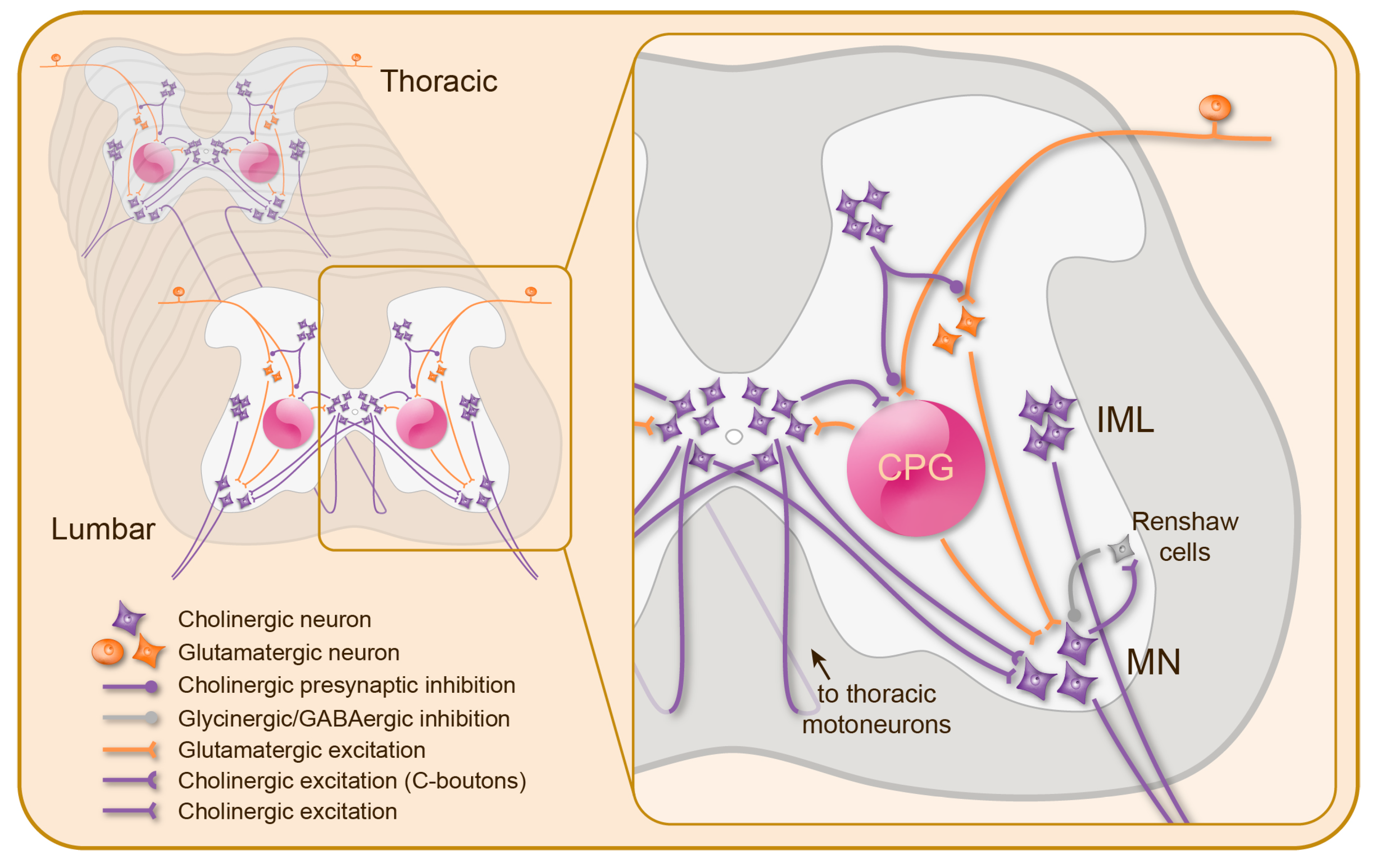
3. Cholinergic Mechanisms in the Spinal Cord
3.1. Nicotinic Modulation of Spinal Motor Circuits
3.2. Muscarinic Modulation of Spinal Motor Circuits
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grillner, S.; El Manira, A. Current Principles of Motor Control, with Special Reference to Vertebrate Locomotion. Physiol. Rev. 2020, 100, 271–320. [Google Scholar] [CrossRef] [PubMed]
- Grillner, S. Evolution: Vertebrate Limb Control over 420 Million Years. Curr. Biol. 2018, 28, R162–R164. [Google Scholar] [CrossRef] [PubMed]
- Grillner, S. Control of Locomotion in Bipeds, Tetrapods and Fish. In Handbook of Physiology. The Nervous System II. Motor Control; American Physiological Society: Rockville, MD, USA; Waverly Press: Baltimore, MD, USA, 1981; pp. 1179–1236. [Google Scholar]
- Grillner, S. Neurobiological Bases of Rhythmic Motor Acts in Vertebrates. Science 1985, 228, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Kiehn, O. Decoding the Organization of Spinal Circuits That Control Locomotion. Nat. Rev. Neurosci. 2016, 17, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Wyart, C. Taking a Big Step towards Understanding Locomotion. Trends Neurosci. 2018, 41, 869–870. [Google Scholar] [CrossRef]
- Grillner, S.; Kozlov, A. The CPGs for Limbed Locomotion—Facts and Fiction. IJMS 2021, 22, 5882. [Google Scholar] [CrossRef] [PubMed]
- Grillner, S. Biological Pattern Generation: The Cellular and Computational Logic of Networks in Motion. Neuron 2006, 52, 751–766. [Google Scholar] [CrossRef]
- Juvin, L.; Grätsch, S.; Trillaud-Doppia, E.; Gariépy, J.-F.; Büschges, A.; Dubuc, R. A Specific Population of Reticulospinal Neurons Controls the Termination of Locomotion. Cell Rep. 2016, 15, 2377–2386. [Google Scholar] [CrossRef]
- Grätsch, S.; Auclair, F.; Demers, O.; Auguste, E.; Hanna, A.; Büschges, A.; Dubuc, R. A Brainstem Neural Substrate for Stopping Locomotion. J. Neurosci. 2019, 39, 1044–1057. [Google Scholar] [CrossRef]
- Deliagina, T.G.; Musienko, P.E.; Zelenin, P.V. Nervous Mechanisms of Locomotion in Different Directions. Curr. Opin. Physiol. 2019, 8, 7–13. [Google Scholar] [CrossRef]
- Clarac, F. How Do Sensory and Motor Signals Interact during Locomotion? In Motor Control: Concepts and Issues; Wiley: New York, NY, USA, 1991; pp. 199–221. [Google Scholar]
- Rossignol, S.; Dubuc, R.; Gossard, J.-P. Dynamic Sensorimotor Interactions in Locomotion. Physiol. Rev. 2006, 86, 89–154. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, S.; Bouyer, L. Adaptive Mechanisms of Spinal Locomotion in Cats. Integr. Comp. Biol. 2004, 44, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Daghfous, G.; Green, W.W.; Alford, S.T.; Zielinski, B.S.; Dubuc, R. Sensory Activation of Command Cells for Locomotion and Modulatory Mechanisms: Lessons from Lampreys. Front. Neural Circuits 2016, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, J.T. Spinal Locomotor Inputs to Individually Identified Reticulospinal Neurons in the Lamprey. J. Neurophysiol. 2011, 106, 2346–2357. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, J.T.; Cohen, A.H. Activities of Identified Interneurons, Motoneurons, and Muscle Fibers during Fictive Swimming in the Lamprey and Effects of Reticulospinal and Dorsal Cell Stimulation. J. Neurophysiol. 1982, 47, 948–960. [Google Scholar] [CrossRef]
- Bouvier, J.; Caggiano, V.; Leiras, R.; Caldeira, V.; Bellardita, C.; Balueva, K.; Fuchs, A.; Kiehn, O. Descending Command Neurons in the Brainstem That Halt Locomotion. Cell 2015, 163, 1191–1203. [Google Scholar] [CrossRef]
- Capelli, P.; Pivetta, C.; Soledad Esposito, M.; Arber, S. Locomotor Speed Control Circuits in the Caudal Brainstem. Nature 2017, 551, 373–377. [Google Scholar] [CrossRef]
- Grätsch, S.; Büschges, A.; Dubuc, R. Descending Control of Locomotor Circuits. Curr. Opin. Physiol. 2019, 8, 94–98. [Google Scholar] [CrossRef]
- Shik, M.L.; Orlovsky, G.N. Neurophysiology of Locomotor Automatism. Physiol. Rev. 1976, 56, 465–501. [Google Scholar] [CrossRef]
- McClellan, A.D. Command Systems for Initiating Locomotion in Fish and Amphibians: Parallels to Initiation Systems in Mammals. In Neurobiology of Vertebrate Locomotion: Proceedings of an International Symposium Held at The Wenner-Gren Center, Stockholm, 17–19 June 1985; Grillner, S., Stein, P.S.G., Stuart, D.G., Forssberg, H., Herman, R.M., Eds.; Wenner-Gren Center International Symposium Series; Palgrave Macmillan: London, UK, 1986; pp. 3–20. ISBN 978-1-349-09148-5. [Google Scholar]
- Sirota, M.G.; Di Prisco, G.V.; Dubuc, R. Stimulation of the Mesencephalic Locomotor Region Elicits Controlled Swimming in Semi-Intact Lampreys: Brainstem-Induced Swimming. Eur. J. Neurosci. 2000, 12, 4081–4092. [Google Scholar] [CrossRef]
- Cabelguen, J.-M.; Bourcier-Lucas, C.; Dubuc, R. Bimodal Locomotion Elicited by Electrical Stimulation of the Midbrain in the Salamander Notophthalmus Viridescens. J. Neurosci. 2003, 23, 2434–2439. [Google Scholar] [CrossRef] [PubMed]
- Ryczko, D.; Auclair, F.; Cabelguen, J.-M.; Dubuc, R. The Mesencephalic Locomotor Region Sends a Bilateral Glutamatergic Drive to Hindbrain Reticulospinal Neurons in a Tetrapod: MLR Downstream Connectivity in Salamanders. J. Comp. Neurol. 2016, 524, 1361–1383. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.; Sinnamon, H. Forward Locomotion Elicited by Electrical Stimulation in the Diencephalon and Mesencephalon of the Awake Rat. Physiol. Behav. 1983, 31, 581–587. [Google Scholar] [PubMed]
- El Manira, A.; Pombal, M.A.; Grillner, S. Diencephalic Projection to Reticulospinal Neurons Involved in the Initiation of Locomotion in Adult Lampreys Lampetra Fluviatilis. J. Comp. Neurol. 1997, 389, 603–616. [Google Scholar] [CrossRef]
- Shik, M.L.; Severin, F.V.; Orlovsky, G.N. Control of Walking and Running by Means of Electric Stimulation of the Midbrain. Biofizika 1966, 11, 659–666. [Google Scholar]
- Brocard, F.; Ryczko, D.; Fenelon, K.; Hatem, R.; Gonzales, D.; Auclair, F.; Dubuc, R. The Transformation of a Unilateral Locomotor Command into a Symmetrical Bilateral Activation in the Brainstem. J. Neurosci. 2010, 30, 523–533. [Google Scholar] [CrossRef]
- Garcia-Rill, E.; Skinner, R.D. The Mesencephalic Locomotor Region. II. Projections to Reticulospinal Neurons. Brain Res. 1987, 411, 13–20. [Google Scholar] [CrossRef]
- Skinner, R.D.; Kinjo, N.; Ishikawa, Y.; Biedermann, J.A.; Garcia-Rill, E. Locomotor Projections from the Pedunculopontine Nucleus to the Medioventral Medulla. Neuroreport 1990, 1, 207–210. [Google Scholar] [CrossRef]
- Brownstone, R.M.; Chopek, J.W. Reticulospinal Systems for Tuning Motor Commands. Front. Neural Circuits 2018, 12, 30. [Google Scholar] [CrossRef]
- Ryczko, D.; Grätsch, S.; Auclair, F.; Dubé, C.; Bergeron, S.; Alpert, M.H.; Cone, J.J.; Roitman, M.F.; Alford, S.; Dubuc, R. Forebrain Dopamine Neurons Project down to a Brainstem Region Controlling Locomotion. Proc. Natl. Acad. Sci. USA 2013, 110, E3235–E3242. [Google Scholar] [CrossRef]
- Grillner, S.; Wallén, P.; Saitoh, K.; Kozlov, A.; Robertson, B. Neural Bases of Goal-Directed Locomotion in Vertebrates—An Overview. Brain Res. Rev. 2008, 57, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Le Ray, D.; Juvin, L.; Ryczko, D.; Dubuc, R. Supraspinal Control of Locomotion. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2011; Volume 188, pp. 51–70. ISBN 978-0-444-53825-3. [Google Scholar]
- Ryczko, D.; Cone, J.J.; Alpert, M.H.; Goetz, L.; Auclair, F.; Dubé, C.; Parent, M.; Roitman, M.F.; Alford, S.; Dubuc, R. A Descending Dopamine Pathway Conserved from Basal Vertebrates to Mammals. Proc. Natl. Acad. Sci. USA 2016, 113, E2440–E2449. [Google Scholar] [CrossRef] [PubMed]
- Ryczko, D.; Dubuc, R. Dopamine and the Brainstem Locomotor Networks: From Lamprey to Human. Front. Neurosci. 2017, 11, 295. [Google Scholar] [CrossRef] [PubMed]
- Dubuc, R.; Brocard, F.; Antri, M.; Fénelon, K.; Gariépy, J.-F.; Smetana, R.; Ménard, A.; Le Ray, D.; Viana Di Prisco, G.; Pearlstein, É.; et al. Initiation of Locomotion in Lampreys. Brain Res. Rev. 2008, 57, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Yeomans, J.S.; Li, L.; Scott, B.W.; Frankland, P.W. Tactile, Acoustic and Vestibular Systems Sum to Elicit the Startle Reflex. Neurosci. Biobehav. Rev. 2002, 26, 1–11. [Google Scholar] [CrossRef]
- Viana Di Prisco, G.; Pearlstein, E.; Robitaille, R.; Dubuc, R. Role of Sensory-Evoked NMDA Plateau Potentials in the Initiation of Locomotion. Science 1997, 278, 1122–1125. [Google Scholar] [CrossRef]
- Viana Di Prisco, G.; Pearlstein, E.; Le Ray, D.; Robitaille, R.; Dubuc, R. A Cellular Mechanism for the Transformation of a Sensory Input into a Motor Command. J. Neurosci. 2000, 20, 8169–8176. [Google Scholar] [CrossRef]
- Karayannidou, A.; Zelenin, P.V.; Orlovsky, G.N.; Deliagina, T.G. Responses of Reticulospinal Neurons in the Lamprey to Lateral Turns. J. Neurophysiol. 2007, 97, 512–521. [Google Scholar] [CrossRef][Green Version]
- Derjean, D.; Moussaddy, A.; Atallah, E.; St-Pierre, M.; Auclair, F.; Chang, S.; Ren, X.; Zielinski, B.; Dubuc, R. A Novel Neural Substrate for the Transformation of Olfactory Inputs into Motor Output. PLoS Biol. 2010, 8, e1000567. [Google Scholar] [CrossRef]
- Suzuki, D.; Perez-Fernandez, J.; Wibble, T.; Kardamakis, A.; Grillner, S. The Role of the Optic Tectum for Visually Evoked Orienting and Evasive Movements. Proc. Natl. Acad. Sci. USA 2019, 116, 15272–15281. [Google Scholar] [CrossRef]
- Lauder, J.M.; Schambra, U.B. Morphogenetic Roles of Acetylcholine. Environ. Health Perspect. 1999, 107, 5. [Google Scholar]
- Lichtensteiger, W.; Ribary, U.; Schlumpf, M.; Odermatt, B.; Widmer, H.R. Prenatal Adverse Effects of Nicotine on the Developing Brain. Prog. Brain Res. 1988, 73, 137–157. [Google Scholar] [CrossRef] [PubMed]
- Schlumpf, M.; Palacios, J.M.; Cortes, R.; Lichtensteiger, W. Regional Development of Muscarinic Cholinergic Binding Sites in the Prenatal Rat Brain. Neuroscience 1991, 45, 347–357. [Google Scholar] [CrossRef]
- Hasselmo, M.E.; Sarter, M. Modes and Models of Forebrain Cholinergic Neuromodulation of Cognition. Neuropsychopharmacology 2011, 36, 52–73. [Google Scholar] [CrossRef]
- Mena-Segovia, J.; Winn, P.; Bolam, J.P. Cholinergic Modulation of Midbrain Dopaminergic Systems. Brain Res. Rev. 2008, 58, 265–271. [Google Scholar] [CrossRef]
- Takakusaki, K.; Chiba, R.; Nozu, T.; Okumura, T. Brainstem Control of Locomotion and Muscle Tone with Special Reference to the Role of the Mesopontine Tegmentum and Medullary Reticulospinal Systems. J. Neural Transm. 2016, 123, 695–729. [Google Scholar] [CrossRef]
- Morel, C.; Fattore, L.; Pons, S.; Hay, Y.A.; Marti, F.; Lambolez, B.; De Biasi, M.; Lathrop, M.; Fratta, W.; Maskos, U.; et al. Nicotine Consumption Is Regulated by a Human Polymorphism in Dopamine Neurons. Mol. Psychiatry 2014, 19, 930–936. [Google Scholar] [CrossRef]
- Steidl, S. Muscarinic Cholinergic Receptor Antagonists in the VTA and RMTg Have Opposite Effects on Morphine-Induced Locomotion in Mice. Behav. Brain Res. 2017, 323, 111–116. [Google Scholar] [CrossRef]
- Tattersall, T.L.; Stratton, P.G.; Coyne, T.J.; Cook, R.; Silberstein, P.; Silburn, P.A.; Windels, F.; Sah, P. Imagined Gait Modulates Neuronal Network Dynamics in the Human Pedunculopontine Nucleus. Nat. Neurosci. 2014, 17, 449–454. [Google Scholar] [CrossRef]
- Ryczko, D.; Dubuc, R. The Multifunctional Mesencephalic Locomotor Region. CPD 2013, 19, 4448–4470. [Google Scholar] [CrossRef]
- Garcia-Rill, E.; Skinner, R.D.; Conrad, C.; Mosley, D.; Campbell, C. Projections of the Mesencephalic Locomotor Region in the Rat. Brain Res. Bull. 1986, 17, 33–40. [Google Scholar] [CrossRef]
- Kimura, H.; McGeer, P.L.; Peng, J.H.; McGeer, E.G. The Central Cholinergic System Studied by Choline Acetyltransferase Immunohistochemistry in the Cat. J. Comp. Neurol. 1981, 200, 151–201. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rill, E.; Skinner, R.D.; Gilmore, S.A. Pallidal Projections to the Mesencephalic Locomotor Region (MLR) in the Cat. Am. J. Anat. 1981, 161, 311–321. [Google Scholar] [CrossRef]
- Garcia-Rill, E.; Skinner, R.D.; Jackson, M.B.; Smith, M.M. Connections of the Mesencephalic Locomotor Region (MLR) I. Substantia Nigra Afferents. Brain Res. Bull. 1983, 10, 57–62. [Google Scholar] [CrossRef]
- Garcia-Rill, E. The Basal Ganglia and the Locomotor Regions. Brain Res. 1986, 396, 47–63. [Google Scholar] [CrossRef]
- Garcia-Rill, E.; Houser, C.R.; Skinner, R.D.; Smith, W.; Woodward, D.J. Locomotion-Inducing Sites in the Vicinity of the Pedunculopontine Nucleus. Brain Res. Bull. 1987, 18, 731–738. [Google Scholar] [CrossRef]
- Garcia-Rill, E. The Pedunculopontine Nucleus. Prog. Neurobiol. 1991, 36, 363–389. [Google Scholar] [CrossRef]
- Garcia-Rill, E.; Mahaffey, S.; Hyde, J.R.; Urbano, F.J. Bottom-up Gamma Maintenance in Various Disorders. Neurobiol. Dis. 2019, 128, 31–39. [Google Scholar] [CrossRef]
- Virmani, T.; Urbano, F.J.; Bisagno, V.; Garcia-Rill, E. The Pedunculopontine Nucleus: From Posture and Locomotion to Neuroepigenetics. AIMS Neurosci. 2019, 6, 219–230. [Google Scholar] [CrossRef]
- Le Ray, D.; Brocard, F.; Bourcier-Lucas, C.; Auclair, F.; Lafaille, P.; Dubuc, R. Nicotinic Activation of Reticulospinal Cells Involved in the Control of Swimming in Lampreys: Nicotinic Inputs to Reticulospinal Cells. Eur. J. Neurosci. 2003, 17, 137–148. [Google Scholar] [CrossRef]
- Coles, S.K.; Iles, J.F.; Nicolopoulos-Stournaras, S. The Mesencephalic Centre Controlling Locomotion in the Rat. Neuroscience 1989, 28, 149–157. [Google Scholar] [CrossRef]
- Goetz, L.; Bhattacharjee, M.; Ferraye, M.U.; Fraix, V.; Maineri, C.; Nosko, D.; Fenoy, A.J.; Piallat, B.; Torres, N.; Krainik, A.; et al. Deep Brain Stimulation of the Pedunculopontine Nucleus Area in Parkinson Disease: MRI-Based Anatomoclinical Correlations and Optimal Target. Neurosurgery 2019, 84, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.E.; Webster, H.H. Neurotoxic Lesions of the Dorsolateral Pontomesencephalic Tegmentum-Cholinergic Cell Area in the Cat. I. Effects upon the Cholinergic Innervation of the Brain. Brain Res. 1988, 451, 13–32. [Google Scholar] [CrossRef]
- Lai, Y.Y.; Clements, J.R.; Wu, X.Y.; Shalita, T.; Kuo, J.S.; Siegel, J.M. Brainstem Projections to the Ventromedial Medulla in Cat: Retrograde Transport Horseradish Peroxidase and Immunohistochemical Studies. J. Comp. Neurol. 1999, 408, 419–436. [Google Scholar] [CrossRef]
- Mesulam, M.M.; Mufson, E.J.; Wainer, B.H.; Levey, A.I. Central Cholinergic Pathways in the Rat: An Overview Based on an Alternative Nomenclature (Ch1-Ch6). Neuroscience 1983, 10, 1185–1201. [Google Scholar] [CrossRef]
- Pombal, M.A.; Marin, O.; Lez, A.G. Distribution of Choline Acetyltransferase-Immunoreactive Structures in the Lamprey Brain. J. Comp. Neurol. 2001, 431, 105–126. [Google Scholar] [CrossRef]
- Wang, H.-L.; Morales, M. Pedunculopontine and Laterodorsal Tegmental Nuclei Contain Distinct Populations of Cholinergic, Glutamatergic and GABAergic Neurons in the Rat. Eur. J. Neurosci. 2009, 29, 340–358. [Google Scholar] [CrossRef]
- Karachi, C.; Grabli, D.; Bernard, F.A.; Tandé, D.; Wattiez, N.; Belaid, H.; Bardinet, E.; Prigent, A.; Nothacker, H.-P.; Hunot, S.; et al. Cholinergic Mesencephalic Neurons Are Involved in Gait and Postural Disorders in Parkinson Disease. J. Clin. Investig. 2010, 120, 2745–2754. [Google Scholar] [CrossRef]
- Lee, M.S.; Rinne, J.O.; Marsden, C.D. The Pedunculopontine Nucleus: Its Role in the Genesis of Movement Disorders. Yonsei Med. J. 2000, 41, 167–184. [Google Scholar] [CrossRef]
- Janickova, H.; Rosborough, K.; Al-Onaizi, M.; Kljakic, O.; Guzman, M.S.; Gros, R.; Prado, M.A.M.; Prado, V.F. Deletion of the Vesicular Acetylcholine Transporter from Pedunculopontine/Laterodorsal Tegmental Neurons Modifies Gait. J. Neurochem. 2017, 140, 787–798. [Google Scholar] [CrossRef]
- Gut, N.K.; Winn, P. Deep Brain Stimulation of Different Pedunculopontine Targets in a Novel Rodent Model of Parkinsonism. J. Neurosci. 2015, 35, 4792–4803. [Google Scholar] [CrossRef] [PubMed]
- Forster, G.L.; Blaha, C.D. Pedunculopontine Tegmental Stimulation Evokes Striatal Dopamine Efflux by Activation of Acetylcholine and Glutamate Receptors in the Midbrain and Pons of the Rat. Eur. J. Neurosci. 2003, 17, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Woolf, N.J.; Hernit, M.C.; Butcher, L.L. Cholinergic and Non-Cholinergic Projections from the Rat Basal Forebrain Revealed by Combined Choline Acetyltransferase and Phaseolus Vulgaris Leucoagglutinin Immunohistochemistry. Neurosci. Lett. 1986, 66, 281–286. [Google Scholar] [CrossRef]
- Dautan, D.; Souza, A.S.; Huerta-Ocampo, I.; Valencia, M.; Assous, M.; Witten, I.B.; Deisseroth, K.; Tepper, J.M.; Bolam, J.P.; Gerdjikov, T.V.; et al. Segregated Cholinergic Transmission Modulates Dopamine Neurons Integrated in Distinct Functional Circuits. Nat. Neurosci. 2016, 19, 1025–1033. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, X.; Zhou, P.; Zhang, J.; He, W.; Yuan, T.-F. Cholinergic Tone in Ventral Tegmental Area: Functional Organization and Behavioral Implications. Neurochem. Int. 2018, 114, 127–133. [Google Scholar] [CrossRef]
- Xiang, H.-B.; Zhu, W.-Z.; Guan, X.-H.; Ye, D.-W. The Cuneiform Nucleus May Be Involved in the Regulation of Skeletal Muscle Tone by Motor Pathway: A Virally Mediated Trans-Synaptic Tracing Study in Surgically Sympathectomized Mice. Brain 2013, 136, e251. [Google Scholar] [CrossRef][Green Version]
- Ztaou, S.; Amalric, M. Contribution of Cholinergic Interneurons to Striatal Pathophysiology in Parkinson’s Disease. Neurochem Int 2019, 126, 1–10. [Google Scholar] [CrossRef]
- Lindroos, R.; Kotaleski, J.H. Predicting Complex Spikes in Striatal Projection Neurons of the Direct Pathway Following Neuromodulation by Acetylcholine and Dopamine. Eur. J. Neurosci. 2021, 53, 2117–2134. [Google Scholar] [CrossRef]
- Mena-Segovia, J.; Bolam, J.P. Rethinking the Pedunculopontine Nucleus: From Cellular Organization to Function. Neuron 2017, 94, 7–18. [Google Scholar] [CrossRef]
- Morris, R.; Martini, D.N.; Madhyastha, T.; Kelly, V.E.; Grabowski, T.J.; Nutt, J.; Horak, F. Overview of the Cholinergic Contribution to Gait, Balance and Falls in Parkinson’s Disease. Parkinsonism Relat. Disord. 2019, 63, 20–30. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, C.; van Andel, J.; Bolam, J.P.; Mena-Segovia, J. Divergent Motor Projections from the Pedunculopontine Nucleus Are Differentially Regulated in Parkinsonism. Brain Struct. Funct. 2014, 219, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Rye, D.B.; Lee, H.J.; Saper, C.B.; Wainer, B.H. Medullary and Spinal Efferents of the Pedunculopontine Tegmental Nucleus and Adjacent Mesopontine Tegmentum in the Rat. J. Comp. Neurol. 1988, 269, 315–341. [Google Scholar] [CrossRef] [PubMed]
- Shafei, M.N.; Niazmand, S.; Enayatfard, L.; Hosseini, M.; Daloee, M.H. Pharmacological Study of Cholinergic System on Cardiovascular Regulation in the Cuneiform Nucleus of Rat. Neurosci. Lett. 2013, 549, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Roseberry, T.K.; Lee, A.M.; Lalive, A.L.; Wilbrecht, L.; Bonci, A.; Kreitzer, A.C. Cell-Type-Specific Control of Brainstem Locomotor Circuits by Basal Ganglia. Cell 2016, 164, 526–537. [Google Scholar] [CrossRef]
- Caggiano, V.; Leiras, R.; Goñi-Erro, H.; Masini, D.; Bellardita, C.; Bouvier, J.; Caldeira, V.; Fisone, G.; Kiehn, O. Midbrain Circuits That Set Locomotor Speed and Gait Selection. Nature 2018, 553, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Josset, N.; Roussel, M.; Lemieux, M.; Lafrance-Zoubga, D.; Rastqar, A.; Bretzner, F. Distinct Contributions of Mesencephalic Locomotor Region Nuclei to Locomotor Control in the Freely Behaving Mouse. Curr. Biol. 2018, 28, 884–901.e3. [Google Scholar] [CrossRef]
- van der Zouwen, C.I.; Boutin, J.; Fougère, M.; Flaive, A.; Vivancos, M.; Santuz, A.; Akay, T.; Sarret, P.; Ryczko, D. Freely Behaving Mice Can Brake and Turn During Optogenetic Stimulation of the Mesencephalic Locomotor Region. Front. Neural Circuits 2021, 15, 18. [Google Scholar] [CrossRef]
- Dautan, D.; Kovács, A.; Bayasgalan, T.; Diaz-Acevedo, M.A.; Pal, B.; Mena-Segovia, J. Modulation of Motor Behavior by the Mesencephalic Locomotor Region. Cell Rep. 2021, 36, 109594. [Google Scholar] [CrossRef]
- Carvalho, M.M.; Tanke, N.; Kropff, E.; Witter, M.P.; Moser, M.-B.; Moser, E.I. A Brainstem Locomotor Circuit Drives the Activity of Speed Cells in the Medial Entorhinal Cortex. Cell Rep. 2020, 32, 108123. [Google Scholar] [CrossRef]
- Leiras, R.; Cregg, J.M.; Kiehn, O. Brainstem Circuits for Locomotion. Annu. Rev. Neurosci. 2022, 45, 63–85. [Google Scholar] [CrossRef]
- Webster, H.H.; Jones, B.E. Neurotoxic Lesions of the Dorsolateral Pontomesencephalic Tegmentum-Cholinergic Cell Area in the Cat. II. Effects upon Sleep-Waking States. Brain Res. 1988, 458, 285–302. [Google Scholar] [CrossRef]
- Jordan, L.M.; McVagh, J.R.; Noga, B.R.; Cabaj, A.M.; Majczyński, H.; Sławińska, U.; Provencher, J.; Leblond, H.; Rossignol, S. Cholinergic Mechanisms in Spinal Locomotion—Potential Target for Rehabilitation Approaches. Front. Neural Circuits 2014, 8, 132. [Google Scholar] [CrossRef]
- Garcia-Rill, E.; Skinner, R.D. The Mesencephalic Locomotor Region. I. Activation of a Medullary Projection Site. Brain Res. 1987, 411, 1–12. [Google Scholar] [CrossRef]
- Sholomenko, G.N.; Funk, G.D.; Steeves, J.D. Avian Locomotion Activated by Brainstem Infusion of Neurotransmitter Agonists and Antagonists. Exp. Brain Res. 1991, 85, 659–673. [Google Scholar] [CrossRef] [PubMed]
- Homma, Y.; Skinner, R.D.; Garcia-Rill, E. Effects of Pedunculopontine Nucleus (PPN) Stimulation on Caudal Pontine Reticular Formation (PnC) Neurons In Vitro. J. Neurophysiol. 2002, 87, 3033–3047. [Google Scholar] [CrossRef]
- Chang, S.J.; Santamaria, A.J.; Sanchez, F.J.; Villamil, L.M.; Saraiva, P.P.; Benavides, F.; Nunez-Gomez, Y.; Solano, J.P.; Opris, I.; Guest, J.D.; et al. Deep Brain Stimulation of Midbrain Locomotor Circuits in the Freely Moving Pig. Brain Stimul. 2021, 14, 467–476. [Google Scholar] [CrossRef]
- Ryczko, D.; Grätsch, S.; Alpert, M.H.; Cone, J.J.; Kasemir, J.; Ruthe, A.; Beauséjour, P.-A.; Auclair, F.; Roitman, M.F.; Alford, S.; et al. Descending Dopaminergic Inputs to Reticulospinal Neurons Promote Locomotor Movements. J. Neurosci. 2020, 40, 8478–8490. [Google Scholar] [CrossRef]
- Brocard, F.; Dubuc, R. Differential Contribution of Reticulospinal Cells to the Control of Locomotion Induced By the Mesencephalic Locomotor Region. J. Neurophysiol. 2003, 90, 1714–1727. [Google Scholar] [CrossRef]
- Smetana, R.; Juvin, L.; Dubuc, R.; Alford, S. A Parallel Cholinergic Brainstem Pathway for Enhancing Locomotor Drive. Nat. Neurosci. 2010, 13, 731–738. [Google Scholar] [CrossRef]
- Smetana, R.W.; Alford, S.; Dubuc, R. Muscarinic Receptor Activation Elicits Sustained, Recurring Depolarizations in Reticulospinal Neurons. J. Neurophysiol. 2007, 97, 3181–3192. [Google Scholar] [CrossRef]
- Mamiya, K.; Bay, K.; Skinner, R.D.; Garcia-Rill, E. Induction of Long-Lasting Depolarization in Medioventral Medulla Neurons by Cholinergic Input from the Pedunculopontine Nucleus. J. Appl. Physiol. 2005, 99, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Kow, L.; Pfaff, D.W. Responses of Medullary Reticulospinal and Other Reticular Neurons to Somatosensory and Brainstem Stimulation in Anesthetized or Freely-Moving Ovariectomized Rats with or without Estrogen Treatment. Exp. Brain Res. 1982, 47, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Drew, T.; Dubuc, R.; Rossignol, S. Discharge Patterns of Reticulospinal and Other Reticular Neurons in Chronic, Unrestrained Cats Walking on a Treadmill. J. Neurophysiol. 1986, 55, 375–401. [Google Scholar] [CrossRef]
- Le Ray, D.; Juvin, L.; Boutin, T.; Auclair, F.; Dubuc, R. A Neuronal Substrate for a State-Dependent Modulation of Sensory Inputs in the Brainstem: MLR-Induced Depression of Sensory Inputs. Eur. J. Neurosci. 2010, 32, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Viana Di Prisco, G.; Boutin, T.; Petropoulos, D.; Brocard, F.; Dubuc, R. The Trigeminal Sensory Relay to Reticulospinal Neurones in Lampreys. Neuroscience 2005, 131, 535–546. [Google Scholar] [CrossRef]
- Le Ray, D.; Brocard, F.; Dubuc, R. Muscarinic Modulation of the Trigemino-Reticular Pathway in Lampreys. J. Neurophysiol. 2004, 92, 926–938. [Google Scholar] [CrossRef]
- Dubuc, R.; Cabelguen, J.M.; Rossignol, S. Rhythmic Fluctuations of Dorsal Root Potentials and Antidromic Discharges of Primary Afferents during Fictive Locomotion in the Cat. J. Neurophysiol. 1988, 60, 2014–2036. [Google Scholar] [CrossRef]
- Barber, R.P.; Phelps, P.E.; Houser, C.R.; Crawford, G.D.; Salvaterra, P.M.; Vaughn, J.E. The Morphology and Distribution of Neurons Containing Choline Acetyltransferase in the Adult Rat Spinal Cord: An Immunocytochemical Study. J. Comp. Neurol. 1984, 229, 329–346. [Google Scholar] [CrossRef]
- Falgairolle, M.; O’Donovan, M.J. Feedback Regulation of Locomotion by Motoneurons in the Vertebrate Spinal Cord. Curr. Opin. Physiol. 2019, 8, 50–55. [Google Scholar] [CrossRef]
- Hanson, M.G.; Landmesser, L.T. Increasing the Frequency of Spontaneous Rhythmic Activity Disrupts Pool-Specific Axon Fasciculation and Pathfinding of Embryonic Spinal Motoneurons. J. Neurosci. 2006, 26, 12769–12780. [Google Scholar] [CrossRef]
- Hanson, M.G.; Landmesser, L.T. Normal Patterns of Spontaneous Activity Are Required for Correct Motor Axon Guidance and the Expression of Specific Guidance Molecules. Neuron 2004, 43, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Myers, C.P.; Lewcock, J.W.; Hanson, M.G.; Gosgnach, S.; Aimone, J.B.; Gage, F.H.; Lee, K.-F.; Landmesser, L.T.; Pfaff, S.L. Cholinergic Input Is Required during Embryonic Development to Mediate Proper Assembly of Spinal Locomotor Circuits. Neuron 2005, 46, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Rima, M.; Lattouf, Y.; Abi Younes, M.; Bullier, E.; Legendre, P.; Mangin, J.M.; Hong, E. Dynamic Regulation of the Cholinergic System in the Spinal Central Nervous System. Sci. Rep. 2020, 10, 15338. [Google Scholar] [CrossRef] [PubMed]
- Alkaslasi, M.R.; Piccus, Z.E.; Hareendran, S.; Silberberg, H.; Chen, L.; Zhang, Y.; Petros, T.J.; Le Pichon, C.E. Single Nucleus RNA-Sequencing Defines Unexpected Diversity of Cholinergic Neuron Types in the Adult Mouse Spinal Cord. Nat. Commun. 2021, 12, 2471. [Google Scholar] [CrossRef]
- Quinlan, K.A.; Buchanan, J.T. Cellular and Synaptic Actions of Acetylcholine in the Lamprey Spinal Cord. J. Neurophysiol. 2008, 100, 1020–1031. [Google Scholar] [CrossRef][Green Version]
- Roberts, A.; Li, W.-C.; Soffe, S.R.; Wolf, E. Origin of Excitatory Drive to a Spinal Locomotor Network. Brain Res. Rev. 2008, 57, 22–28. [Google Scholar] [CrossRef]
- Stepien, A.E.; Tripodi, M.; Arber, S. Monosynaptic Rabies Virus Reveals Premotor Network Organization and Synaptic Specificity of Cholinergic Partition Cells. Neuron 2010, 68, 456–472. [Google Scholar] [CrossRef]
- Zagoraiou, L.; Akay, T.; Martin, J.F.; Brownstone, R.M.; Jessell, T.M.; Miles, G.B. A Cluster of Cholinergic Premotor Interneurons Modulates Mouse Locomotor Activity. Neuron 2009, 64, 645–662. [Google Scholar] [CrossRef]
- Matzner, H.; Zelinger, M.; Cherniak, M.; Anglister, L.; Lev-Tov, A. Rhythmogenic Networks Are Potently Modulated by Activation of Muscarinic Acetylcholine Receptors in the Rodent Spinal Cord. J. Neurochem. 2021, 158, 1263–1273. [Google Scholar] [CrossRef]
- Perrins, R.; Roberts, A. Nicotinic and Muscarinic ACh Receptors in Rhythmically Active Spinal Neurones in the Xenopus Laevis Embryo. J. Physiol. 1994, 478, 221–228. [Google Scholar] [CrossRef]
- Perrins, R.; Roberts, A. Cholinergic Contribution to Excitation in a Spinal Locomotor Central Pattern Generator in Xenopus Embryos. J. Neurophysiol. 1995, 73, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, K.A.; Placas, P.G.; Buchanan, J.T. Cholinergic Modulation of the Locomotor Network in the Lamprey Spinal Cord. J. Neurophysiol. 2004, 92, 1536–1548. [Google Scholar] [CrossRef] [PubMed]
- Blake, J.F.; Evans, R.H.; Smith, D.A. The Effect of Nicotine on Motoneurones of the Immature Rat Spinal Cord in Vitro. Br. J. Pharmacol. 1987, 90, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Perrins, R.; Roberts, A. Cholinergic and Electrical Motoneuron-to-Motoneuron Synapses Contribute to on-Cycle Excitation during Swimming in Xenopus Embryos. J. Neurophysiol. 1995, 73, 1005–1012. [Google Scholar] [CrossRef]
- Homma, S. Physiology and Pharmacology of Putative Transmitters in Lamprey Central Nervous System. Prog. Neurobiol. 1983, 20, 287–311. [Google Scholar] [CrossRef]
- Martin, A.R.; Wickelgren, W.O.; Berànek, R. Effects of Iontophoretically Applied Drugs on Spinal Interneurones of the Lamprey. J. Physiol. 1970, 207, 653–665. [Google Scholar] [CrossRef]
- Ogier, R.; Liu, X.; Tribollet, E.; Bertrand, D.; Raggenbass, M. Identified Spinal Motoneurons of Young Rats Possess Nicotinic Acetylcholine Receptors of the Heteromeric Family. Eur. J. Neurosci. 2004, 20, 2591–2597. [Google Scholar] [CrossRef]
- Cullheim, S.; Lipsenthal, L.; Burke, R.E. Direct Monosynaptic Contacts between Type-Identified a-Motoneurons in the Cat. Brain Res. 1984, 308, 196–199. [Google Scholar] [CrossRef]
- Cullheim, S.; Kellerth, J.-O.; Conradi, S. Evidence for Direct Synaptic Interconnections between Cat Spinal A-Motoneurones via the Recurrent Axon Collaterals: A Morphological Study Using Intracellular Injection of Horseradish Peroxidase. Brain Res. 1977, 132, 1–10. [Google Scholar] [CrossRef]
- Perrins, R.; Roberts, A. Cholinergic and Electrical Synapses between Synergistic Spinal Motoneurones in the Xenopus Laevis Embryo. J. Physiol. 1995, 485, 135–144. [Google Scholar] [CrossRef]
- Mentis, G.Z.; Alvarez, F.J.; Bonnot, A.; Richards, D.S.; Gonzalez-Forero, D.; Zerda, R.; O’Donovan, M.J. Noncholinergic Excitatory Actions of Motoneurons in the Neonatal Mammalian Spinal Cord. Proc. Natl. Acad. Sci. USA 2005, 102, 7344–7349. [Google Scholar] [CrossRef] [PubMed]
- Lamotte d’Incamps, B.; Ascher, P. High Affinity and Low Affinity Heteromeric Nicotinic Acetylcholine Receptors at Central Synapses. J. Physiol. 2014, 592, 4131–4136. [Google Scholar] [CrossRef] [PubMed]
- Lamotte d’Incamps, B.; Ascher, P. Four Excitatory Postsynaptic Ionotropic Receptors Coactivated at the Motoneuron-Renshaw Cell Synapse. J. Neurosci. 2008, 28, 14121–14131. [Google Scholar] [CrossRef] [PubMed]
- Szczupak, L. Recurrent Inhibition in Motor Systems, a Comparative Analysis. J. Physiol. Paris 2014, 108, 148–154. [Google Scholar] [CrossRef]
- Naser, P.V.; Kuner, R. Molecular, Cellular and Circuit Basis of Cholinergic Modulation of Pain. Neuroscience 2018, 387, 135–148. [Google Scholar] [CrossRef]
- Ribeiro-da-Silva, A.; Cuello, A.C. Choline Acetyltransferase-Immunoreactive Profiles Are Presynaptic to Primary Sensory Fibers in the Rat Superficial Dorsal Horn. J. Comp. Neurol. 1990, 295, 370–384. [Google Scholar] [CrossRef]
- Steen, K.H.; Reeh, P.W. Actions of Cholinergic Agonists and Antagonists on Sensory Nerve Endings in Rat Skin, in Vitro. J. Neurophysiol. 1993, 70, 397–405. [Google Scholar] [CrossRef]
- Genzen, J.R.; Van Cleve, W.; McGehee, D.S. Dorsal Root Ganglion Neurons Express Multiple Nicotinic Acetylcholine Receptor Subtypes. J. Neurophysiol. 2001, 86, 1773–1782. [Google Scholar] [CrossRef]
- Shreckengost, J.; Halder, M.; Mena-Avila, E.; Garcia-Ramirez, D.L.; Quevedo, J.; Hochman, S. Nicotinic Receptor Modulation of Primary Afferent Excitability with Selective Regulation of Aδ-Mediated Spinal Actions. J. Neurophysiol. 2021, 125, 568–585. [Google Scholar] [CrossRef]
- Nicoll, R.A.; Alger, B. Presynaptic Inhibition: Transmitter and Ionic Mechanisms—PubMed. Int. Rev. Neurobiol. 1979, 21, 217–258. [Google Scholar]
- Rudomin, P.; Schmidt, R.F. Presynaptic Inhibition in the Vertebrate Spinal Cord Revisited. Exp. Brain Res. 1999, 129, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Comitato, A.; Bardoni, R. Presynaptic Inhibition of Pain and Touch in the Spinal Cord: From Receptors to Circuits. Int. J. Mol. Sci. 2021, 22, 414. [Google Scholar] [CrossRef]
- Barron, D.H.; Matthews, B.H.C. Intermittent Conduction in the Spinal Cord. J. Physiol. 1935, 85, 73–103. [Google Scholar] [CrossRef] [PubMed]
- Dubuc, R.; Cabelguen, J.-M.; Rossignol, S. Rhythmic Antidromic Discharges of Single Primary Afferents Recorded in Cut Dorsal Root Filaments during Locomotion in the Cat. Brain Res. 1985, 359, 375–378. [Google Scholar] [CrossRef]
- Decima, E.E.; Goldberg, L.J. Centrifugal Dorsal Root Discharges Induced by Motoneurone Activation. J. Physiol. 1970, 207, 103–118. [Google Scholar] [CrossRef]
- Cattaert, D.; Le Ray, D. Direct Glutamate-Mediated Presynaptic Inhibition of Sensory Afferents by the Postsynaptic Motor Neurons: Glutamate-Mediated Presynaptic Inhibition. Eur. J. Neurosci. 1998, 10, 3737–3746. [Google Scholar] [CrossRef] [PubMed]
- Le Ray, D.; Cattaert, D. Active Motor Neurons Potentiate Their Own Sensory Inputs via Glutamate-Induced Long-Term Potentiation. J. Neurosci. 1999, 19, 1473–1483. [Google Scholar] [CrossRef]
- Mille, T.; Quilgars, C.; Cazalets, J.-R.; Bertrand, S.S. Acetylcholine and Spinal Locomotor Networks: The Insider. Physiol. Rep. 2021, 9, e14736. [Google Scholar] [CrossRef]
- Witts, E.C.; Zagoraiou, L.; Miles, G.B. Anatomy and Function of Cholinergic C Bouton Inputs to Motor Neurons. J. Anat. 2014, 224, 52–60. [Google Scholar] [CrossRef]
- Cowley, K.C.; Schmidt, B.J. A Comparison of Motor Patterns Induced by N-Methyl-D-Aspartate, Acetylcholine and Serotonin in the in Vitro Neonatal Rat Spinal Cord. Neurosci. Lett. 1994, 171, 147–150. [Google Scholar] [CrossRef]
- Nascimento, F.; Spindler, L.R.; Miles, G.B. Balanced Cholinergic Modulation of Spinal Locomotor Circuits via M2 and M3 Muscarinic Receptors. Sci. Rep. 2019, 9, 14051. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, S.S.; Cazalets, J.R. Cholinergic Partition Cells and Lamina X Neurons Induce a Muscarinic-Dependent Short-Term Potentiation of Commissural Glutamatergic Inputs in Lumbar Motoneurons. Front. Neural Circuits 2011, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Bertuzzi, M.; Ampatzis, K. Spinal Cholinergic Interneurons Differentially Control Motoneuron Excitability and Alter the Locomotor Network Operational Range. Sci. Rep. 2018, 8, 1988. [Google Scholar] [CrossRef] [PubMed]
- Alaburda, A.; Perrier, J.-F.; Hounsgaard, J. An M-like Outward Current Regulates the Excitability of Spinal Motoneurones in the Adult Turtle. J. Physiol. 2002, 540, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Chevallier, S.; Nagy, F.; Cabelguen, J.-M. Cholinergic Control of Excitability of Spinal Motoneurones in the Salamander: Cholinergic Control of Motoneurone Excitability in Salamander. J. Physiol. 2006, 570, 525–540. [Google Scholar] [CrossRef] [PubMed]
- Miles, G.B.; Hartley, R.; Todd, A.J.; Brownstone, R.M. Spinal Cholinergic Interneurons Regulate the Excitability of Motoneurons during Locomotion. Proc. Natl. Acad. Sci. USA 2007, 104, 2448–2453. [Google Scholar] [CrossRef]
- Guertin, P.A.; Hounsgaard, J. L-Type Calcium Channels but Not N-Methyl-d-Aspartate Receptor Channels Mediate Rhythmic Activity Induced by Cholinergic Agonist in Motoneurons from Turtle Spinal Cord Slices. Neurosci. Lett. 1999, 261, 81–84. [Google Scholar] [CrossRef]
- Svirskis, G.; Hounsgaard, J. Transmitter Regulation of Plateau Properties in Turtle Motoneurons. J. Neurophysiol. 1998, 79, 45–50. [Google Scholar] [CrossRef]
- Sourioux, M.; Bertrand, S.S.; Cazalets, J.-R. Cholinergic-Mediated Coordination of Rhythmic Sympathetic and Motor Activities in the Newborn Rat Spinal Cord. PLOS Biol. 2018, 16, e2005460. [Google Scholar] [CrossRef]
- Fok, M.; Stein, R.B. Effects of Cholinergic and Noradrenergic Agents on Locomotion in the Mudpuppy (Necturus Maculatus). Exp. Brain Res. 2002, 145, 498–504. [Google Scholar] [CrossRef]
- Lambert, F.M.; Cardoit, L.; Courty, E.; Bougerol, M.; Thoby-Brisson, M.; Simmers, J.; Tostivint, H.; Le Ray, D. Functional Limb Muscle Innervation Prior to Cholinergic Transmitter Specification during Early Metamorphosis in Xenopus. eLife 2018, 7, e30693. [Google Scholar] [CrossRef] [PubMed]
- Arias, H.R.; Targowska-Duda, K.M.; García-Colunga, J.; Ortells, M.O. Is the Antidepressant Activity of Selective Serotonin Reuptake Inhibitors Mediated by Nicotinic Acetylcholine Receptors? Molecules 2021, 26, 2149. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.A.; Bassi, C.; Saunders, M.E.; Nechanitzky, R.; Morgado-Palacin, I.; Zheng, C.; Mak, T.W. Beyond Neurotransmission: Acetylcholine in Immunity and Inflammation. J. Intern. Med. 2020, 287, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Ohkawara, B.; Ito, M.; Ohno, K. Secreted Signaling Molecules at the Neuromuscular Junction in Physiology and Pathology. IJMS 2021, 22, 2455. [Google Scholar] [CrossRef]
- Hamani, C.; Aziz, T.; Bloem, B.R.; Brown, P.; Chabardes, S.; Coyne, T.; Foote, K.; Garcia-Rill, E.; Hirsch, E.C.; Lozano, A.M.; et al. Pedunculopontine Nucleus Region Deep Brain Stimulation in Parkinson Disease: Surgical Anatomy and Terminology. Stereotact Funct. Neurosurg. 2016, 94, 298–306. [Google Scholar] [CrossRef]
- Hamani, C.; Lozano, A.M.; Mazzone, P.A.M.; Moro, E.; Hutchison, W.; Silburn, P.A.; Zrinzo, L.; Alam, M.; Goetz, L.; Pereira, E.; et al. Pedunculopontine Nucleus Region Deep Brain Stimulation in Parkinson Disease: Surgical Techniques, Side Effects, and Postoperative Imaging. Stereotact Funct. Neurosurg. 2016, 94, 307–319. [Google Scholar] [CrossRef]
- Grabli, D.; Karachi, C.; Folgoas, E.; Monfort, M.; Tande, D.; Clark, S.; Civelli, O.; Hirsch, E.C.; Francois, C. Gait Disorders in Parkinsonian Monkeys with Pedunculopontine Nucleus Lesions: A Tale of Two Systems. J. Neurosci. 2013, 33, 11986–11993. [Google Scholar] [CrossRef]
- Müller, M.L.T.M.; Bohnen, N.I. Cholinergic Dysfunction in Parkinson’s Disease. Curr. Neurol. Neurosci. Rep. 2013, 13, 377. [Google Scholar] [CrossRef]
- Stieglitz, L.H.; Hofer, S.; Bolliger, M.; Oertel, M.F.; Filli, L.; Willi, R.; Cathomen, A.; Meyer, C.; Schubert, M.; Hubli, M.; et al. Deep Brain Stimulation for Locomotion in Incomplete Human Spinal Cord Injury (DBS—SCI): Protocol of a Prospective One—Armed Multi—Centre Study. BJM Open 2021, 11, e047670. [Google Scholar] [CrossRef]
- Bachmann, L.C.; Matis, A.; Lindau, N.T.; Felder, P.; Gullo, M.; Schwab, M.E. Deep Brain Stimulation of the Midbrain Locomotor Region Improves Paretic Hindlimb Function After Spinal Cord Injury in Rats. Sci. Transl. Med. 2013, 5, 208ra146. [Google Scholar] [CrossRef]
- Hofer, A.-S.; Scheuber, M.; Sartori, A.; Good, N.; Stalder, S.; Hammer, N.; Fricke, K.; Schalbetter, S.; Engmann, A.; Weber, R.; et al. Stimulation of the Cuneiform Nucleus Enables Training and Boosts Recovery after Spinal Cord Injury. Brain 2022, awac184. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Ray, D.; Bertrand, S.S.; Dubuc, R. Cholinergic Modulation of Locomotor Circuits in Vertebrates. Int. J. Mol. Sci. 2022, 23, 10738. https://doi.org/10.3390/ijms231810738
Le Ray D, Bertrand SS, Dubuc R. Cholinergic Modulation of Locomotor Circuits in Vertebrates. International Journal of Molecular Sciences. 2022; 23(18):10738. https://doi.org/10.3390/ijms231810738
Chicago/Turabian StyleLe Ray, Didier, Sandrine S. Bertrand, and Réjean Dubuc. 2022. "Cholinergic Modulation of Locomotor Circuits in Vertebrates" International Journal of Molecular Sciences 23, no. 18: 10738. https://doi.org/10.3390/ijms231810738
APA StyleLe Ray, D., Bertrand, S. S., & Dubuc, R. (2022). Cholinergic Modulation of Locomotor Circuits in Vertebrates. International Journal of Molecular Sciences, 23(18), 10738. https://doi.org/10.3390/ijms231810738




